Synergistic Anticancer Effects of Fibroblast Growth Factor Receptor Inhibitor and Cannabidiol in Colorectal Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Reagents and Antibodies
2.3. Small Interfering RNA (siRNA)
2.4. WST-1 Assay
2.5. Western Blotting
2.6. Morphological Transformation Analysis
2.7. Flow Cytometry Analysis of Cell Apoptosis
2.8. RNA Interference Assay
2.9. RNA Sequencing
2.10. Statistical Analysis
3. Results
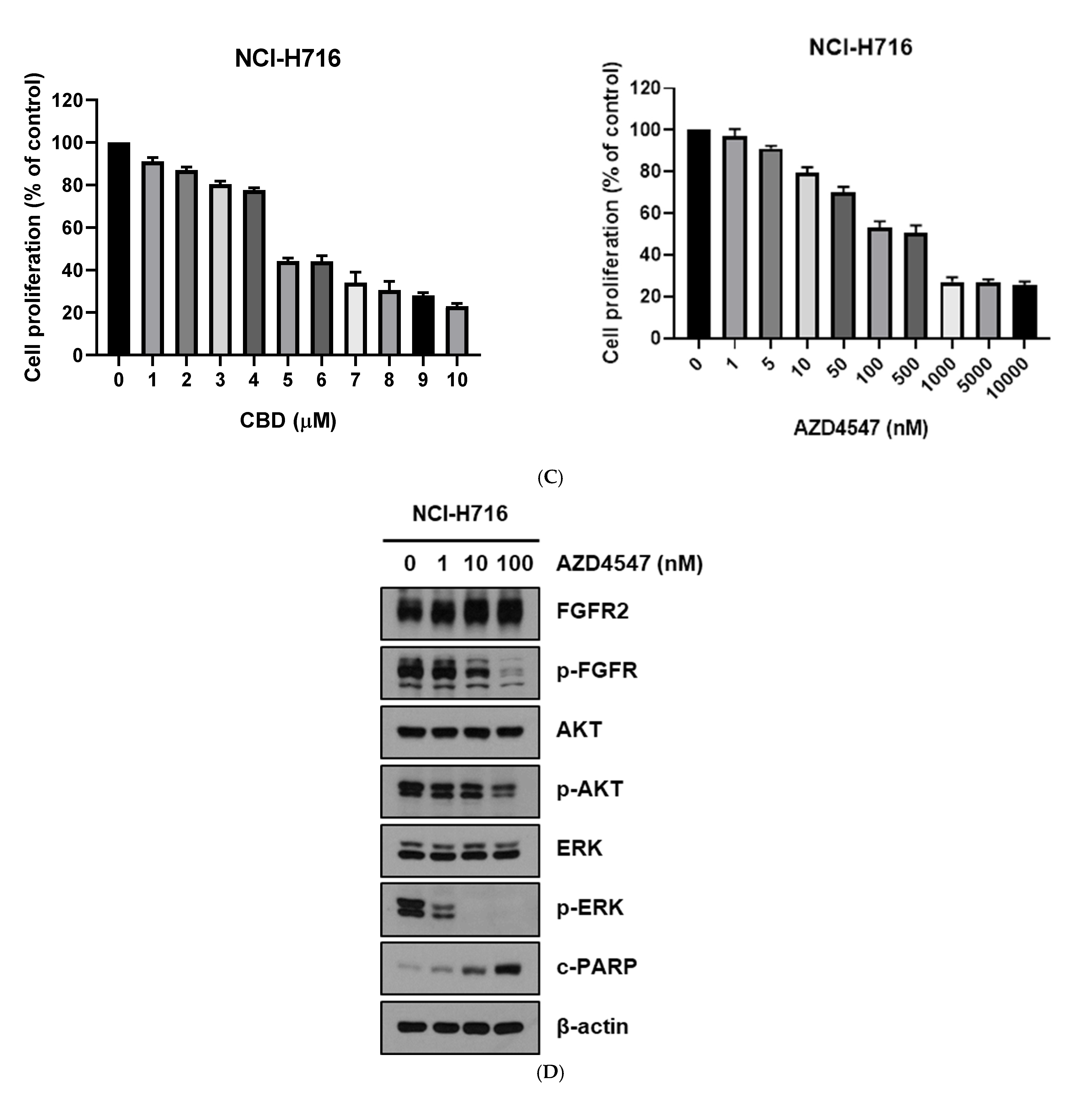
3.1. FGFR2 Expression and Response to CBD and FGFR Inhibitor in Colorectal Cancer Cells
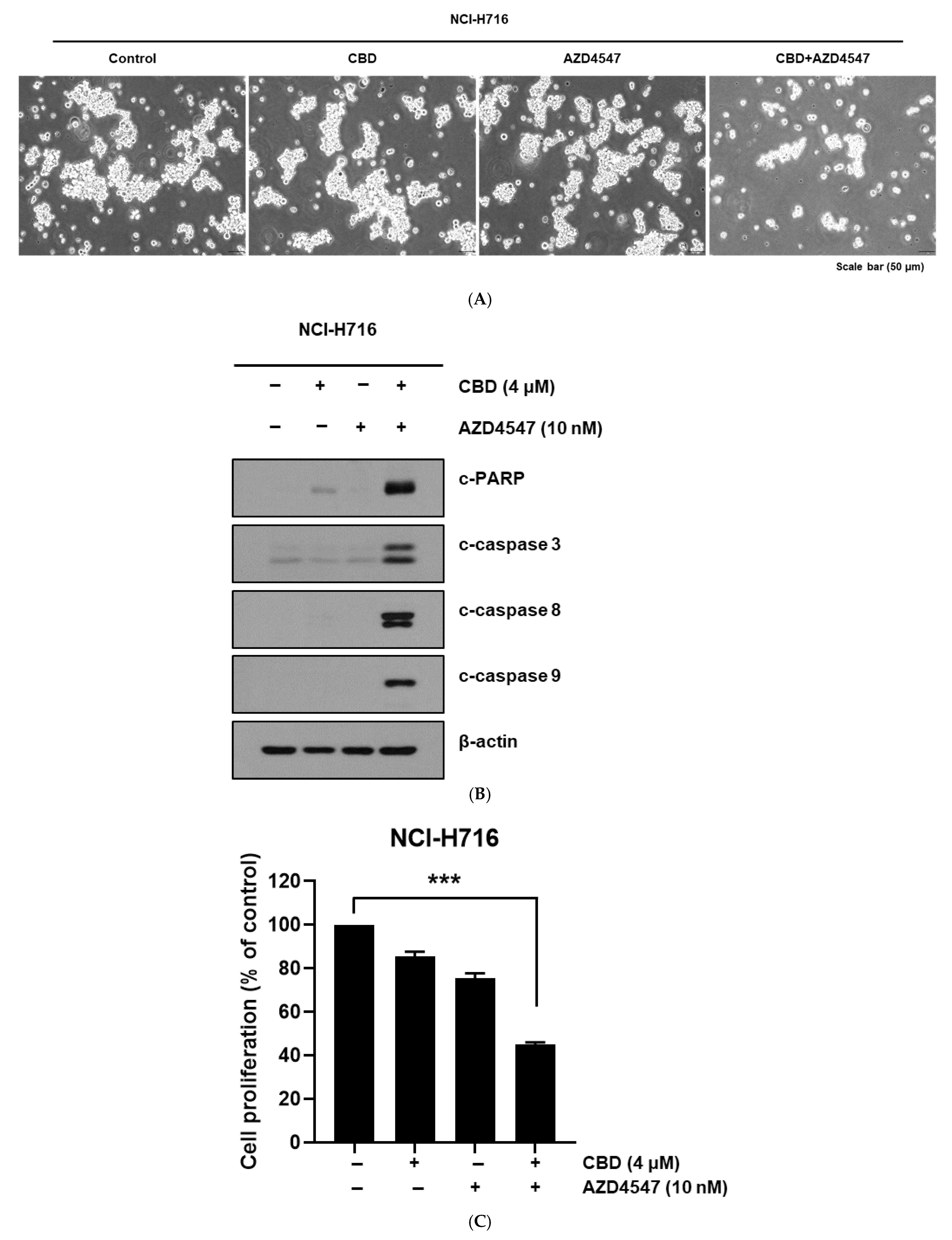
3.2. Cytotoxic Assay of CBD and FGFR Inhibitor in Colorectal Cancer Cells
3.3. Enhanced Apoptotic Response from Combined Treatment
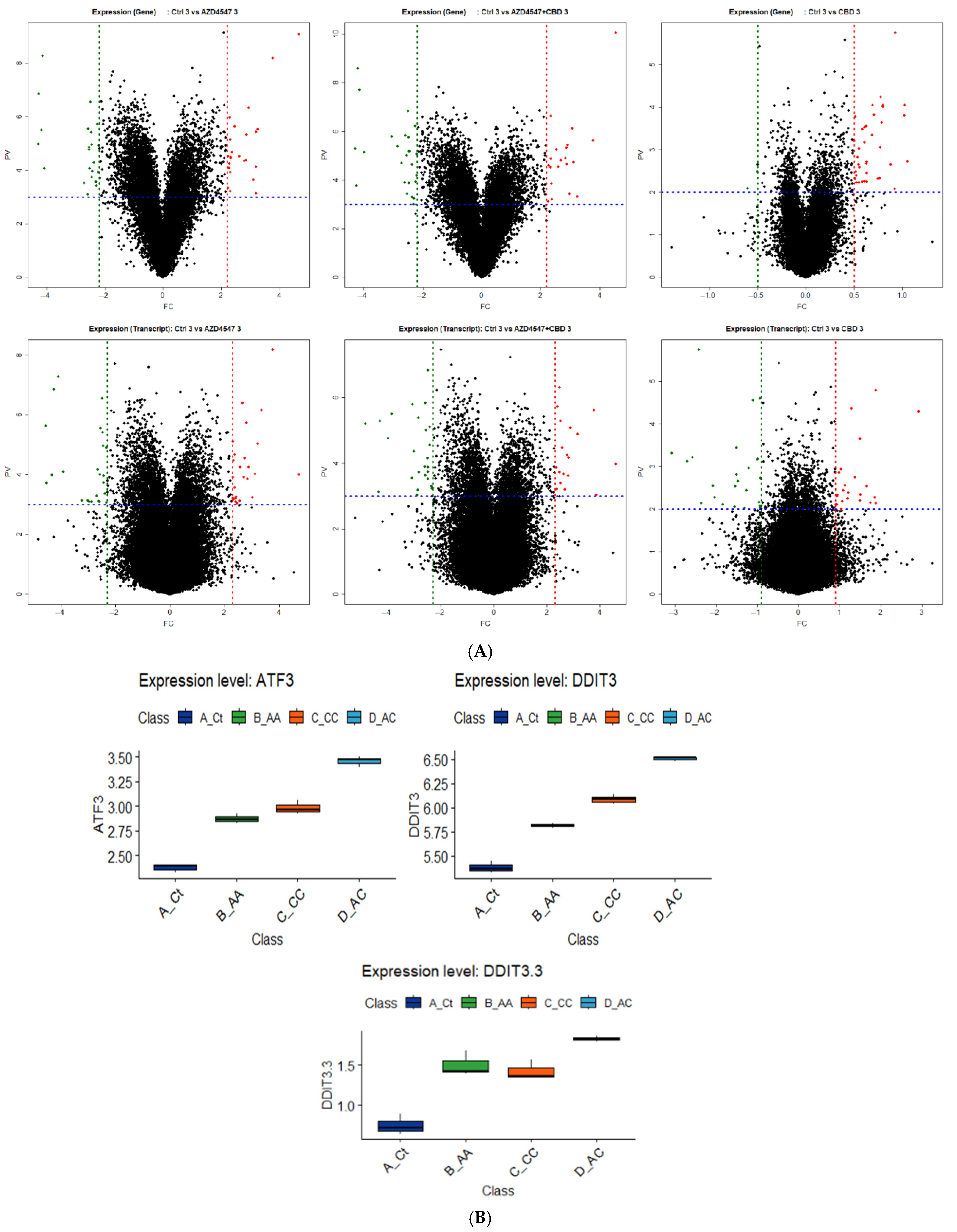
3.4. RNA Sequencing and Analysis Insights to Examine Mechanisms Underlying Combination Synergy in Therapy
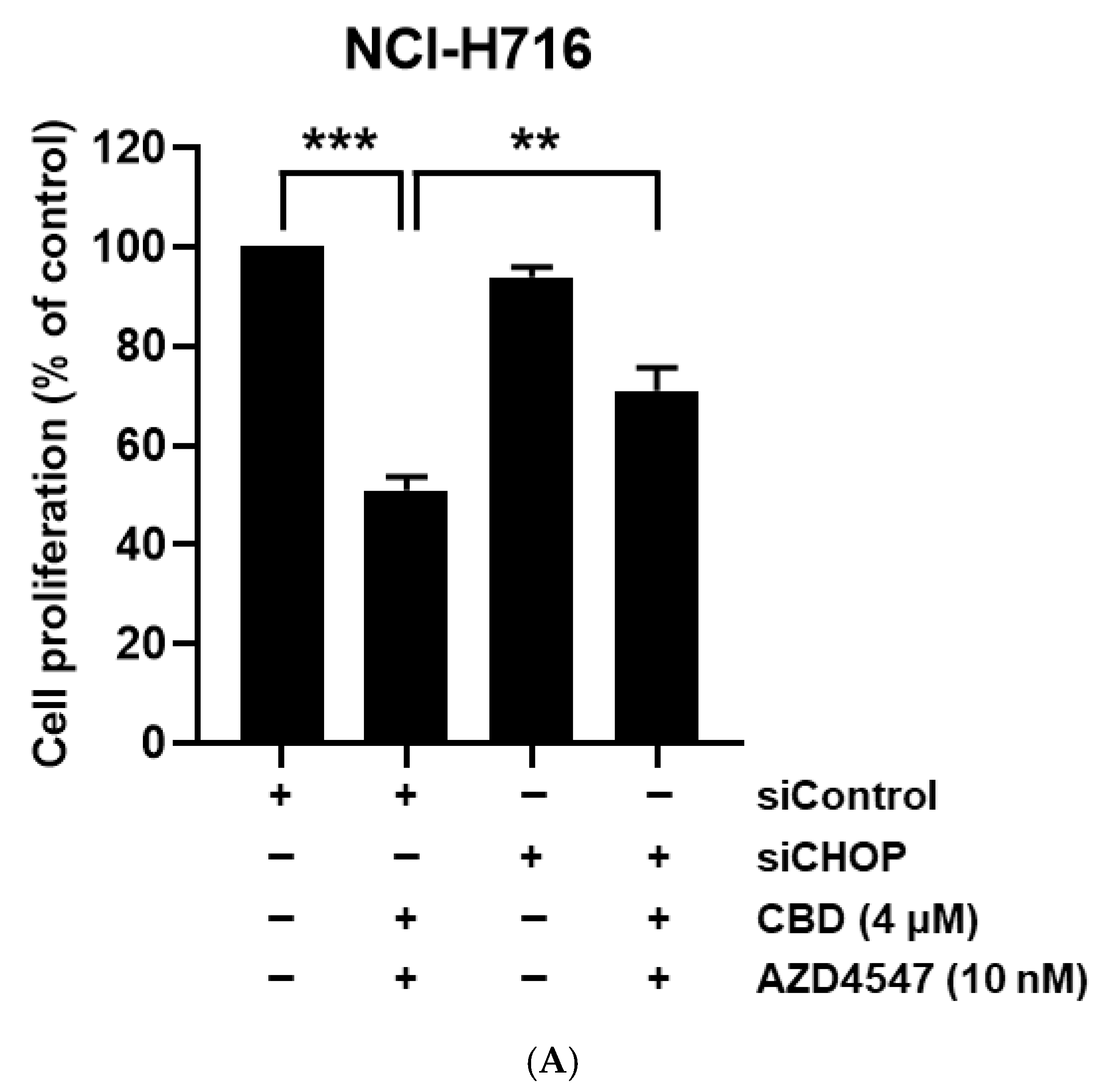
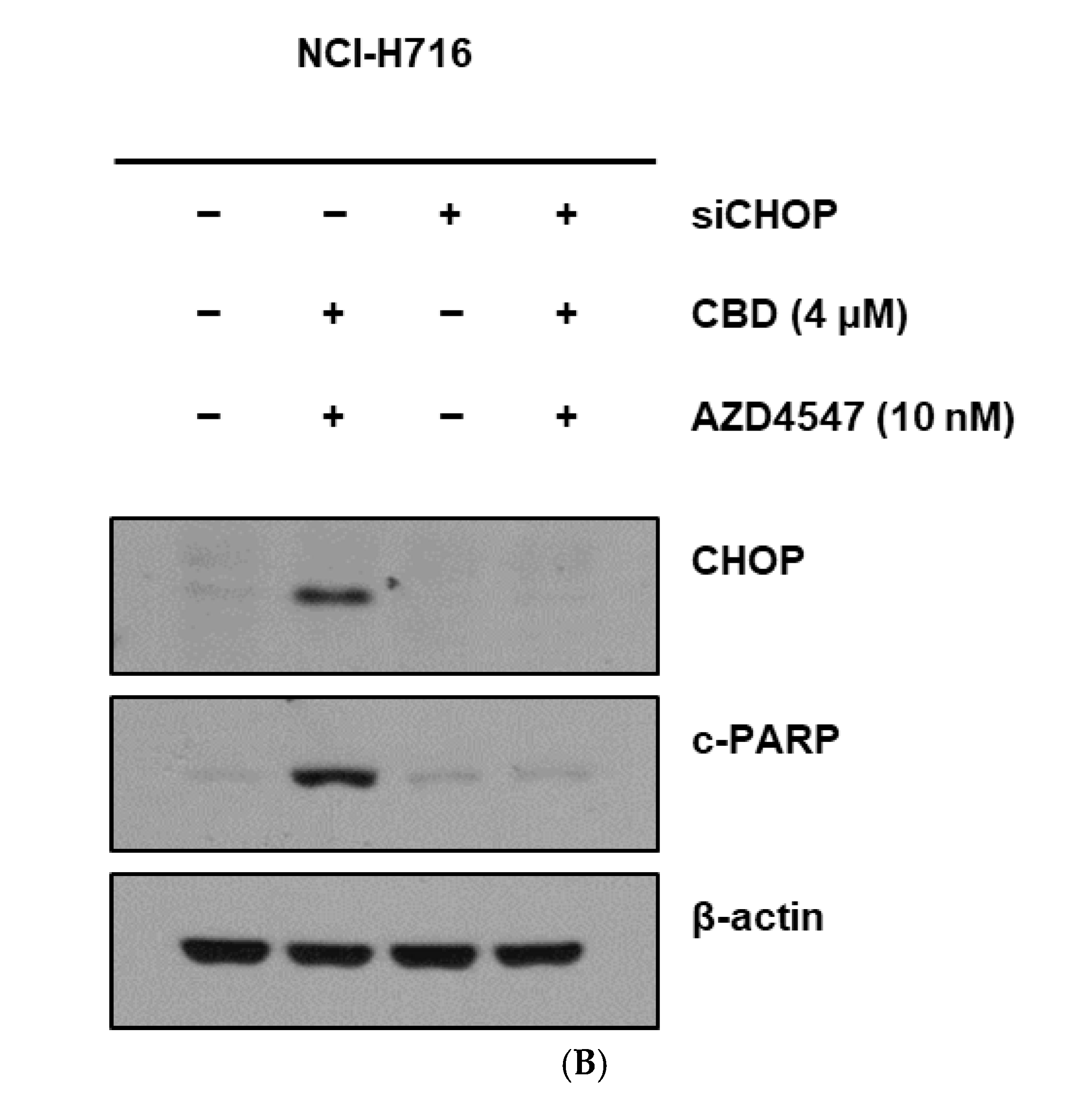
3.5. Synergistic Cell Death Induced by Upstream Activation of ER Stress
4. Discussion
4.1. Comprehensive Cancer Treatment Strategies
4.2. Advances in CRC Molecular Understanding
4.3. FGFR’s Role and Limitations in CRC Treatment
4.4. CBD’s Emerging Role in CRC Treatment
4.5. Rationale for Combining CBD with FGFR Inhibition
4.6. Mechanistic Findings from This Study
4.7. Limitations and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CRC | Colorectal cancer |
| FGF | Fibroblast Growth Factor |
| FGFR | Fibroblast Growth Factor Receptor |
| CBD | Cannabidiol |
| ER | Endoplasmic Reticulum |
| CHOP | C/EBP Homologous Protein (also known as DDIT3) |
| siRNA | Small interfering RNA |
| WST-1 | Water-Soluble Tetrazolium salt-1 |
| FBS | Fetal Bovine Serum |
| PI | Propidium Iodide |
| ATCC | American Type Culture Collection |
| KCLB | Korea Cell Line Bank |
| RIPA | Radioimmunoprecipitation Assay (buffer) |
| RT | Room Temperature |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| NGS | Next-Generation Sequencing |
| FDA | Food and Drug Administration |
| MAPK | Mitogen-Activated Protein Kinase |
| ROS | Reactive Oxygen Species |
| ANOVA | Analysis of Variance |
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Aigner, F.; Pratschke, J.; Schmelzle, M. Oligometastatic Disease in Colorectal Cancer–How to Proceed? Visc. Med. 2017, 33, 23–28. [Google Scholar] [CrossRef]
- Buisman, F.E.; Giardiello, D.; Kemeny, N.E.; Steyerberg, E.W.; Höppener, D.J.; Galjart, B.; Nierop, P.M.H.; Balachandran, V.P.; Cercek, A.; Drebin, J.A.; et al. Predicting 10-year survival after resection of colorectal liver metastases; an international study including biomarkers and perioperative treatment. Eur. J. Cancer 2022, 168, 25–33. [Google Scholar] [CrossRef]
- Gkikas, A.; Kakos, C.; Lampridis, S.; Godolphin, P.J.; Patrini, D. Preoperative prognostic factors for 5-year survival following pulmonary metastasectomy from colorectal cancer: A systematic review and meta-analysis. Eur. J. Cardiothorac. Surg. 2023, 63, ezad059. [Google Scholar] [CrossRef]
- Ohishi, T.; Kaneko, M.K.; Yoshida, Y.; Takashima, A.; Kato, Y.; Kawada, M. Current Targeted Therapy for Metastatic Colorectal Cancer. Int. J. Mol. Sci. 2023, 24, 1702. [Google Scholar] [CrossRef]
- Ciardiello, F.; Ciardiello, D.; Martini, G.; Napolitano, S.; Tabernero, J.; Cervantes, A. Clinical management of metastatic colorectal cancer in the era of precision medicine. CA Cancer J. Clin. 2022, 72, 372–401. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Su, N.; Yang, J.; Tan, Q.; Huang, S.; Jin, M.; Ni, Z.; Zhang, B.; Zhang, D.; Luo, F.; et al. FGF/FGFR signaling in health and disease. Signal Transduct. Target. Ther. 2020, 5, 181. [Google Scholar] [CrossRef]
- Du, S.; Zhang, Y.; Xu, J. Current progress in cancer treatment by targeting FGFR signaling. Cancer Biol. Med. 2023, 20, 490–499. [Google Scholar] [CrossRef]
- Helsten, T.; Elkin, S.; Arthur, E.; Tomson, B.N.; Carter, J.; Kurzrock, R. The FGFR Landscape in Cancer: Analysis of 4853 Tumors by Next-Generation Sequencing. Clin. Cancer Res. 2016, 22, 259–267. [Google Scholar] [CrossRef]
- Szymczyk, J.; Sluzalska, K.D.; Materla, I.; Opalinski, L.; Otlewski, J.; Zakrzewska, M. FGF/FGFR-Dependent Molecular Mechanisms Underlying Anti-Cancer Drug Resistance. Cancers 2021, 13, 5796. [Google Scholar] [CrossRef] [PubMed]
- Touat, M.; Ileana, E.; Postel-Vinay, S.; André, F.; Soria, J.C. Targeting FGFR signaling in cancer. Clin. Cancer Res. 2015, 21, 2684–2694. [Google Scholar] [CrossRef] [PubMed]
- Mangal, N.; Erridge, S.; Habib, N.; Sadanandam, A.; Reebye, V.; Sodergren, M.H. Cannabinoids in the landscape of cancer. J. Cancer Res. Clin. Oncol. 2021, 147, 2507–2534. [Google Scholar] [CrossRef]
- Seltzer, E.S.; Watters, A.K.; MacKenzie, D., Jr.; Granat, L.M.; Zhang, D. Cannabidiol (CBD) as a Promising Anti-Cancer Drug. Cancers 2020, 12, 3203. [Google Scholar] [CrossRef]
- Jeong, S.; Yun, H.K.; Jeong, Y.A.; Jo, M.J.; Kang, S.H.; Kim, J.L.; Kim, D.Y.; Park, S.H.; Kim, B.R.; Na, Y.J.; et al. Cannabidiol-induced apoptosis is mediated by activation of Noxa in human colorectal cancer cells. Cancer Lett. 2019, 447, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.L.; Kim, B.R.; Kim, D.Y.; Jeong, Y.A.; Jeong, S.; Na, Y.J.; Park, S.H.; Yun, H.K.; Jo, M.J.; Kim, B.G.; et al. Cannabidiol Enhances the Therapeutic Effects of TRAIL by Upregulating DR5 in Colorectal Cancer. Cancers 2019, 11, 642. [Google Scholar] [CrossRef]
- Kim, N.Y.; Mohan, C.D.; Sethi, G.; Ahn, K.S. Cannabidiol activates MAPK pathway to induce apoptosis, paraptosis, and autophagy in colorectal cancer cells. J. Cell Biochem. 2024, 125, e30537. [Google Scholar] [CrossRef]
- Li, B.; Pi, Z.; Liu, L.; Zhang, B.; Huang, X.; Hu, P.; Chevet, E.; Yi, P.; Liu, J. FGF-2 prevents cancer cells from ER stress-mediated apoptosis via enhancing proteasome-mediated Nck degradation. Biochem. J. 2013, 452, 139–145. [Google Scholar] [CrossRef]
- Guler, I.; Askan, G.; Klostergaard, J.; Sahin, I.H. Precision medicine for metastatic colorectal cancer: An evolving era. Expert. Rev. Gastroenterol. Hepatol. 2019, 13, 919–931. [Google Scholar] [CrossRef]
- Grothey, A.; Van Cutsem, E.; Sobrero, A.; Siena, S.; Falcone, A.; Ychou, M.; Humblet, Y.; Bouché, O.; Mineur, L.; Barone, C.; et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): An international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013, 381, 303–312. [Google Scholar] [CrossRef]
- Matsuda, Y.; Ishiwata, T.; Yamahatsu, K.; Kawahara, K.; Hagio, M.; Peng, W.-X.; Yamamoto, T.; Nakazawa, N.; Seya, T.; Ohaki, Y.; et al. Overexpressed fibroblast growth factor receptor 2 in the invasive front of colorectal cancer: A potential therapeutic target in colorectal cancer. Cancer Lett. 2011, 309, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Huang, T.; Zou, Q.; Liu, D.; Wang, Y.; Tan, X.; Wei, Y.; Qiu, H. FGFR2 Promotes Expression of PD-L1 in Colorectal Cancer via the JAK/STAT3 Signaling Pathway. J. Immunol. 2019, 202, 3065–3075. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shi, Y.; Zhang, X.; Li, P.; Ma, L.; Hu, P.; Xu, L.; Dai, Y.; Xia, S.; Qiu, H. FGFR2 upregulates PAI-1 via JAK2/STAT3 signaling to induce M2 polarization of macrophages in colorectal cancer. Biochim. Biophys. Acta Mol. Basis Dis. 2023, 1869, 166665. [Google Scholar] [CrossRef]
- Mathur, A.; Ware, C.; Davis, L.; Gazdar, A.; Pan, B.S.; Lutterbach, B. FGFR2 is amplified in the NCI-H716 colorectal cancer cell line and is required for growth and survival. PLoS ONE 2014, 9, e98515. [Google Scholar] [CrossRef]
- Sun, X.; Zhou, L.; Wang, Y.; Deng, G.; Cao, X.; Ke, B.; Wu, X.; Gu, Y.; Cheng, H.; Xu, Q.; et al. Single-cell analyses reveal cannabidiol rewires tumor microenvironment via inhibiting alternative activation of macrophage and synergizes with anti-PD-1 in colon cancer. J. Pharm. Anal. 2023, 13, 726–744. [Google Scholar] [CrossRef]
- Lee, H.-S.; Tamia, G.; Song, H.-J.; Amarakoon, D.; Wei, C.-I.; Lee, S.-H. Cannabidiol exerts anti-proliferative activity via a cannabinoid receptor 2-dependent mechanism in human colorectal cancer cells. Int. Immunopharmacol. 2022, 108, 108865. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Dezfouli, A.B.; Khosravi, M.; Sievert, W.; Stangl, S.; Schwab, M.; Wu, Z.; Steiger, K.; Ma, H.; Multhoff, G. Cannabidiol-induced crosstalk of apoptosis and macroautophagy in colorectal cancer cells involves p53 and Hsp70. Cell Death Discov. 2023, 9, 286. [Google Scholar] [CrossRef]
- Warrier, M.; Paules, E.M.; Silva-Gomez, J.; Friday, W.B.; Bramlett, F.; Kim, H.; Zhang, K.; Trujillo-Gonzalez, I. Homocysteine-induced endoplasmic reticulum stress activates FGF21 and is associated with browning and atrophy of white adipose tissue in Bhmt knockout mice. Heliyon 2023, 9, e13216. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ju, Y.; Kim, B.G.; Gim, J.-A.; Bong, J.W.; Cheong, C.O.; Oh, S.C.; Kang, S.H.; Min, B.W.; Lee, S.I. Synergistic Anticancer Effects of Fibroblast Growth Factor Receptor Inhibitor and Cannabidiol in Colorectal Cancer. Nutrients 2025, 17, 2609. https://doi.org/10.3390/nu17162609
Ju Y, Kim BG, Gim J-A, Bong JW, Cheong CO, Oh SC, Kang SH, Min BW, Lee SI. Synergistic Anticancer Effects of Fibroblast Growth Factor Receptor Inhibitor and Cannabidiol in Colorectal Cancer. Nutrients. 2025; 17(16):2609. https://doi.org/10.3390/nu17162609
Chicago/Turabian StyleJu, Yeonuk, Bu Gyeom Kim, Jeong-An Gim, Jun Woo Bong, Chin Ock Cheong, Sang Cheul Oh, Sang Hee Kang, Byung Wook Min, and Sun Il Lee. 2025. "Synergistic Anticancer Effects of Fibroblast Growth Factor Receptor Inhibitor and Cannabidiol in Colorectal Cancer" Nutrients 17, no. 16: 2609. https://doi.org/10.3390/nu17162609
APA StyleJu, Y., Kim, B. G., Gim, J.-A., Bong, J. W., Cheong, C. O., Oh, S. C., Kang, S. H., Min, B. W., & Lee, S. I. (2025). Synergistic Anticancer Effects of Fibroblast Growth Factor Receptor Inhibitor and Cannabidiol in Colorectal Cancer. Nutrients, 17(16), 2609. https://doi.org/10.3390/nu17162609







