Iron Overload Accelerates Aging-Associated Kidney Injury in Mice: Implications for Iron Supplementation in the Elderly
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Tissue Preparation
2.3. Perls’ Prussian Blue Staining
2.4. Periodic Acid–Schiff (PAS) Stain
2.5. Masson’s Trichrome Staining
2.6. Transmission Electron Microscopy (TEM)
2.7. Statistical Analysis
3. Results
3.1. Similar Increases in Serum Iron Markers Between the Young and Old Iron Overload Groups
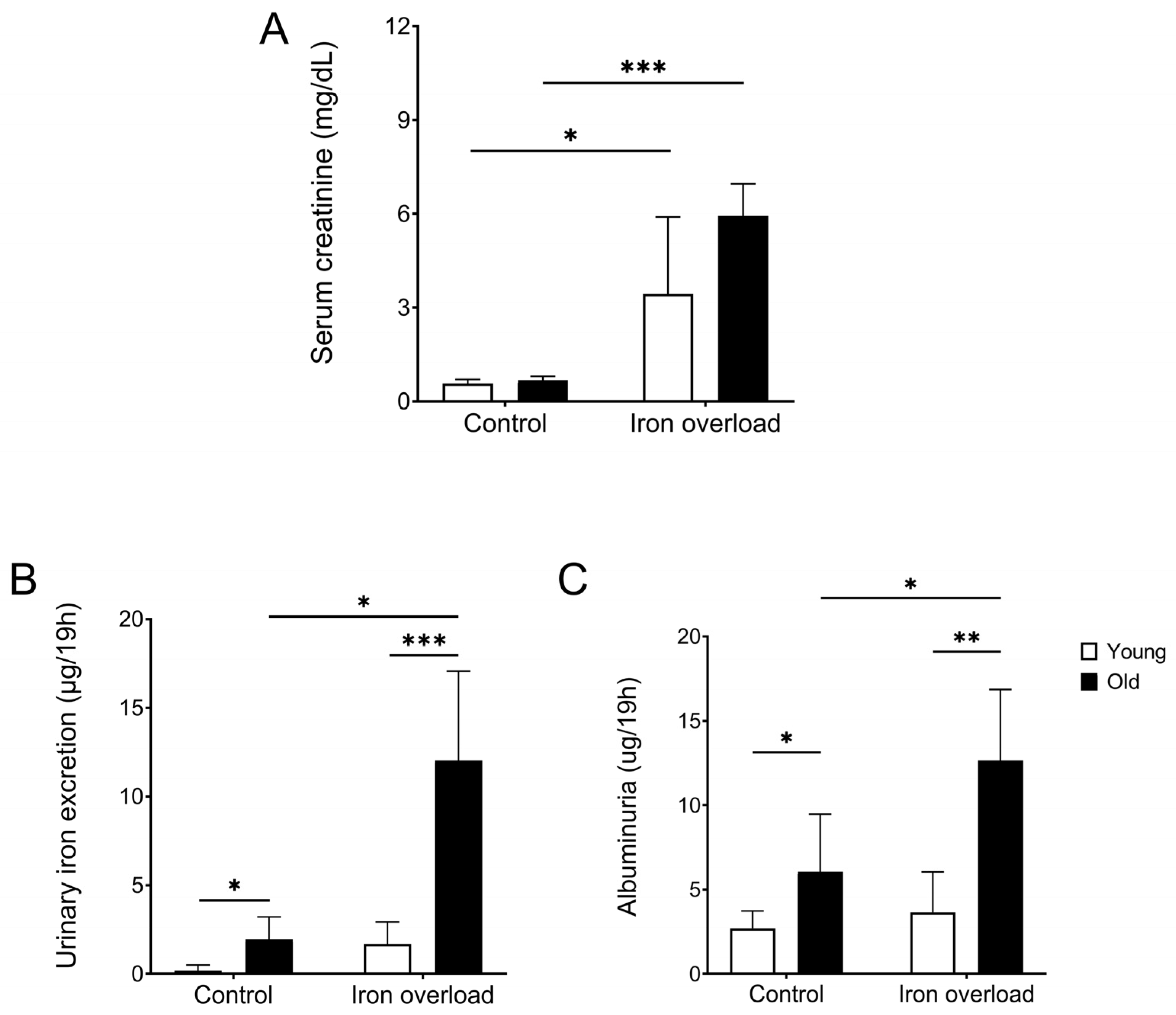
3.2. The Old Iron Overload Group Showed Increased Creatinine, Urinary Iron Excretion, and Albuminuria
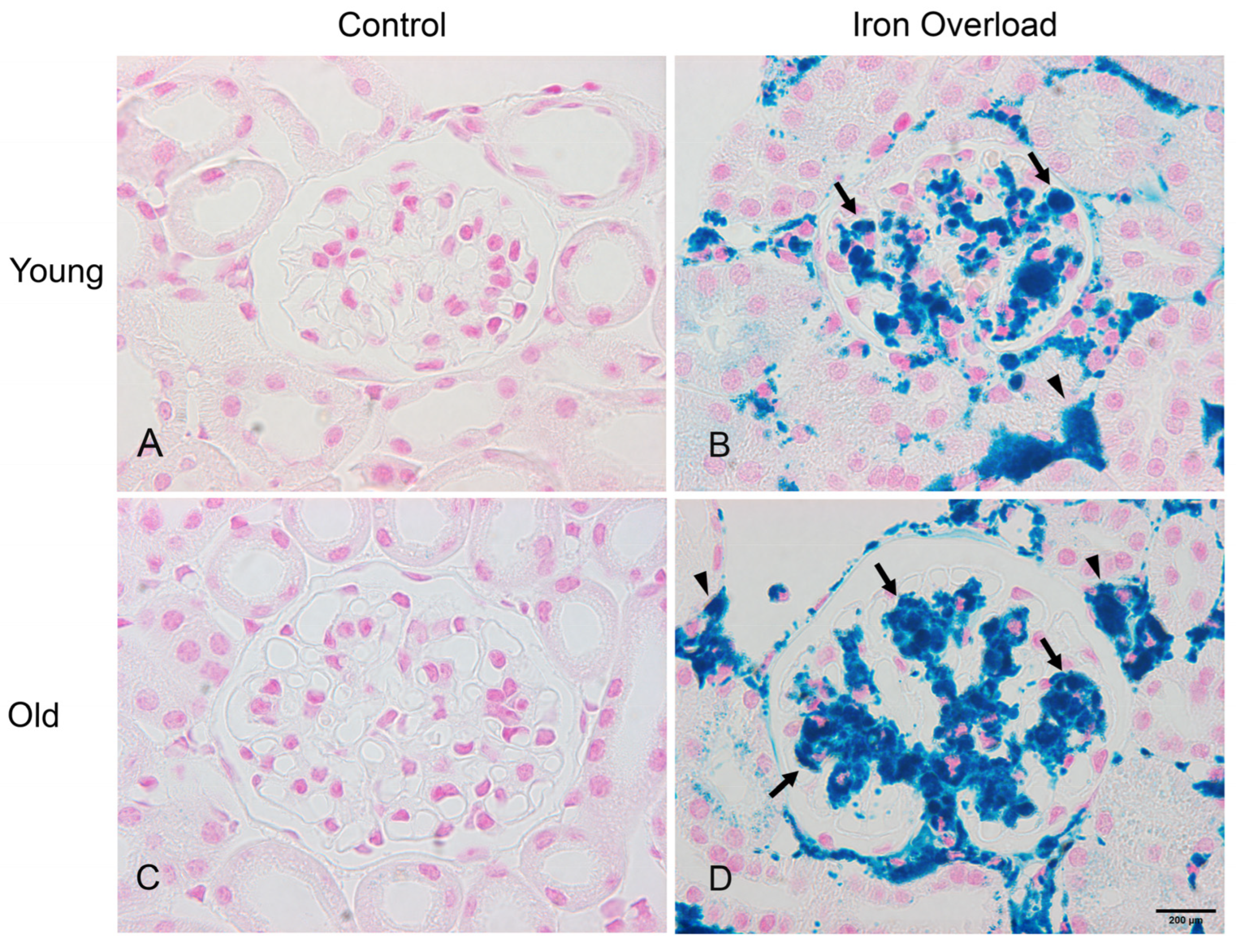
3.3. Iron Deposition Increased in the Old Iron Overload Group than in Other Groups
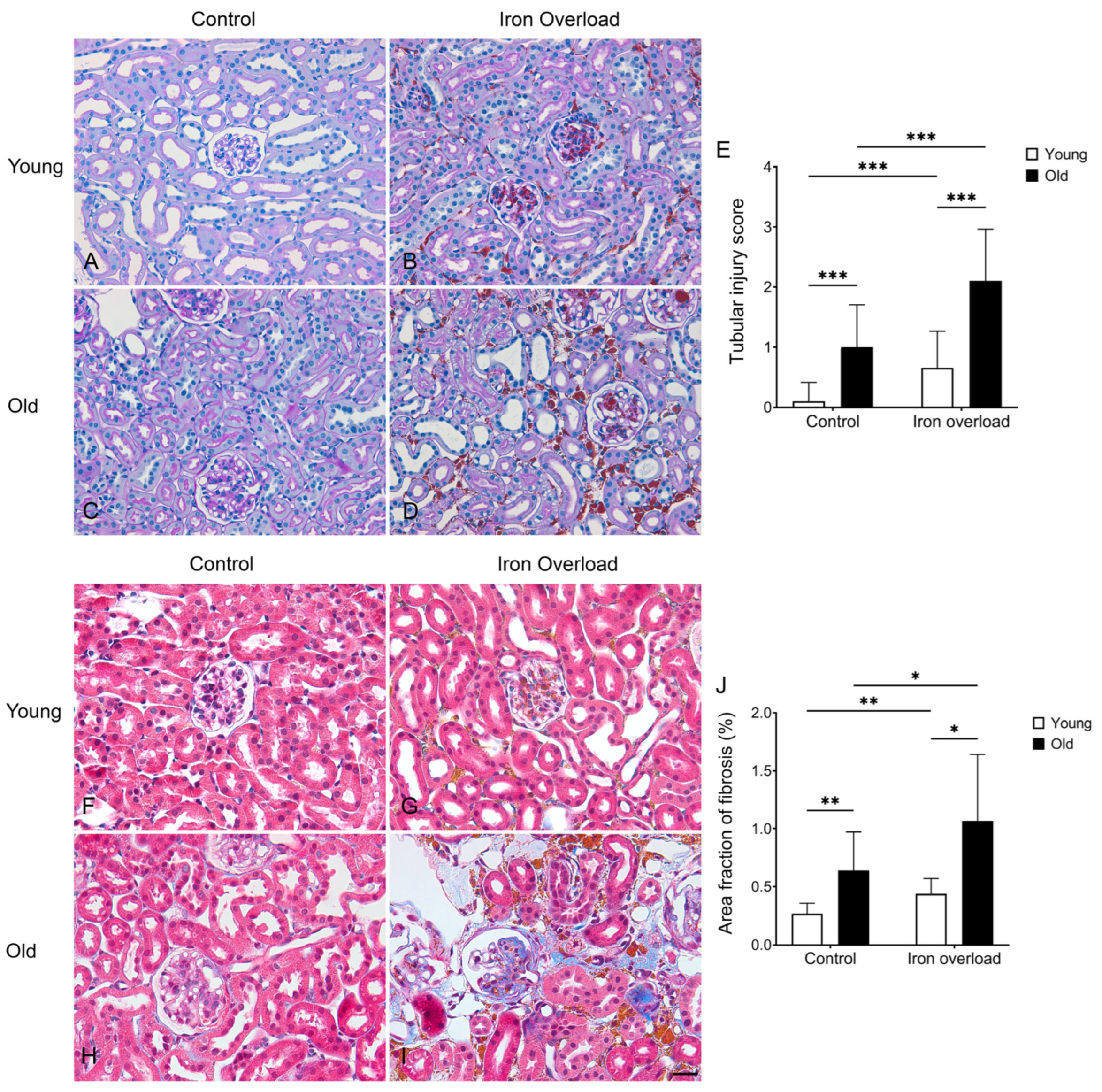
3.4. Renal Structural Changes Were Frequently Observed in the Iron Overload Aged Group
3.5. Renal Fibrosis Was Markedly Exacerbated in Aged Mice Under Iron Overload Conditions
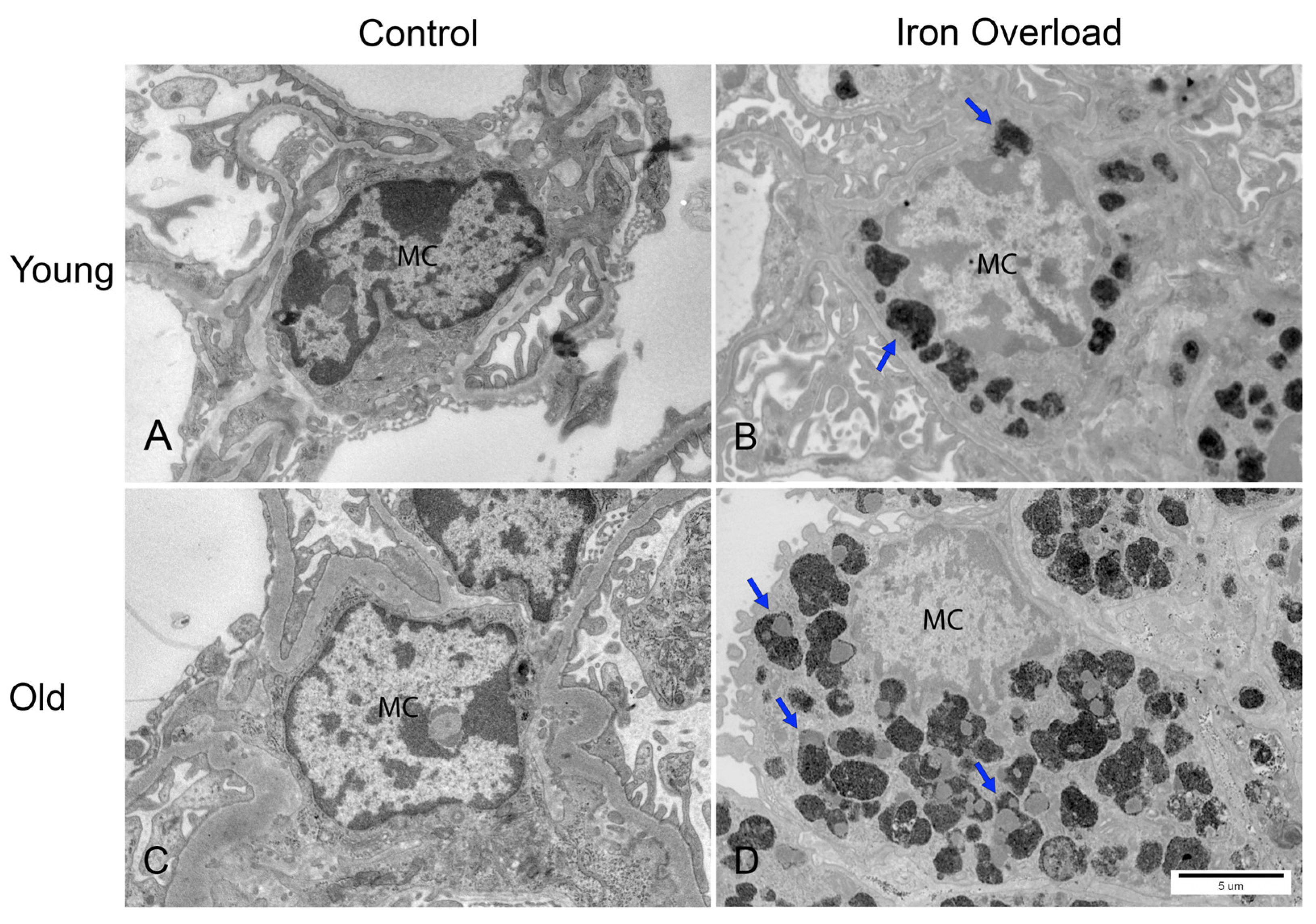
3.6. Greater Iron Deposition in Renal Mesangial Cells Was Observed in the Old Iron Overload Group
3.7. Iron Accumulation Was Increased in Aged Glomerular Endothelial Cells
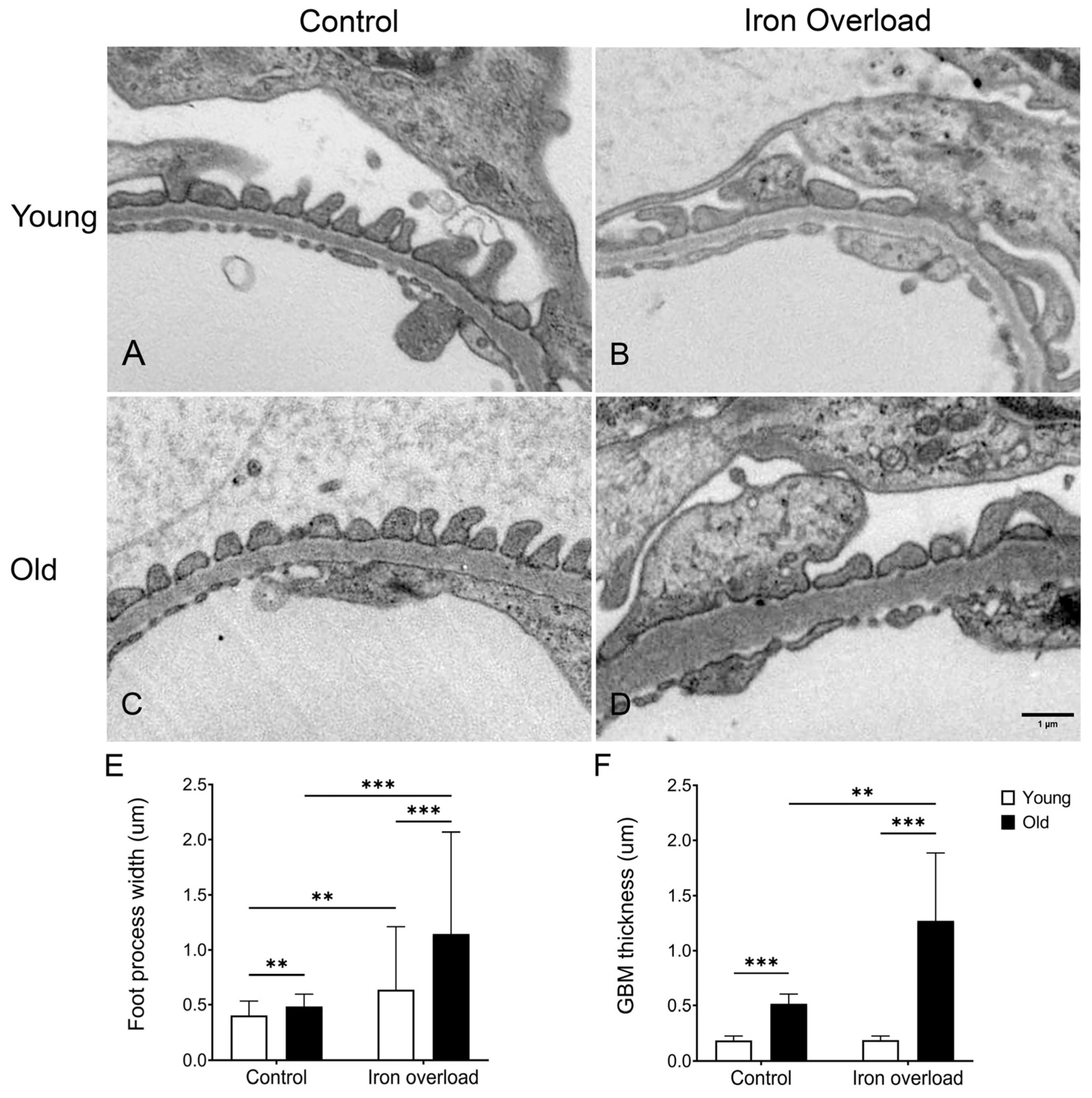
3.8. GBM Thickening and Foot Process Widening Were More Significant in the Old Iron Overload Group
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| GBM | glomerular basement membrane |
| PAS | periodic acid–Schiff |
| TBI | transferrin-bound iron |
| TEM | transmission electron microscopy |
| TF | transferrin |
| TIBC | total iron-binding capacity |
| UIBC | unsaturated iron-binding capacity |
References
- Abbaspour, N.; Hurrell, R.; Kelishadi, R. Review on iron and its importance for human health. J. Res. Med. Sci. 2014, 19, 164–174. [Google Scholar] [PubMed]
- Lieu, P.T.; Heiskala, M.; Peterson, P.A.; Yang, Y. The roles of iron in health and disease. Mol. Asp. Med. 2001, 22, 1–87. [Google Scholar] [CrossRef] [PubMed]
- Muckenthaler, M.U.; Rivella, S.; Hentze, M.W.; Galy, B. A Red Carpet for Iron Metabolism. Cell 2017, 168, 344–361. [Google Scholar] [CrossRef] [PubMed]
- Kohgo, Y.; Ikuta, K.; Ohtake, T.; Torimoto, Y.; Kato, J. Body iron metabolism and pathophysiology of iron overload. Int. J. Hematol. 2008, 88, 7–15. [Google Scholar] [CrossRef]
- Anderson, G.J. Mechanisms of iron loading and toxicity. Am. J. Hematol. 2007, 82 (Suppl. 12), 1128–1131. [Google Scholar] [CrossRef]
- Macdougall, I.C.; Bircher, A.J.; Eckardt, K.-U.; Obrador, G.T.; Pollock, C.A.; Stenvinkel, P.; Swinkels, D.W.; Wanner, C.; Weiss, G.; Chertow, G.M.; et al. Iron management in chronic kidney disease: Conclusions from a “Kidney Disease: Improving Global Outcomes” (KDIGO) Controversies Conference. Kidney Int. 2016, 89, 28–39. [Google Scholar] [CrossRef]
- Ozkurt, S.; Acikalin, M.F.; Temiz, G.; Akay, O.M.; Soydan, M. Renal hemosiderosis and rapidly progressive glomerulonephritis associated with primary hemochromatosis. Ren. Fail. 2014, 36, 814–816. [Google Scholar] [CrossRef]
- Dev, S.; Babitt, J.L. Babitt, Overview of iron metabolism in health and disease. Hemodial. Int. 2017, 21 (Suppl. 1), S6–S20. [Google Scholar] [CrossRef]
- Guan, S.; Ma, J.; Zhang, Y.; Gao, Y.; Zhang, Y.; Zhang, X.; Wang, N.; Xie, Y.; Wang, J.; Zhang, J.; et al. Danshen (Salvia miltiorrhiza) injection suppresses kidney injury induced by iron overload in mice. PLoS ONE 2013, 8, e74318. [Google Scholar] [CrossRef]
- Kellum, J.A.; Romagnani, P.; Ashuntantang, G.; Ronco, C.; Zarbock, A.; Anders, H.J. Acute kidney injury. Nat. Rev. Dis. Primers 2021, 7, 52. [Google Scholar] [CrossRef]
- Knepper, M.A.; Kwon, T.H.; Nielsen, S. Molecular physiology of water balance. N. Engl. J. Med. 2015, 372, 1349–1358. [Google Scholar] [CrossRef]
- Cortinovis, M.; Perico, N.; Ruggenenti, P.; Remuzzi, A.; Remuzzi, G. Glomerular hyperfiltration. Nat. Rev. Nephrol. 2022, 18, 435–451. [Google Scholar] [CrossRef]
- Tryggvason, K.; Wartiovaara, J. How does the kidney filter plasma? Physiology 2005, 20, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Verlander, J.W.; Clapp, W.L. Anatomy of the Kidney. In Brenner and Rector’s the Kidney; Yu, A.S.L., Chertow, G.M., Luyckx, V.A., Marsden, P.A., Skorecki, K., Taal, M.W., Eds.; Elsevier: Philadelphia, PA, USA, 2020. [Google Scholar]
- Schlöndorff, D.; Wyatt, C.M.; Campbell, K.N. Revisiting the determinants of the glomerular filtration barrier: What goes round must come round. Kidney Int. 2017, 92, 533–536. [Google Scholar] [CrossRef] [PubMed]
- Suh, J.H.; Miner, J.H. The glomerular basement membrane as a barrier to albumin. Nat. Rev. Nephrol. 2013, 9, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Scindia, Y.; Leeds, J.; Swaminathan, S. Iron Homeostasis in Healthy Kidney and its Role in Acute Kidney Injury. Semin. Nephrol. 2019, 39, 76–84. [Google Scholar] [CrossRef]
- Smith, C.P.; Thévenod, F. Iron transport and the kidney. Biochim. Biophys. Acta 2009, 1790, 724–730. [Google Scholar] [CrossRef]
- van Raaij, S.E.G.; Rennings, A.J.; Biemond, B.J.; Schols, S.E.M.; Wiegerinck, E.T.G.; Roelofs, H.M.J.; Hoorn, E.J.; Walsh, S.B.; Nijenhuis, T.; Swinkels, D.W.; et al. Iron handling by the human kidney: Glomerular filtration and tubular reabsorption both contribute to urinary iron excretion. Am. J. Physiol. Ren. Physiol. 2019, 316, F606–F614. [Google Scholar] [CrossRef]
- Thévenod, F.; Wolff, N.A. Iron transport in the kidney: Implications for physiology and cadmium nephrotoxicity. Metallomics 2015, 8, 17–42. [Google Scholar] [CrossRef]
- Smith, C.P.; Lee, W.-K.; Haley, M.; Poulsen, S.B.; Thévenod, F.; Fenton, R.A. Proximal tubule transferrin uptake is modulated by cellular iron and mediated by apical membrane megalin-cubilin complex and transferrin receptor 1. J. Biol. Chem. 2019, 294, 7025–7036. [Google Scholar] [CrossRef]
- Veuthey, T.; D’Anna, M.C.; Roque, M.E. Role of the kidney in iron homeostasis: Renal expression of Prohepcidin, Ferroportin, and DMT1 in anemic mice. Am. J. Physiol. Ren. Physiol. 2008, 295, F1213–F1221. [Google Scholar] [CrossRef]
- Starzyński, R.R.; Canonne-Hergaux, F.; Lenartowicz, M.; Krzeptowski, W.; Willemetz, A.; Styś, A.; Bierła, J.; Pietrzak, P.; Dziaman, T.; Lipiński, P. Ferroportin expression in haem oxygenase 1-deficient mice. Biochem. J. 2012, 449, 69–78. [Google Scholar] [CrossRef]
- van Raaij, S.; van Swelm, R.; Bouman, K.; Cliteur, M.; van den Heuvel, M.C.; Pertijs, J.; Patel, D.; Bass, P.; van Goor, H.; Unwin, R.; et al. Tubular iron deposition and iron handling proteins in human healthy kidney and chronic kidney disease. Sci. Rep. 2018, 8, 9353. [Google Scholar] [PubMed]
- van Swelm, R.P.L.; Wetzels, J.F.M.; Swinkels, D.W. The multifaceted role of iron in renal health and disease. Nat. Rev. Nephrol. 2020, 16, 77–98. [Google Scholar] [CrossRef] [PubMed]
- Martines, A.M.; Masereeuw, R.; Tjalsma, H.; Hoenderop, J.G.; Wetzels, J.F.M.; Swinkels, D.W. Iron metabolism in the pathogenesis of iron-induced kidney injury. Nat. Rev. Nephrol. 2013, 9, 385–398. [Google Scholar] [CrossRef] [PubMed]
- Nankivell, B.J.; Boadle, R.A.; Harris, D.C. Iron accumulation in human chronic renal disease. Am. J. Kidney Dis. 1992, 20, 580–584. [Google Scholar] [CrossRef]
- Richter, G.W. Iron overload nephropathy in rats. Pathol. Res. Pract. 1980, 168, 84–106. [Google Scholar] [CrossRef]
- Sterzel, R.B.; Perfetto, M.; Biemesderfer, D.; Kashgarian, M. Disposal of ferritin in the glomerular mesangium of rats. Kidney Int. 1983, 23, 480–488. [Google Scholar] [CrossRef]
- Denic, A.; Glassock, R.J.; Rule, A.D. Structural and Functional Changes With the Aging Kidney. Adv. Chronic Kidney Dis. 2016, 23, 19–28. [Google Scholar] [CrossRef]
- Kazama, I. Pathological and functional significance of aging mouse kidneys: Clinical implications to reduce the risk of hyper- or hypokalemia in the elderly. Kidney Res. Clin. Pract. 2024, 43, 703–708. [Google Scholar] [CrossRef]
- Hommos, M.S.; Glassock, R.J.; Rule, A.D. Structural and Functional Changes in Human Kidneys with Healthy Aging. J. Am. Soc. Nephrol. 2017, 28, 2838–2844. [Google Scholar] [CrossRef]
- O’Sullivan, E.D.; Hughes, J.; Ferenbach, D.A. Renal Aging: Causes and Consequences. J. Am. Soc. Nephrol. 2017, 28, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.J.; Rakheja, D.; Yu, X.; Saxena, R.; Vaziri, N.D.; Silva, F.G. The aging kidney. Kidney Int. 2008, 74, 710–720. [Google Scholar] [CrossRef] [PubMed]
- Shu, W.; Baumann, B.H.; Song, Y.; Liu, Y.; Wu, X.; Dunaief, J.L. Iron Accumulates in Retinal Vascular Endothelial Cells But Has Minimal Retinal Penetration After IP Iron Dextran Injection in Mice. Investig. Ophthalmol. Vis. Sci. 2019, 60, 4378–4387. [Google Scholar] [CrossRef] [PubMed]
- Mandala, A.; Chen, W.J.; Armstrong, A.; Malhotra, M.R.; Chavalmane, S.; McCommis, K.S.; Chen, A.; Carpenter, D.; Biswas, P.; Gnana-Prakasam, J.P. PPARα agonist fenofibrate attenuates iron-induced liver injury in mice by modulating the Sirt3 and β-catenin signaling. Am. J. Physiol. Gastrointest. Liver Physiol. 2021, 321, G262–G269. [Google Scholar] [CrossRef]
- Park, S.H.; Song, S.; Lee, J.; Shin, J. Effects of Aging on the Severity of Liver Injury in Mice With Iron Overload. J. Gastroenterol. Hepatol. 2025, 40, 1016–1025. [Google Scholar] [CrossRef]
- Wu, A.; Feng, B.; Yu, J.; Yan, L.; Che, L.; Zhuo, Y.; Luo, Y.; Yu, B.; Wu, D.; Chen, D. Fibroblast growth factor 21 attenuates iron overload-induced liver injury and fibrosis by inhibiting ferroptosis. Redox Biol. 2021, 46, 102131. [Google Scholar] [CrossRef]
- Tsay, J.; Yang, Z.; Ross, F.P.; Cunningham-Rundles, S.; Lin, H.; Coleman, R.; Mayer-Kuckuk, P.; Doty, S.B.; Grady, R.W.; Giardina, P.J.; et al. Bone loss caused by iron overload in a murine model: Importance of oxidative stress. Blood 2010, 116, 2582–2589. [Google Scholar] [CrossRef]
- Chultemsuren, M.; Lee, S.-Y.; Jee, M.J.; Park, K.M.; Han, K.-H. Ultrastructural Study of Ciliary Fragmentation in Renal Tubules During Ischemia-Reperfusion Injury. Microsc. Microanal. 2025, 31, ozaf042. [Google Scholar] [CrossRef]
- Zoja, C.; Corna, D.; Camozzi, D.; Cattaneo, D.; Rottoli, D.; Batani, C.; Zanchi, C.; Abbate, M.; Remuzzi, G. How to fully protect the kidney in a severe model of progressive nephropathy: A multidrug approach. J. Am. Soc. Nephrol. 2002, 13, 2898–2908. [Google Scholar] [CrossRef]
- Marquez-Exposito, L.; Tejedor-Santamaria, L.; Valentijn, F.A.; Tejera-Muñoz, A.; Rayego-Mateos, S.; Marchant, V.; Rodrigues-Diez, R.R.; Rubio-Soto, I.; Knoppert, S.N.; Ortiz, A.; et al. Oxidative Stress and Cellular Senescence Are Involved in the Aging Kidney. Antioxidants 2022, 11, 301. [Google Scholar] [CrossRef]
- Lee, S.Y.; Lee, S.J.; Piao, H.L.; Yang, S.Y.; Weiner, I.D.; Kim, J.; Han, K.H. Hydration status affects osteopontin expression in the rat kidney. J. Vet. Sci. 2016, 17, 269–277. [Google Scholar] [CrossRef]
- Weinstein, J.R.; Anderson, S. The aging kidney: Physiological changes. Adv. Chronic Kidney Dis. 2010, 17, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Wiggins, J.E.; Goyal, M.; Sanden, S.K.; Wharram, B.L.; Shedden, K.A.; Misek, D.E.; Kuick, R.D.; Wiggins, R.C. Podocyte hypertrophy, “adaptation,” and “decompensation” associated with glomerular enlargement and glomerulosclerosis in the aging rat: Prevention by calorie restriction. J. Am. Soc. Nephrol. 2005, 16, 2953–2966. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Yang, X.; Gong, H.; Li, X. Time- and Gender-Dependent Alterations in Mice during the Aging Process. Int. J. Mol. Sci. 2023, 24, 12790. [Google Scholar] [CrossRef] [PubMed]
- Musso, C.G.; Oreopoulos, D.G. Aging and physiological changes of the kidneys including changes in glomerular filtration rate. Nephron Physiol. 2011, 119 (Suppl. 1), 1–5. [Google Scholar] [CrossRef]
- Chung, K.W.; Jeong, H.O.; Lee, B.; Park, D.; Kim, D.H.; Choi, Y.J.; Lee, E.K.; Kim, K.M.; Park, J.W.; Yu, B.P.; et al. Involvement of NF-κBIZ and related cytokines in age-associated renal fibrosis. Oncotarget 2017, 8, 7315–7327. [Google Scholar] [CrossRef]
- Yang, M.; Liu, Y.; Luo, S.-L.; Liu, C.-B.; Jiang, N.; Li, C.-R.; Zhao, H.; Han, Y.-C.; Chen, W.; Li, L.; et al. DsbA-L ameliorates renal aging and renal fibrosis by maintaining mitochondrial homeostasis. Acta Pharmacol. Sin. 2024, 45, 777–789. [Google Scholar] [CrossRef]
- Ballermann, B.J. Glomerular endothelial cell differentiation. Kidney Int. 2005, 67, 1668–1671. [Google Scholar] [CrossRef]
- Jiang, T.; Liebman, S.E.; Lucia, M.S.; Li, J.; Levi, M. Role of altered renal lipid metabolism and the sterol regulatory element binding proteins in the pathogenesis of age-related renal disease. Kidney Int. 2005, 68, 2608–2620. [Google Scholar] [CrossRef]
- Neumann, K.H.; Kellner, C.; KüHn, K.; Stolte, H.; Schurek, H.-J. Age-dependent thickening of glomerular basement membrane has no major effect on glomerular hydraulic conductivity. Nephrol. Dial. Transplant. 2004, 19, 805–811. [Google Scholar] [CrossRef]
- Ru, Q.; Li, Y.; Chen, L.; Wu, Y.; Min, J.; Wang, F. Iron homeostasis and ferroptosis in human diseases: Mechanisms and therapeutic prospects. Signal Transduct. Target. Ther. 2024, 9, 271. [Google Scholar] [CrossRef] [PubMed]
- Fleming, R.E.; Ponka, P. Iron overload in human disease. N. Engl. J. Med. 2012, 366, 348–359. [Google Scholar] [CrossRef] [PubMed]
- Naito, Y.; Fujii, A.; Sawada, H.; Oboshi, M.; Iwasaku, T.; Okuhara, Y.; Morisawa, D.; Eguchi, A.; Hirotani, S.; Masuyama, T. Association between renal iron accumulation and renal interstitial fibrosis in a rat model of chronic kidney disease. Hypertens. Res. 2015, 38, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Meyron-Holtz, E.; Rouault, T.A. Renal iron metabolism: Transferrin iron delivery and the role of iron regulatory proteins. J. Am. Soc. Nephrol. 2007, 18, 401–406. [Google Scholar] [CrossRef]
- Bolton, W.K.; Benton, F.R.; Maclay, J.G.; Sturgill, B.C. Spontaneous glomerular sclerosis in aging Sprague-Dawley rats. I. Lesions associated with mesangial IgM deposits. Am. J. Pathol. 1976, 85, 277–302. [Google Scholar]
- Merle, N.S.; Grunenwald, A.; Figueres, M.-L.; Chauvet, S.; Daugan, M.; Knockaert, S.; Robe-Rybkine, T.; Noe, R.; May, O.; Frimat, M.; et al. Characterization of Renal Injury and Inflammation in an Experimental Model of Intravascular Hemolysis. Front. Immunol. 2018, 9, 179. [Google Scholar] [CrossRef]
- Ebefors, K.; Bergwall, L.; Nyström, J. The Glomerulus According to the Mesangium. Front. Med. 2021, 8, 740527. [Google Scholar] [CrossRef]
- Ross, M.H.; Pawlina, W. Urinary system. In Histology: A Text and Atlas: With Correlated Cell and Molecular Biology; Ross, M.H., Pawlina, W., Eds.; Wolters Kluwer: Philadelphia, PA, USA, 2016; pp. 698–742. [Google Scholar]
- Shankland, S.J. The podocyte’s response to injury: Role in proteinuria and glomerulosclerosis. Kidney Int. 2006, 69, 2131–2147. [Google Scholar] [CrossRef]
- Miner, J.H. The glomerular basement membrane. Exp. Cell Res. 2012, 318, 973–978. [Google Scholar] [CrossRef]
- Kawashima, N.; Naito, S.; Nagane, M.; Yamashita, T.; Nakayama, K.-I. Progression of albuminuria and podocyte injury in focal segmental glomerulosclerosis inhibited by enhanced glycosphingolipid GM3 via valproic acid. Sci. Rep. 2023, 13, 22487. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chultemsuren, M.; Song, S.-J.; Han, K.-H.; Shin, J.-A. Iron Overload Accelerates Aging-Associated Kidney Injury in Mice: Implications for Iron Supplementation in the Elderly. Nutrients 2025, 17, 2580. https://doi.org/10.3390/nu17162580
Chultemsuren M, Song S-J, Han K-H, Shin J-A. Iron Overload Accelerates Aging-Associated Kidney Injury in Mice: Implications for Iron Supplementation in the Elderly. Nutrients. 2025; 17(16):2580. https://doi.org/10.3390/nu17162580
Chicago/Turabian StyleChultemsuren, Mungunchimeg, Soo-Jin Song, Ki-Hwan Han, and Jung-A Shin. 2025. "Iron Overload Accelerates Aging-Associated Kidney Injury in Mice: Implications for Iron Supplementation in the Elderly" Nutrients 17, no. 16: 2580. https://doi.org/10.3390/nu17162580
APA StyleChultemsuren, M., Song, S.-J., Han, K.-H., & Shin, J.-A. (2025). Iron Overload Accelerates Aging-Associated Kidney Injury in Mice: Implications for Iron Supplementation in the Elderly. Nutrients, 17(16), 2580. https://doi.org/10.3390/nu17162580




