Exploring the Association Between Multidimensional Dietary Patterns and Non-Scarring Hair Loss Using Mendelian Randomization
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Sources
2.3. Instrumental Variable (IV)
2.4. MR Analysis
2.5. Meta-Analysis
3. Results
3.1. SNP Selection
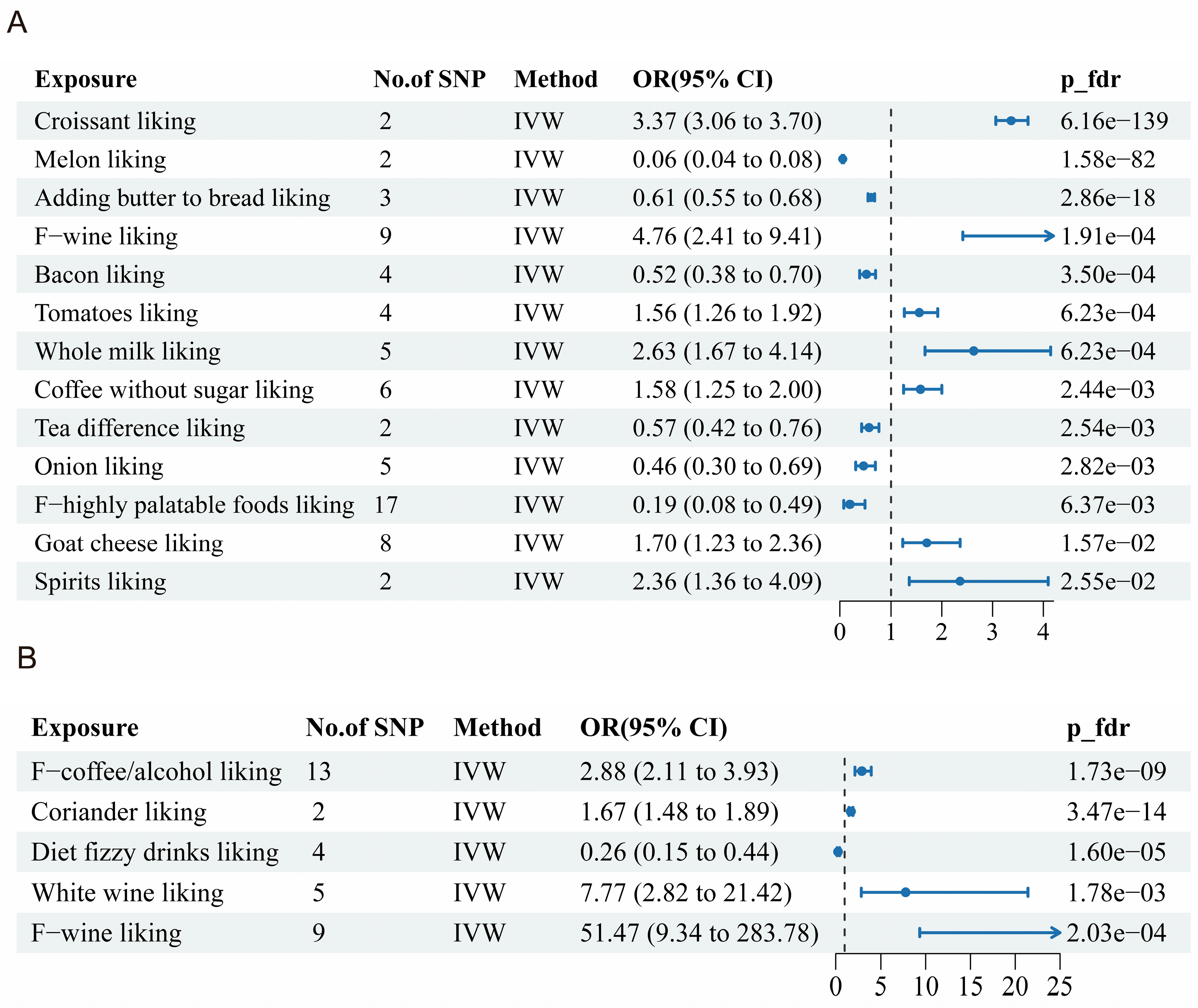
3.2. Dietary Liking and Alopecia Areata
3.3. Dietary Liking and Androgenetic Alopecia
3.4. Sensitivity Analyses
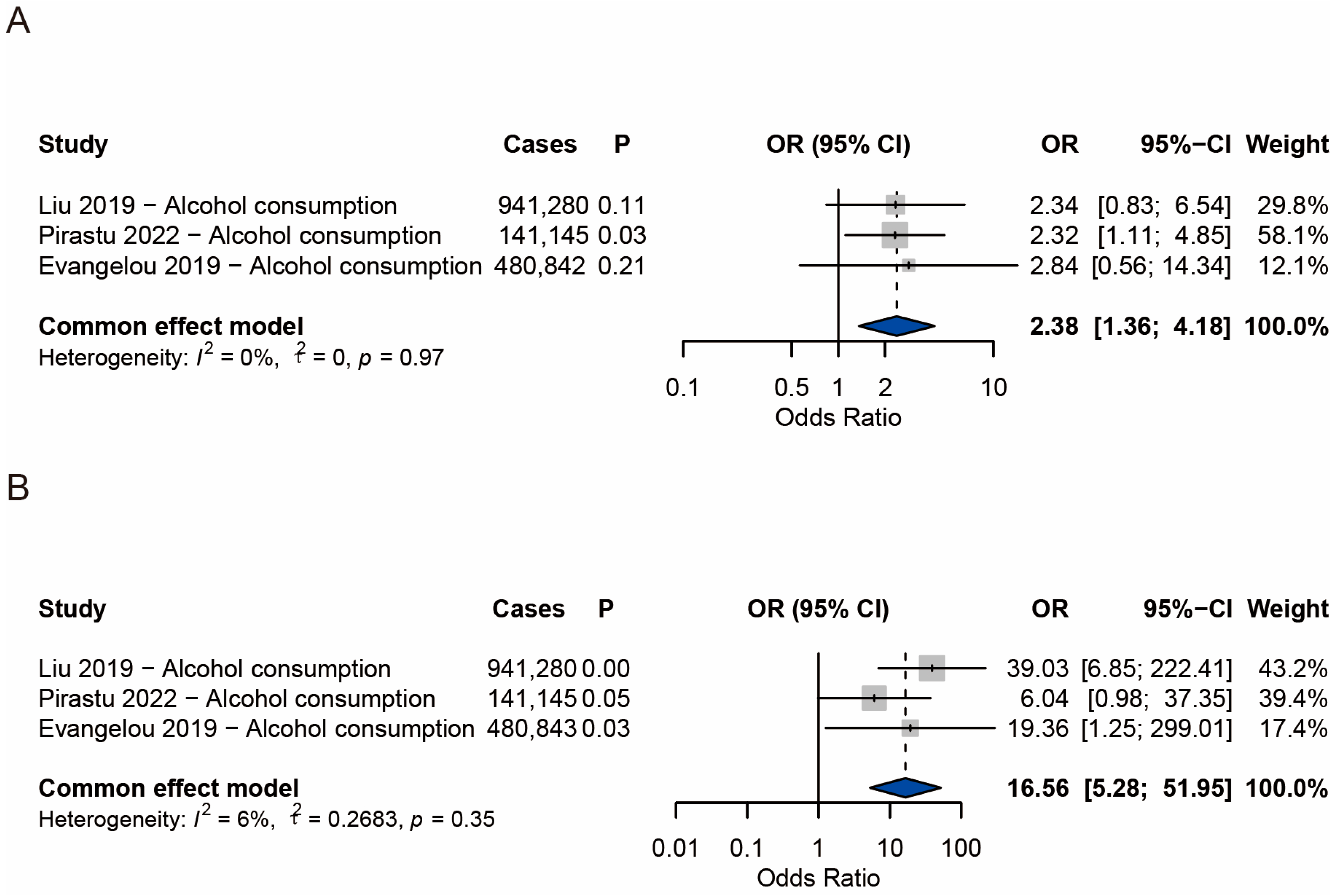
3.5. Meta-Analysis Results of Alopecia Areata
3.6. Meta-Analysis Results of Androgenetic Alopecia
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adil, A.; Godwin, M. The Effectiveness of Treatments for Androgenetic Alopecia: A Systematic Review and Meta-Analysis. J. Am. Acad. Dermatol. 2017, 77, 136–141.e5. [Google Scholar] [CrossRef]
- Starace, M.; Orlando, G.; Alessandrini, A.; Piraccini, B.M. Female Androgenetic Alopecia: An Update on Diagnosis and Management. Am. J. Clin. Dermatol. 2020, 21, 69–84. [Google Scholar] [CrossRef]
- Okhovat, J.-P.; Marks, D.H.; Manatis-Lornell, A.; Hagigeorges, D.; Locascio, J.J.; Senna, M.M. Association between Alopecia Areata, Anxiety, and Depression: A Systematic Review and Meta-Analysis. J. Am. Acad. Dermatol. 2023, 88, 1040–1050. [Google Scholar] [CrossRef]
- Tzur Bitan, D.; Berzin, D.; Kridin, K.; Cohen, A. The Association between Alopecia Areata and Anxiety, Depression, Schizophrenia, and Bipolar Disorder: A Population-Based Study. Arch. Dermatol. Res. 2022, 314, 463–468. [Google Scholar] [CrossRef]
- Toussi, A.; Barton, V.R.; Le, S.T.; Agbai, O.N.; Kiuru, M. Psychosocial and Psychiatric Comorbidities and Health-Related Quality of Life in Alopecia Areata: A Systematic Review. J. Am. Acad. Dermatol. 2021, 85, 162–175. [Google Scholar] [CrossRef]
- Ma, Y.; Chachin, M.; Hirose, T.; Nakamura, K.; Shi, N.; Hiro, S.; Imafuku, S. Prevalence and Incidence of Comorbidities in Patients with Atopic Dermatitis, Psoriasis, Alopecia Areata, and Vitiligo Using a Japanese Claims Database. J. Dermatol. 2025, 52, 841–854. [Google Scholar] [CrossRef]
- Vañó-Galván, S.; Camacho, F. New Treatments for Hair Loss. Actas Dermosifiliogr. 2017, 108, 221–228. [Google Scholar] [CrossRef]
- Lee, S.; Lee, Y.B.; Choe, S.J.; Lee, W.-S. Adverse Sexual Effects of Treatment with Finasteride or Dutasteride for Male Androgenetic Alopecia: A Systematic Review and Meta-Analysis. Acta Derm. Venereol. 2019, 99, 12–17. [Google Scholar] [CrossRef]
- Finner, A.M. Nutrition and Hair: Deficiencies and Supplements. Dermatol. Clin. 2013, 31, 167–172. [Google Scholar] [CrossRef]
- Thompson, J.M.; Mirza, M.A.; Park, M.K.; Qureshi, A.A.; Cho, E. The Role of Micronutrients in Alopecia Areata: A Review. Am. J. Clin. Dermatol. 2017, 18, 663–679. [Google Scholar] [CrossRef]
- Pham, C.T.; Romero, K.; Almohanna, H.M.; Griggs, J.; Ahmed, A.; Tosti, A. The Role of Diet as an Adjuvant Treatment in Scarring and Nonscarring Alopecia. Ski. Appendage Disord. 2020, 6, 88–96. [Google Scholar] [CrossRef]
- Wróblewska-Kończalik, K.; Pawlaczyk, M.; Kolasiński, J.; Kolenda, M.; Miechowicz, I.; Seraszek-Jaros, A.; Kroma-Szal, A.; Gornowicz-Porowska, J. Non-Cicatricial Alopecia and Its Association with Anthropometric Measurements and Nutritional Laboratory Markers. Life 2024, 14, 609. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho Vidigal, F.; Guedes Cocate, P.; Gonçalves Pereira, L.; de Cássia Gonçalves Alfenas, R. The Role of Hyperglycemia in the Induction of Oxidative Stress and Inflammatory Process. Nutr. Hosp. 2012, 27, 1391–1398. [Google Scholar] [CrossRef] [PubMed]
- Bazmi, S.; Sepehrinia, M.; Pourmontaseri, H.; Bazyar, H.; Vahid, F.; Farjam, M.; Dehghan, A.; Hébert, J.R.; Homayounfar, R.; Shakouri, N. Androgenic Alopecia Is Associated with Higher Dietary Inflammatory Index and Lower Antioxidant Index Scores. Front. Nutr. 2024, 11, 1433962. [Google Scholar] [CrossRef]
- Darmon, N.; Drewnowski, A. Contribution of Food Prices and Diet Cost to Socioeconomic Disparities in Diet Quality and Health: A Systematic Review and Analysis. Nutr. Rev. 2015, 73, 643–660. [Google Scholar] [CrossRef]
- D’Onofrio, B.M.; Sjölander, A.; Lahey, B.B.; Lichtenstein, P.; Öberg, A.S. Accounting for Confounding in Observational Studies. Annu. Rev. Clin. Psychol. 2020, 16, 25–48. [Google Scholar] [CrossRef]
- Skrivankova, V.W.; Richmond, R.C.; Woolf, B.A.R.; Yarmolinsky, J.; Davies, N.M.; Swanson, S.A.; VanderWeele, T.J.; Higgins, J.P.T.; Timpson, N.J.; Dimou, N.; et al. Strengthening the Reporting of Observational Studies in Epidemiology Using Mendelian Randomization: The STROBE-MR Statement. JAMA 2021, 326, 1614–1621. [Google Scholar] [CrossRef]
- Ba, Y.; Shen, L.; Peng, X.; Zhang, Y.; Wang, J. Elucidating Causal Relationships of Diet-Derived Circulating Antioxidants and the Risk of Non-Scarring Alopecia: A Mendelian Randomization Study. Medicine 2024, 103, e38426. [Google Scholar] [CrossRef]
- Drake, L.; Reyes-Hadsall, S.; Martinez, J.; Heinrich, C.; Huang, K.; Mostaghimi, A. Evaluation of the Safety and Effectiveness of Nutritional Supplements for Treating Hair Loss: A Systematic Review. JAMA Dermatol. 2023, 159, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Petermann-Rocha, F.; Wirth, M.D.; Boonpor, J.; Parra-Soto, S.; Zhou, Z.; Mathers, J.C.; Livingstone, K.; Forrest, E.; Pell, J.P.; Ho, F.K.; et al. Associations between an Inflammatory Diet Index and Severe Non-Alcoholic Fatty Liver Disease: A Prospective Study of 171,544 UK Biobank Participants. BMC Med. 2023, 21, 123. [Google Scholar] [CrossRef]
- Schatzkin, A.; Kipnis, V.; Carroll, R.J.; Midthune, D.; Subar, A.F.; Bingham, S.; Schoeller, D.A.; Troiano, R.P.; Freedman, L.S. A Comparison of a Food Frequency Questionnaire with a 24-Hour Recall for Use in an Epidemiological Cohort Study: Results from the Biomarker-Based Observing Protein and Energy Nutrition (OPEN) Study. Int. J. Epidemiol. 2003, 32, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- May-Wilson, S.; Matoba, N.; Wade, K.H.; Hottenga, J.-J.; Concas, M.P.; Mangino, M.; Grzeszkowiak, E.J.; Menni, C.; Gasparini, P.; Timpson, N.J.; et al. Large-Scale GWAS of Food Liking Reveals Genetic Determinants and Genetic Correlations with Distinct Neurophysiological Traits. Nat. Commun. 2022, 13, 2743. [Google Scholar] [CrossRef]
- Pirastu, N.; McDonnell, C.; Grzeszkowiak, E.J.; Mounier, N.; Imamura, F.; Merino, J.; Day, F.R.; Zheng, J.; Taba, N.; Concas, M.P.; et al. Using Genetic Variation to Disentangle the Complex Relationship between Food Intake and Health Outcomes. PLoS Genet. 2022, 18, e1010162. [Google Scholar] [CrossRef]
- Liu, M.; Jiang, Y.; Wedow, R.; Li, Y.; Brazel, D.M.; Chen, F.; Datta, G.; Davila-Velderrain, J.; McGuire, D.; Tian, C.; et al. Association Studies of up to 1.2 Million Individuals Yield New Insights into the Genetic Etiology of Tobacco and Alcohol Use. Nat. Genet. 2019, 51, 237–244. [Google Scholar] [CrossRef]
- Evangelou, E.; Gao, H.; Chu, C.; Ntritsos, G.; Blakeley, P.; Butts, A.R.; Pazoki, R.; Suzuki, H.; Koskeridis, F.; Yiorkas, A.M.; et al. New Alcohol-Related Genes Suggest Shared Genetic Mechanisms with Neuropsychiatric Disorders. Nat. Hum. Behav. 2019, 3, 950–961. [Google Scholar] [CrossRef]
- Byrska-Bishop, M.; Evani, U.S.; Zhao, X.; Basile, A.O.; Abel, H.J.; Regier, A.A.; Corvelo, A.; Clarke, W.E.; Musunuri, R.; Nagulapalli, K.; et al. High-Coverage Whole-Genome Sequencing of the Expanded 1000 Genomes Project Cohort Including 602 Trios. Cell 2022, 185, 3426–3440.e19. [Google Scholar] [CrossRef]
- Kurki, M.I.; Karjalainen, J.; Palta, P.; Sipilä, T.P.; Kristiansson, K.; Donner, K.M.; Reeve, M.P.; Laivuori, H.; Aavikko, M.; Kaunisto, M.A.; et al. FinnGen Provides Genetic Insights from a Well-Phenotyped Isolated Population. Nature 2023, 613, 508–518. [Google Scholar] [CrossRef]
- Burgess, S.; Thompson, S.G.; CRP CHD Genetics Collaboration. Avoiding Bias from Weak Instruments in Mendelian Randomization Studies. Int. J. Epidemiol. 2011, 40, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Burgess, S.; Butterworth, A.; Thompson, S.G. Mendelian Randomization Analysis with Multiple Genetic Variants Using Summarized Data. Genet. Epidemiol. 2013, 37, 658–665. [Google Scholar] [CrossRef] [PubMed]
- Bowden, J.; Davey Smith, G.; Burgess, S. Mendelian Randomization with Invalid Instruments: Effect Estimation and Bias Detection through Egger Regression. Int. J. Epidemiol. 2015, 44, 512–525. [Google Scholar] [CrossRef]
- Bowden, J.; Davey Smith, G.; Haycock, P.C.; Burgess, S. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet. Epidemiol. 2016, 40, 304–314. [Google Scholar] [CrossRef]
- Xue, H.; Shen, X.; Pan, W. Constrained Maximum Likelihood-Based Mendelian Randomization Robust to Both Correlated and Uncorrelated Pleiotropic Effects. Am. J. Hum. Genet. 2021, 108, 1251–1269. [Google Scholar] [CrossRef]
- Verbanck, M.; Chen, C.-Y.; Neale, B.; Do, R. Detection of Widespread Horizontal Pleiotropy in Causal Relationships Inferred from Mendelian Randomization between Complex Traits and Diseases. Nat. Genet. 2018, 50, 693–698. [Google Scholar] [CrossRef]
- Burgess, S.; Scott, R.A.; Timpson, N.J.; Davey Smith, G.; Thompson, S.G.; EPIC-InterAct Consortium. Using Published Data in Mendelian Randomization: A Blueprint for Efficient Identification of Causal Risk Factors. Eur. J. Epidemiol. 2015, 30, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Pagoni, P.; Dimou, N.L.; Murphy, N.; Stergiakouli, E. Using Mendelian Randomisation to Assess Causality in Observational Studies. Evid. Based Ment. Health 2019, 22, 67–71. [Google Scholar] [CrossRef]
- Hemani, G.; Tilling, K.; Davey Smith, G. Orienting the Causal Relationship between Imprecisely Measured Traits Using GWAS Summary Data. PLoS Genet. 2017, 13, e1007081. [Google Scholar] [CrossRef]
- Migliavaca, C.B.; Stein, C.; Colpani, V.; Barker, T.H.; Ziegelmann, P.K.; Munn, Z.; Falavigna, M.; Prevalence Estimates Reviews-Systematic Review Methodology Group (PERSyst). Meta-Analysis of Prevalence: I2 Statistic and How to Deal with Heterogeneity. Res. Synth. Methods 2022, 13, 363–367. [Google Scholar] [CrossRef]
- Dai, Y.-X.; Yeh, F.-Y.; Shen, Y.-J.; Tai, Y.-H.; Chou, Y.-J.; Chang, Y.-T.; Chen, T.-J.; Li, C.-P.; Wu, C.-Y. Cigarette Smoking, Alcohol Consumption, and Risk of Alopecia Areata: A Population-Based Cohort Study in Taiwan. Am. J. Clin. Dermatol. 2020, 21, 901–911. [Google Scholar] [CrossRef]
- Joshi, T.P.; Zhu, H.; Tomaras, M.; Terrell, M.; Strouphauer, E.; Stafford, H.; Okundia, F.; Iacobucci, A.; Holla, S.; Hinson, D.; et al. Association of Alopecia Areata with Alcohol Use Disorder, Attention-Deficit Hyperactivity Disorder and Insomnia: A Case-Control Analysis Using the All of Us Research Programme. Clin. Exp. Dermatol. 2023, 48, 797–799. [Google Scholar] [CrossRef] [PubMed]
- Murugan, M.; Sadasivam, I.P.; Manoharan, A.; Jayakumar, S.; Vetriselvan, Y.; Samuel, M.S.; Sambandam, R. Association between PITX2 Polymorphism and Androgenetic Alopecia in the Indian Population. Indian J. Dermatol. Venereol. Leprol. 2024, 91, 158–162. [Google Scholar] [CrossRef]
- Clemmesen, M.E.R.; Gren, S.T.; Frøstrup, A.G.; Thomsen, S.F.; Egeberg, A.; Thein, D. Psychosocial and Mental Impact of Alopecia Areata: Analysis of the Danish Skin Cohort. J. Eur. Acad. Dermatol. Venereol. JEADV 2024, 39, 688–697. [Google Scholar] [CrossRef]
- Butts, M.; Sundaram, V.L.; Murughiyan, U.; Borthakur, A.; Singh, S. The Influence of Alcohol Consumption on Intestinal Nutrient Absorption: A Comprehensive Review. Nutrients 2023, 15, 1571. [Google Scholar] [CrossRef]
- Das, S.K.; Vasudevan, D.M. Alcohol-Induced Oxidative Stress. Life Sci. 2007, 81, 177–187. [Google Scholar] [CrossRef]
- Peivasteh-Roudsari, L.; Karami, M.; Barzegar-Bafrouei, R.; Samiee, S.; Karami, H.; Tajdar-Oranj, B.; Mahdavi, V.; Alizadeh, A.M.; Sadighara, P.; Oliveri Conti, G.; et al. Toxicity, Metabolism, and Mitigation Strategies of Acrylamide: A Comprehensive Review. Int. J. Environ. Health Res. 2024, 34, 1–29. [Google Scholar] [CrossRef]
- Mollakhalili-Meybodi, N.; Khorshidian, N.; Nematollahi, A.; Arab, M. Acrylamide in Bread: A Review on Formation, Health Risk Assessment, and Determination by Analytical Techniques. Environ. Sci. Pollut. Res. Int. 2021, 28, 15627–15645. [Google Scholar] [CrossRef]
- Rondinella, D.; Raoul, P.C.; Valeriani, E.; Venturini, I.; Cintoni, M.; Severino, A.; Galli, F.S.; Mora, V.; Mele, M.C.; Cammarota, G.; et al. The Detrimental Impact of Ultra-Processed Foods on the Human Gut Microbiome and Gut Barrier. Nutrients 2025, 17, 859. [Google Scholar] [CrossRef]
- Dahabreh, D.; Jung, S.; Renert-Yuval, Y.; Bar, J.; Del Duca, E.; Guttman-Yassky, E. Alopecia Areata: Current Treatments and New Directions. Am. J. Clin. Dermatol. 2023, 24, 895–912. [Google Scholar] [CrossRef]
- Morinaga, H.; Mohri, Y.; Grachtchouk, M.; Asakawa, K.; Matsumura, H.; Oshima, M.; Takayama, N.; Kato, T.; Nishimori, Y.; Sorimachi, Y.; et al. Obesity Accelerates Hair Thinning by Stem Cell-Centric Converging Mechanisms. Nature 2021, 595, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Jin, R.; Zeng, J.; Hua, Y.; Yorek, M.S.; Liu, L.; Mandal, A.; Li, J.; Zheng, H.; Sun, Y.; et al. Consumption of Fish Oil High-Fat Diet Induces Murine Hair Loss via Epidermal Fatty Acid Binding Protein in Skin Macrophages. Cell Rep. 2022, 41, 111804. [Google Scholar] [CrossRef]
- Palmer, M.A.; Blakeborough, L.; Harries, M.; Haslam, I.S. Cholesterol Homeostasis: Links to Hair Follicle Biology and Hair Disorders. Exp. Dermatol. 2020, 29, 299–311. [Google Scholar] [CrossRef]
- Lattouf, C.; Jimenez, J.J.; Tosti, A.; Miteva, M.; Wikramanayake, T.C.; Kittles, C.; Herskovitz, I.; Handler, M.Z.; Fabbrocini, G.; Schachner, L.A. Treatment of Alopecia Areata with Simvastatin/Ezetimibe. J. Am. Acad. Dermatol. 2015, 72, 359–361. [Google Scholar] [CrossRef]
- Cervantes, J.; Jimenez, J.J.; DelCanto, G.M.; Tosti, A. Treatment of Alopecia Areata with Simvastatin/Ezetimibe. J. Investig. Dermatol. Symp. Proc. 2018, 19, S25–S31. [Google Scholar] [CrossRef]
- Wang, S.; Moustaid-Moussa, N.; Chen, L.; Mo, H.; Shastri, A.; Su, R.; Bapat, P.; Kwun, I.; Shen, C.-L. Novel Insights of Dietary Polyphenols and Obesity. J. Nutr. Biochem. 2014, 25, 1–18. [Google Scholar] [CrossRef]
- Kwon, O.S.; Han, J.H.; Yoo, H.G.; Chung, J.H.; Cho, K.H.; Eun, H.C.; Kim, K.H. Human Hair Growth Enhancement in Vitro by Green Tea Epigallocatechin-3-Gallate (EGCG). Phytomedicine Int. J. Phytother. Phytopharm. 2007, 14, 551–555. [Google Scholar] [CrossRef]
- Zhou, S.; Huang, G.; Huang, H. Extraction, Derivatization and Antioxidant Activities of Onion Polysaccharide. Food Chem. 2022, 388, 133000. [Google Scholar] [CrossRef]
- Mlcek, J.; Jurikova, T.; Skrovankova, S.; Sochor, J. Quercetin and Its Anti-Allergic Immune Response. Molecules 2016, 21, 623. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, G.S.; Santos, L.S.; Vieira, G.P.; Espírito-Santo, D.A.; Teixeira, R.S.; Matos, R.J.B.; Costa, C.A.S.; Deiró, T.C.B.J.; Barreto-Medeiros, J.M. Antioxidant, Anti-Inflammatory and Anti-Obesity Effects of Onion and Its by-Products in High-Fat Fed Rodents: A Systematic Review. Braz. J. Biol. Rev. Brasleira Biol. 2023, 83, e266108. [Google Scholar] [CrossRef]
- Grzelak-Błaszczyk, K.; Milala, J.; Kosmala, M.; Kołodziejczyk, K.; Sójka, M.; Czarnecki, A.; Klewicki, R.; Juśkiewicz, J.; Fotschki, B.; Jurgoński, A. Onion Quercetin Monoglycosides Alter Microbial Activity and Increase Antioxidant Capacity. J. Nutr. Biochem. 2018, 56, 81–88. [Google Scholar] [CrossRef]
- Yue, Z.; Li, C.; Liu, Y.; Liu, M.; Zhao, M.; Li, F.; Liu, L. Vitamin A Alleviates Heat Stress-Induced Damage to Hair Follicle Development in Rex Rabbits. J. Sci. Food Agric. 2022, 102, 2291–2299. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir, C.; Kucuksezer, U.C.; Ogulur, I.; Pat, Y.; Yazici, D.; Ardicli, S.; Akdis, M.; Nadeau, K.; Akdis, C.A. Lifestyle Changes and Industrialization in the Development of Allergic Diseases. Curr. Allergy Asthma Rep. 2024, 24, 331–345. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Nan, F.; Liang, H.; Shu, P.; Fan, X.; Song, X.; Hou, Y.; Zhang, D. Excessive Intake of Sugar: An Accomplice of Inflammation. Front. Immunol. 2022, 13, 988481. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.J.; Lee, J.Y.; Ko, D.S.; Son, E.; Shin, K.; Kim, W.K.; Kim, K.; Kim, Y.H. Exploring the Association between Alcohol Consumption and Androgenic Alopecia: A Systematic Review and Meta-Analysis. Alcohol Alcohol. 2024, 59, agae076. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, L.; Moog, P.; Li, C.; Steinbacher, L.; Knoedler, S.; Kükrek, H.; Dornseifer, U.; Machens, H.-G.; Jiang, J. Exploring the Association Between Multidimensional Dietary Patterns and Non-Scarring Hair Loss Using Mendelian Randomization. Nutrients 2025, 17, 2569. https://doi.org/10.3390/nu17152569
Pan L, Moog P, Li C, Steinbacher L, Knoedler S, Kükrek H, Dornseifer U, Machens H-G, Jiang J. Exploring the Association Between Multidimensional Dietary Patterns and Non-Scarring Hair Loss Using Mendelian Randomization. Nutrients. 2025; 17(15):2569. https://doi.org/10.3390/nu17152569
Chicago/Turabian StylePan, Lingfeng, Philipp Moog, Caihong Li, Leonard Steinbacher, Samuel Knoedler, Haydar Kükrek, Ulf Dornseifer, Hans-Günther Machens, and Jun Jiang. 2025. "Exploring the Association Between Multidimensional Dietary Patterns and Non-Scarring Hair Loss Using Mendelian Randomization" Nutrients 17, no. 15: 2569. https://doi.org/10.3390/nu17152569
APA StylePan, L., Moog, P., Li, C., Steinbacher, L., Knoedler, S., Kükrek, H., Dornseifer, U., Machens, H.-G., & Jiang, J. (2025). Exploring the Association Between Multidimensional Dietary Patterns and Non-Scarring Hair Loss Using Mendelian Randomization. Nutrients, 17(15), 2569. https://doi.org/10.3390/nu17152569