Comparative Effectiveness of Nutritional Supplements in the Treatment of Knee Osteoarthritis: A Network Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources and Searches
2.2. Study Selection
2.3. Data Extraction and Outcome Measures
2.4. Quality Evaluation and Risk of Bias Assessment
2.5. Data Synthesis and Statistical Analysis
3. Results
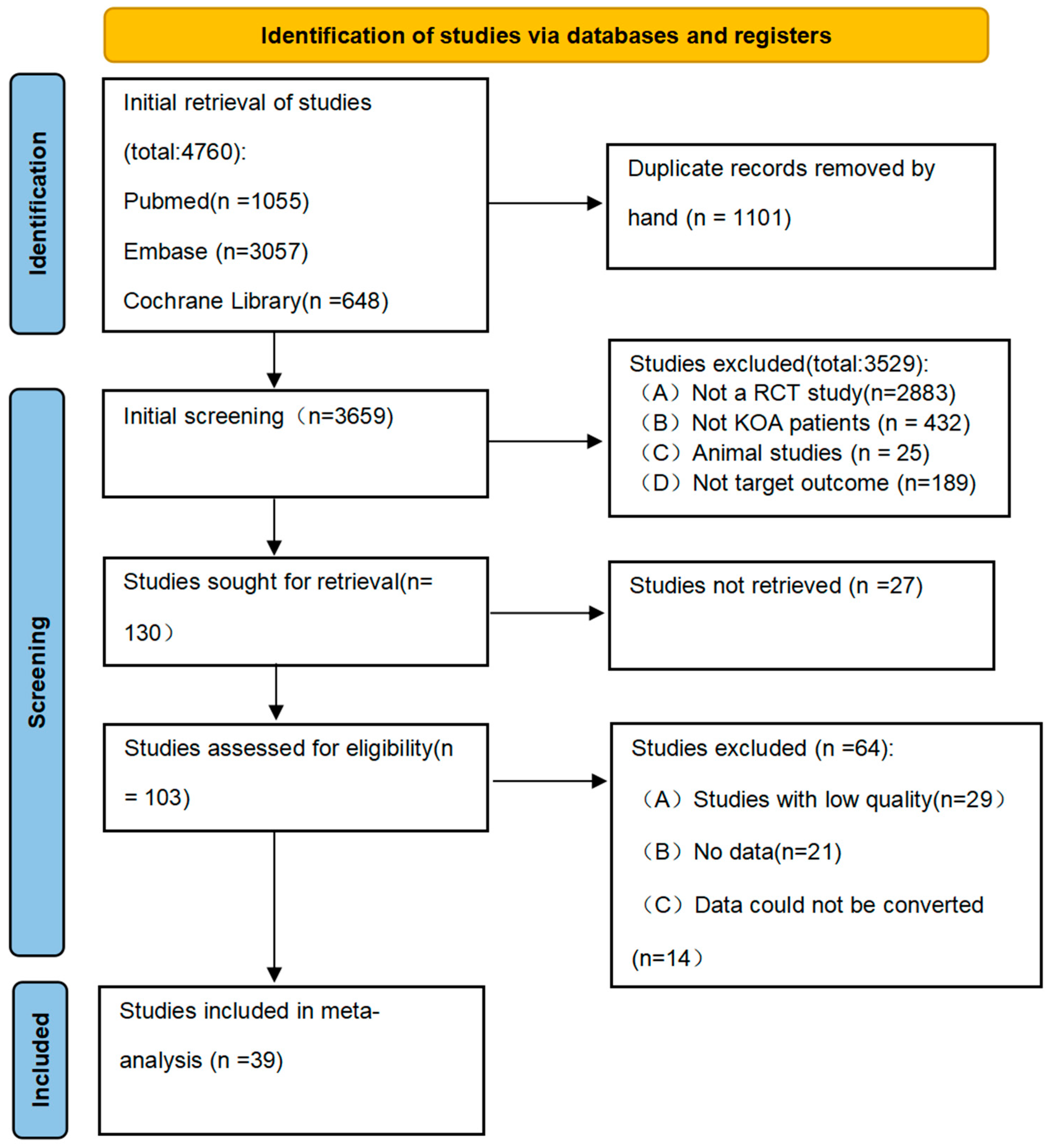
3.1. Literature Search and Screening
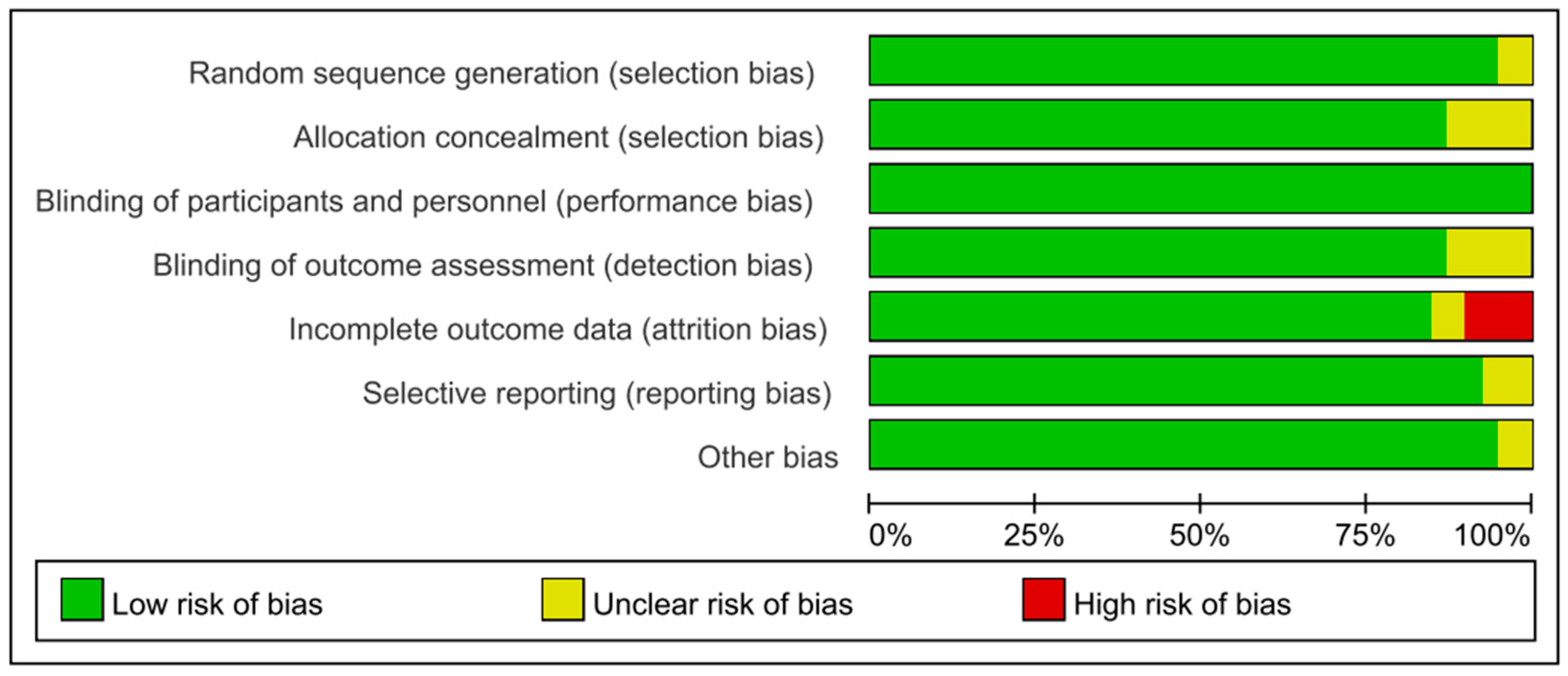
3.2. Quality Assessment of the Included Studies
3.3. Characteristics of the Eligible Studies
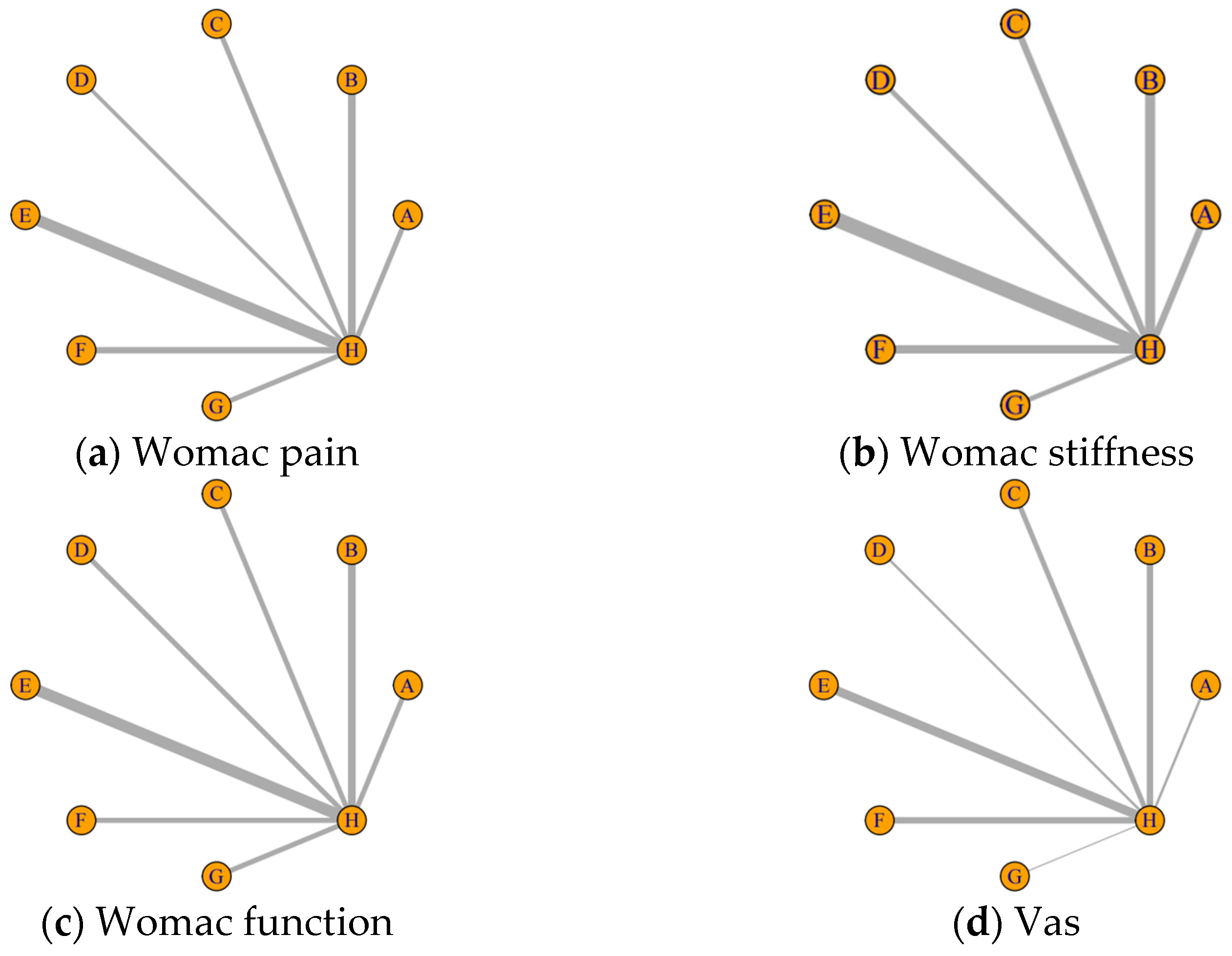
3.4. Results from Network Meta-Analysis
3.5. Adverse Events
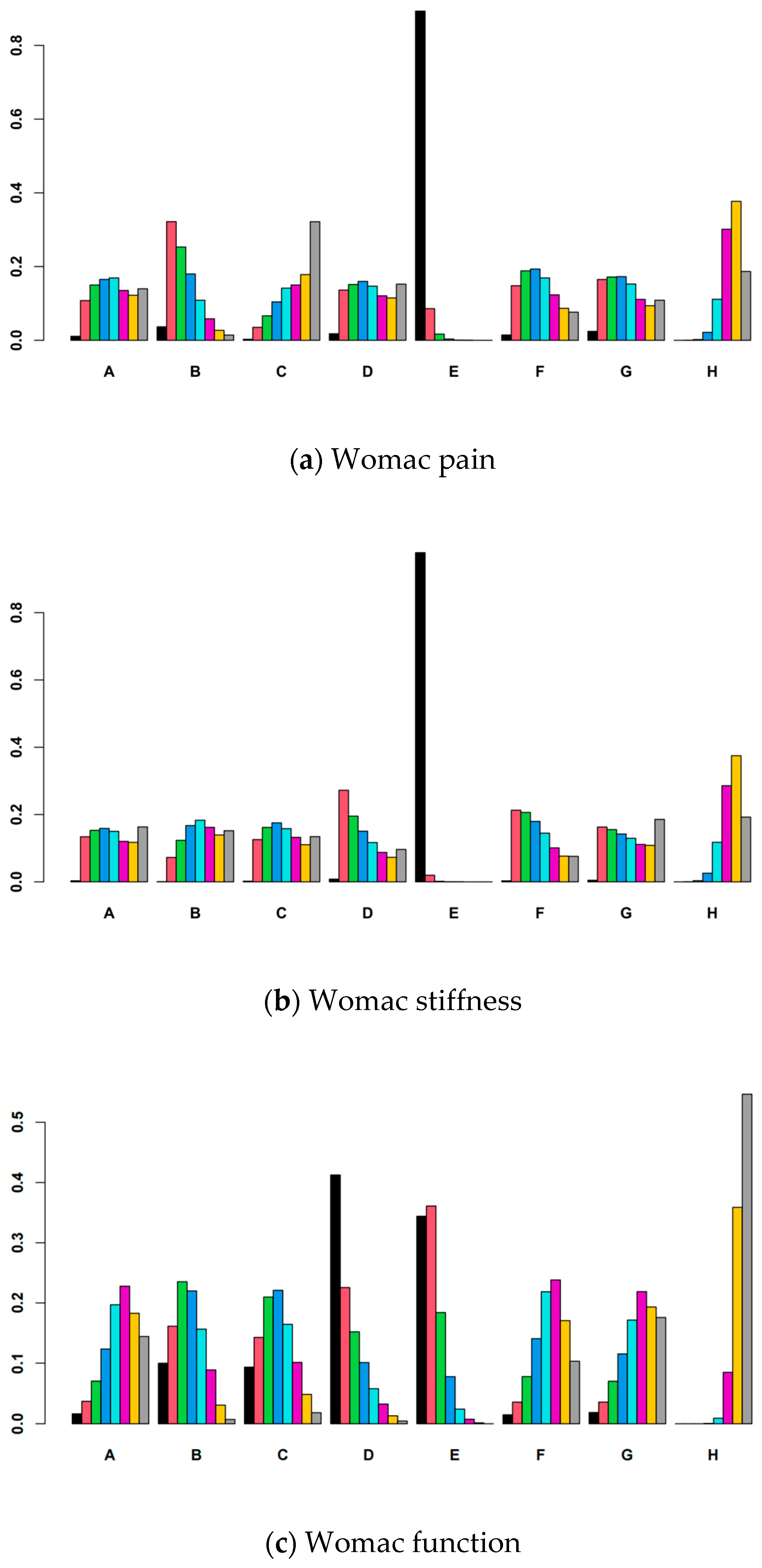
3.6. Ranking of Interventions
3.7. Publication Bias
3.8. Convergence Assessment
3.9. Assessment of Inconsistency
4. Discussion
4.1. Study Strengthens and Limitations
4.2. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Motta, F.; Barone, E.; Sica, A.; Selmi, C. Inflammaging and Osteoarthritis. Clin. Rev. Allergy Immunol. 2023, 64, 222–238. [Google Scholar] [CrossRef]
- Zhu, S.; Qu, W.; He, C. Evaluation and management of knee osteoarthritis. J. Evid.-Based Med. 2024, 17, 675–687. [Google Scholar] [CrossRef]
- McAlindon, T.E.; Bannuru, R.R.; Sullivan, M.C.; Arden, N.K.; Berenbaum, F.; Bierma-Zeinstra, S.M.; Hawker, G.A.; Henrotin, Y.; Hunter, D.J.; Kawaguchi, H.; et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthr. Cartil. 2014, 22, 363–388. [Google Scholar] [CrossRef] [PubMed]
- Kolasinski, S.L.; Neogi, T.; Hochberg, M.C.; Oatis, C.; Guyatt, G.; Block, J.; Callahan, L.; Copenhaver, C.; Dodge, C.; Felson, D.; et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Management of Osteoarthritis of the Hand, Hip, and Knee. Arthritis Rheumatol. 2020, 72, 220–233. [Google Scholar] [CrossRef] [PubMed]
- da Costa, B.R.; Pereira, T.V.; Saadat, P.; Rudnicki, M.; Iskander, S.M.; Bodmer, N.S.; Bobos, P.; Gao, L.; Kiyomoto, H.D.; Montezuma, T.; et al. Effectiveness and safety of non-steroidal anti-inflammatory drugs and opioid treatment for knee and hip osteoarthritis: Network meta-analysis. BMJ 2021, 375, n2321. [Google Scholar] [CrossRef] [PubMed]
- Aweid, O.; Haider, Z.; Saed, A.; Kalairajah, Y. Treatment modalities for hip and knee osteoarthritis: A systematic review of safety. J. Orthop. Surg. 2018, 26, 2309499018808669. [Google Scholar] [CrossRef]
- Fuggle, N.R.; Cooper, C.; Oreffo, R.O.C.; Price, A.J.; Kaux, J.F.; Maheu, E.; Cutolo, M.; Honvo, G.; Conaghan, P.G.; Berenbaum, F.; et al. Alternative and complementary therapies in osteoarthritis and cartilage repair. Aging Clin. Exp. Res. 2020, 32, 547–560. [Google Scholar] [CrossRef]
- Sellam, J.; Courties, A.; Eymard, F.; Ferrero, S.; Latourte, A.; Ornetti, P.; Bannwarth, B.; Baumann, L.; Berenbaum, F.; Chevalier, X.; et al. French Society of Rheumatology Recommendations of the French Society of Rheumatology on pharmacological treatment of knee osteoarthritis. Jt. Bone Spine 2020, 87, 548–555. [Google Scholar] [CrossRef]
- Marriott, K.A.; Birmingham, T.B. Fundamentals of osteoarthritis. Rehabilitation: Exercise, diet, biomechanics, and physical therapist-delivered interventions. Osteoarthr. Cartil. 2023, 31, 1312–1326. [Google Scholar] [CrossRef]
- Asadi, S.; Grafenauer, S.; Burley, C.V.; Fitzgerald, C.; Humburg, P.; Parmenter, B.J. The effectiveness of dietary intervention in osteoarthritis management: A systematic review and meta-analysis of randomized clinical trials. Eur. J. Clin. Nutr. 2025. [Google Scholar] [CrossRef]
- Bideshki, M.V.; Jourabchi-Ghadim, N.; Radkhah, N.; Behzadi, M.; Asemani, S.; Jamilian, P.; Zarezadeh, M. The efficacy of curcumin in relieving osteoarthritis: A meta-analysis of meta-analyses. Phytother. Res. 2024, 38, 2875–2891. [Google Scholar] [CrossRef] [PubMed]
- Bartels, E.M.; Folmer, V.N.; Bliddal, H.; Altman, R.D.; Juhl, C.; Tarp, S.; Zhang, W.; Christensen, R. Efficacy and safety of ginger in osteoarthritis patients: A meta-analysis of randomized placebo-controlled trials. Osteoarthr. Cartil. 2015, 23, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Araya-Quintanilla, F.; Gutierrez-Espinoza, H.; Munoz-Yanez, M.J.; Sanchez-Montoya, U.; Lopez-Jeldes, J. Effectiveness of Ginger on Pain and Function in Knee Osteoarthritis: A PRISMA Systematic Review and Meta-Analysis. Pain Physician 2020, 23, E151–E161. [Google Scholar] [CrossRef]
- Dalmonte, T.; Andreani, G.; Rudelli, C.; Isani, G. Efficacy of Extracts of Oleogum Resin of Boswellia in the Treatment of Knee Osteoarthritis: A Systematic Review and Meta-Analysis. Phytother. Res. 2024, 38, 5672–5689. [Google Scholar] [CrossRef] [PubMed]
- García-Coronado, J.M.; Martínez-Olvera, L.; Elizondo-Omaña, R.E.; Acosta-Olivo, C.A.; Vilchez-Cavazos, F.; Simental-Mendía, L.E.; Simental-Mendía, M. Effect of collagen supplementation on osteoarthritis symptoms: A meta-analysis of randomized placebo-controlled trials. Int. Orthop. 2019, 43, 531–538. [Google Scholar] [CrossRef]
- García-Muñoz, A.M.; Abellán-Ruiz, M.S.; García-Guillén, A.I.; Victoria-Montesinos, D. Efficacy of Eggshell Membrane in Knee Osteoarthritis: A Systematic Review and Meta-Analysis. Nutrients 2024, 16, 2640. [Google Scholar] [CrossRef]
- Pimentel, T.; Queiroz, I.; Florêncio de Mesquita, C.; Gallo Ruelas, M.; Leandro, G.N.; Ribeiro Monteiro, A.; Nunes Pimentel, F. Krill oil supplementation for knee pain: A systematic review and meta-analysis with trial sequential analysis of randomized controlled trials. Inflammopharmacology 2024, 32, 3109–3118. [Google Scholar] [CrossRef]
- Hill, W.S.; Dohnalek, M.H.; Ha, Y.; Kim, S.J.; Jung, J.C.; Kang, S.B. A Multicenter, Randomized, Double-Blinded, Placebo-Controlled Clinical Trial to Evaluate the Efficacy and Safety of a Krill Oil, Astaxanthin, and Oral Hyaluronic Acid Complex on Joint Health in People with Mild Osteoarthritis. Nutrients 2023, 15, 3769. [Google Scholar] [CrossRef]
- McAlindon, T.; LaValley, M.; Schneider, E.; Nuite, M.; Lee, J.Y.; Price, L.L.; Lo, G.; Dawson-Hughes, B. Effect of vitamin D supplementation on progression of knee pain and cartilage volume loss in patients with symptomatic osteoarthritis: A randomized controlled trial. JAMA 2013, 309, 155–162. [Google Scholar] [CrossRef]
- Hutton, B.; Salanti, G.; Caldwell, D.M.; Chaimani, A.; Schmid, C.H.; Cameron, C.; Ioannidis, J.P.; Straus, S.; Thorlund, K.; Jansen, J.P.; et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: Checklist and explanations. Ann. Intern. Med. 2015, 162, 777–784. [Google Scholar] [CrossRef]
- Woolacott, N.F.; Corbett, M.S.; Rice, S.J. The use and reporting of WOMAC in the assessment of the benefit of physical therapies for the pain of osteoarthritis of the knee: Findings from a systematic review of clinical trials. Rheumatology 2012, 51, 1440–1446. [Google Scholar] [CrossRef]
- Alghadir, A.H.; Anwer, S.; Iqbal, A.; Iqbal, Z.A. Test-retest reliability, validity, and minimum detectable change of visual analog, numerical rating, and verbal rating scales for measurement of osteoarthritic knee pain. J. Pain Res. 2018, 11, 851–856. [Google Scholar] [CrossRef]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Ruff, K.J.; Winkler, A.; Jackson, R.W.; DeVore, D.P.; Ritz, B.W. Eggshell membrane in the treatment of pain and stiffness from osteoarthritis of the knee: A randomized, multicenter, double-blind, placebo-controlled clinical study. Clin. Rheumatol. 2009, 28, 907–914. [Google Scholar] [CrossRef] [PubMed]
- Cánovas, F.; Abellán-Ruíz, M.S.; García-Muñoz, A.M.; Luque-Rubia, A.J.; Victoria-Montesinos, D.; Pérez-Piñero, S.; Sánchez-Macarro, M.; López-Román, F.J. Randomised Clinical Trial to Analyse the Efficacy of Eggshell Membrane to Improve Joint Functionality in Knee Osteoarthritis. Nutrients 2022, 14, 2340. [Google Scholar] [CrossRef]
- Hewlings, S.; Kalman, D.; Schneider, L.V. A Randomized, Double-Blind, Placebo-Controlled, Prospective Clinical Trial Evaluating Water-Soluble Chicken Eggshell Membrane for Improvement in Joint Health in Adults with Knee Osteoarthritis. J. Med. Food 2019, 22, 875–884. [Google Scholar] [CrossRef]
- Eskiyurt, N.; Sarıdoğan, M.; Senel, K.; Günaydin, R.; Erdal, A.; Özyiğit, E.; Akarrırmak, Ü.; Şendur, Ö.F.; Barut, K.; Akyüz, G.; et al. Efficacy and safety of natural eggshell membrane (NEM®) in patients with grade 2/3 knee osteoarthritis: A multi-center, randomized, double-blind, placebo-controlled, single-crossover clinical study. J. Arthritis 2019, 8, 1000285. [Google Scholar]
- Park, S.; Ko, S.-H.; Yoon, N.-K.; Kim, B.-K.; Kim, J.; Kang, E.-B.; Oh, M.; Son, C.-G.; Lee, E.-J. Efficacy of natural eggshell membrane for knee osteoarthritis: A randomized, double-blind, placebo-controlled clinical trial. J. Funct. Foods 2024, 121, 106449. [Google Scholar] [CrossRef]
- Hashemzadeh, K.; Davoudian, N.; Jaafari, M.R.; Mirfeizi, Z. The Effect of Nanocurcumin in Improvement of Knee Osteoarthritis: A Randomized Clinical Trial. Curr. Rheumatol. Rev. 2020, 16, 158–164. [Google Scholar] [CrossRef]
- Madhu, K.; Chanda, K.; Saji, M.J. Safety and efficacy of Curcuma longa extract in the treatment of painful knee osteoarthritis: A randomized placebo-controlled trial. Inflammopharmacology 2013, 21, 129–136. [Google Scholar] [CrossRef]
- Panahi, Y.; Rahimnia, A.R.; Sharafi, M.; Alishiri, G.; Saburi, A.; Sahebkar, A. Curcuminoid treatment for knee osteoarthritis: A randomized double-blind placebo-controlled trial. Phytother. Res. 2014, 28, 1625–1631. [Google Scholar] [CrossRef]
- Srivastava, S.; Saksena, A.K.; Khattri, S.; Kumar, S.; Dagur, R.S. Curcuma longa extract reduces inflammatory and oxidative stress biomarkers in osteoarthritis of knee: A four-month, double-blind, randomized, placebo-controlled trial. Inflammopharmacology 2016, 24, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Jones, G.; Winzenberg, T.; Cai, G.; Laslett, L.L.; Aitken, D.; Hopper, I.; Singh, A.; Jones, R.; Fripp, J.; et al. Effectiveness of Curcuma longa Extract for the Treatment of Symptoms and Effusion-Synovitis of Knee Osteoarthritis: A Randomized Trial. Ann. Intern. Med. 2020, 173, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Atabaki, M.; Shariati-Sarabi, Z.; Tavakkol-Afshari, J.; Mohammadi, M. Significant immunomodulatory properties of curcumin in patients with osteoarthritis; a successful clinical trial in Iran. Int. Immunopharmacol. 2020, 85, 106607. [Google Scholar] [CrossRef] [PubMed]
- Haroyan, A.; Mukuchyan, V.; Mkrtchyan, N.; Minasyan, N.; Gasparyan, S.; Sargsyan, A.; Narimanyan, M.; Hovhannisyan, A. Efficacy and safety of curcumin and its combination with boswellic acid in osteoarthritis: A comparative, randomized, double-blind, placebo-controlled study. BMC Complement. Altern. Med. 2018, 18, 7. [Google Scholar] [CrossRef]
- Panda, S.K.; Nirvanashetty, S.; Parachur, V.A.; Mohanty, N.; Swain, T. A Randomized, Double Blind, Placebo Controlled, Parallel-Group Study to Evaluate the Safety and Efficacy of Curene® versus Placebo in Reducing Symptoms of Knee OA. Biomed Res. Int. 2018, 2018, 5291945. [Google Scholar] [CrossRef]
- Benito-Ruiz, P.; Camacho-Zambrano, M.M.; Carrillo-Arcentales, J.N.; Mestanza-Peralta, M.A.; Vallejo-Flores, C.A.; Vargas-López, S.V.; Villacís-Tamayo, R.A.; Zurita-Gavilanes, L.A. A randomized controlled trial on the efficacy and safety of a food ingredient, collagen hydrolysate, for improving joint comfort. Int. J. Food Sci. Nutr. 2009, 60 (Suppl. 2), 99–113. [Google Scholar] [CrossRef]
- Kumar, S.; Sugihara, F.; Suzuki, K.; Inoue, N.; Venkateswarathirukumara, S. A double-blind, placebo-controlled, randomised, clinical study on the effectiveness of collagen peptide on osteoarthritis. J. Sci. Food Agric. 2015, 95, 702–707. [Google Scholar] [CrossRef]
- Lugo, J.P.; Saiyed, Z.M.; Lane, N.E. Efficacy and tolerability of an undenatured type II collagen supplement in modulating knee osteoarthritis symptoms: A multicenter randomized, double-blind, placebo-controlled study. Nutr. J. 2016, 15, 14. [Google Scholar] [CrossRef]
- McAlindon, T.E.; Nuite, M.; Krishnan, N.; Ruthazer, R.; Price, L.L.; Burstein, D.; Griffith, J.; Flechsenhar, K. Change in knee osteoarthritis cartilage detected by delayed gadolinium enhanced magnetic resonance imaging following treatment with collagen hydrolysate: A pilot randomized controlled trial. Osteoarthr. Cartil. 2011, 19, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Schauss, A.G.; Stenehjem, J.; Park, J.; Endres, J.R.; Clewell, A. Effect of the novel low molecular weight hydrolyzed chicken sternal cartilage extract, BioCell Collagen, on improving osteoarthritis-related symptoms: A randomized, double-blind, placebo-controlled trial. J. Agric. Food Chem. 2012, 60, 4096–4101. [Google Scholar] [CrossRef] [PubMed]
- Stonehouse, W.; Benassi-Evans, B.; Bednarz, J.; Vincent, A.D.; Hall, S.; Hill, C.L. Krill oil improved osteoarthritic knee pain in adults with mild to moderate knee osteoarthritis: A 6-month multicenter, randomized, double-blind, placebo-controlled trial. Am. J. Clin. Nutr. 2022, 116, 672–685. [Google Scholar] [CrossRef] [PubMed]
- Laslett, L.L.; Scheepers, L.E.J.M.; Antony, B.; Wluka, A.E.; Cai, G.; Hill, C.L.; March, L.; Keen, H.I.; Otahal, P.; Cicuttini, F.M.; et al. Krill Oil for Knee Osteoarthritis: A Randomized Clinical Trial. JAMA 2024, 331, 1997–2006. [Google Scholar] [CrossRef]
- Deutsch, L. Evaluation of the effect of Neptune Krill Oil on chronic inflammation and arthritic symptoms. J. Am. Coll. Nutr. 2007, 26, 39–48. [Google Scholar] [CrossRef]
- Karlapudi, V.; Prasad Mungara, A.V.V.; Sengupta, K.; Davis, B.A.; Raychaudhuri, S.P. A Placebo-Controlled Double-Blind Study Demonstrates the Clinical Efficacy of a Novel Herbal Formulation for Relieving Joint Discomfort in Human Subjects with Osteoarthritis of Knee. J. Med. Food 2018, 21, 511–520. [Google Scholar] [CrossRef]
- Karlapudi, V.; Sunkara, K.B.; Konda, P.R.; Sarma, K.V.; Rokkam, M.P. Efficacy and Safety of Aflapin®, a Novel Boswellia Serrata Extract, in the Treatment of Osteoarthritis of the Knee: A Short-Term 30-Day Randomized, Double-Blind, Placebo-Controlled Clinical Study. J. Am. Nutr. Assoc. 2023, 42, 159–168. [Google Scholar] [CrossRef]
- Sengupta, K.; Alluri, K.V.; Satish, A.R.; Mishra, S.; Golakoti, T.; Sarma, K.V.; Dey, D.; Raychaudhuri, S.P. A double blind, randomized, placebo controlled study of the efficacy and safety of 5-Loxin for treatment of osteoarthritis of the knee. Arthritis Res. Ther. 2008, 10, R85. [Google Scholar] [CrossRef]
- Kumar, B.; Ghaytidak, A.B.; Pandey, A.K.; Somepalli, R.R.; Sarda, P.; Raychaudhuri, S.P.; Rokkam, M.P. A Standardized Boswellia serrata Extract Improves Knee Joint Function and Cartilage Morphology in Human Volunteers with Mild to Moderate Osteoarthritis in a Randomized Placebo-Controlled Study. J. Am. Nutr. Assoc. 2025, 44, 375–386. [Google Scholar] [CrossRef]
- Sengupta, K.; Krishnaraju, A.V.; Vishal, A.A.; Mishra, A.; Trimurtulu, G.; Sarma, K.V.; Raychaudhuri, S.K.; Raychaudhuri, S.P. Comparative efficacy and tolerability of 5-Loxin and AflapinAgainst osteoarthritis of the knee: A double blind, randomized, placebo controlled clinical study. Int. J. Med. Sci. 2010, 7, 366–377. [Google Scholar] [CrossRef]
- Vishal, A.A.; Mishra, A.; Raychaudhuri, S.P. A double blind, randomized, placebo controlled clinical study evaluates the early efficacy of aflapin in subjects with osteoarthritis of knee. Int. J. Med. Sci. 2011, 8, 615–622. [Google Scholar] [CrossRef]
- Majeed, M.; Majeed, S.; Narayanan, N.K.; Nagabhushanam, K. A pilot, randomized, double-blind, placebo-controlled trial to assess the safety and efficacy of a novel Boswellia serrata extract in the management of osteoarthritis of the knee. Phytother. Res. 2019, 33, 1457–1468. [Google Scholar] [CrossRef]
- Haghighi, M.; Khalvat, A.; Toliat, T.; Jallaei, S. Comparing the effects of ginger (zingiber officinale) extract and ibuprofen on patients with osteoarthritis. Arch. Iran. Med. 2005, 8, 267–271. [Google Scholar]
- Zakeri, Z.; Izadi, S.; Bari, Z.; Soltani, F.; Narouie, B.; Ghasemi-rad, M. Evaluating the effects of ginger extract on knee pain, stiffness and difficulty in patients with knee osteoarthritis. J. Med. Plants Res. 2011, 5, 3375–3379. [Google Scholar]
- Altman, R.D.; Marcussen, K.C. Effects of a ginger extract on knee pain in patients with osteoarthritis. Arthritis Rheum. 2001, 44, 2531–2538. [Google Scholar] [CrossRef]
- Wigler, I.; Grotto, I.; Caspi, D.; Yaron, M. The effects of Zintona EC (a ginger extract) on symptomatic gonarthritis. Osteoarthr. Cartil. 2003, 11, 783–789. [Google Scholar] [CrossRef]
- Afshar, F.; Abdolahi, N.; Amin, G.; Esmaily, H.; Ziayie, S.; Azimi, S.; Darvishi, B.; Afshar, S. A randomized, double-blind placebo-controlled phase I clinical study on safety and efficacy of the G-Rup® syrup (a mixture of ginger extract and honey) in symptomatic treatment of knee osteoarthritis. J. Clin. Pharm. Ther. 2022, 47, 2295–2301. [Google Scholar] [CrossRef] [PubMed]
- Baek, H.I.; Shen, L.; Ha, K.C.; Park, Y.K.; Kim, C.S.; Kwon, J.E.; Park, S.J. Effectiveness and safety of steamed ginger extract on mild osteoarthritis: A randomized, double-blind, placebo-controlled clinical trial. Food Funct. 2024, 15, 9512–9523. [Google Scholar] [CrossRef] [PubMed]
- Arden, N.K.; Cro, S.; Sheard, S.; Doré, C.J.; Bara, A.; Tebbs, S.A.; Hunter, D.J.; James, S.; Cooper, C.; O’Neill, T.W.; et al. The effect of vitamin D supplementation on knee osteoarthritis, the VIDEO study: A randomised controlled trial. Osteoarthr. Cartil. 2016, 24, 1858–1866. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Jones, G.; Cicuttini, F.; Wluka, A.; Zhu, Z.; Han, W.; Antony, B.; Wang, X.; Winzenberg, T.; Blizzard, L.; et al. Effect of Vitamin D Supplementation on Tibial Cartilage Volume and Knee Pain Among Patients with Symptomatic Knee Osteoarthritis: A Randomized Clinical Trial. JAMA 2016, 315, 1005–1013. [Google Scholar] [CrossRef]
- Sanghi, D.; Mishra, A.; Sharma, A.C.; Singh, A.; Natu, S.M.; Agarwal, S.; Srivastava, R.N. Does vitamin D improve osteoarthritis of the knee: A randomized controlled pilot trial. Clin. Orthop. Relat. Res. 2013, 471, 3556–3562. [Google Scholar] [CrossRef] [PubMed]
- Primorac, D.; Molnar, V.; Rod, E.; Jeleč, Ž.; Čukelj, F.; Matišić, V.; Vrdoljak, T.; Hudetz, D.; Hajsok, H.; Borić, I. Knee Osteoarthritis: A Review of Pathogenesis and State-Of-The-Art Non-Operative Therapeutic Considerations. Genes 2020, 11, 854. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Yin, J.; Gao, J.; Cheng, T.S.; Pavlos, N.J.; Zhang, C.; Zheng, M.H. Subchondral bone in osteoarthritis: Insight into risk factors and microstructural changes. Arthritis Res. Ther. 2013, 15, 223. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Zhao, X.; Wang, C.; Geng, Y.; Zhao, J.; Xu, J.; Zuo, B.; Zhao, C.; Wang, C.; Zhang, X. MicroRNA-145 attenuates TNF-α-driven cartilage matrix degradation in osteoarthritis via direct suppression of MKK4. Cell Death Dis. 2017, 8, e3140. [Google Scholar] [CrossRef]
- Mathiessen, A.; Conaghan, P.G. Synovitis in osteoarthritis: Current understanding with therapeutic implications. Arthritis Res. Ther. 2017, 19, 18. [Google Scholar] [CrossRef]
- Dai, Z.; Yang, T.; Liu, J. Contralateral knee osteoarthritis is a risk factor for ipsilateral knee osteoarthritis progressing: A case control study. BMC Musculoskelet. Disord. 2024, 25, 190. [Google Scholar] [CrossRef]
- Tsonga, T.; Michalopoulou, M.; Malliou, P.; Godolias, G.; Kapetanakis, S.; Gkasdaris, G.; Soucacos, P. Analyzing the History of Falls in Patients with Severe Knee Osteoarthritis. Clin. Orthop. Surg. 2015, 7, 449–456. [Google Scholar] [CrossRef]
- Yang, W.; Ma, G.; Li, J.; Guan, T.; He, D.; Yang, D.; Wang, G.; Shi, H. Anxiety and depression as potential risk factors for limited pain management in patients with elderly knee osteoarthritis: A cross-lagged study. BMC Musculoskelet. Disord. 2024, 25, 995. [Google Scholar] [CrossRef]
- Messier, S.P.; Beavers, D.P.; Queen, K.; Mihalko, S.L.; Miller, G.D.; Losina, E.; Katz, J.N.; Loeser, R.F.; DeVita, P.; Hunter, D.J.; et al. Effect of Diet and Exercise on Knee Pain in Patients with Osteoarthritis and Overweight or Obesity: A Randomized Clinical Trial. JAMA 2022, 328, 2242–2251. [Google Scholar] [CrossRef]
- Luan, L.; El-Ansary, D.; Adams, R.; Wu, S.; Han, J. Knee osteoarthritis pain and stretching exercises: A systematic review and meta-analysis. Physiotherapy 2022, 114, 16–29. [Google Scholar] [CrossRef]
- Salis, Z.; Sainsbury, A. Association of long-term use of non-steroidal anti-inflammatory drugs with knee osteoarthritis: A prospective multi-cohort study over 4-to-5 years. Sci. Rep. 2024, 14, 6593. [Google Scholar] [CrossRef]
- Cheung, A.; Goh, S.K.; Tang, A.; Tay, B. Complications of total knee arthroplasty. Curr. Orthop. 2008, 22, 274–283. [Google Scholar] [CrossRef]
- Aghamohammadi, D.; Dolatkhah, N.; Bakhtiari, F.; Eslamian, F.; Hashemian, M. Nutraceutical supplements in management of pain and disability in osteoarthritis: A systematic review and meta-analysis of randomized clinical trials. Sci. Rep. 2020, 10, 20892. [Google Scholar] [CrossRef]
- Wu, D.; Lewis, E.D.; Pae, M.; Meydani, S.N. Nutritional Modulation of Immune Function: Analysis of Evidence, Mechanisms, and Clinical Relevance. Front. Immunol. 2019, 9, 3160. [Google Scholar] [CrossRef] [PubMed]
- D’Adamo, S.; Cetrullo, S.; Panichi, V.; Mariani, E.; Flamigni, F.; Borzì, R.M. Nutraceutical Activity in Osteoarthritis Biology: A Focus on the Nutrigenomic Role. Cells 2020, 9, 1232. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Jung, J.I.; Bae, J.; Lee, J.K.; Kim, E.J. Evaluating the Anti-Osteoarthritis Potential of Standardized Boswellia serrata Gum Resin Extract in Alleviating Knee Joint Pathology and Inflammation in Osteoarthritis-Induced Models. Int. J. Mol. Sci. 2024, 25, 3218. [Google Scholar] [CrossRef] [PubMed]
- Poeckel, D.; Werz, O. Boswellic acids: Biological actions and molecular targets. Curr. Med. Chem. 2006, 13, 3359–3369. [Google Scholar] [CrossRef]
- Ammon, H.P. Modulation of the immune system by Boswellia serrata extracts and boswellic acids. Phytomedicine Int. J. Phytother. Phytopharm. 2010, 17, 862–867. [Google Scholar] [CrossRef]
- Bannuru, R.R.; Osani, M.C.; Al-Eid, F.; Wang, C. Efficacy of curcumin and Boswellia for knee osteoarthritis: Systematic review and meta-analysis. Semin. Arthritis Rheum. 2018, 48, 416–429. [Google Scholar] [CrossRef]
- Yu, G.; Xiang, W.; Zhang, T.; Zeng, L.; Yang, K.; Li, J. Effectiveness of Boswellia and Boswellia extract for osteoarthritis patients: A systematic review and meta-analysis. BMC Complement. Med. Ther. 2020, 20, 225. [Google Scholar] [CrossRef]
- Lopresti, A.L.; Smith, S.J.; Jackson-Michel, S.; Fairchild, T. An Investigation into the Effects of a Curcumin Extract (Curcugen®) on Osteoarthritis Pain of the Knee: A Randomised, Double-Blind, Placebo-Controlled Study. Nutrients 2021, 14, 41. [Google Scholar] [CrossRef] [PubMed]
- Koroljević, Z.D.; Jordan, K.; Ivković, J.; Bender, D.V.; Perić, P. Curcuma as an anti-inflammatory component in treating osteoarthritis. Rheumatol. Int. 2023, 43, 589–616. [Google Scholar] [CrossRef]
- Razavi, B.M.; Ghasemzadeh Rahbardar, M.; Hosseinzadeh, H. A review of therapeutic potentials of turmeric (Curcuma longa) and its active constituent, curcumin, on inflammatory disorders, pain, and their related patents. Phytother. Res. 2021, 35, 6489–6513. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Singh, A.; Jones, G.; Winzenberg, T.; Ding, C.; Chopra, A.; Das, S.; Danda, D.; Laslett, L.; Antony, B. Efficacy and Safety of Turmeric Extracts for the Treatment of Knee Osteoarthritis: A Systematic Review and Meta-analysis of Randomised Controlled Trials. Curr. Rheumatol. Rep. 2021, 23, 11. [Google Scholar] [CrossRef] [PubMed]
- Lao, C.D.; Ruffin, M.T., IV; Normolle, D.; Heath, D.D.; Murray, S.I.; Bailey, J.M.; Boggs, M.E.; Crowell, J.; Rock, C.L.; Brenner, D.E. Dose escalation of a curcuminoid formulation. BMC Complement. Altern. Med. 2006, 6, 10. [Google Scholar] [CrossRef]
- Baharizade, M.; Ghetmiri, S.I.; Mohammady, M.; Mohammadi-Samani, S.; Yousefi, G. Revolutionizing Knee Osteoarthritis Treatment: Innovative Self-Nano-Emulsifying Polyethylene Glycol Organogel of Curcumin for Effective Topical Delivery. AAPS PharmSciTech 2024, 25, 80. [Google Scholar] [CrossRef]
- Zeng, L.; Yu, G.; Hao, W.; Yang, K.; Chen, H. The efficacy and safety of Curcuma longa extract and curcumin supplements on osteoarthritis: A systematic review and meta-analysis. Biosci. Rep. 2021, 41, BSR20210817. [Google Scholar] [CrossRef]
- Mao, Q.Q.; Xu, X.Y.; Cao, S.Y.; Gan, R.Y.; Corke, H.; Beta, T.; Li, H.B. Bioactive Compounds and Bioactivities of Ginger (Zingiber officinale Roscoe). Foods 2019, 8, 185. [Google Scholar] [CrossRef]
- Paramdeep, G. Efficacy and tolerability of ginger (Zingiber officinale) in patients of osteoarthritis of knee. Indian J. Physiol. Pharmacol. 2013, 57, 177–183. [Google Scholar]
- Mashhadi, N.S.; Ghiasvand, R.; Askari, G.; Hariri, M.; Darvishi, L.; Mofid, M.R. Anti-oxidative and anti-inflammatory effects of ginger in health and physical activity: Review of current evidence. Int. J. Prev. Med. 2013, 4 (Suppl. 1), S36–S42. [Google Scholar]
- Shen, C.L.; Hong, K.J.; Kim, S.W. Comparative effects of ginger root (Zingiber officinale Rosc.) on the production of inflammatory mediators in normal and osteoarthrotic sow chondrocytes. J. Med. Food 2005, 8, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Rondanelli, M.; Fossari, F.; Vecchio, V.; Gasparri, C.; Peroni, G.; Spadaccini, D.; Riva, A.; Petrangolini, G.; Iannello, G.; Nichetti, M.; et al. Clinical trials on pain lowering effect of ginger: A narrative review. Phytother. Res. 2020, 34, 2843–2856. [Google Scholar] [CrossRef] [PubMed]
- Sulejmanović, M.; Panić, M.; Redovniković, I.R.; Milić, N.; Drljača, J.; Damjanović, A.; Vidović, S. Sustainable isolation of ginger (Zingiber officinale) herbal dust bioactive compounds with favorable toxicological profile employing natural deep eutectic solvents (NADES). Food Chem. 2025, 464 Pt 1, 141545. [Google Scholar] [CrossRef] [PubMed]
- Tou, J.C.; Jaczynski, J.; Chen, Y.C. Krill for human consumption: Nutritional value and potential health benefits. Nutr. Rev. 2007, 65, 63–77. [Google Scholar] [CrossRef]
- Wang, K.; Han, L.; Zhu, Y.; Liu, Y.; Wang, J.; Xue, C. Antarctic krill oil improves articular cartilage degeneration via activating chondrocyte autophagy and inhibiting apoptosis in osteoarthritis mice. J. Funct. Foods 2018, 46, 413–422. [Google Scholar] [CrossRef]
- Lee, M.; Kim, D.; Park, S.J.; Yun, J.M.; Oh, D.H.; Lee, J. Antarctic Krill Oil Ameliorates Monosodium Iodoacetate-Induced Irregularities in Articular Cartilage and Inflammatory Response in the Rat Models of Osteoarthritis. Nutrients 2020, 12, 3550. [Google Scholar] [CrossRef]
- Meng, J.; Wang, X.; Li, Y.; Xiang, Y.; Wu, Y.; Xiong, Y.; Liu, P.; Gao, S. Krill oil for knee osteoarthritis: A meta-analysis of randomized controlled trials. Medicine 2025, 104, e41566. [Google Scholar] [CrossRef]
- Siadat, S.M.; Ruberti, J.W. Mechanochemistry of collagen. Acta Biomater. 2023, 163, 50–62. [Google Scholar] [CrossRef]
- Virgilio, N.; Schön, C.; Mödinger, Y.; van der Steen, B.; Vleminckx, S.; van Holthoon, F.L.; Kleinnijenhuis, A.J.; Silva, C.I.F.; Prawitt, J. Absorption of bioactive peptides following collagen hydrolysate intake: A randomized, double-blind crossover study in healthy individuals. Front. Nutr. 2024, 11, 1416643. [Google Scholar] [CrossRef]
- Castrogiovanni, P.; Trovato, F.M.; Loreto, C.; Nsir, H.; Szychlinska, M.A.; Musumeci, G. Nutraceutical Supplements in the Management and Prevention of Osteoarthritis. Int. J. Mol. Sci. 2016, 17, 2042. [Google Scholar] [CrossRef]
- Broere, F.; Wieten, L.; Klein Koerkamp, E.I.; van Roon, J.A.; Guichelaar, T.; Lafeber, F.P.; van Eden, W. Oral or nasal antigen induces regulatory T cells that suppress arthritis and proliferation of arthritogenic T cells in joint draining lymph nodes. J. Immunol. 2008, 181, 899–906. [Google Scholar] [CrossRef]
- Müller, R.D.; John, T.; Kohl, B.; Oberholzer, A.; Gust, T.; Hostmann, A.; Hellmuth, M.; Laface, D.; Hutchins, B.; Laube, G.; et al. IL-10 overexpression differentially affects cartilage matrix gene expression in response to TNF-alpha in human articular chondrocytes in vitro. Cytokine 2008, 44, 377–385. [Google Scholar] [CrossRef]
- van Meegeren, M.E.; Roosendaal, G.; Jansen, N.W.; Wenting, M.J.; van Wesel, A.C.; van Roon, J.A.; Lafeber, F.P. IL-4 alone and in combination with IL-10 protects against blood-induced cartilage damage. Osteoarthr. Cartil. 2012, 20, 764–772. [Google Scholar] [CrossRef]


| Study | Country Year | N | Treatment | Control | Treatment Duration | Age (Years) | Female | Dosage | Outcome | |
|---|---|---|---|---|---|---|---|---|---|---|
| T | C | |||||||||
| Ruff et al. [25] | USA 2009 | 60 | Eggshell | Placebo | 60 days | NR | NR | NR | NEM® 500 mg/day | WOMAC |
| Cánovas et al. [26] | Spain 2022 | 51 | Eggshell | Placebo | 8 weeks | 36.36 ± 13.54 | 41.31 ± 14.36 | 27/51 | ESM® 500 mg/day | VAS |
| Hewlings et al. [27] | USA 2019 | 88 | Eggshell | Placebo | 12 weeks | NR | NR | 63/88 | BiovaFlex® 450 mg/day | WOMAC |
| Eskiyurt et al. [28] | Turkey 2019 | 166 | Eggshell | Placebo | 90 days | 55.9 ± 11.9 | 58.5 ± 9.7 | 134/166 | NEM® 500 mg/day | WOMAC |
| Park et al. [29] | Korea 2024 | 99 | Eggshell | Placebo | 12 weeks | 57.73 ± 7.75 | 58.54 ± 8.28 | 79/99 | NEM® 500 mg/day | WOMAC; VAS |
| Hashemzadeh et al. [30] | Iran 2024 | 71 | Curcumin | Placebo | 6 weeks | 54.11 ± 5.80 | 56.54 ± 5.77 | 60/71 | SinaCurcumin™ 80 mg/day | WOMAC |
| Madhu et al. [31] | India 2013 | 60 | Curcumin | Placebo | 6 weeks | 56.63 ± 10.58 | 56.77 ± 9.98 | 34/60 | Turmacin™ 1000 mg/day | VAS |
| Panahi et al. [32] | Iran 2014 | 40 | Curcumin | Placebo | 6 weeks | 57.32 ± 8.78 | 57.57 ± 9.05 | 31/40 | C3 Complex® 1500 mg/day | WOMAC; VAS |
| Srivastava et al. [33] | India 2016 | 160 | Curcumin | Placebo | 16 weeks | 50.23 ± 8.08 | 50.27 ± 8.63 | 103/160 | Haridra® 1000 mg/day | WOMAC; VAS |
| Wang et al. [34] | Australia 2020 | 70 | Curcumin | Placebo | 12 weeks | 61.3 ± 8.5 | 62.4 ± 8.8 | 39/70 | Turmacin™ 1000 mg/day | WOMAC; VAS |
| Atabaki et al. [35] | Iran 2020 | 30 | Curcumin | Placebo | 12 weeks | 49.13 ± 8.87 | 48.26 ± 7.81 | 30/30 | SinaCurcumin® 80 mg/day | VAS |
| Haroyan et al. [36] | Armenia 2018 | 134 | Curcumin | Placebo | 12 weeks | 54.65 ± 8.84 | 56.04 ± 8.55 | 127/134 | CuraMed® 1500 mg | WOMAC |
| Panda et al. [37] | India 2018 | 50 | Curcumin | Placebo | 12 weeks | 55.20 ± 8.58 | 53.12 ± 8.25 | NR | Curene® 500 mg | WOMAC; VAS |
| Benito-Ruiz et al. [38] | Spain 2009 | 207 | Collagen | Placebo | 24 weeks | 58.7 ± 10.4 | 59.1 ± 11.6 | 192/207 | Colnatur® 10 g/day | WOMAC; VAS |
| Kumar et al. [39] | India 2014 | 60 | Collagen | Placebo | 13 weeks | NR | NR | BCP:18/30 PCP:27/30 | Pork Collagen Peptide 10 g/day Bovine Collagen Peptide 10 g/day | VAS |
| Lugo et al. [40] | USA 2016 | 121 | Collagen | Placebo | 24 weeks | 53.5 ± 7.9 | 53.1 ± 7.8 | 60/121 | UC-II® 40 mg/day | WOMAC; VAS |
| McAllindon et al. [41] | USA 2011 | 30 | Collagen | Placebo | 48 weeks | 58.9 ± 8.0 | 60.3 ± 8.5 | 18/30 | Fortigel® 10 g/day | WOMAC |
| Schauss et al. [42] | USA 2012 | 88 | Collagen | Placebo | 10 weeks | 54.3 ± 8.7 | 54.5 ± 9.8 | 41/88 | BioCell Collagen® 2000 mg/day | WOMAC |
| Stonehouse et al. [43] | Australia 2022 | 235 | Krill oil | Placebo | 24 weeks | 59.9 ± 6.3 | 59.3 ± 6.6 | 77/235 | Superba Boost™ 4 g/day | WOMAC |
| Laslet et al. [44] | Australian 2024 | 262 | Krill oil | Placebo | 24 weeks | 61.7 ± 9.3 | 61.4 ± 9.9 | 122/262 | krill oil softgel 2 g/day | WOMAC; VAS |
| Hill et al. [18] | Korean 2023 | 75 | Krill oil | Placebo | 12 weeks | 57.0 ± 10.28 | 59.0 ± 11.82 | 44/75 | FlexPro MD® 600 mg/day | WOMAC; VAS |
| Deutsch et al. [45] | Canada 2007 | 90 | Krill oil | Placebo | 30 days | 54.6 ± 14.8 | 55.3 ± 14.3 | 43/90 | NKO™ 300 mg/day | WOMAC |
| Karlapudi et al. [46] | India 2018 | 70 | Boswellia | Placebo | 90 days | 48.7 ± 1.13 | 50.3 ± 1.34 | 43/70 | LI73014F2 400 mg/day | WOMAC; VAS |
| Karlapudi et al. [47] | India2021 | 67 | Boswellia | Placebo | 30 days | 51.60 ± 8.48 | 51.81 ± 7.21 | 50/67 | Aflapin® 100 mg/day | WOMAC; VAS |
| Sengupta et al. [48] | India 2008 | 46 | Boswellia | Placebo | 90 days | 53.22 ± 8.73 | 52.43 ± 9.65 | 33/46 | 5-Loxin® 250 mg/day | WOMAC; VAS |
| Kumar et al. [49] | India 2024 | 80 | Boswellia | Placebo | 180 days | 48.60 ± 7.39 | 47.93 ± 7.89 | 47/80 | Aflapin® 100 mg/day | WOMAC; VAS |
| Sengupta et al. [50] | India 2010 | 76 | Boswellia | Placebo | 90 days | Aflapin®; 53.2 ± 7.9 5-Loxin® 51.6 ± 9.9 | 52.4 ± 7.5 | Aflapin®; 22/38 5-Loxin®; 26/38 | 5-Loxin® 100 mg/day Aflapin® 100 mg/day | WOMAC; VAS |
| Vishal et al. [51] | India 2011 | 59 | Boswellia | Placebo | 30 days | 53.2 ± 6.5 | 55.3 ± 8.8 | 37/59 | Aflapin® 100 mg/day | WOMAC VAS |
| Haroyan et al. [36] | Armenia 2018 | 135 | Boswellia | Placebo | 12 weeks | 57.91 ± 9.02 | 56.04 ± 8.55 | 127/135 | Curamin® 1500 mg/day | WOMAC |
| Majeed et al. [52] | India 2019 | 48 | Boswellia | Placebo | 120 days | NR | NR | 31/48 | Boswellin® 338.66 mg/day | WOMAC; VAS |
| Haghighi et al. [53] | Iran 2005 | 80 | Ginger | Placebo | 4 weeks | 58.3 ± 0.33 | 58.4 ± 0.36 | 23/80 | Zingiber officinale 30 mg/day | VAS |
| Zakeri et al. [54] | Iran 2011 | 204 | Ginger | Placebo | 6 weeks | 48.4 ± 11.1 | 45.74 ± 12.5 | 164/204 | Zintoma® 500 mg/day | WOMAC; VAS |
| Altman et al. [55] | USA 2001 | 247 | Ginger | Placebo | 6 weeks | 64.0 6 11.5 | 66.3 6 11.6 | 152/247 | EV.EXT 77 510 mg/day | WOMAC; VAS |
| Wigler et al. [56] | Israel 2003 | 29 | Ginger | Placebo | 12 weeks | 64.7 (47–85) | 59.3 (42–81) | 23/29 | Zintona EC® 1000 mg/day | WOMAC |
| Afshar et al. [57] | Iran 2022 | 43 | Ginger | Placebo | 12 weeks | 55.62 ± 8.646 | 54.86 ± 6.63 | 29/43 | G-Rup® 60 mL/day | WOMAC; VAS |
| Baek et al. [58] | Korea 2024 | 100 | Ginger | Placebo | 8 weeks | 60.66 ± 6.87 | 60.54 ± 6.34 | 78/100 | GGE03 1600 mg/day | WOMAC; VAS |
| Ardne NK et al. [59] | UK 2016 | 474 | Vitamin D | Placebo | 36 month | 64.0 ± 8.0 | 64.0 ± 8.0 | 289/474 | Cholecalciferol 800 IU/day | WOMAC |
| Jin XZ et al. [60] | Australia 2016 | 413 | Vitamin D | Placebo | 24 month | 63.5 ± 6.9 | 62.9 ± 7.2 | 208/413 | cholecalciferol 50,000 IU/day | WOMAC; VAS |
| McAlindon T et al. [19] | USA 2013 | 146 | Vitamin D | Placebo | 24 month | 61.8 ± 7.7 | 63 ± 9.3 | 89/146 | Cholecalciferol 2000 IU/day | WOMAC |
| Sanghi et al. [61] | India 2013 | 103 | Vitamin D | Placebo | 12 month | 53.24 ± 9.64 | 53.00 ± 7.44 | 66/103 | Cholecalciferol 60,000 IU/day | WOMAC |
| A | |||||||
| −2.47 (−11.18, 5.8) | B | ||||||
| 1.88 (−7.05, 10.97) | 4.32 (−3.8, 12.86) | C | |||||
| −0.11 (−9.81, 9.45) | 2.32 (−6.37, 11.46) | −2 (−11.4, 7.32) | D | ||||
| −8.56 (−16.27, −0.7) | −6.02 (−12.71, 0.97) | −10.34 (−17.89, −2.92) | −8.35 (−16.73, −0.11) | E | |||
| −0.71 (−9.49, 8.21) | 1.75 (−6.09, 10.04) | −2.54 (−11.19, 6.03) | −0.53 (−9.94, 8.77) | 7.82 (0.51, 15.06) | F | ||
| −0.7 (−10.24, 8.71) | 1.84 (−6.89, 10.49) | −2.53 (−11.8, 6.47) | −0.5 (−10.77, 9.18) | 7.78 (−0.28, 15.77) | 0.02 (−9.15, 8.8) | G | |
| 2.08 (−4.33, 8.71) | 4.55 (−0.57, 10.15) | 0.22 (−5.87, 6.44) | 2.23 (−4.9, 9.32) | 10.58 (6.45, 14.78) | 2.78 (−3.15, 8.75) | 2.77 (−3.93, 9.76) | H |
| A | |||||||
| 0.32 (−5.45, 6.12) | B | ||||||
| −0.07 (−6.12, 6.2) | −0.38 (−5.82, 5.21) | C | |||||
| −1.01 (−7.7, 5.81) | −1.31 (−7.53, 4.84) | −0.93 (−7.45, 5.54) | D | ||||
| −8.13 (−13.74, −2.64) | −8.46 (−13.21, −3.74) | −8.06 (−13.52, −2.97) | −7.11 (−13.21, −1.33) | E | |||
| −0.77 (−6.85, 5.29) | −1.07 (−6.63, 4.45) | −0.7 (−6.65, 5.03) | 0.25 (−6.43, 6.7) | 7.37 (2.12, 12.65) | F | ||
| −0.1 (−6.86, 6.98) | −0.36 (−6.73, 6.04) | 0.03 (−6.63, 6.73) | 0.94 (−6.26, 8.14) | 8.07 (2.1, 14.31) | 0.68 (−5.77, 7.47) | G | |
| 1.32 (−3.13, 6.06) | 1.02 (−2.54, 4.78) | 1.4 (−2.78, 5.5) | 2.33 (−2.66, 7.36) | 9.47 (6.39, 12.74) | 2.1 (−1.99, 6.41) | 1.37 (−3.79, 6.69) | H |
| A | |||||||
| −5.4 (−18.82, 7.65) | B | ||||||
| −4.87 (−18.75, 8.88) | 0.53 (−12.14, 13.66) | C | |||||
| −9.46 (−24.1, 5.1) | −4.12 (−17.58, 10) | −4.59 (−18.99, 9.77) | D | ||||
| −9.42 (−21.19, 2.53) | −4.08 (−14.46, 6.96) | −4.55 (−15.75, 7) | 0.06 (−12.45, 12.66) | E | |||
| −0.54 (−14.14, 13.39) | 4.9 (−7.53, 17.5) | 4.43 (−8.94, 17.59) | 8.96 (−5.33, 23.24) | 8.89 (−2.27, 20.15) | F | ||
| 0.34 (−14.42, 14.64) | 5.78 (−8.12, 19.31) | 5.25 (−9.34, 19.16) | 9.85 (−5.81, 24.68) | 9.74 (−2.84, 21.84) | 0.86 (−13.35, 14.49) | G | |
| 4.51 (−5.42, 14.59) | 9.96 (1.44, 18.79) | 9.42 (0.02, 19) | 14.01 (3.37, 24.93) | 14 (7.74, 20.21) | 5.05 (−4.24, 14.45) | 4.21 (−6.04, 15.16) | H |
| A | |||||||
| −4.01 (−24.14, 16.25) | B | ||||||
| −8.43 (−29.11, 12.55) | −4.37 (−20.82, 12.4) | C | |||||
| 3.35 (−20.83, 27.84) | 7.4 (−13.35, 28.44) | 11.65 (−9.64, 33.05) | D | ||||
| −8.94 (−28.14, 10.08) | −4.92 (−19.24, 9.39) | −0.61 (−16.03, 14.94) | −12.21 (−32.3, 7.75) | E | |||
| −3.57 (−23.43, 16.36) | 0.43 (−14.72, 16.08) | 4.76 (−11.53, 21.43) | −6.96 (−27.22, 13.61) | 5.4 (−8.82, 19.57) | F | ||
| 2.86 (−26.46, 31.87) | 6.84 (−20.02, 34.24) | 11.13 (−15.88, 38.52) | −0.5 (−30.85, 29.73) | 11.86 (−14.15, 37.82) | 6.48 (−20.14, 33.17) | G | |
| 8.26 (−8.33, 25.17) | 12.34 (1.59, 23.34) | 16.65 (4.32, 29.09) | 4.96 (−12.72, 22.35) | 17.26 (8.06, 26.52) | 11.89 (1.01, 22.49) | 5.41 (−18.87, 29.73) | H |
| Literature Source | Experimental Group Intervention | Adverse Reactions in the Control Group | Adverse Reactions in the Experimental Group |
|---|---|---|---|
| Hewlings et al., 2019 [27] | Eggshell Membrane | One case of headache and one case of poor sleep quality. | N |
| Park et al., 2024 [29] | Eggshell Membrane | One case of rash and itching occurring on the limbs and back. | N |
| Wang et al., 2020 [34] | Curcumin | One case each of nausea and vomiting, bloating, fatigue, drowsiness, sore throat with fever, and a sensation of fullness in the upper abdomen. | N |
| Wigler et al., 2003 [56] | Ginger | Two cases of heartburn | N |
| Jin XZ et al., 2016 [60] | Vitamin D | Four cases of hypercalcemia | Four cases of hypercalcemia |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Gui, Y.; Adams, R.; Farragher, J.; Itsiopoulos, C.; Bow, K.; Cai, M.; Han, J. Comparative Effectiveness of Nutritional Supplements in the Treatment of Knee Osteoarthritis: A Network Meta-Analysis. Nutrients 2025, 17, 2547. https://doi.org/10.3390/nu17152547
Zhang Y, Gui Y, Adams R, Farragher J, Itsiopoulos C, Bow K, Cai M, Han J. Comparative Effectiveness of Nutritional Supplements in the Treatment of Knee Osteoarthritis: A Network Meta-Analysis. Nutrients. 2025; 17(15):2547. https://doi.org/10.3390/nu17152547
Chicago/Turabian StyleZhang, Yuntong, Yunfei Gui, Roger Adams, Joshua Farragher, Catherine Itsiopoulos, Keegan Bow, Ming Cai, and Jia Han. 2025. "Comparative Effectiveness of Nutritional Supplements in the Treatment of Knee Osteoarthritis: A Network Meta-Analysis" Nutrients 17, no. 15: 2547. https://doi.org/10.3390/nu17152547
APA StyleZhang, Y., Gui, Y., Adams, R., Farragher, J., Itsiopoulos, C., Bow, K., Cai, M., & Han, J. (2025). Comparative Effectiveness of Nutritional Supplements in the Treatment of Knee Osteoarthritis: A Network Meta-Analysis. Nutrients, 17(15), 2547. https://doi.org/10.3390/nu17152547






