Role of Micronutrient Supplementation in Promoting Cognitive Healthy Aging in Latin America: Evidence-Based Consensus Statement
Abstract
1. Introduction
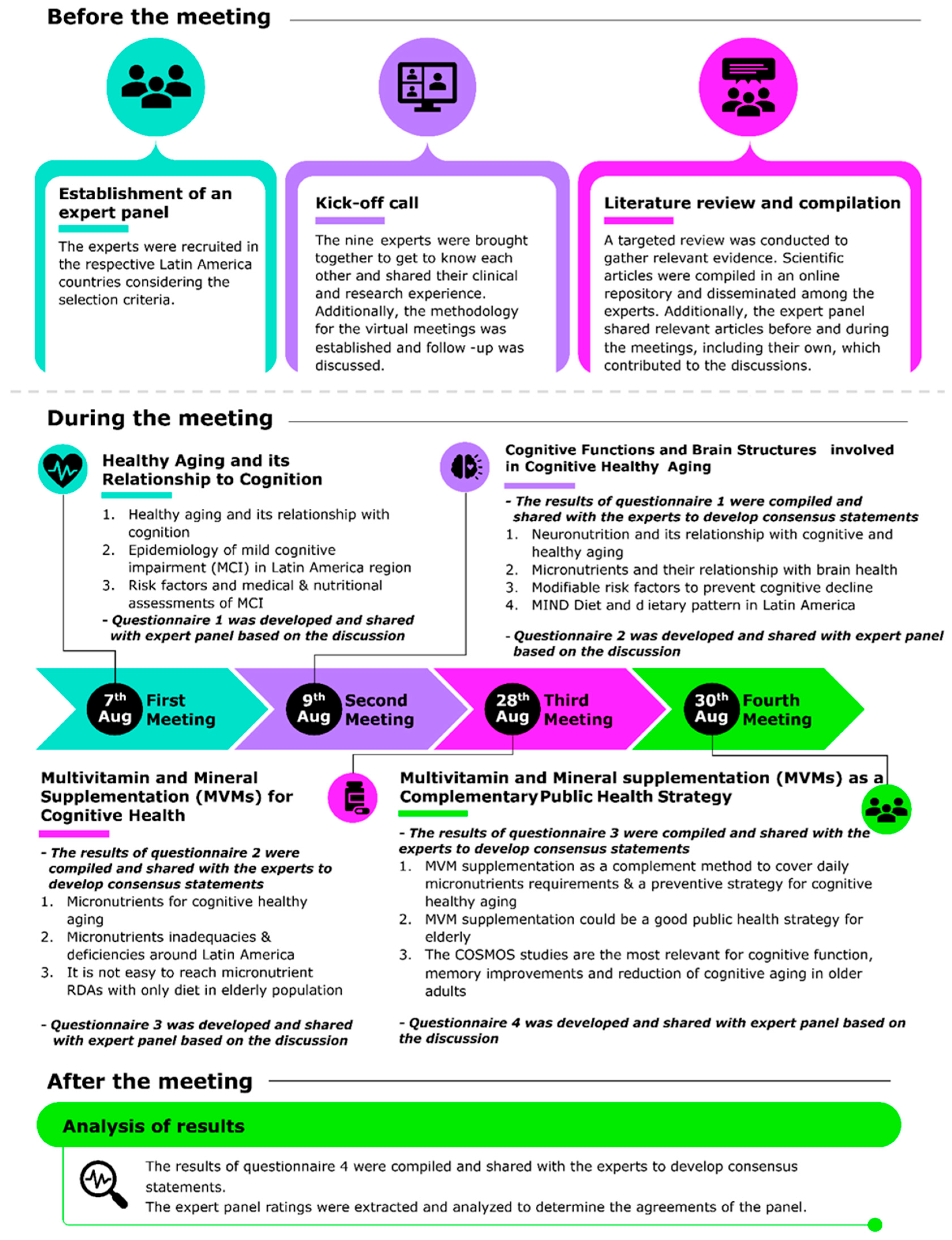
2. Methods
2.1. Expert Panel Formation and Study Design
2.2. Literature Review and Evidence Gathering
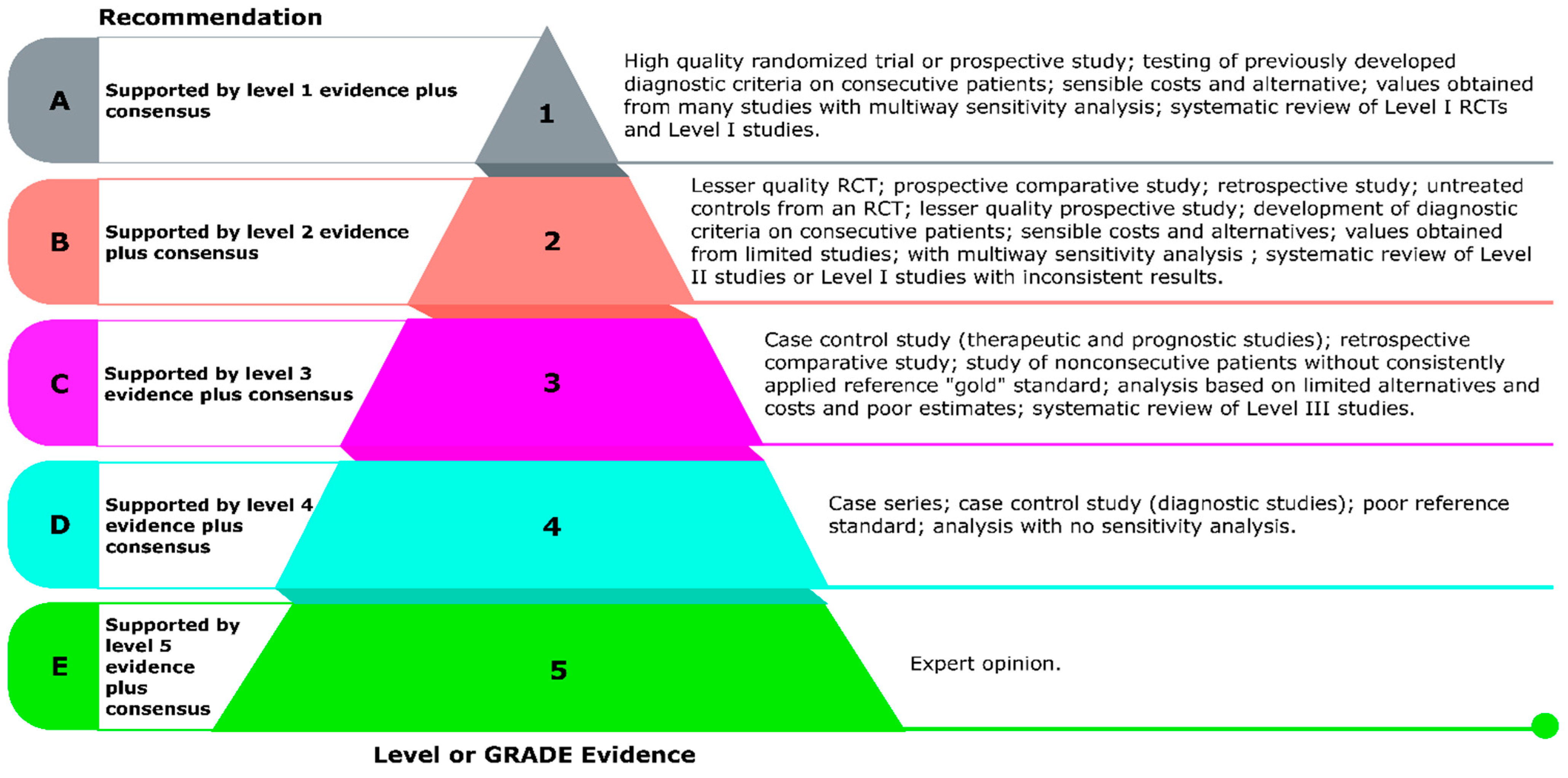
2.3. Delphi Process and Consensus Development
3. Results
3.1. Consensus Statement Development
3.1.1. (A) Healthy Aging and Its Relationship to Cognition
- Statement 1: Cognitive impairment has increased in Latin America in the elderly over the past few years.
- Agreement: Strongly Agree: 6, Agree: 3; Level of evidence: C
- Statement 2: As people age, cognitive impairment generally becomes more pronounced, and typically noticeable between the ages of 60 and 70 years. However, underlying neurobiological changes may begin as early as 40 years old.
- Agreement: Strongly Agree: 4, Agree: 5; Level of evidence: A
- Statement 3: The most significant barriers to addressing cognitive impairment in the elderly in the Latin America region are underdiagnosis, time constraints, and resource limitations.
- Agreement: Strongly Agree: 6, Agree: 3; Level of evidence: A
- Statement 4: Several risk factors such as poor nutrition, unhealthy lifestyle, smoking, excess alcohol consumption, obesity, and lack of awareness contribute to cognitive decline in the elderly population.
- Agreement: Strongly Agree: 9; Level of evidence: A
3.1.2. (B) Cognitive Functions and Brain Structures Involved in Cognitive Healthy Aging
- Statement 5: Vitamins and minerals are neuronutrients. Vitamin C, D, E, B-complex, chromium, copper, iron, magnesium, selenium, and zinc are micronutrients that have specific roles in brain structures and global cognitive functions.
- Agreement: Strongly Agree: 9, Level of evidence: A
- B vitamins (B6, B9, B12) regulate homocysteine metabolism: deficiencies are linked to increased brain atrophy and cognitive impairment [43].
- Vitamin D modulates neurotrophic factors such as Brain-Derived Neurotrophic Factor (BDNF) and demonstrates neuroprotective properties, with low levels associated with dementia risk [44].
- Vitamin C supports dopamine synthesis and acts as a potent antioxidant [45].
- Vitamin E stabilizes neuronal membranes by preventing lipid peroxidation, implicated in Alzheimer’s pathology [46].
- Zinc is crucial for synaptic transmission and hippocampal function [47].
- Magnesium modulates N-methyl-D-aspartate (NMDA) receptors, essential for learning and memory [48].
- Iron supports myelination and dopamine production: its early-life deficiency causes long-term cognitive deficits [49].
- Selenium, through glutathione peroxidase, enhances antioxidant defenses and its deficiency correlates with cognitive decline [50].
- Copper is required for neurotransmitter synthesis, while its dysregulation is implicated in neurodegeneration [51].
- Chromium indirectly enhances neuronal glucose utilization by improving insulin sensitivity [52].
- Statement 6: Dietary pattern from the MIND diet is effective in preventing cognitive decline.
- Agreement: Strongly Agree: 3, Agree: 6; Level of evidence: A
- Statement 7: Adherence to the MIND diet is challenging in Latin America due to economic, cultural, and structural barriers.
- Agreement: Strongly Agree: 4, Agree: 5; Level of evidence: A
- Statement 8: Food insecurity in Latin America should be considered a risk factor for cognitive impairment.
- Agreement: Strongly Agree: 4, Agree: 5; Level of evidence: A
3.1.3. Multivitamin and Mineral Supplementation (MVM) for Cognitive Health
- Statement 9: The elderly Latin American population has a high insufficiency of vitamins and minerals.
- Agreement: Strongly Agree: 4, Agree: 5; Level of Evidence: A
- Statement 10: Meeting micronutrient RDAs through diet alone is challenging for older adults.
- Agreement: Strongly Agree: 3, Agree: 6; Level of evidence: A
- Statement 11: Daily MVM use promotes cognitive healthy aging.
- Agreement: Strongly agree: 4, Agree: 5; Level of evidence: A
3.1.4. MVMs as a Complementary Public Health Strategy
- Statement 12: Considering that the MIND diet pattern is difficult to cover in Latin America’s elderly population, an appropriate strategy for cognitive healthy aging is using MVMs as a preventive method.
- Agreement: Strongly Agree: 9; Level of evidence: A
- Statement 13: MVM use could be a good public health strategy for the elderly.
- Agreement: Strongly Agree: 4, Agree: 5; Level of evidence: A
- Statement 14: COSMOS-Mind studies are the most relevant ones related to MVMs and healthy cognitive aging.
- Agreement: Strongly Agree: 6, Agree: 3; Level of evidence: A
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MVMs | Multivitamin and Mineral Supplements |
| MCI | Mild Cognitive Impairment |
| GRADE | Grades of Recommendation, Assessment, Development, and Evaluation |
| HCPs | Healthcare Professionals |
| LatAm-FINGERS | Latin America has launched several initiatives including Lifestyle Intervention to Prevent Cognitive Decline |
| MIND | Mediterranean-DASH Intervention for Neurodegenerative Delay |
| DASH | Dietary Approaches to Stop Hypertension |
| MoCA | Montreal Cognitive Assessment |
| MMSE | Mini-Mental State Examination |
| COSMOS-Mind | Cocoa Supplement and Multivitamin Outcomes Study for the Mind |
References
- World Health Organization. Available online: https://www.who.int/publications/i/item/global-strategy-and-action-plan-on-ageing-and-health-summary (accessed on 10 November 2024).
- Cruz-Góngora, V.D.l.; Palazuelos-González, R.; Domínguez-Flores, O. Micronutrient Deficiencies in Older Adults in Latin America: A Narrative Review. Food Nutr. Bull. 2024, 45, S26–S38. [Google Scholar] [CrossRef] [PubMed]
- ECLAC Examines Current Outlook for Aging in the Region as Well as Progress and Challenges for Older Persons’ Inclusion and the Exercise of Their Rights. Available online: https://www.cepal.org/en/news/eclac-examines-current-outlook-ageing-region-well-progress-and-challenges-older-persons#:~:text=The%20report%20presents%20the%20region%E2%80%99s%20progress%20and%20achievements,remain%20for%20fulfilling%20regional%20agreements%20on%20this%20issue (accessed on 24 September 2024).
- Gichu, M.; Harwood, R.H. Measurement of Healthy Ageing. Age Ageing 2023, 52, iv3–iv5. [Google Scholar] [CrossRef]
- Oberlin, L.E.; Jaywant, A.; Wolff, A.; Gunning, F.M. Strategies to Promote Cognitive Health in Aging: Recent Evidence and Innovations. Curr. Psychiatry Rep. 2022, 24, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Brito, D.V.C.; Esteves, F.; Rajado, A.T.; Silva, N.; Araújo, I.; Bragança, J.; Castelo-Branco, P.; Nóbrega, C. Assessing Cognitive Decline in the Aging Brain: Lessons from Rodent and Human Studies. NPJ Aging 2023, 9, 23. [Google Scholar] [CrossRef] [PubMed]
- Phillips, C. Lifestyle Modulators of Neuroplasticity: How Physical Activity, Mental Engagement, and Diet Promote Cognitive Health during Aging. Neural. Plast. 2017, 2017, 3589271. [Google Scholar] [CrossRef]
- Vyas, C.M.; Manson, J.E.; Sesso, H.D.; Cook, N.R.; Rist, P.M.; Weinberg, A.; Moorthy, M.V.; Baker, L.D.; Espeland, M.A.; Yeung, L.-K.; et al. Effect of Multivitamin-Mineral Supplementation versus Placebo on Cognitive Function: Results from the Clinic Subcohort of the COcoa Supplement and Multivitamin Outcomes Study (COSMOS) Randomized Clinical Trial and Meta-Analysis of 3 Cognitive Studies within COSMOS. Am. J. Clin. Nutr. 2024, 119, 692–701. [Google Scholar] [CrossRef]
- Yeung, L.-K.; Alschuler, D.M.; Wall, M.; Luttmann-Gibson, H.; Copeland, T.; Hale, C.; Sloan, R.P.; Sesso, H.D.; Manson, J.E.; Brickman, A.M. Multivitamin Supplementation Improves Memory in Older Adults: A Randomized Clinical Trial. Am. J. Clin. Nutr. 2023, 118, 273–282. [Google Scholar] [CrossRef]
- Baker, L.D.; Manson, J.E.; Rapp, S.R.; Sesso, H.D.; Gaussoin, S.A.; Shumaker, S.A.; Espeland, M.A. Effects of Cocoa Extract and a Multivitamin on Cognitive Function: A Randomized Clinical Trial. Alzheimers Dement. 2023, 19, 1308–1319. [Google Scholar] [CrossRef]
- Kaufman, H.; Howell, S.; Stolow, J.; Andrinopoulos, K.; Anglewicz, P.; Burt, M.; Castro, A. Self-Perceived Health of Older Adults in Latin America and the Caribbean: A Scoping Review. Rev. Panam. Salud. Publica. 2023, 47, e105. [Google Scholar] [CrossRef] [PubMed]
- Passarelli, S.; Free, C.M.; Shepon, A.; Beal, T.; Batis, C.; Golden, C.D. Global Estimation of Dietary Micronutrient Inadequacies: A Modelling Analysis. Lancet Glob. Health 2024, 12, e1590–e1599. [Google Scholar] [CrossRef]
- Rentería, M.A.; Manly, J.J.; Vonk, J.M.J.; Arango, S.M.; Obregon, A.M.; Samper-Ternent, R.; Wong, R.; Barral, S.; Tosto, G. Midlife Vascular Factors and Prevalence of Mild Cognitive Impairment in Late-Life in Mexico. J. Int. Neuropsychol. Soc. 2022, 28, 351–361. [Google Scholar] [CrossRef]
- Blumberg, J.B.; Cena, H.; Barr, S.I.; Biesalski, H.K.; Dagach, R.U.; Delaney, B.; Frei, B.; González, M.I.M.; Hwalla, N.; Lategan-Potgieter, R.; et al. The Use of Multivitamin/Multimineral Supplements: A Modified Delphi Consensus Panel Report. Clin. Ther. 2018, 40, 640–657. [Google Scholar] [CrossRef]
- Hanley, D.A.; Cranney, A.; Jones, G.; Whiting, S.J.; Leslie, W.D. Vitamin D in Adult Health and Disease: A Review and Guideline Statement from Osteoporosis Canada (Summary). CMAJ 2010, 182, 1315–1319. [Google Scholar] [CrossRef]
- Levels of Evidence in Research. Available online: https://scientific-publishing.webshop.elsevier.com/research-process/levels-of-evidence-in-research/ (accessed on 10 November 2024).
- Ribeiro, F.S.; Teixeira-Santos, A.C.; Leist, A.K. The Prevalence of Mild Cognitive Impairment in Latin America and the Caribbean: A Systematic Review and Meta-Analysis. Aging Ment. Health 2022, 26, 1710–1720. [Google Scholar] [CrossRef] [PubMed]
- Magallón-Zertuche, V.; Garrido-Dzib, A.G.; Salazar-Gonzalez, E.; González-Castro, D.G.; Chávez-Loría, G.; Avila-Nava, A.; Gutiérrez-Solis, A.L. A Systematic Review and Meta-Analysis on the Prevalence of Mild Cognitive Impairment and Dementia in Mexico. Dement. Geriatr. Cogn. Disord. 2024, 53, 274–288. [Google Scholar] [CrossRef] [PubMed]
- González-Carballo, C.; Kuri-Morales, P.; Chiquete, E.; Rojas-Russell, M.; Santacruz-Benitez, R.; Ramirez-Reyes, R.; Garcilazo-Ávila, A.; Berumen, J.; Trichia, E.; Friedrichs, L.G.; et al. Cognitive Impairment at Older Ages among 8000 Men and Women Living in Mexico City: A Cross-Sectional Analyses of a Prospective Study. BMC Public Health 2024, 24, 3620. [Google Scholar] [CrossRef]
- Paradela, R.S.; Calandri, I.; Castro, N.P.; Garat, E.; Delgado, C.; Crivelli, L.; Yaffe, K.; Ferri, C.P.; Mukadam, N.; Livingston, G.; et al. Population Attributable Fractions for Risk Factors for Dementia in Seven Latin American Countries: An Analysis Using Cross-Sectional Survey Data. Lancet Glob. Health 2024, 12, e1600–e1610. [Google Scholar] [CrossRef] [PubMed]
- Pais, R.; Ruano, L.; Carvalho, O.P.; Barros, H. Global Cognitive Impairment Prevalence and Incidence in Community Dwelling Older Adults-A Systematic Review. Geriatrics 2020, 5, 84. [Google Scholar] [CrossRef]
- Kennedy, K.M.; Rodrigue, K.M.; Bischof, G.N.; Hebrank, A.C.; Reuter-Lorenz, P.A.; Park, D.C. Age Trajectories of Functional Activation under Conditions of Low and High Processing Demands: An Adult Lifespan fMRI Study of the Aging Brain. NeuroImage 2015, 104, 21–34. [Google Scholar] [CrossRef]
- Flanagan, E.; Lamport, D.; Brennan, L.; Burnet, P.; Calabrese, V.; Cunnane, S.C.; Wilde, M.C.d.; Dye, L.; Farrimond, J.A.; Lombardo, N.E.; et al. Nutrition and the Ageing Brain: Moving towards Clinical Applications. Ageing Res. Rev. 2020, 62, 101079. [Google Scholar] [CrossRef]
- Salthouse, T.A. When Does Age-Related Cognitive Decline Begin? Neurobiol. Aging 2009, 30, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Santiago, J.A.; Bernstein, M.; Potashkin, J.A. Diet and Lifestyle Impact the Development and Progression of Alzheimer’s Dementia. Front. Nutr. 2023, 10, 1213223. [Google Scholar] [CrossRef]
- Mónica, H. Developing Telemedicine for Rural and Marginal Suburban Locations in Latin America; Nova Science Publishers: Hauppauge, NY, USA, 2015. [Google Scholar]
- Borson, S.; Small, G.W.; O’Brien, Q.; Morrello, A.; Boustani, M. Understanding Barriers to and Facilitators of Clinician-Patient Conversations About Brain Health and Cognitive Concerns in Primary Care: A Systematic Review and Practical Considerations for the Clinician. BMC Prim. Care 2023, 24, 233. [Google Scholar] [CrossRef]
- Tsai, Y.I.-P.; Beh, J.; Ganderton, C.; Pranata, A. Digital Interventions for Healthy Ageing and Cognitive Health in Older Adults: A Systematic Review of Mixed Method Studies and Meta-Analysis. BMC Geriatr. 2024, 24, 217. [Google Scholar] [CrossRef]
- Lilia, G.A.; Alejandro, G.-M.; Salvador, V.-R.; Roberto, M.; Raúl, R.; Manuel, C.; Luis, V. Current Situation and Challenges for mHealth in the Latin America Region. In mHealth Multidisciplinary Verticals; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Soto-Añari, M.; Camargo, L.; Ramos-Henderson, M.; Rivera-Fernández, C.; Denegri-Solís, L.; Calle, U.; Mori, N.; Ocampo-Barbá, N.; López, F.; Porto, M.; et al. Prevalence of Dementia and Associated Factors among Older Adults in Latin America during the COVID-19 Pandemic. Dement. Geriatr. Cogn Dis. Extra 2021, 11, 213–221. [Google Scholar] [CrossRef]
- Amber, H. Loneliness and Social Isolation in Older Adults: The Effects of a Pandemic. Perspect ASHA Spec. Interest Groups 2021, 6, 1729–1736. [Google Scholar]
- Paula, V.A.; Jardim, S.E.; Claudia, B.A.; Claudine, L.; Solange, A.; Roberto, R.L. Effects of Combined Digital Inclusion and Physical Activity Intervention on the Cognition of Older Adults in Brazil. Gerontechnology 2020, 19, 1–10. [Google Scholar] [CrossRef]
- Concha-Cisternas, Y.; Lanuza, F.; Waddell, H.; Sillars, A.; Leiva, A.M.; Troncoso, C.; Martinez, M.A.; Villagrán, M.; Mardones, L.; Martorell, M.; et al. Association between Adiposity Levels and Cognitive Impairment in the Chilean Older Adult Population. J. Nutr. Sci. 2019, 8, e33. [Google Scholar] [CrossRef]
- Ramos-Henderson, M.; Soto-Añari, M.; Herrera-Pino, J.; Porto, M.F.; Camargo, L.; Hesse, H.; Ferrel-Ortega, R.; Quispe-Ayala, C.; Cadena, C.G.D.L.; Mendoza-Ruvalcaba, N.; et al. Factors Associated with Cognitive Impairment in Latin American Older Adults: A Cross-Sectional Observational Study of COVID-19 Confinement. Alzheimers Dement. 2023, 15, e12427. [Google Scholar] [CrossRef] [PubMed]
- Carlos, A.R.T.; Enrique, C.-P.; Alonso, R.-R. Dieta MIND Y Condiciones Neurodegenerativas: Una Revisión Narrativa. Red Nutr. 2022, 13, 972–977. [Google Scholar]
- Garrido-Dzib, A.G.; Chávez-Loría, G.; Magallón-Zertuche, V.; Avila-Nava, A.; Palacios-González, B.; Gutiérrez-Solis, A.L. Micro- and Macronutrient Intake and Food Group Frequency Consumed by Subjects with Cognitive Impairment and Dementia in Latin America: A Systematic Review. J. Alzheimers Dis. 2023, 94, 425–439. [Google Scholar] [CrossRef]
- Boumenna, T.; Scott, T.M.; Lee, J.-S.; Zhang, X.; Kriebel, D.; Tucker, K.L.; Palacios, N. MIND Diet and Cognitive Function in Puerto Rican Older Adults. J. Gerontol. A Biol. Sci. Med. Sci. 2022, 77, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Maria, S.-C.R.; Graciela, M.-G.J.; Ana, V.-M.; Tomas, C.-V.; Cristina, G.I.; Luisa, S.-O.A.; FINGERS, L. MIND Diet Consumption Pattern in a Cohort of Cognitively Healthy Mexican Older Adults. LatAm FINGERS Mexico. Alzheimers Dement. 2023, 19, e076739. [Google Scholar]
- van Soest, A.P.; Beers, S.; van de Rest, O.; de Groot, L.C. The Mediterranean-Dietary Approaches to Stop Hypertension Intervention for Neurodegenerative Delay (MIND) Diet for the Aging Brain: A Systematic Review. Adv. Nutr. 2024, 15, 100184. [Google Scholar] [CrossRef] [PubMed]
- Diana, M.; Jacqueline, G.; Yuliana, S.; Susan, A. The Mediterranean and MIND Dietary Patterns: Associations with Cognition and Psychological Distress among Latinos. In Older Mexicans and Latinos in the United States: Where Worlds Meet; Springer: Berlin/Heidelberg, Germany, 2023; pp. 151–165. [Google Scholar]
- Crivelli, L.; Calandri, I.L.; Suemoto, C.K.; Salinas, R.M.; Velilla, L.M.; Yassuda, M.S.; Caramelli, P.; Lopera, F.; Nitrini, R.; Sevlever, G.E.; et al. Latin American Initiative for Lifestyle Intervention to Prevent Cognitive Decline (LatAm-FINGERS): Study Design and Harmonization. Alzheimers Dement. 2023, 19, 4046–4060. [Google Scholar] [CrossRef]
- Kivipelto, M.; Mangialasche, F.; Snyder, H.M.; Allegri, R.; Andrieu, S.; Arai, H.; Baker, L.; Belleville, S.; Brodaty, H.; Brucki, S.M.; et al. World-Wide FINGERS Network: A Global Approach to Risk Reduction and Prevention of Dementia. Alzheimers Dement. 2020, 16, 1078–1094. [Google Scholar] [CrossRef]
- Smith, A.D.; Smith, S.M.; Jager, C.A.D.; Whitbread, P.; Johnston, C.; Agacinski, G.; Oulhaj, A.; Bradley, K.M.; Jacoby, R.; Refsum, H. Homocysteine-Lowering by B Vitamins Slows the Rate of Accelerated Brain Atrophy in Mild Cognitive Impairment: A Randomized Controlled Trial. PLoS ONE 2010, 5, e12244. [Google Scholar] [CrossRef]
- Annweiler, C.; Dursun, E.; Féron, F.; Gezen-Ak, D.; Kalueff, A.V.; Littlejohns, T.; Llewellyn, D.J.; Millet, P.; Scott, T.; Tucker, K.L.; et al. ‘Vitamin D and Cognition in Older Adults’: Updated International Recommendations. J. Intern. Med. 2015, 277, 45–57. [Google Scholar] [CrossRef]
- Harrison, F.E.; May, J.M. Vitamin C Function in the Brain: Vital Role of the Ascorbate Transporter SVCT2. Free Radic. Biol. Med. 2009, 46, 719–730. [Google Scholar] [CrossRef]
- Mangialasche, F.; Xu, W.; Kivipelto, M.; Costanzi, E.; Ercolani, S.; Pigliautile, M.; Cecchetti, R.; Baglioni, M.; Simmons, A.; Soininen, H.; et al. Tocopherols and Tocotrienols Plasma Levels Are Associated with Cognitive Impairment. Neurobiol. Aging 2012, 33, 2282–2290. [Google Scholar] [CrossRef]
- Takeda, A. Zinc Homeostasis and Functions of Zinc in the Brain. Biometals 2001, 14, 343–351. [Google Scholar] [CrossRef]
- Kirkland, A.E.; Sarlo, G.L.; Holton, K.F. The Role of Magnesium in Neurological Disorders. Nutrients 2018, 10, 730. [Google Scholar] [CrossRef]
- Beard, J.L. Iron Biology in Immune Function, Muscle Metabolism and Neuronal Functioning. J. Nutr. 2001, 131, 568S–579S; discussion 580S. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.E.; Souza, J.V.; Galiciolli, M.E.A.; Sare, F.; Vieira, G.S.; Kruk, I.L.; Oliveira, C.S. Effects of Selenium Supplementation in Patients with Mild Cognitive Impairment or Alzheimer’s Disease: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 3205. [Google Scholar] [CrossRef] [PubMed]
- Opazo, C.M.; Greenough, M.A.; Bush, A.I. Copper: From Neurotransmission to Neuroproteostasis. Front. Aging Neurosci. 2014, 6, 143. [Google Scholar] [CrossRef] [PubMed]
- Althuis, M.D.; Jordan, N.E.; Ludington, E.A.; Wittes, J.T. Glucose and Insulin Responses to Dietary Chromium Supplements: A Meta-Analysis. Am. J. Clin. Nutr. 2002, 76, 148–155. [Google Scholar] [CrossRef]
- The Nutrition Source. Available online: https://nutritionsource.hsph.harvard.edu/healthy-weight/diet-reviews/mind-diet/ (accessed on 24 September 2024).
- Gaona-Pineda, E.B.; Rodríguez-Ramírez, S.; Medina-Zacarías, M.C.; Valenzuela-Bravo, D.G.; Martinez-Tapia, B.; Arango-Angarita, A. Consumidores de grupos de alimentos en población mexicana. Ensanut Continua 2020–2022. Salud Publica Mex. 2023, 65, s248–s258. [Google Scholar] [CrossRef] [PubMed]
- Secretaría de Gobierno de Salud. 2° Encuesta Nacional de Nutrición y Salud (Ennys 2); Ministerio de Salud y Desarrollo Social: Argentina, Spain, 2025. [Google Scholar]
- FAO Regional Office for Latin America and the Caribbean. New UN Report: 43.2 Million People Suffer from Hunger in Latin America and the Caribbean, and the Region Has Higher Levels of Overweight and Obesity than the Global Estimate; FAO Regional Office for Latin America and the Caribbean: Santiago, Chile, 2025. [Google Scholar]
- Saenz, J.; Avila, J.C. Late-Life Food Insecurity and Cognition: Exploring Timing, Duration, and Mechanisms among Older Mexican Adults. BMC Geriatr. 2023, 23, 788. [Google Scholar] [CrossRef]
- Melzer, T.M.; Manosso, L.M.; Yau, S.-Y.; Gil-Mohapel, J.; Brocardo, P.S. In Pursuit of Healthy Aging: Effects of Nutrition on Brain Function. Int. J. Mol. Sci. 2021, 22, 5026. [Google Scholar] [CrossRef]
- Cavagnari, B.M.; Favieri, A.; Zonis, L.; Guajardo, V.; Gerardi, A.; Fisberg, M.; Kovalskys, I. Inadecuación de Micronutrientes En Adolescentes Y Adultos Argentinos de Población Urbana. Result. De Estud. Latinoam. De Nutr. Y Salud (ELANS). Actual. Nutr. 2021, 22, 71–79. [Google Scholar]
- Junior, E.V.; Marchioni, D.M.; Araujo, M.C.; De Carli, E.; de Oliveira, D.C.R.S.; Yokoo, E.M.; Sichieri, R.; Pereira, R.A. Evolution of Energy and Nutrient Intake in Brazil between 2008–2009 and 2017–2018. Rev. Saude Publica 2021, 55, 5s. [Google Scholar]
- Laura, J.; Tobias, P. Can Non-Contributory Pensions Decrease Food Vulnerability? the Case of Mexico. Empir. Econ. 2020, 59, 1865–1882. [Google Scholar]
- Hirsch, S.; Maza, P.d.l.; Barrera, G.; Gattás, V.; Petermann, M.; Bunout, D. The Chilean Flour Folic Acid Fortification Program Reduces Serum Homocysteine Levels and Masks Vitamin B-12 Deficiency in Elderly People. J. Nutr. 2002, 132, 289–291. [Google Scholar] [CrossRef]
- David, S.A.; Helga, R.; Teodoro, B.; Michael, F.; Babak, H.; Andrew, M.; Miller, J.W.; Rosenberg, I.H.; Obeid, R. Homocysteine and Dementia: An International Consensus Statement. J. Alzheimers Dis. 2018, 62, 561–570. [Google Scholar]
- Ibáñez, A.; Pina-Escudero, S.D.; Possin, K.L.; Quiroz, Y.T.; Peres, F.A.; Slachevsky, A.; Sosa, A.L.; Brucki, S.M.D.; Miller, B.L. Dementia Caregiving across Latin America and the Caribbean and Brain Health Diplomacy. Lancet Healthy Longev. 2021, 2, e222–e231. [Google Scholar] [CrossRef]
- Solomons, N.W. Perspective on Emerging Micronutrient Deficiencies in Latin America and the Caribbean. Food Nutr. Bull. 2024, 45, S39–S46. [Google Scholar] [CrossRef]
- McRae, I.; Zheng, L.; Bourke, S.; Cherbuin, N.; Anstey, K.J. Cost-Effectiveness of Dementia Prevention Interventions. J. Prev. Alzheimers Dis. 2021, 8, 210–217. [Google Scholar] [CrossRef]
- Martinho, K.O.; Tinôco, A.L.A.; Ribeiro, A.Q. Prevalence and Factors Associated with Vitamin B12 Deficiency in Elderly from Viçosa/Mg, Brasil. Nutr. Hosp. 2015, 32, 2162–2168. [Google Scholar] [CrossRef]
- Hamer, D.H.; Sempértegui, F.; Estrella, B.; Tucker, K.L.; Rodríguez, A.; Egas, J.; Dallal, G.E.; Selhub, J.; Griffiths, J.K.; Meydani, S.N. Micronutrient Deficiencies Are Associated with Impaired Immune Response and Higher Burden of Respiratory Infections in Elderly Ecuadorians. J. Nutr. 2009, 139, 113–119. [Google Scholar] [CrossRef]
- Lee, J.; Kim, H.-J. Normal Aging Induces Changes in the Brain and Neurodegeneration Progress: Review of the Structural, Biochemical, Metabolic, Cellular, and Molecular Changes. Front. Aging Neurosci. 2022, 14, 931536. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Ge, B.; Wu, S.; Li, H.; Lin, L. Optimal Cut-off MoCA Score for Screening for Mild Cognitive Impairment in Elderly Individuals in China: A Systematic Review and Meta-Analysis. Asian J. Psychiatr. 2023, 87, 103691. [Google Scholar] [CrossRef] [PubMed]
- Martorell, R.; Romaña, D.L.d. Components of Successful Staple Food Fortification Programs: Lessons from Latin America. Food Nutr. Bull. 2017, 38, 384–404. [Google Scholar] [CrossRef] [PubMed]
- Osendarp, S.J.M.; Martinez, H.; Garrett, G.S.; Neufeld, L.M.; De-Regil, L.M.; Vossenaar, M.; Darnton-Hill, I. Large-Scale Food Fortification and Biofortification in Low- and Middle-Income Countries: A Review of Programs, Trends, Challenges, and Evidence Gaps. Food. Nutr. Bull. 2018, 39, 315–331. [Google Scholar] [CrossRef]
- Fu, Q.; DeJager, J.; Gardner, E.M. Supplementation and mitigating cognitive decline in older adults with or without mild cognitive impairment or dementia: A systematic review. Nutrients 2024, 16, 3567. [Google Scholar] [CrossRef]
- Olivares, M.; Hertrampf, E.; Capurro, M.T.; Wegner, D. Prevalence of Anemia in Elderly Subjects Living at Home: Role of Micronutrient Deficiency and Inflammation. Eur. J. Clin. Nutr. 2000, 54, 834–839. [Google Scholar] [CrossRef] [PubMed]
- Hugo, S.; Cecilia, A.; Eva, H.F.; Renato, V.; Manuel, L.; Luis, C.J.; Lydia, L.; Ricardo, U. Prevalence of Vitamin B-12 Deficiency in Older Adults. Rev. Med. Chil. 2010, 138, 44–52. [Google Scholar]
- Castillo-Lancellotti, C.; Margozzini, P.; Valdivia, G.; Padilla, O.; Uauy, R.; Rozowski, J.; Tur, J.A. Serum Folate and Vitamin B12 in Older People: Results from the Chilean National Health Survey 2009–2010. Rev. Med. Chil. 2013, 141, 1107–1116. [Google Scholar] [CrossRef][Green Version]
- Luis, D.-M.J.; Alejandra, V.-L. Are Older Adults with Hip Fractures a Specific Risk Group for Vitamin B12 Deficiency? JCSM Clin. Rep. 2022, 7, 44–52. [Google Scholar] [CrossRef]
- Ministerio de Salud de Costa Rica. Encuesta Nacional de Nutrición 2008–2009. Fasculo 2. Micronutrientes/Ministerio de Salud, Instituto Cstarricense de Investigación y Enseñanza en Nutrición y Salud, Caja Costarricense de Seguro Social, Instituto Costarricense de Estadística y Censos, Instituto Costarricense Sobre Drogas; Ministerio de Salud de Costa Rica: San Jose, CA, USA, 2012. [Google Scholar]
- Veloz, A.F.V.; Arias, T.V.C.; Mejía, J.S.V.; Veloz, E.C.T.; Andrade, J.S.P.; Cifuentes, T.M.N.; Aguirre, S.I.H.; Veloz, M.F.V. Cognitive Function and Vitamin B12 and D among Community-Dwelling Elders: A Cross-Sectional Study. Clin. Nutr. ESPEN 2022, 50, 270–276. [Google Scholar] [CrossRef]
- Pereda, A.R.; Pacheco, B.I.; Astiazarán-García, H.; Esparza-Romero, J.; Alemán-Mateo, H. Vitamin B12 and Folate in Non-Institutionalized Urban Older People. Arch. Latinoam. Nutr. 2006, 56, 135–140. [Google Scholar]
- De la Cruz-Góngora, V.; Salinas-Rodríguez, A.; Flores-Aldana, M.; Villalpando, S. Etiology of Anemia in Older Mexican Adults: The Role of Hepcidin, Vitamin A and Vitamin D. Nutrients 2021, 13, 3814. [Google Scholar] [CrossRef] [PubMed]
- Diaz, M.M.; Custodio, N.; Montesinos, R.; Lira, D.; Herrera-Perez, E.; Pintado-Caipa, M.; Cuenca-Alfaro, J.; Gamboa, C.; Lanata, S. Thyroid Dysfunction, Vitamin B12, and Folic Acid Deficiencies Are Not Associated with Cognitive Impairment in Older Adults in Lima, Peru. Front. Public Health 2021, 9, 676518. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Santos, M.; Santos, J.Y.G.D.; Carvalho, G.Q.; Santos, D.B.D.; Oliveira, A.M. Epidemiology of Vitamin D Insufficiency and Deficiency in a Population in a Sunny Country: Geospatial Meta-Analysis in Brazil. Crit. Rev. Food Sci. Nutr. 2019, 59, 2102–2109. [Google Scholar] [CrossRef]
- Lima-Costa, M.F.; Mambrini, J.V.M.; Souza-Junior, P.R.B.D.; Andrade, F.B.D.; Peixoto, S.V.; Vidigal, C.M.; Oliveira, C.D.; Vidigal, P.G. Nationwide Vitamin D Status in Older Brazilian Adults and Its Determinants: The Brazilian Longitudinal Study of Aging (ELSI). Sci. Rep. 2020, 10, 13521. [Google Scholar] [CrossRef]
- Carrasco, G.M.; De, L.A.D.; Martínez, F.G.; Ihle, S.S.; Rojas, Á.V.; Foradori, C.A.; Marín, L.P.P. Vitamin D Levels in Older Healthy Chilean Adults and Their Association with Functional Performance. Rev. Med. Chil. 2014, 142, 1385–1391. [Google Scholar] [CrossRef][Green Version]
- Orces, C.H. Vitamin D Status among Older Adults Residing in the Littoral and Andes Mountains in Ecuador. Sci. World J. 2015, 2015, 545297. [Google Scholar] [CrossRef]
- Carrillo-Vega, M.F.; García-Peña, C.; Gutiérrez-Robledo, L.M.; Pérez-Zepeda, M.U. Vitamin D Deficiency in Older Adults and Its Associated Factors: A Cross-Sectional Analysis of the Mexican Health and Aging Study. Arch. Osteoporos. 2017, 12, 8. [Google Scholar] [CrossRef]
- Carrazco-Peña, K.B.; Farías-Moreno, K.; Toro-Equihua, M.D.; Aguilar-Mancilla, Z.C.; Trujillo-Magallón, M.; Solórzano-Rodríguez, M.A.; Trujillo-Hernández, B. Components of Frailty, Sarcopenia and Their Association with Vitamin D Deficiency. Cross-Sectional, Analytical Study. Gac. Med. Mex. 2022, 158, 343–348. [Google Scholar] [CrossRef]
- Mendoza-Garcés, L.; Velázquez-Alva, M.C.; Cabrer-Rosales, M.F.; Arrieta-Cruz, I.; Gutiérrez-Juárez, R.; Irigoyen-Camacho, M.E. Vitamin D Deficiency Is Associated with Handgrip Strength, Nutritional Status and T2DM in Community-Dwelling Older Mexican Women: A Cross-Sectional Study. Nutrients 2021, 13, 736. [Google Scholar] [CrossRef]
- González, R.P.; Cruz-Góngora, V.D.l.; Rodríguez, A.S. Serum Retinol Levels Are Associated with Cognitive Function among Community-Dwelling Older Mexican Adults. Nutr. Neurosci. 2022, 25, 1881–1888. [Google Scholar] [CrossRef]
- Sales, C.H.; Rogero, M.M.; Sarti, F.M.; Fisberg, R.M. Prevalence and Factors Associated with Iron Deficiency and Anemia among Residents of Urban Areas of São Paulo, Brazil. Nutrients 2021, 13, 1888. [Google Scholar] [CrossRef]
- Contreras-Manzano, A.; Cruz, V.d.l.; Villalpando, S.; Rebollar, R.; Shamah-Levy, T. Anemia and Iron Deficiency in Mexican Elderly Population: Results from the Ensanut 2012. Salud Publica Mex. 2015, 57, 394–402. [Google Scholar] [CrossRef][Green Version]
- Cruz-Góngora, V.D.l.; Rivera-Pasquel, M.; Shamah-Levy, T.; Villalpando-Hernández, S. Iron deficiency is not the main contributor to anemia in older Mexican adults: Results from the National Health and Nutrition Survey 2018-19. Salud Publica Mex. 2021, 63, 412–421. [Google Scholar] [CrossRef]
- Marchetti, M.F.; Silva, G.M.d.; Freiria, C.N.; Borim, F.S.A.; Brito, T.R.P.d.; Milanski, M.; Corona, L.P. Association between Zinc Deficiency and Cognitive Decline in Community-Dwelling Older Adults. Cien. Saude. Colet. 2022, 27, 2805–2816. [Google Scholar] [CrossRef]
- Chengxiang, Z.; Qi, H.; Shifen, L.; Feifei, D.; Wen, Q.; Susan, H.; Ting, Y.; Yubang, W. A Magtein®, Magnesium L-Threonate,-Based Formula Improves Brain Cognitive Functions in Healthy Chinese Adults. Nutrients 2022, 14, 5235. [Google Scholar]
- Gibson, G.E.; Luchsinger, J.A.; Cirio, R.; Chen, H.; Franchino-Elder, J.; Hirsch, J.A.; Bettendorff, L.; Chen, Z.; Flowers, S.A.; Gerber, L.M.; et al. Benfotiamine and Cognitive Decline in Alzheimer’s Disease: Results of a Randomized Placebo-Controlled Phase IIa Clinical Trial. J. Alzheimers Dis. 2020, 78, 989–1010. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Li, Q.; Zhou, X.; Zhao, J.; Song, A.; Li, W.; Liu, H.; Xu, W.; Huang, G. Effects of Folic Acid Supplementation on Cognitive Function and Aβ-Related Biomarkers in Mild Cognitive Impairment: A Randomized Controlled Trial. Eur. J. Nutr. 2019, 58, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Kwok, T.; Wu, Y.; Lee, J.; Lee, R.; Yung, C.Y.; Choi, G.; Lee, V.; Harrison, J.; Lam, L.; Mok, V. A Randomized Placebo-Controlled Trial of Using B Vitamins to Prevent Cognitive Decline in Older Mild Cognitive Impairment Patients. Clin. Nutr. 2020, 39, 2399–2405. [Google Scholar] [CrossRef]
- Jia, J.; Hu, J.; Huo, X.; Miao, R.; Zhang, Y.; Ma, F. Effects of Vitamin D Supplementation on Cognitive Function and Blood Aβ-Related Biomarkers in Older Adults with Alzheimer’s Disease: A Randomised, Double-Blind, Placebo-Controlled Trial. J. Neurol. Neurosurg. Psychiatry 2019, 90, 1347–1352. [Google Scholar] [CrossRef]
- Bischoff-Ferrari, H.A.; Vellas, B.; Rizzoli, R.; Kressig, R.W.; Silva, J.A.P.d.; Blauth, M.; Felson, D.T.; McCloskey, E.V.; Watzl, B.; Hofbauer, L.C.; et al. Effect of Vitamin D Supplementation, Omega-3 Fatty Acid Supplementation, or a Strength-Training Exercise Program on Clinical Outcomes in Older Adults: The DO-HEALTH Randomized Clinical Trial. JAMA 2020, 324, 1855–1868. [Google Scholar] [CrossRef]
- Yang, T.; Wang, H.; Xiong, Y.; Chen, C.; Duan, K.; Jia, J.; Ma, F. Vitamin D Supplementation Improves Cognitive Function through Reducing Oxidative Stress Regulated by Telomere Length in Older Adults with Mild Cognitive Impairment: A 12-Month Randomized Controlled Trial. J. Alzheimers Dis. 2020, 78, 1509–1518. [Google Scholar] [CrossRef] [PubMed]
- Castle, M.; Fiedler, N.; Pop, L.C.; Schneider, S.J.; Schlussel, Y.; Sukumar, D.; Hao, L.; Shapses, S.A. Three Doses of Vitamin D and Cognitive Outcomes in Older Women: A Double-Blind Randomized Controlled Trial. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Ghaderi, A.; Rasouli-Azad, M.; Farhadi, M.H.; Mirhosseini, N.; Motmaen, M.; Pishyareh, E.; Omidi, A.; Asemi, Z. Exploring the Effects of Vitamin D Supplementation on Cognitive Functions and Mental Health Status in Subjects under Methadone Maintenance Treatment. J. Addict. Med. 2020, 14, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Jia, J.; Zhang, Y.; Miao, R.; Huo, X.; Ma, F. Effects of Vitamin D(3) Supplementation on Cognition and Blood Lipids: A 12-Month Randomised, Double-Blind, Placebo-Controlled Trial. J. Neurol. Neurosurg. Psychiatry 2018, 89, 1341–1347. [Google Scholar] [CrossRef]
- Olivier, B.; Launay, C.P.; Kevin, G.; Christine, V.; Flore, D.-P.; Brigitte, R.; Valérie, B.; Gilles, A. Effects of Vitamin D and Calcium Fortified Yogurts on Gait, Cognitive Performances, and Serum 25-Hydroxyvitamin D Concentrations in Older Community-Dwelling Females: Results from the GAit, MEmory, Dietary and Vitamin D (GAME-D2) Randomized Controlled Trial. Nutrients 2019, 11, 2880. [Google Scholar]
| S. No. | Consensus | Agreement | Published Evidence | Level of Evidence |
|---|---|---|---|---|
| Healthy Aging and its Relationship to Cognition in elderly in Latin America | ||||
| 1. | Cognitive impairment has increased in Latin America in the elderly over the past few years. |  Strongly agree: 6, Agree: 3 | 1 SR of CSS, 4 CSSs | C |
| 2. | As people age, cognitive impairment generally becomes more pronounced, and typically noticeable between the ages of 60 and 70 years. However, underlying neurobiological changes may begin as early as 40 years old. |  Strongly agree: 4, Agree: 5 | 1 SR, 1 review on RCTs, 1 clinical study | A |
| 3. | The most significant barriers to addressing cognitive impairment in the elderly in the Latin America region are underdiagnosis, time constraints, and resource limitations. |  Strongly agree: 6, Agree: 3 | 1 SR, 6 CSSs, 1 RCT | A |
| 4. | Several risk factors such as poor nutrition, unhealthy lifestyle, smoking, excess alcohol consumption, obesity, and lack of awareness contribute to cognitive decline in the elderly population. |  Strongly agree: 9 | 4 CSSs, 1 RCT | A |
| Cognitive Functions and Brain Structures involved in Cognitive Healthy Aging | ||||
| 5. | Vitamins and minerals are neuronutrients. Vitamin C, D, E, B-complex, chromium, copper, iron, magnesium, selenium, and zinc are micronutrients that have specific roles in brain structures and global cognitive functions. |  Strongly agree: 9 | 3 RCTs, 7 CSSs | A |
| 6. | Dietary pattern from the MIND diet is effective in preventing cognitive decline. |  Strongly agree: 3, Agree: 6 | 6 CSSs, 1 SR on RCTs and CSSs | A |
| 7. | Adherence to the MIND diet is challenging in Latin America due to economic, cultural, and structural barriers. |  Strongly agree: 4, Agree: 5 | 2 CSSs | A |
| 8. | Food insecurity in Latin America should be considered a risk factor for cognitive impairment. |  Strongly agree: 4, Agree: 5 | 1 SR, 3 CSSs | A |
| Multivitamin and Mineral Supplementation (MVM) for Cognitive Health | ||||
| 9. | The elderly Latin American population has a high insufficiency of vitamins and minerals. |  Strongly agree: 4, Agree: 5 | 21 CSSs, 1 review of RCTs | A |
| 10. | Meeting micronutrient RDAs through diet alone is challenging for older adults. |  Strongly agree: 3, Agree: 6 | 2 reviews of RCTs and CSSs and 2 CSSs | A |
| 11. | Daily MVM use promotes cognitive healthy aging. |  Strongly agree: 4, Agree: 5 | 1 review of RCTs and CSSs, 3 CSSs | A |
| MVMs as a Complementary Public Health Strategy | ||||
| 12. | Considering that the MIND diet pattern is difficult to cover in Latin America’s elderly population, an appropriate strategy for cognitive healthy aging is using MVMs as a preventive method. |  Strongly Agree: 9 | 2 CSSs, 2 RCTs, and 1 systematic review and meta-analysis | A |
| 13. | MVM use could be a good public health strategy for the elderly. |  Strongly agree: 4, Agree: 5 | 3 RCTs | A |
| 14. | COSMOS-Mind studies are the most relevant ones related to MVMs and healthy cognitive aging. |  Strongly agree: 6, Agree: 3 | 2 RCTs and 1 meta-analysis | A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nogueira-de-Almeida, C.A.; Gutiérrez, C.A.C.; Ramos, L.R.; Katz, M.; Gonzalez, M.M.; Angel Badillo, B.; Gómez Santa María, O.A.; Reyes Torres, C.A.; O’Neill, S.; Reyes, M.G.; et al. Role of Micronutrient Supplementation in Promoting Cognitive Healthy Aging in Latin America: Evidence-Based Consensus Statement. Nutrients 2025, 17, 2545. https://doi.org/10.3390/nu17152545
Nogueira-de-Almeida CA, Gutiérrez CAC, Ramos LR, Katz M, Gonzalez MM, Angel Badillo B, Gómez Santa María OA, Reyes Torres CA, O’Neill S, Reyes MG, et al. Role of Micronutrient Supplementation in Promoting Cognitive Healthy Aging in Latin America: Evidence-Based Consensus Statement. Nutrients. 2025; 17(15):2545. https://doi.org/10.3390/nu17152545
Chicago/Turabian StyleNogueira-de-Almeida, Carlos Alberto, Carlos A. Cano Gutiérrez, Luiz R. Ramos, Mónica Katz, Manuel Moreno Gonzalez, Bárbara Angel Badillo, Olga A. Gómez Santa María, Carlos A. Reyes Torres, Santiago O’Neill, Marine Garcia Reyes, and et al. 2025. "Role of Micronutrient Supplementation in Promoting Cognitive Healthy Aging in Latin America: Evidence-Based Consensus Statement" Nutrients 17, no. 15: 2545. https://doi.org/10.3390/nu17152545
APA StyleNogueira-de-Almeida, C. A., Gutiérrez, C. A. C., Ramos, L. R., Katz, M., Gonzalez, M. M., Angel Badillo, B., Gómez Santa María, O. A., Reyes Torres, C. A., O’Neill, S., Reyes, M. G., & Mustapic, L. (2025). Role of Micronutrient Supplementation in Promoting Cognitive Healthy Aging in Latin America: Evidence-Based Consensus Statement. Nutrients, 17(15), 2545. https://doi.org/10.3390/nu17152545







