Gut Hormones and Postprandial Metabolic Effects of Isomaltulose vs. Saccharose Consumption in People with Metabolic Syndrome
Abstract
1. Introduction
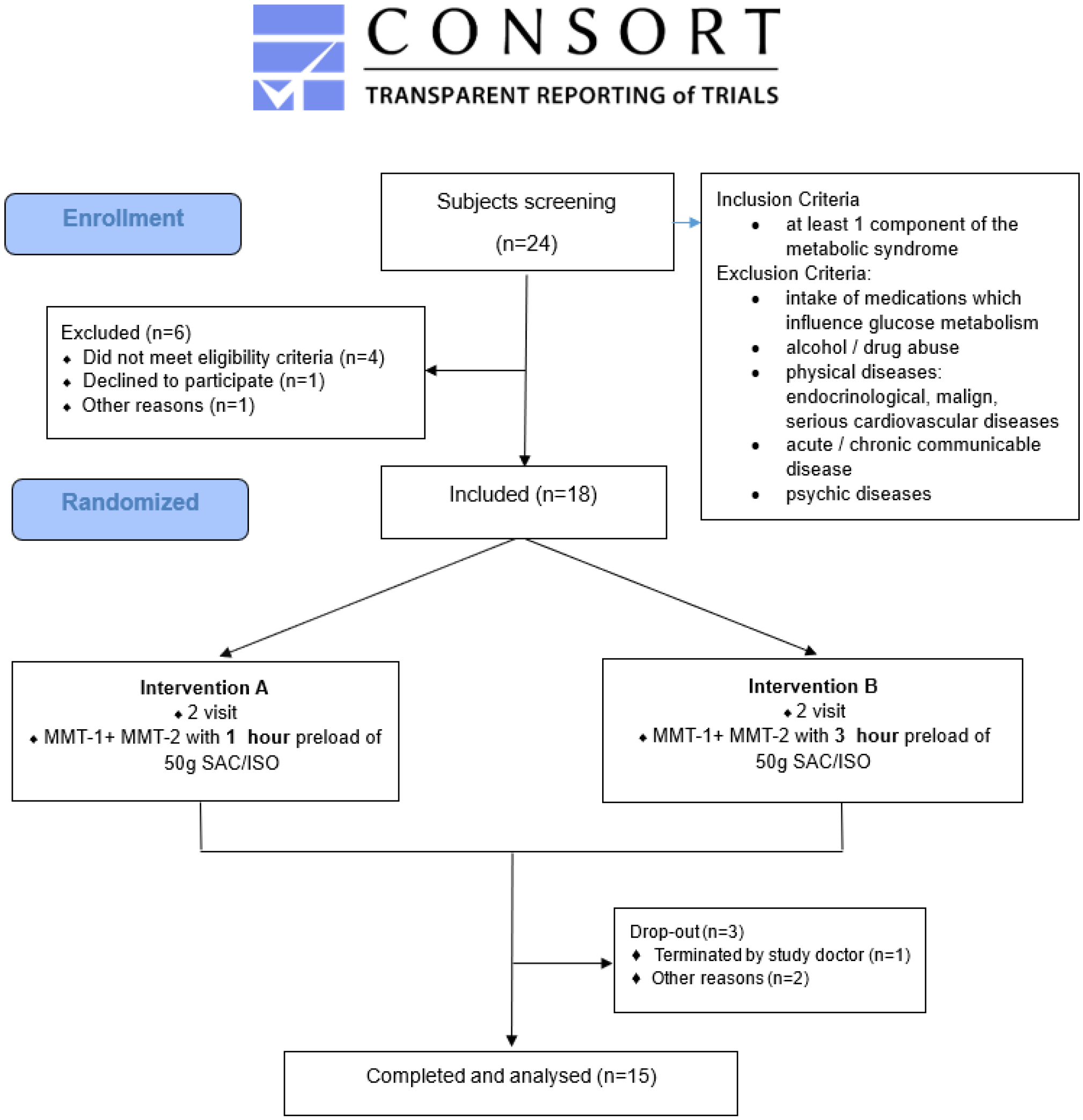
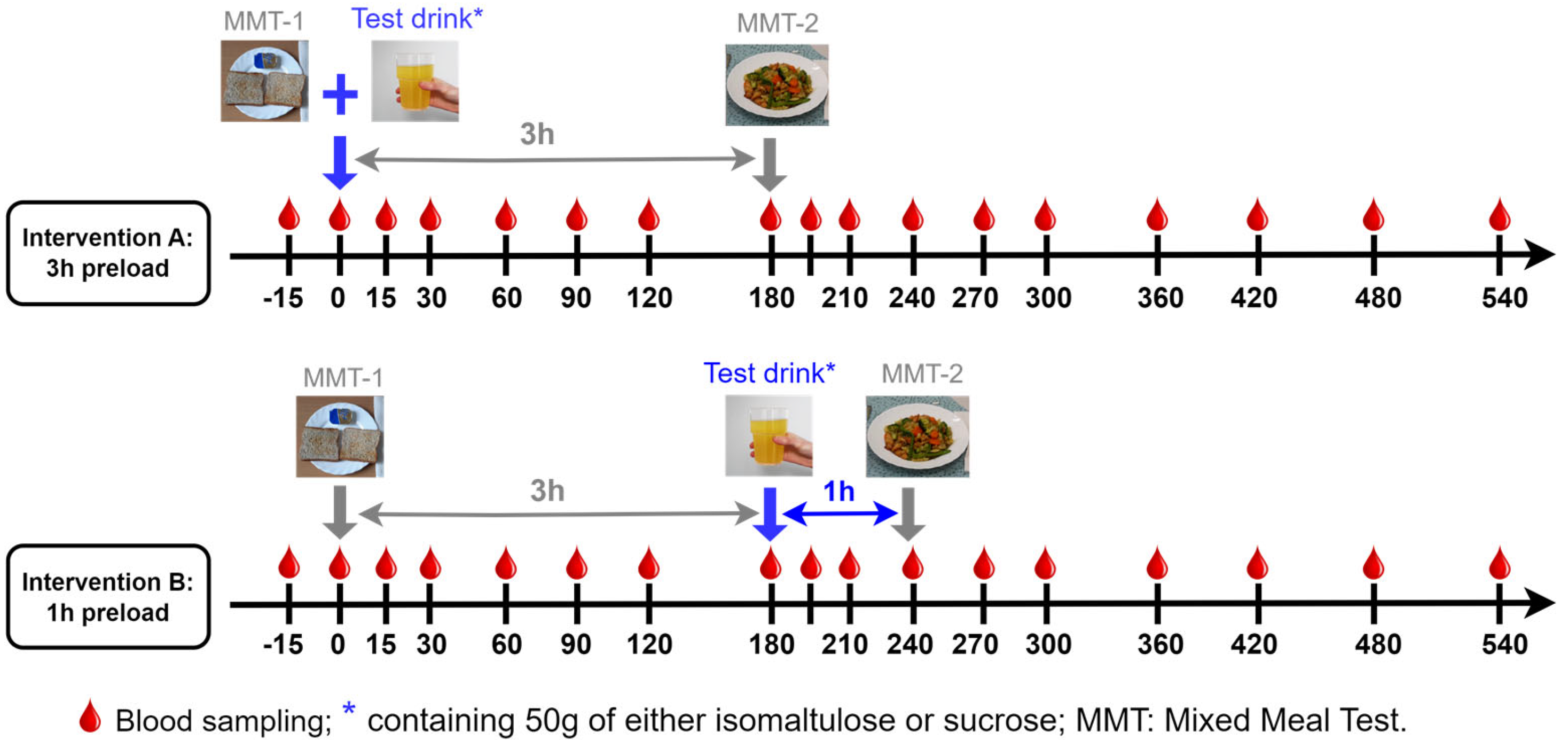
2. Materials and Methods
2.1. Ethics and Study Design
2.2. Biomarkers Measurements
2.3. Calculations and Statistical Analysis
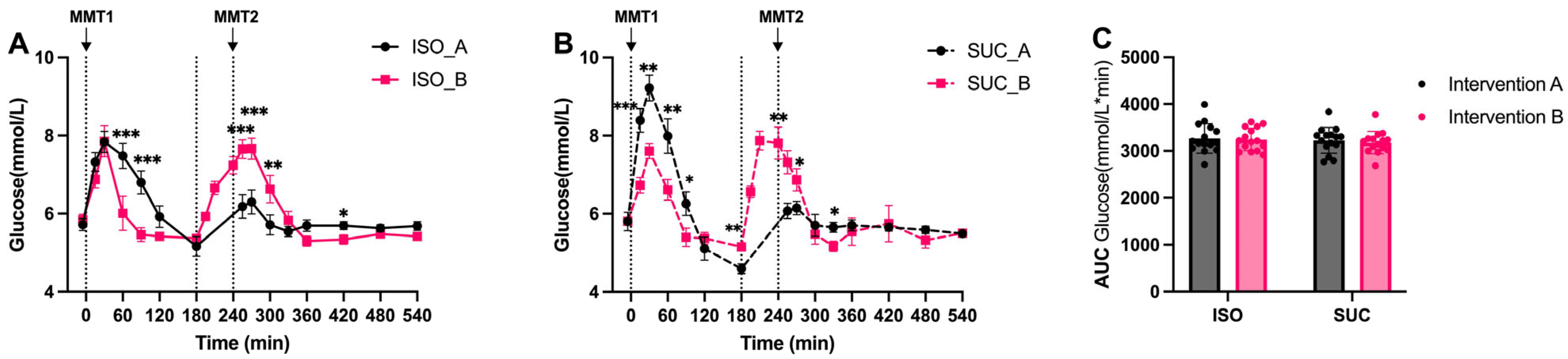
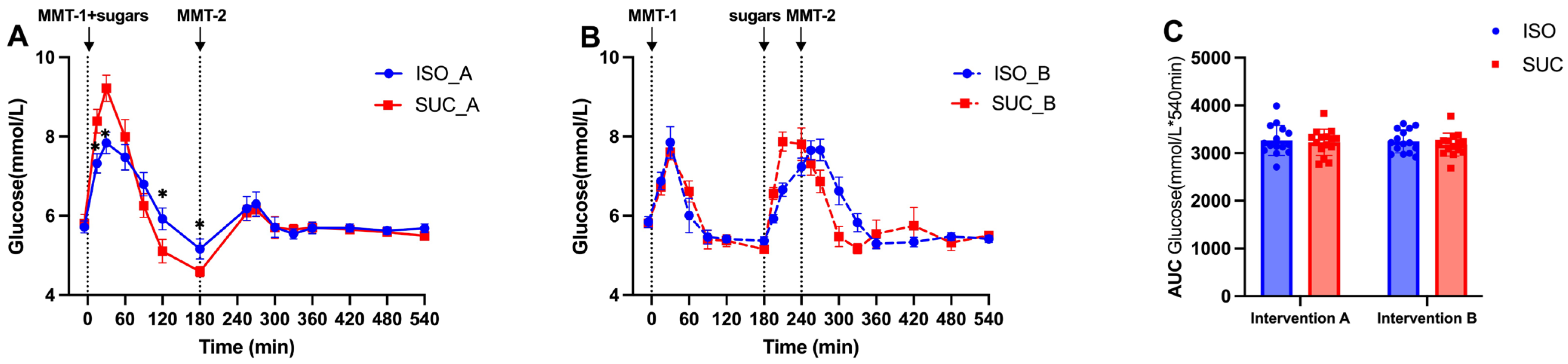
3. Results
3.1. Metabolic Parameter Responses to ISO/SUC Preload
3.1.1. Glucose
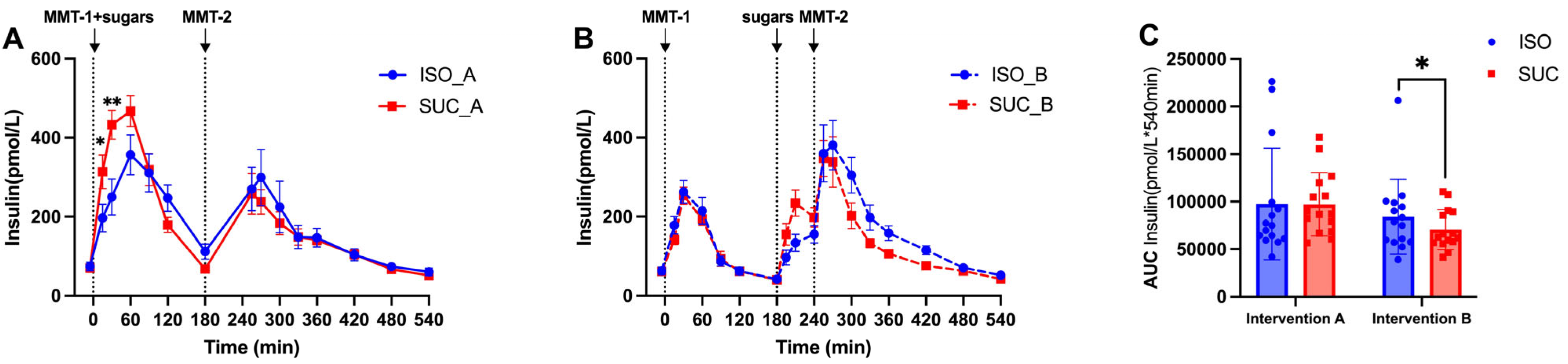
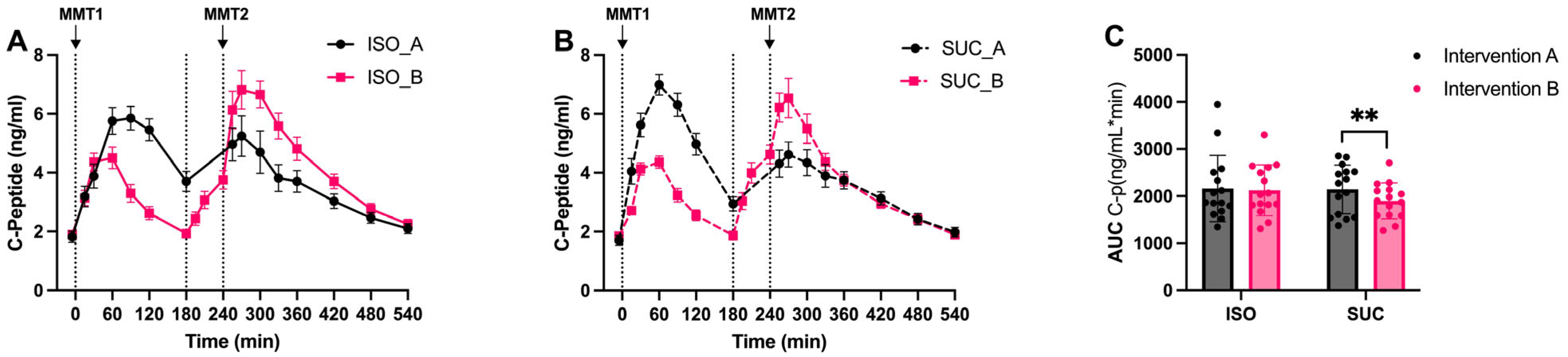
3.1.2. Insulin
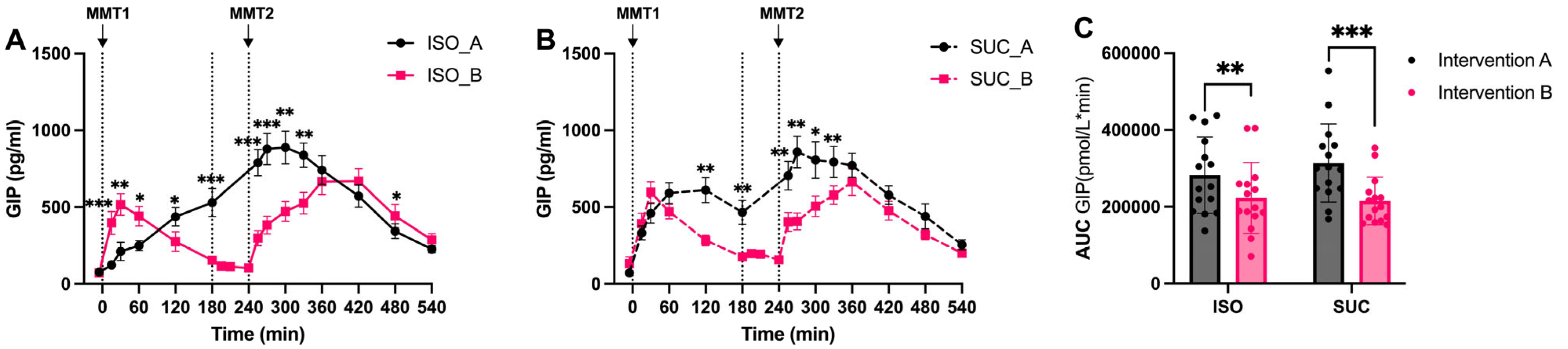
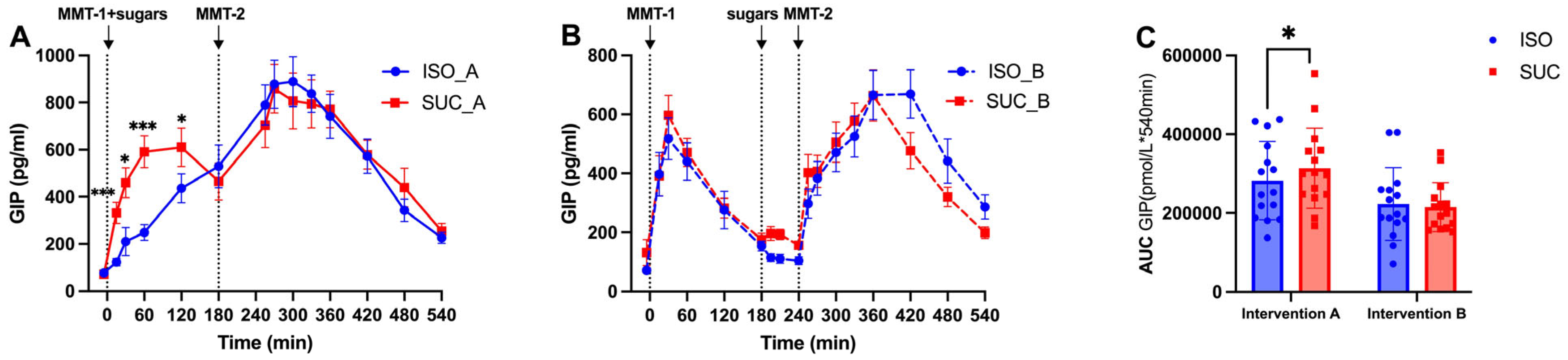
3.1.3. Gut Hormones (GIP, GLP-1, PYY)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AUC | Area Under the Curve |
| DPP-4i | Dipeptidyl Peptidase-4 inhibitor |
| FFAR2/3 | Free Fatty Acid Receptor 2/3 |
| GI | Glycemic Index |
| GIP | Glucose-dependent Insulinotropic Polypeptide |
| GLP-1 | Glucagon-Like Peptide-1 |
| HOMA-IR | Homeostatic Model Assessment for Insulin Resistance |
| ISO | Isomaltulose |
| MetS | Metabolic Syndrome |
| MMT | Mixed Meal Test |
| OGTT | Oral Glucose Tolerance Test |
| PYY | Peptide YY |
| SUC | Saccharose |
| SCFA | Short-Chain Fatty Acids |
| SGLT1 | Sodium-Glucose Cotransporter-1 |
| SME | Second Meal Effect |
| T2DM | Type 2 Diabetes |
Appendix A
| Quantity (g) | Energy (kcal) | CH (g) | F (g) | P (g) | Fiber (g) | |
|---|---|---|---|---|---|---|
| Dinner the evening before | ||||||
| Chicken meal (Frosta) | 500 | 520 | 62.0 | 14.0 | 32.5 | 7.5 |
| Breakfast fixed component (MMT-1) | ||||||
| Toast whole grain (REWE) | 50 | 128 | 24.0 | 2.0 | 4.0 | 1.5 |
| Butter (Meggle) | 17 | 126 | 0.0 | 14.0 | 0.1 | 0.0 |
| Total | 67 | 254 | 24.0 | 16.0 | 4.1 | 1.5 |
| Lunch (MMT-2) | ||||||
| “Spätzle” meal (Frosta) | 500 | 530 | 65.0 | 14.0 | 33.0 | 9.0 |
| Olive oil (Henry Lamotte) | 15 | 133 | 0.0 | 15.0 | 0.0 | 0.0 |
| Total | 515 | 663 | 65.0 | 29.0 | 33.0 | 9.0 |
| Test Meal (ISO/SUC) | ||||||
| Citrus drink (BENEO) | 400 | 202.5 | 50.0 | 0.0 | 0.0 | 0.0 |
References
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.T.; Loria, C.M.; Smith, S.C., Jr. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar]
- Brunner, S.; Holub, I.; Theis, S.; Gostner, A.; Melcher, R.; Wolf, P.; Amann-Gassner, U.; Scheppach, W.; Hauner, H. Metabolic effects of replacing sucrose by isomaltulose in subjects with type 2 diabetes: A randomized double-blind trial. Diabetes Care 2012, 35, 1249–1251. [Google Scholar] [CrossRef] [PubMed]
- Keyhani-Nejad, F.; Kemper, M.; Schueler, R.; Pivovarova, O.; Rudovich, N.; Pfeiffer, A.F. Effects of Palatinose and Sucrose Intake on Glucose Metabolism and Incretin Secretion in Subjects With Type 2 Diabetes. Diabetes Care 2016, 39, e38–e39. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, A.F.H.; Keyhani-Nejad, F. High Glycemic Index Metabolic Damage—A Pivotal Role of GIP and GLP-1. Trends Endocrinol. Metab. 2018, 29, 289–299. [Google Scholar] [CrossRef]
- Keyhani-Nejad, F.; Yanez, R.L.B.; Kemper, M.; Schueler, R.; Pivovarova-Ramich, O.; Rudovich, N.; Pfeiffer, A.F. Endogenously released GIP reduces and GLP-1 increases hepatic insulin extraction. Peptides 2020, 125, 170231. [Google Scholar] [CrossRef]
- Tian, Y.; Deng, Y.; Zhang, W.; Mu, W. Sucrose isomers as alternative sweeteners: Properties, production, and applications. Appl. Microbiol. Biotechnol. 2019, 103, 8677–8687. [Google Scholar] [CrossRef]
- Ludwig, D.S. The glycemic index: Physiological mechanisms relating to obesity, diabetes, and cardiovascular disease. JAMA 2002, 287, 2414–2423. [Google Scholar] [CrossRef]
- Wolever, T.M.; Jenkins, D.J.; Ocana, A.M.; Rao, V.A.; Collier, G.R. Second-meal effect: Low-glycemic-index foods eaten at dinner improve subsequent breakfast glycemic response. Am. J. Clin. Nutr. 1988, 48, 1041–1047. [Google Scholar] [CrossRef]
- Ballon, A.; Neuenschwander, M.; Schlesinger, S. Breakfast Skipping Is Associated with Increased Risk of Type 2 Diabetes among Adults: A Systematic Review and Meta-Analysis of Prospective Cohort Studies. J. Nutr. 2019, 149, 106–113. [Google Scholar] [CrossRef]
- Salgin, B.; Marcovecchio, M.L.; Humphreys, S.M.; Hill, N.; Chassin, L.J.; Lunn, D.J.; Hovorka, R.; Dunger, D.B. Effects of prolonged fasting and sustained lipolysis on insulin secretion and insulin sensitivity in normal subjects. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E454–E461. [Google Scholar] [CrossRef]
- Antoni, R.; Johnston, K.L.; Collins, A.L.; Robertson, M.D. Investigation into the acute effects of total and partial energy restriction on postprandial metabolism among overweight/obese participants. Br. J. Nutr. 2016, 115, 951–959. [Google Scholar] [CrossRef]
- Willard, F.S.; Douros, J.D.; Gabe, M.B.; Showalter, A.D.; Wainscott, D.B.; Suter, T.M.; Capozzi, M.E.; van der Velden, W.J.; Stutsman, C.; Cardona, G.R.; et al. Tirzepatide is an imbalanced and biased dual GIP and GLP-1 receptor agonist. JCI Insight 2020, 5, e140532. [Google Scholar] [CrossRef]
- Frias, J.P.; Nauck, M.A.; Van, J.; Kutner, M.E.; Cui, X.; Benson, C.; Urva, S.; Gimeno, R.E.; Milicevic, Z.; Robins, D.; et al. Efficacy and safety of LY3298176, a novel dual GIP and GLP-1 receptor agonist, in patients with type 2 diabetes: A randomised, placebo-controlled and active comparator-controlled phase 2 trial. Lancet 2018, 392, 2180–2193. [Google Scholar] [CrossRef] [PubMed]
- Frias, J.P.; Nauck, M.A.; Van, J.; Benson, C.; Bray, R.; Cui, X.; Milicevic, Z.; Urva, S.; Haupt, A.; Robins, D.A. Efficacy and tolerability of tirzepatide, a dual glucose-dependent insulinotropic peptide and glucagon-like peptide-1 receptor agonist in patients with type 2 diabetes: A 12-week, randomized, double-blind, placebo-controlled study to evaluate different dose-escalation regimens. Diabetes Obes. Metab. 2020, 22, 938–946. [Google Scholar] [PubMed]
- Habib, A.M.; Richards, P.; Rogers, G.J.; Reimann, F.; Gribble, F.M. Co-localisation and secretion of glucagon-like peptide 1 and peptide YY from primary cultured human L cells. Diabetologia 2013, 56, 1413–1416. [Google Scholar] [CrossRef] [PubMed]
- Boey, D.; Lin, S.; Karl, T.; Baldock, P.; Lee, N.; Enriquez, R.; Couzens, M.; Slack, K.; Dallmann, R.; Sainsbury, A.; et al. Peptide YY ablation in mice leads to the development of hyperinsulinaemia and obesity. Diabetologia 2006, 49, 1360–1370. [Google Scholar] [CrossRef]
- Hjerpsted, J.B.; Flint, A.; Brooks, A.; Axelsen, M.B.; Kvist, T.; Blundell, J. Semaglutide improves postprandial glucose and lipid metabolism, and delays first-hour gastric emptying in subjects with obesity. Diabetes Obes. Metab. 2018, 20, 610–619. [Google Scholar] [CrossRef]
- Nauck, M.A.; Quast, D.R.; Wefers, J.; Meier, J.J. GLP-1 receptor agonists in the treatment of type 2 diabetes—State-of-the-art. Mol. Metab. 2021, 46, 101102. [Google Scholar] [CrossRef]
- Pérez-Arana, G.-M.; Díaz-Gómez, A.; Camacho-Ramírez, A.; Ribelles-García, A.; Almorza-Gomar, D.; Gracia-Romero, M.; Prada-Oliveira, J.-A. Peptide Tyrosine-Tyrosine Triggers GLP-2-Mediated Intestinal Hypertrophy After Roux-en-Y Gastric Bypass. Obes. Surg. 2022, 32, 4023–4032. [Google Scholar] [CrossRef]
- Wu, T.; Rayner, C.K.; Jones, K.L.; Horowitz, M.; Feinle-Bisset, C.; Standfield, S.D.; Xie, C.; Deacon, C.F.; Holst, J.J.; Albrechtsen, N.J.W. Measurement of plasma glucagon in humans: A shift in the performance of a current commercially available radioimmunoassay kit. Diabetes Obes. Metab. 2022, 24, 1182–1184. [Google Scholar] [CrossRef]
- Baggio, L.L.; Drucker, D.J. Biology of incretins: GLP-1 and GIP. Gastroenterology 2007, 132, 2131–2157. [Google Scholar] [CrossRef]
- Conwell, L.S.; Trost, S.G.; Brown, W.J.; Batch, J.A. Indexes of insulin resistance and secretion in obese children and adolescents: A validation study. Diabetes Care 2004, 27, 314–319. [Google Scholar] [CrossRef]
- Matsuda, M.; DeFronzo, R.A. Insulin sensitivity indices obtained from oral glucose tolerance testing: Comparison with the euglycemic insulin clamp. Diabetes Care 1999, 22, 1462–1470. [Google Scholar] [CrossRef]
- Zhang, J.; Sonnenburg, D.; Tricò, D.; Kabisch, S.; Mari, A.; Theis, S.; Kemper, M.; Pivovarova-Ramich, O.; Rohn, S.; Pfeiffer, A.F.H. Isomaltulose Enhances GLP-1 and PYY Secretion to a Mixed Meal in People With or Without Type 2 Diabetes as Compared to Saccharose. Mol. Nutr. Food Res. 2024, 68, e2300086. [Google Scholar] [CrossRef]
- Tong, J.; D’Alessio, D. Give the receptor a brake: Slowing gastric emptying by GLP-1. Diabetes 2014, 63, 407–409. [Google Scholar] [CrossRef]
- Gandasi, N.R.; Gao, R.; Kothegala, L.; Pearce, A.; Santos, C.; Acreman, S.; Basco, D.; Benrick, A.; Chibalina, M.V.; Clark, A.; et al. GLP-1 metabolite GLP-1(9-36) is a systemic inhibitor of mouse and human pancreatic islet glucagon secretion. Diabetologia 2024, 67, 528–546. [Google Scholar] [CrossRef]
- Zheng, Z.; Zong, Y.; Ma, Y.; Tian, Y.; Pang, Y.; Zhang, C.; Gao, J. Glucagon-like peptide-1 receptor: Mechanisms and advances in therapy. Signal Transduct. Target. Ther. 2024, 9, 234. [Google Scholar] [CrossRef] [PubMed]




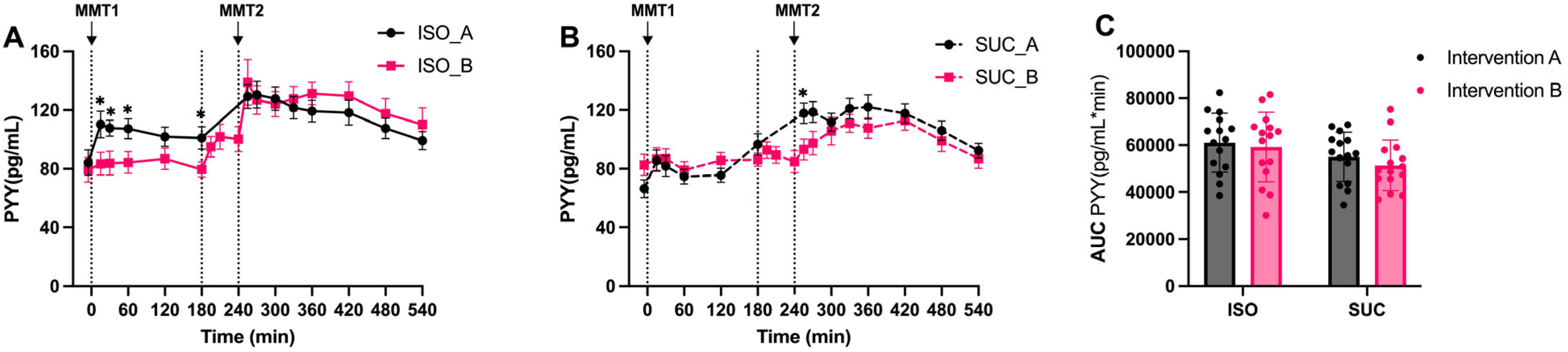
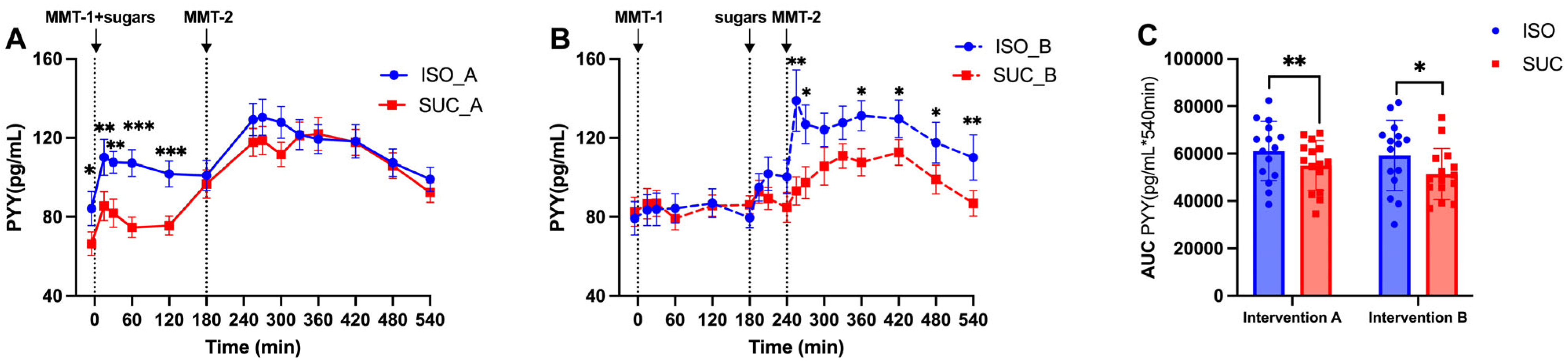
| Characteristics (n = 15) | Mets |
|---|---|
| Male:Female | 9:6 |
| Age (years) | 61.9 ± 8.5 |
| BMI (kg/m2) | 30.8 ± 4.0 |
| WHR | 0.96 ± 0.1 |
| Fat mass (%) | 31.8 ± 17.0 |
| Fat-free mass (%) | 61.1 ± 7.2 |
| HbA1c (%) | 5.6 ± 0.1 |
| HOMA-IR | 2.9 ± 0.2 |
| Fasting glucose (mmol/L) | 5.8 ± 0.5 |
| Fasting insulin (pmol/L) | 60.9 ± 23.0 |
| Fasting C-peptide (pmol/L) | 2.0 ± 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Sonnenburg, D.; Kabisch, S.; Theis, S.; Kemper, M.; Pivovarova-Ramich, O.; Tricò, D.; Rohn, S.; Pfeiffer, A.F.H. Gut Hormones and Postprandial Metabolic Effects of Isomaltulose vs. Saccharose Consumption in People with Metabolic Syndrome. Nutrients 2025, 17, 2539. https://doi.org/10.3390/nu17152539
Zhang J, Sonnenburg D, Kabisch S, Theis S, Kemper M, Pivovarova-Ramich O, Tricò D, Rohn S, Pfeiffer AFH. Gut Hormones and Postprandial Metabolic Effects of Isomaltulose vs. Saccharose Consumption in People with Metabolic Syndrome. Nutrients. 2025; 17(15):2539. https://doi.org/10.3390/nu17152539
Chicago/Turabian StyleZhang, Jiudan, Dominik Sonnenburg, Stefan Kabisch, Stephan Theis, Margrit Kemper, Olga Pivovarova-Ramich, Domenico Tricò, Sascha Rohn, and Andreas F. H. Pfeiffer. 2025. "Gut Hormones and Postprandial Metabolic Effects of Isomaltulose vs. Saccharose Consumption in People with Metabolic Syndrome" Nutrients 17, no. 15: 2539. https://doi.org/10.3390/nu17152539
APA StyleZhang, J., Sonnenburg, D., Kabisch, S., Theis, S., Kemper, M., Pivovarova-Ramich, O., Tricò, D., Rohn, S., & Pfeiffer, A. F. H. (2025). Gut Hormones and Postprandial Metabolic Effects of Isomaltulose vs. Saccharose Consumption in People with Metabolic Syndrome. Nutrients, 17(15), 2539. https://doi.org/10.3390/nu17152539





.png)


