Not All Weight Loss Is Equal: Divergent Patterns and Prognostic Roles in Head and Neck Cancer Versus High-Grade B-Cell Lymphoma
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis and Data Handling
3. Results
3.1. Clinical Characteristics
3.2. Weight Loss Is a Common Event in Cancer Patients
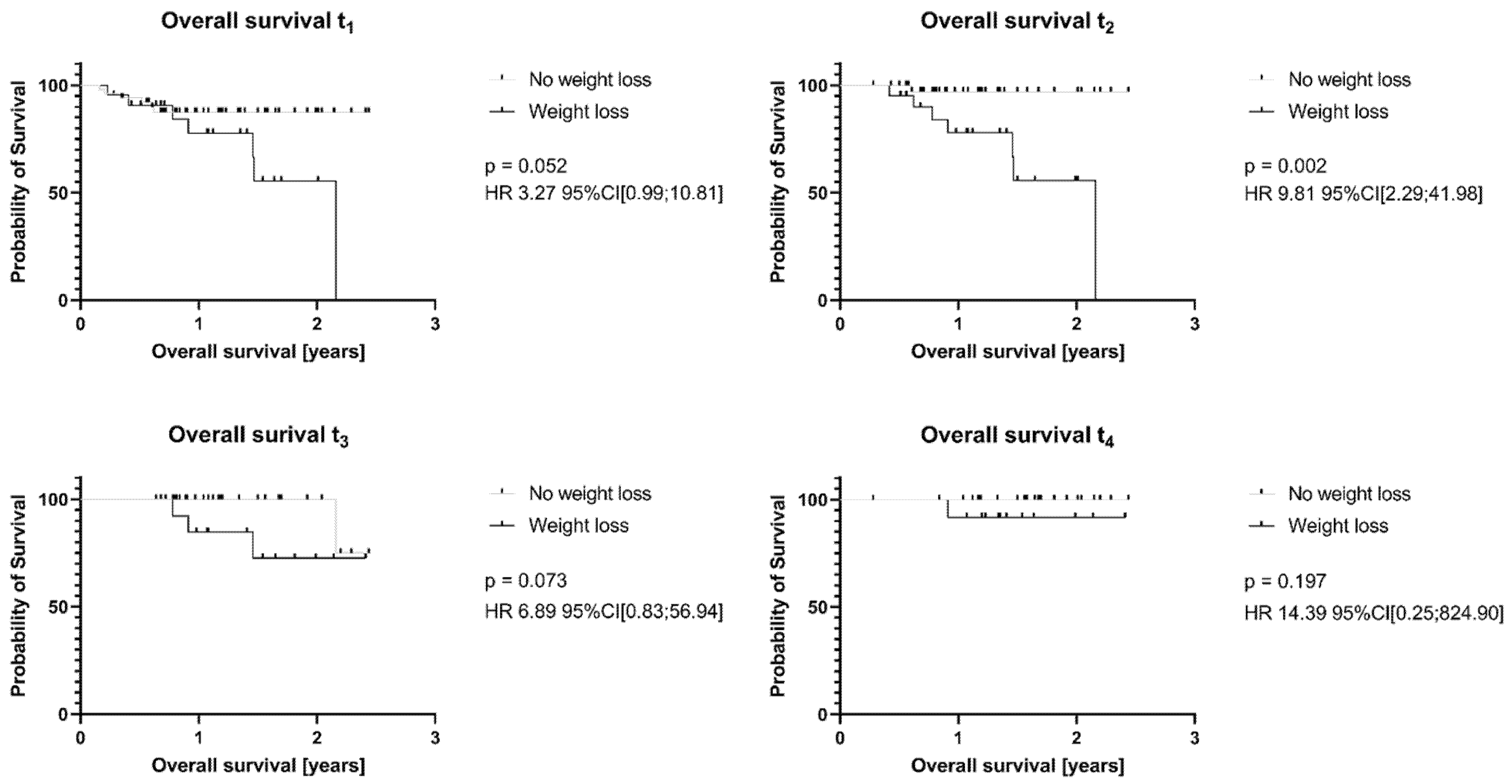
3.3. Weight Loss Has No Influence on Recurrent Disease in HNC or HGBCL Patients, but on Survival
3.4. CrP and Albumin as Indicators for Systemic Inflammation in HGBCL Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HNC | Head and neck cancer |
| HGBCL | High grade B-cell lymphoma |
| HR | Hazard ratio |
| CrP | C-reactive protein |
| STROBE | Strengthening the Reporting of Observational Studies in Epidemiology |
| CTCAE | Common Terminology Criteria for Adverse Events |
| mPGS | modified Prognostic Glasgow Score |
Appendix A
| Item No | Recommendation | Page No | |
|---|---|---|---|
| Title and abstract | 1 | (a) Indicate the study’s design with a commonly used term in the title or the abstract | 1 |
| (b) Provide in the abstract an informative and balanced summary of what was done and what was found | 1 | ||
| Introduction | |||
| Background/rationale | 2 | Explain the scientific background and rationale for the investigation being reported | 2 |
| Objectives | 3 | State specific objectives, including any prespecified hypotheses | 2 |
| Methods | |||
| Study design | 4 | Present key elements of study design early in the paper | 3 |
| Setting | 5 | Describe the setting, locations, and relevant dates, including periods of recruitment, exposure, follow-up, and data collection | 3 |
| Participants | 6 | Cross-sectional study—Give the eligibility criteria, and the sources and methods of selection of participants | 3 |
| Variables | 7 | Clearly define all outcomes, exposures, predictors, potential confounders, and effect modifiers. Give diagnostic criteria, if applicable | 3 |
| Data sources/measurement | 8 * | For each variable of interest, give sources of data and details of methods of assessment (measurement). Describe comparability of assessment methods if there is more than one group | 3 |
| Bias | 9 | Describe any efforts to address potential sources of bias | 3 |
| Study size | 10 | Explain how the study size was arrived at | 3 |
| Quantitative variables | 11 | Explain how quantitative variables were handled in the analyses. If applicable, describe which groupings were chosen and why | 3 |
| Statistical methods | 12 | (a) Describe all statistical methods, including those used to control for confounding | 3 |
| (b) Describe any methods used to examine subgroups and interactions | 3 | ||
| (c) Explain how missing data were addressed | 3 | ||
| (d) Cross-sectional study—If applicable, describe analytical methods taking account of sampling strategy | n. a. | ||
| (e) Describe any sensitivity analyses | n. a. | ||
| Results | |||
| Participants | 13 * | (a) Report numbers of individuals at each stage of study—e.g., numbers potentially eligible, examined for eligibility, confirmed eligible, included in the study, completing follow-up, and analysed | 3ff |
| (b) Give reasons for non-participation at each stage | n. a. | ||
| (c) Consider use of a flow diagram | n. a. | ||
| Descriptive data | 14 * | (a) Give characteristics of study participants (e.g., demographic, clinical, social) and information on exposures and potential confounders | 3ff |
| (b) Indicate number of participants with missing data for each variable of interest | 5 | ||
| Outcome data | 15 * | Cohort study—Report numbers of outcome events or summary measures over time | n. a. |
| Case-control study—Report numbers in each exposure category, or summary measures of exposure | n. a. | ||
| Cross-sectional study—Report numbers of outcome events or summary measures | 3ff | ||
| Main results | 16 | (a) Give unadjusted estimates and, if applicable, confounder-adjusted estimates and their precision (e.g., 95% confidence interval). Make clear which confounders were adjusted for and why they were included | 3ff |
| (b) Report category boundaries when continuous variables were categorized | 3ff | ||
| (c) If relevant, consider translating estimates of relative risk into absolute risk for a meaningful time period | n. a. | ||
| Other analyses | 17 | Report other analyses done—e.g., analyses of subgroups and interactions, and sensitivity analyses | 3ff |
| Discussion | |||
| Key results | 18 | Summarise key results with reference to study objectives | 9ff |
| Limitations | 19 | Discuss limitations of the study, taking into account sources of potential bias or imprecision. Discuss both direction and magnitude of any potential bias | 10f |
| Interpretation | 20 | Give a cautious overall interpretation of results considering objectives, limitations, multiplicity of analyses, results from similar studies, and other relevant evidence | 9ff |
| Generalisability | 21 | Discuss the generalisability (external validity) of the study results | 9ff |
| Other information | |||
| Funding | 22 | Give the source of funding and the role of the funders for the present study and, if applicable, for the original study on which the present article is based | 11 |
| * Give information separately for cases and controls in case-control studies and, if applicable, for exposed and unexposed groups in cohort and cross-sectional studies. | |||
References
- Büthe, L.; Westhofen, G.; Hille, A.; Büntzel, J. Symptom Burden and Dietary Changes Among Older Adults with Cancer: A Cross-Sectional Study. Curr. Oncol. 2024, 31, 7663–7685. [Google Scholar] [CrossRef]
- Aktas, A.; Kadakia, K.C.; Waldman, J.; Walsh, D. Weight Loss in Cancer and the 2017 Common Terminology Criteria for Adverse Events—Dangerous and Misleading. J. Cachexia Sarcopenia Muscle 2025, 16, e13754. [Google Scholar] [CrossRef] [PubMed]
- Bossi, P.; Delrio, P.; Mascheroni, A.; Zanetti, M. The Spectrum of Malnutrition/Cachexia/Sarcopenia in Oncology According to Different Cancer Types and Settings: A Narrative Review. Nutrients 2021, 13, 1980. [Google Scholar] [CrossRef] [PubMed]
- Bozzetti, F. Forcing the Vicious Circle: Sarcopenia Increases Toxicity, Decreases Response to Chemotherapy and Worsens with Chemotherapy. Ann. Oncol. 2017, 28, 2107–2118. [Google Scholar] [CrossRef] [PubMed]
- Büntzel, J.; Micke, O.; Kisters, K.; Büntzel, J.; Mücke, R. Malnutrition and Survival—Bioimpedance Data in Head Neck Cancer Patients. In Vivo 2019, 33, 979–982. [Google Scholar] [CrossRef]
- Muscaritoli, M.; Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN Practical Guideline: Clinical Nutrition in Cancer. Clin. Nutr. Edinb. Scotl. 2021, 40, 2898–2913. [Google Scholar] [CrossRef]
- Nishikawa, H.; Goto, M.; Fukunishi, S.; Asai, A.; Nishiguchi, S.; Higuchi, K. Cancer Cachexia: Its Mechanism and Clinical Significance. Int. J. Mol. Sci. 2021, 22, 8491. [Google Scholar] [CrossRef]
- Bossi, P.; De Luca, R.; Ciani, O.; D’Angelo, E.; Caccialanza, R. Malnutrition Management in Oncology: An Expert View on Controversial Issues and Future Perspectives. Front. Oncol. 2022, 12, 910770. [Google Scholar] [CrossRef]
- Demark-Wahnefried, W.; Rogers, L.Q.; Alfano, C.M.; Thomson, C.A.; Courneya, K.S.; Meyerhardt, J.A.; Stout, N.L.; Kvale, E.; Ganzer, H.; Ligibel, J.A. Practical Clinical Interventions for Diet, Physical Activity, and Weight Control in Cancer Survivors. CA Cancer J. Clin. 2015, 65, 167–189. [Google Scholar] [CrossRef]
- Siwik, C.J.; Jhaveri, K.; Cohen, J.A.; Barulich, M.; Chang, A.; Levin, A.O.; Goyal, N.G.; Melisko, M.; Chesney, M.A.; Shumay, D. Survivorship Wellness: A Multidisciplinary Group Program for Cancer Survivors. Support. Care Cancer 2023, 31, 655. [Google Scholar] [CrossRef]
- Arends, J.; Baracos, V.; Bertz, H.; Bozzetti, F.; Calder, P.C.; Deutz, N.E.P.; Erickson, N.; Laviano, A.; Lisanti, M.P.; Lobo, D.N.; et al. ESPEN Expert Group Recommendations for Action against Cancer-Related Malnutrition. Clin. Nutr. Edinb. Scotl. 2017, 36, 1187–1196. [Google Scholar] [CrossRef]
- Rock, C.L.; Thomson, C.A.; Sullivan, K.R.; Howe, C.L.; Kushi, L.H.; Caan, B.J.; Neuhouser, M.L.; Bandera, E.V.; Wang, Y.; Robien, K.; et al. American Cancer Society Nutrition and Physical Activity Guideline for Cancer Survivors. CA Cancer J. Clin. 2022, 72, 230–262. [Google Scholar] [CrossRef]
- Luan, C.-W.; Kuo, L.-T.; Wang, Y.-T.; Liao, C.-T.; Kang, C.-J.; Lee, Y.-C.; Chen, K.-Y.; Lai, C.-H.; Tsai, Y.-H.; Huang, E.I.; et al. Utility of Modified Glasgow Prognostic Score for Head and Neck Squamous Cell Carcinoma: Systematic Review and Meta-Analysis. Head Neck 2023, 45, 1856–1867. [Google Scholar] [CrossRef]
- Wu, T.-H.; Tsai, Y.-T.; Chen, K.-Y.; Yap, W.-K.; Luan, C.-W. Utility of High-Sensitivity Modified Glasgow Prognostic Score in Cancer Prognosis: A Systemic Review and Meta-Analysis. Int. J. Mol. Sci. 2023, 24, 1318. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.-J.; Chen, C.; Li, B.-F.; Gao, J.; Xia, Y.-F. High Weight Loss during Radiation Treatment Changes the Prognosis in Under-/Normal Weight Nasopharyngeal Carcinoma Patients for the Worse: A Retrospective Analysis of 2433 Cases. PLoS ONE 2013, 8, e68660. [Google Scholar] [CrossRef]
- Park, S.; Han, B.; Cho, J.W.; Woo, S.-Y.; Kim, S.; Kim, S.J.; Kim, W.S. Effect of Nutritional Status on Survival Outcome of Diffuse Large B-Cell Lymphoma Patients Treated with Rituximab-CHOP. Nutr. Cancer 2014, 66, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.-N.; Xia, T.-L.; Mai, D.-M.; Yao, J.-J.; Jiang, C.; He, W.-Z.; Xia, L.-P. The Prognostic Value of Weight Loss during Radiotherapy among Patients with Nasopharyngeal Carcinoma: A Large-Scale Cohort Study. BMC Cancer 2022, 22, 505. [Google Scholar] [CrossRef]
- Gürsoy, V.; Hunutlu, F.-Ç.; Pinar, I.-E.; Göktuğ, M.-R.; Ali, R.; Özkocaman, V.; Özkalemkaş, F. The Clinical Impacts of the Controlling Nutritional Status Score on Patients with Hodgkin Lymphoma. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 9916–9927. [Google Scholar] [CrossRef]
- Khan, M.S.; Butler, J.; Anker, M. Weight Gain Among Cancer Patients Receiving Chemotherapy—Facts and Numbers. J. Cachexia Sarcopenia Muscle 2025, 16, e13694. [Google Scholar] [CrossRef]
- Xiao, D.Y.; Luo, S.; O’Brian, K.; Sanfilippo, K.M.; Ganti, A.; Riedell, P.; Lynch, R.C.; Liu, W.; Kahl, B.S.; Cashen, A.F.; et al. Longitudinal Body Composition Changes in Diffuse Large B-Cell Lymphoma Survivors: A Retrospective Cohort Study of United States Veterans. JNCI J. Natl. Cancer Inst. 2016, 108, djw145. [Google Scholar] [CrossRef]
- Campbell, N.J.; Barton, C.; Cutress, R.I.; Copson, E.R. Impact of Obesity, Lifestyle Factors and Health Interventions on Breast Cancer Survivors. Proc. Nutr. Soc. 2023, 82, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.; Joy, A.A.; Vos, L.J.; Stenson, T.H.; Mackey, J.R.; Jovel, J.; Kao, D.; Madsen, K.L.; Wong, G.K.-S. Chemotherapy-Induced Weight Gain in Early-Stage Breast Cancer: A Prospective Matched Cohort Study Reveals Associations with Inflammation and Gut Dysbiosis. BMC Med. 2023, 21, 178. [Google Scholar] [CrossRef] [PubMed]
- Cvetković, Z.; Marković, O.; Marinković, G.; Pejić, S.; Vučić, V. Tumor Microenvironment, Inflammation, and Inflammatory Prognostic Indices in Diffuse Large B-Cell Lymphomas: A Narrative Review. Int. J. Mol. Sci. 2025, 26, 5670. [Google Scholar] [CrossRef] [PubMed]
- Diffuses Großzelliges B-Zell-Lymphom. Available online: https://www.onkopedia.com/de/onkopedia/guidelines/diffuses-grosszelliges-b-zell-lymphom (accessed on 28 June 2025).
- Ackerman, D.; Laszlo, M.; Provisor, A.; Yu, A. Nutrition Management for the Head and Neck Cancer Patient. Cancer Treat. Res. 2018, 174, 187–208. [Google Scholar] [CrossRef]
- Vandenbroucke, J.P.; von Elm, E.; Altman, D.G.; Gøtzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.J.; Egger, M. STROBE Initiative Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and Elaboration. PLoS Med. 2007, 4, e297. [Google Scholar] [CrossRef]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and Classification of Cancer Cachexia: An International Consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef]
- Common Terminology Criteria for Adverse Events (CTCAE)|Protocol Development|CTEP. Available online: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm (accessed on 21 June 2025).
- Jin, S.; Lu, Q.; Sun, Y.; Xiao, S.; Zheng, B.; Pang, D.; Yang, P. Nutrition Impact Symptoms and Weight Loss in Head and Neck Cancer during Radiotherapy: A Longitudinal Study. BMJ Support. Palliat. Care 2021, 11, 17–24. [Google Scholar] [CrossRef]
- Han, X.; Stevens, J.; Bradshaw, P.T. Body Mass Index, Weight Change and Survival in Non-Hodgkin Lymphoma Patients in Connecticut Women. Nutr. Cancer 2013, 65, 43–50. [Google Scholar] [CrossRef]
- Ottosson, S.; Zackrisson, B.; Kjellén, E.; Nilsson, P.; Laurell, G. Weight Loss in Patients with Head and Neck Cancer during and after Conventional and Accelerated Radiotherapy. Acta Oncol. Stockh. Swed. 2013, 52, 711–718. [Google Scholar] [CrossRef]
- Abu Zaid, Z.; Kay Neoh, M.; Mat Daud, Z.A.; Md Yusop, N.B.; Ibrahim, Z.; Abdul Rahman, Z.; Jamhuri, N.; Abdul Azim, A.Z. Weight Loss in Post-Chemoradiotherapy Head and Neck Cancer Patients. Nutrients 2022, 14, 548. [Google Scholar] [CrossRef]
- Albano, D.; Dondi, F.; Treglia, G.; Tucci, A.; Ravanelli, M.; Farina, D.; Bertagna, F. Longitudinal Body Composition Changes Detected by [18F]FDG PET/CT during and after Chemotherapy and Their Prognostic Role in Elderly Hodgkin Lymphoma. Cancers 2022, 14, 5147. [Google Scholar] [CrossRef] [PubMed]
- McGovern, J.; Dolan, R.D.; Skipworth, R.J.; Laird, B.J.; McMillan, D.C. Cancer Cachexia: A Nutritional or a Systemic Inflammatory Syndrome? Br. J. Cancer 2022, 127, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.; Ebadi, M.; Vo, K.; Novak, J.; Govindarajan, A.; Amini, A. An Updated Review on Head and Neck Cancer Treatment with Radiation Therapy. Cancers 2021, 13, 4912. [Google Scholar] [CrossRef]
- Verhaegen, A.A.; Van Gaal, L.F. Drugs That Affect Body Weight, Body Fat Distribution, and Metabolism. In Endotext; Feingold, K.R., Ahmed, S.F., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Pinto, B.M.; Trunzo, J.J. Health Behaviors during and after a Cancer Diagnosis. Cancer 2005, 104, 2614–2623. [Google Scholar] [CrossRef]
- Anderson, A.S.; Martin, R.M.; Renehan, A.G.; Cade, J.; Copson, E.R.; Cross, A.J.; Grimmett, C.; Keaver, L.; King, A.; Riboli, E.; et al. Cancer Survivorship, Excess Body Fatness and Weight-Loss Intervention-Where Are We in 2020? Br. J. Cancer 2021, 124, 1057–1065. [Google Scholar] [CrossRef]
- Ganzer, H.; Touger-Decker, R.; Byham-Gray, L.; Murphy, B.A.; Epstein, J.B. The Eating Experience after Treatment for Head and Neck Cancer: A Review of the Literature. Oral Oncol. 2015, 51, 634–642. [Google Scholar] [CrossRef]
- Van Liew, J.R.; Brock, R.L.; Christensen, A.J.; Karnell, L.H.; Pagedar, N.A.; Funk, G.F. Weight Loss after Head and Neck Cancer: A Dynamic Relationship with Depressive Symptoms. Head Neck 2017, 39, 370–379. [Google Scholar] [CrossRef]
- Camus, V.; Lanic, H.; Kraut, J.; Modzelewski, R.; Clatot, F.; Picquenot, J.M.; Contentin, N.; Lenain, P.; Groza, L.; Lemasle, E.; et al. Prognostic Impact of Fat Tissue Loss and Cachexia Assessed by Computed Tomography Scan in Elderly Patients with Diffuse Large B-Cell Lymphoma Treated with Immunochemotherapy. Eur. J. Haematol. 2014, 93, 9–18. [Google Scholar] [CrossRef]
- Go, S.-I.; Park, S.; Kang, M.H.; Kim, H.-G.; Kim, H.R.; Lee, G.-W. Clinical Impact of Prognostic Nutritional Index in Diffuse Large B Cell Lymphoma. Ann. Hematol. 2019, 98, 401–411. [Google Scholar] [CrossRef]
- Jhaveri, K.; Thapa, R.; Ercan, D.; Saha, A.; Noble, J.; Singh, P.; Fahrmann, J.; Saini, N.; Wu, R.; Dennison, J.B.; et al. Sarcopenia and Skeletal Muscle Loss after CAR T-Cell Therapy in Diffuse Large B Cell Lymphoma. Clin. Cancer Res. 2025, 31, 2756–2766. [Google Scholar] [CrossRef]



| HNC + HGBCL | HNC | HGBCL | p-Value | |
|---|---|---|---|---|
| N [patients] | 145 | 48 | 97 | |
| Sex | ||||
| Male | 101 | 41 | 60 | |
| Female | 44 | 7 | 37 | |
| Age [years] +/− SD | 63.08 +/− 12.77 | 61.89 +/− 9.73 | 63.67 +/− 13.99 | 0.43 |
| Body-Mass-Index [kg/m2] +/− SD | 25.78 +/− 7.31 | 26.42 +/− 5.87 | 27.23 +/− 2.18 | 0.46 |
| T1 (3 months) | ||||
| Stable weight, N [patients] | 76/118 (64.41%) | 29/45 (64.44%) | 47/73 (64.38%) | |
| Weight loss, N [patients] | 38/118 (32.20%) | 16/45 (35.56%) | 22/73 (30.14%) | 0.69 |
| Weight gain, N [patients] | 4/118 (3.39%) | 0/45 (0.00%) | 4/73 (5.48%) | 0.29 |
| T2 (6 months) | ||||
| Stable weight, N [patients] | 50/106 (47.17%) | 20/46 (43.48%) | 30/60 (50.00%) | |
| Weight loss, N [patients] | 48/106 (45.28%) | 26/46 (56.52%) | 22/60 (36.67%) | 0.22 |
| Weight gain, N [patients] | 8/106 (7.55%) | 0/46 (0.00%) | 8/60 (13.33%) | 0.04 |
| T3 (9 months) | ||||
| Stable weight, N [patients] | 38/87 (43.68%) | 18/44 (40.91%) | 20/43 (46.51%) | |
| Weight loss, N [patients] | 40/87 (45.98%) | 24/44 (54.55%) | 16/43 (37.21%) | 0.26 |
| Weight gain, N [patients] | 9/87 (10.34%) | 2/44 (4.55%) | 7/43 (16.28%) | 0.27 |
| T4 (12 months) | ||||
| Stable weight, N [patients] | 34/78 (43.59%) | 21/44 (47.73%) | 13/34 (38.24%) | |
| Weight loss, N [patients] | 33/78 (42.31%) | 21/44 (47.73%) | 12/34 (35.29%) | 1.00 |
| Weight gain, N [patients] | 11/78 (14.10%) | 2/44 (4.55%) | 9/34 (26.47%) | 0.02 |
| B-symptoms | ||||
| B symptoms, N [patients] | 33/130 | 0/48 | 33/81 | 0.0001 |
| Nutritional intervention t0 N [patients] | 27/140 | 26/48 | 1/92 | 0.0001 |
| Nutritional intervention t1 N [patients] | 12/140 | 11/48 | 1/92 | 0.0001 |
| HNC + HGBCL | HNC | HGBCL | p-Value | |
|---|---|---|---|---|
| t0 (diagnosis of cancer) | ||||
| CrP [mg/dL] | 26.65 ± 51.17 | 5.64 ± 8.38 | 37.03 ± 59.60 | <0.001 |
| albumin [mg/L] | 35.03 ± 10.64 | 44.15 ± 4.36 | 30.01 ± 9.70 | <0.001 |
| t1 (3 months) | ||||
| CrP [mg/dL] | 18.92 ± 33.72 | 16.51 ± 27.29 | 20.13 ± 36.45 | 0.99 |
| albumin [mg/L] | 33.39 ± 11.47 | 41.48 ± 4.92 | 30.08 ± 11.74 | <0.001 |
| t2 (6 months) | ||||
| CrP [mg/dL] | 24.84 ± 62.45 | 8.87 ± 14.42 | 32.13 ± 73.59 | 0.18 |
| albumin [mg/L] | 34.47 ± 10.56 | 41.16 ± 5.67 | 31.31 ± 10.85 | <0.001 |
| t3 (9 months) | ||||
| CrP [mg/dL] | 10.48 ± 16.81 | 15.05 ± 12.18 | 9.28 ± 17.63 | 0.12 |
| albumin [mg/L] | 29.73 ± 13.01 | 38.78 ±6.79 | 27.14 ± 13.21 | 0.75 |
| t4 (12 months) | ||||
| CrP [mg/dL] | 24.75 ± 37.67 | 14.68 ± 18.02 | 31.72 ± 45.36 | 0.35 |
| albumin [mg/L] | 37.46 ± 10.51 | 44.65 ± 2.34 | 31.70 ± 10.95 | 0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Büntzel, J.; Westhofen, G.; Harms, W.; Maulhardt, M.; Angleitner, A.C.; Büntzel, J. Not All Weight Loss Is Equal: Divergent Patterns and Prognostic Roles in Head and Neck Cancer Versus High-Grade B-Cell Lymphoma. Nutrients 2025, 17, 2530. https://doi.org/10.3390/nu17152530
Büntzel J, Westhofen G, Harms W, Maulhardt M, Angleitner AC, Büntzel J. Not All Weight Loss Is Equal: Divergent Patterns and Prognostic Roles in Head and Neck Cancer Versus High-Grade B-Cell Lymphoma. Nutrients. 2025; 17(15):2530. https://doi.org/10.3390/nu17152530
Chicago/Turabian StyleBüntzel, Judith, Gina Westhofen, Wilken Harms, Markus Maulhardt, Alexander Casimir Angleitner, and Jens Büntzel. 2025. "Not All Weight Loss Is Equal: Divergent Patterns and Prognostic Roles in Head and Neck Cancer Versus High-Grade B-Cell Lymphoma" Nutrients 17, no. 15: 2530. https://doi.org/10.3390/nu17152530
APA StyleBüntzel, J., Westhofen, G., Harms, W., Maulhardt, M., Angleitner, A. C., & Büntzel, J. (2025). Not All Weight Loss Is Equal: Divergent Patterns and Prognostic Roles in Head and Neck Cancer Versus High-Grade B-Cell Lymphoma. Nutrients, 17(15), 2530. https://doi.org/10.3390/nu17152530





