Impact of Omega-3 and Vitamin D Supplementation on Bone Turnover Markers in Children with Leukemia: Follow-Up During and After Supplementation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patients
2.3. Sample Size
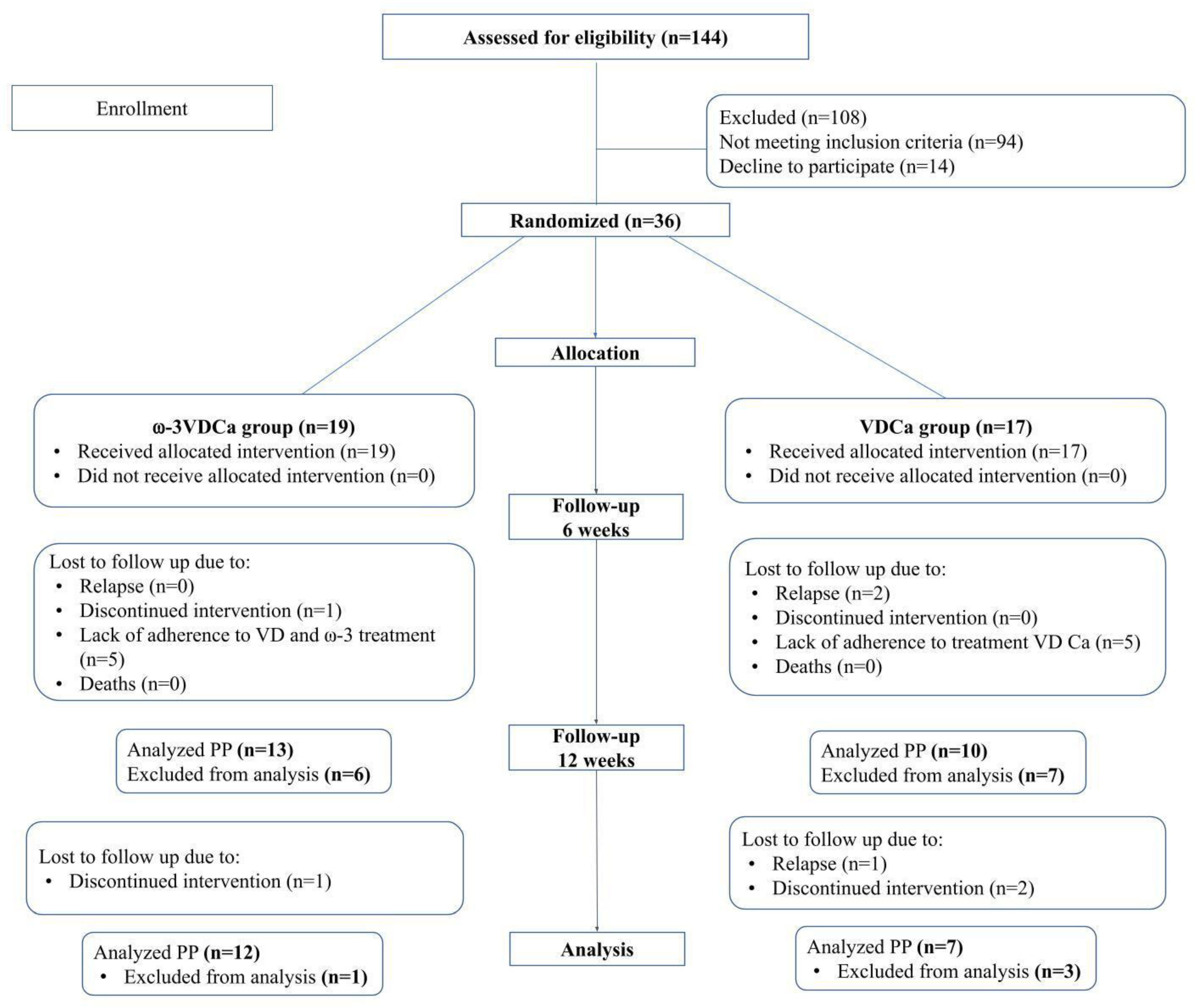
2.4. Recruitment and Allocation
2.5. Intervention
2.6. Supplementation Compliance
2.7. Procedures
2.7.1. Anthropometry
2.7.2. Analytical Methods
2.7.3. Fatty Acid Analyses Using Gas Chromatography
2.7.4. Evaluation of Bone Mineral Density
2.7.5. Statistical Analysis
3. Results
3.1. Demographic and Clinical Characteristics of All Randomized and Study-Completing Children by Protocol
3.2. Compliance with Supplementation
3.2.1. Vitamin D
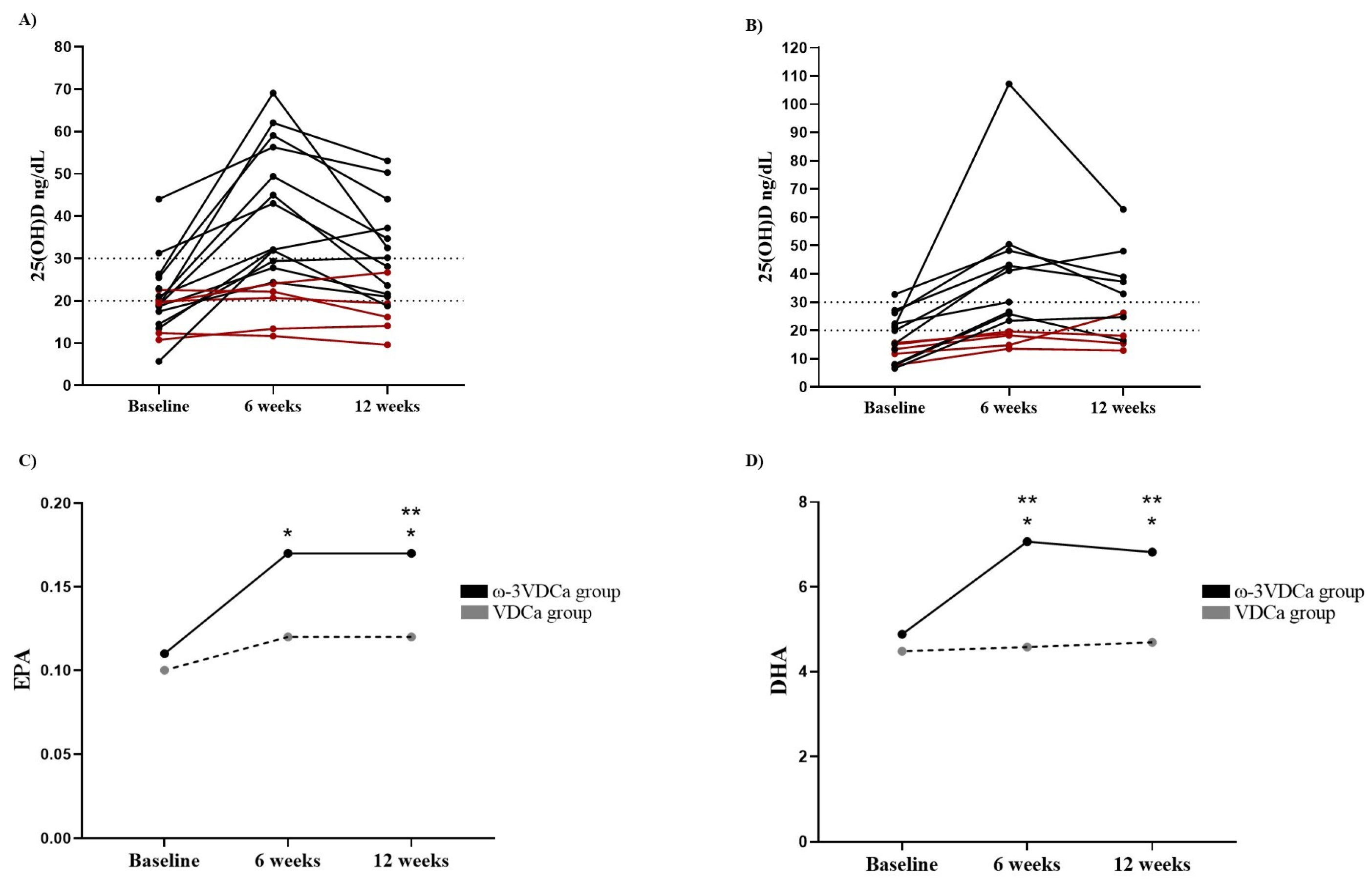
3.2.2. Omega-3 Long-Chain Polyunsaturated Fatty Acids
3.2.3. Omega-3 Long-Chain Polyunsaturated Fatty Acids After Supplementation (12 Weeks)
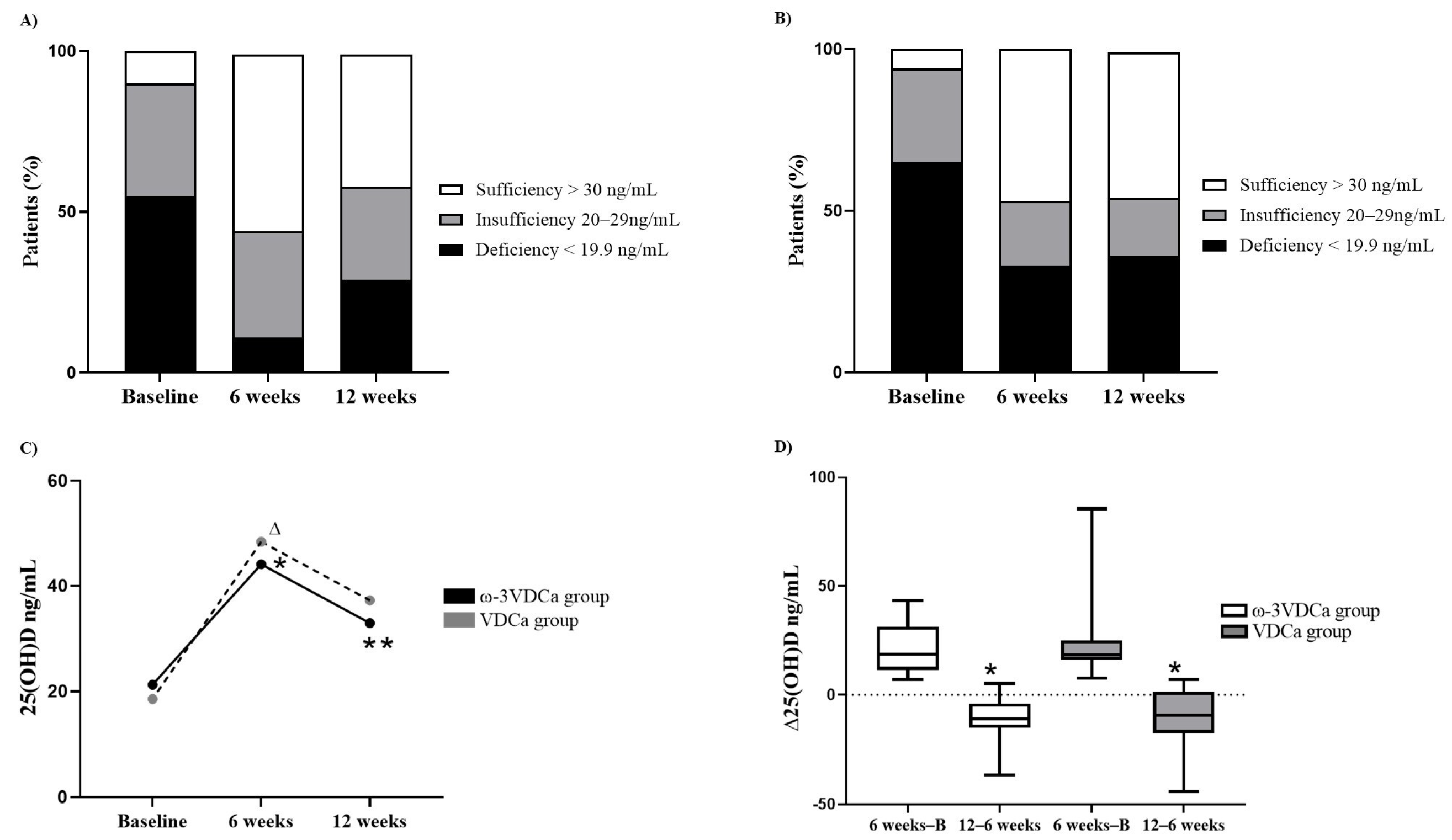
3.3. Follow-Up of Changes in Vitamin D Status During and After Supplementation
3.4. BMD and Vitamin D Nutritional Status
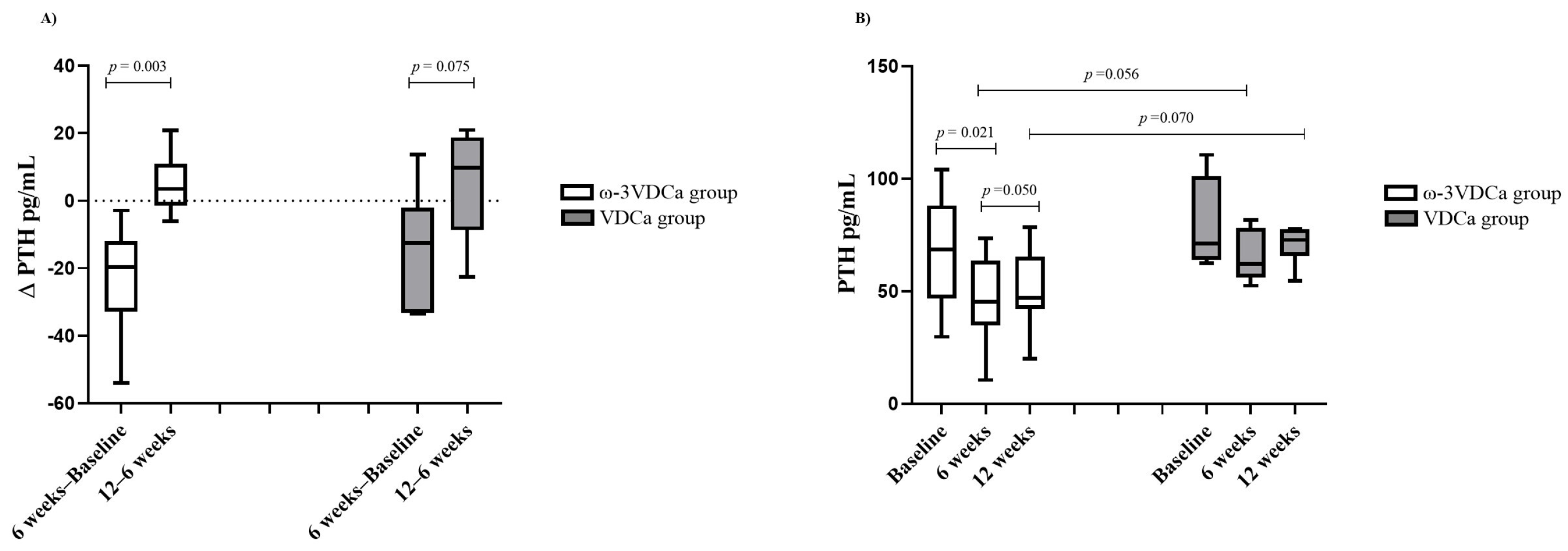
3.5. Follow-Up of Changes in PTH Status During and After Supplementation
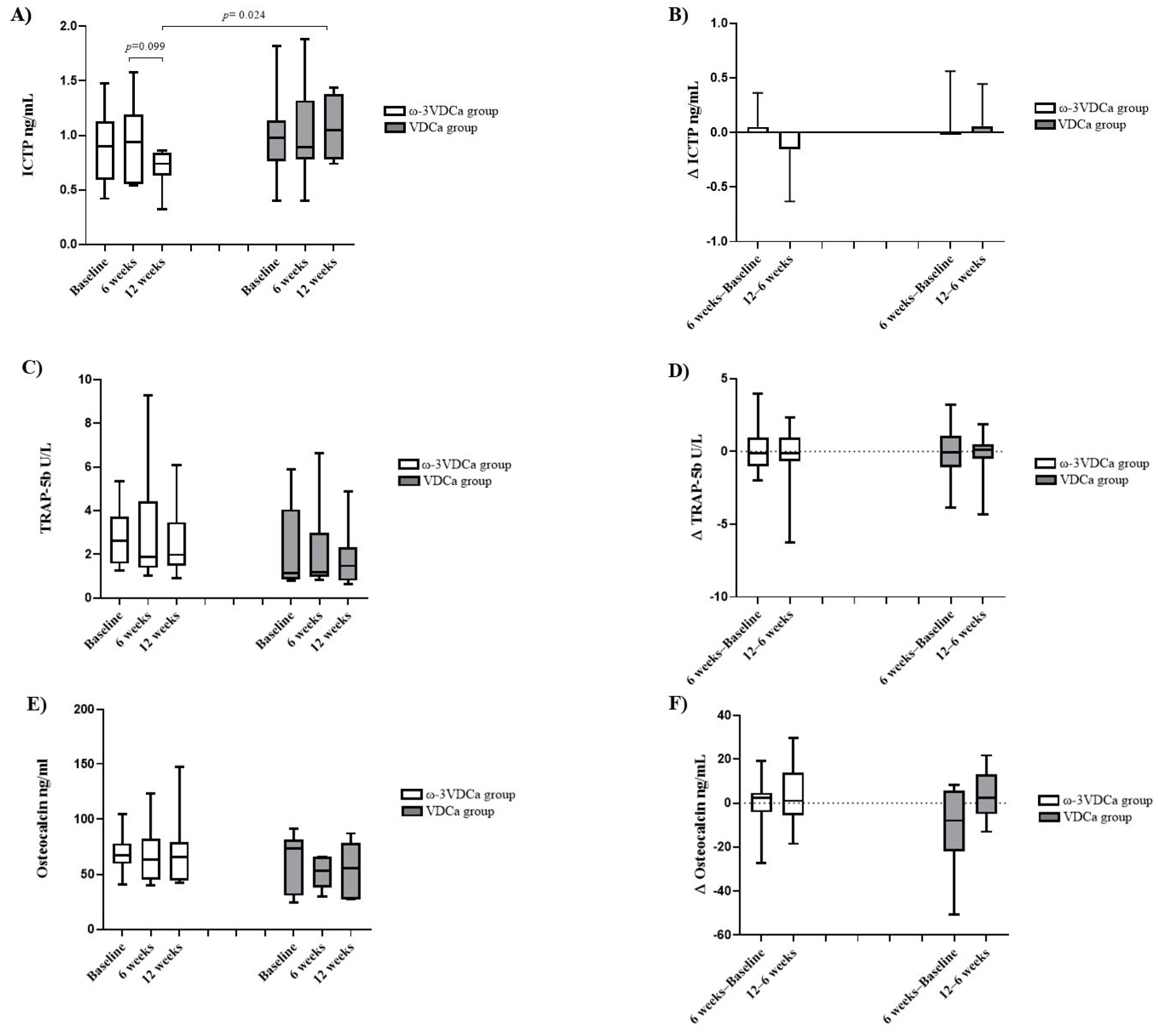
3.6. Bone Turnover Markers
3.7. Diet
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALL | Acute lymphoblastic leukemia |
| LCPUFAs-ω3 | Omega-3 long-chain polyunsaturated fatty acids |
| ω3VDCa | Omega-3 long-chain polyunsaturated fatty acids, vitamin D, and calcium group |
| VDCa | Vitamin D and calcium group |
| 25(OH)D | 25-hydroxyvitamin D |
| Ca | Calcium |
| PTH | Parathormone |
| ICTPs | Human cross-linked C-terminal telopeptides of type I collagen |
| TRAP-5b | Human tartrate-resistant acid phosphatase 5b |
| OC | Osteocalcin |
| MSCs | Mesenchymal stem cells |
| RANKL | Receptor Activator of Nuclear Factor κB Ligand |
| OPG | Osteoprotegerin |
| DHA | Docosahexaenoic acid |
| EPA | Eicosapentaenoic acid |
| M-CSF | Macrophage colony-stimulating factor |
| MITF | Microphthalmia-associated transcription factor |
| RANK | Receptor Activator of Nuclear Factor κB |
| SR | Standard risk |
| IR | Intermediate risk |
| HR | High risk |
| BMI | Body mass index |
| DXA | Dual-energy X-ray absorptiometry |
| BMD | Bone mineral density |
| SD | Standard deviation |
References
- Compston, J. Glucocorticoid-induced osteoporosis: An update. Endocrine 2018, 61, 7–16. [Google Scholar] [PubMed]
- Velentza, L.; Zaman, F.; Sävendahl, L. Bone health in glucocorticoid-treated childhood acute lymphoblastic leukemia. Crit. Rev. Oncol. Hematol. 2021, 168, 103492. [Google Scholar] [PubMed]
- Zhang, Y.; Huang, X.; Li, C.; Zhang, J.; Yu, X.; Li, Y.; Zhou, W.; Yu, F. Broad application prospects of bone turnover markers in pediatrics. J. Clin. Lab. Anal. 2022, 36, e24656. [Google Scholar] [PubMed]
- Lee, A.W.; Romanowski, G.L.; Proudfoot, J.A.; Kuo, D.J. Effect of vitamin D supplementation on delayed hyperphosphatemia in pediatric acute lymphoblastic leukemia patients during induction chemotherapy. J. Pediatr. Pharmacol. Ther. 2017, 22, 102–105. [Google Scholar]
- Atilano-Miguel, S.; Barbosa-Cortés, L.; Ortiz-Muñiz, R.; Maldonado-Hernández, J.; Martin-Trejo, J.A.; Rodríguez-Cruz, M.; Balcázar-Hernández, L.; Solís-Labastida, K.A.; Bautista-Martínez, B.A.; Juárez-Moya, A.; et al. Changes in RANKL, OPG, and 25(OH)D Levels in Children with Leukemia from Diagnosis to Remission. Cancers 2024, 16, 2811. [Google Scholar] [CrossRef]
- Anderson, P.H. Vitamin D Activity and Metabolism in Bone. Curr. Osteoporos. Rep. 2017, 15, 443–449. [Google Scholar]
- Delvin, E.; Alos, N.; Rauch, F.; Marcil, V.; Morel, S.; Boisvert, M.; Lecours, M.A.; Laverdière, C.; Sinnett, D.; Krajinovic, M.; et al. Vitamin D nutritional status and bone turnover markers in childhood acute lymphoblastic leukemia survivors: A PETALE study. Clin. Nutr. 2019, 38, 912–919. [Google Scholar]
- Bhattacharya, S.; Verma, N.; Kumar, A. Prevalence of vitamin D deficiency in childhood acute lymphoblastic leukemia and its association with adverse outcomes during induction phase of treatment. Nutr. Cancer 2020, 72, 1321–1325. [Google Scholar]
- Kaste, S.C.; Qi, A.; Smith, K.; Surprise, H.; Lovorn, E.; Boyett, J.; Ferry, R.J.; Relling, M.V.; Shurtleff, S.A.; Pui, C.H.; et al. Calcium and cholecalciferol supplementation provides no added benefit to nutritional counseling to improve bone mineral density in survivors of childhood acute lymphoblastic leukemia (ALL). Pediatr Blood Cancer. 2014, 61, 885–893. [Google Scholar]
- Demirsoy, U.; Sarper, N.; Aylan Gelen, S.; Zengin, E.; Kum, T.; Demir, H. The Association of Oral Vitamin D and Calcium Supplementation with Bone Mineral Density in Pediatric Acute Lymphoblastic Leukemia Patients. J. Pediatr. Hematol. Oncol. 2017, 39, 287–292. [Google Scholar]
- Orgel, E.; Mueske, N.M.; Sposto, R.; Gilsanz, V.; Wren, T.A.L.; Freyer, D.R.; Butturini, A.M.; Mittelman, S.D. A randomized controlled trial testing an adherence-optimized Vitamin D regimen to mitigate bone change in adolescents being treated for acute lymphoblastic leukemia. Leuk. Lymphoma 2017, 58, 2370–2378. [Google Scholar] [PubMed]
- Solmaz, I.; Ozdemir, M.A.; Unal, E.; Abdurrezzak, U.; Muhtaroglu, S.; Karakukcu, M. Effect of vitamin K2 and vitamin D3 on bone mineral density in children with acute lymphoblastic leukemia: A prospective cohort study. J. Pediatr. Endocrinol. Metab. 2021, 34, 441–447. [Google Scholar] [PubMed]
- Sharma, T.; Mandal, C.C. Omega-3 fatty acids in pathological calcification and bone health. J. Food Biochem. 2020, 44, e13333. [Google Scholar]
- Barbosa-Cortés, L.; Martínez-Vieyra, X.; Mejía-Aranguré, J.M.; López-Alarcón, M.; Martin-Trejo, J.; Delgadillo-Portillo, S.; Guzmán-Castro, B.; Delgadillo-Portillo, J.; Atilano-Miguel, S.; Rodríguez-Cruz, M.; et al. Pilot study on the effect of supplementation with long-chain ω-3 polyunsaturated fatty acids on body composition in children with acute lymphoblastic leukemia: Randomized clinical trial. Clin. Nutr. 2023, 42, 1759–1769. [Google Scholar]
- Barbosa-Cortes, L.; Atilano-Miguel, S.; Martin-Trejo, J.A.; Jiménez-Aguayo, E.; Martínez-Becerril, F.I.; López-Alarcón, M.; Mejia-Arangure, J.M.; Maldonado-Hernández, J.; Delgadillo-Portillo, S.; Guzmán-Castro, B.; et al. Effect of long-chain omega-3 polyunsaturated fatty acids on cardiometabolic factors in children with acute lymphoblastic leukemia undergoing treatment: A secondary analysis of a randomized controlled trial. Front Endocrinol. 2023, 14, 1120364. [Google Scholar]
- Laumann, R.D.; Iversen, T.; Mogensen, P.R.; Lauritzen, L.; Mølgaard, C.; Frandsen, T.L. Effect of Fish Oil Supplementation on Hyperlipidemia during Childhood Acute Lymphoblastic Leukemia Treatment–A Pilot Study. Nutr. Cancer 2021, 73, 1816–1820. [Google Scholar]
- Salvador, C.; Entenmann, A.; Salvador, R.; Niederwanger, A.; Crazzolara, R.; Kropshofer, G. Combination therapy of omega-3 fatty acids and acipimox for children with hypertriglyceridemia and acute lymphoblastic leukemia. J. Clin. Lipidol. 2018, 12, 1260–1266. [Google Scholar]
- WMA Declaration of Helsinki—Ethical Principles for Medical Research Involving Human Participants—WMA—The World Medical Association [Internet]. Available online: https://www.wma.net/policies-post/wma-declaration-of-helsinki/ (accessed on 18 June 2025).
- Möricke, A.; Reiter, A.; Zimmermann, M.; Gadner, H.; Stanulla, M.; Dördelmann, M.; Löning, L.; Beier, R.; Ludwig, W.-D.; Ratei, R.; et al. Risk-adjusted therapy of acute lymphoblastic leukemia can decrease treatment burden and improve survival: Treatment results of 2169 unselected pediatric and adolescent patients enrolled in the trial ALL-BFM 95. Blood 2008, 111, 4477–4489. [Google Scholar]
- Saghaei, M. Random allocation software for parallel group randomized trials. BMC Med. Res. Methodol. 2004, 4, 26. [Google Scholar]
- Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. Available online: https://www.ncbi.nlm.nih.gov/books/NBK56050/ (accessed on 18 June 2025).
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M.; Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar]
- Hossein-Nezhad, A.; Holick, M.F. Vitamin D for health: A global perspective. Mayo Clin. Proc. 2013, 88, 720–755. [Google Scholar]
- van Atteveld, J.E.; Verhagen, I.E.; van den Heuvel-Eibrink, M.M.; van Santen, H.M.; van der Sluis, I.M.; Di Iorgi, N.; Simmons, J.H.; Ward, L.M.; Neggers, S.J.C.M.M. Vitamin D supplementation for children with cancer: A systematic review and consensus recommendations. Cancer Med. 2021, 10, 4177–4194. [Google Scholar]
- WHO Child Growth Standards: Length/Height-for-Age, Weight-for-Age, Weight-for-Length, Weight-for-Height and Body Mass Index-for-Age: Methods and Development. Available online: https://www.who.int/publications/i/item/924154693X (accessed on 18 June 2025).
- De la Cruz-Góngora, V.; García-Guerra, A.; Shamah-Levy, T.; Villalpando, S.; Valdez-Echeverría, R.; Mejía-Rodríguez, F. Micronutrient status in Mexican children: Analysis of the Ensanut 2022. Salud Publica Mex 2023, 65, s231–s237. [Google Scholar] [PubMed]
- Bhatia, S.; Landier, W.; Hageman, L.; Kim, H.; Chen, Y.; Crews, K.R.; Evans, W.E.; Bostrom, B.; Casillas, J.; Dickens, D.S.; et al. 6MP adherence in a multiracial cohort of children with acute lymphoblastic leukemia: A Children’s Oncology Group study. Blood 2014, 124, 2345–2353. [Google Scholar] [PubMed]
- Dawson-Hughes, B.; Mithal, A.; Bonjour, J.P.; Boonen, S.; Burckhardt, P.; Fuleihan, G.E.H.; Josse, R.G.; Lips, P.; Morales-Torres, J.; Yoshimura, N. IOF position statement: Vitamin D recommendations for older adults. Osteoporos. Int. 2010, 21, 1151–1154. [Google Scholar]
- Charoenngam, N.; Holick, M.F. Immunologic effects of vitamin d on human health and disease. Nutrients 2020, 12, 2097. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.W.; Lee, H.C. Vitamin D and health—The missing vitamin in humans. Pediatr. Neonatol. 2019, 60, 237–244. [Google Scholar]
- Partan, R.U.; Hidayat, R.; Saputra, N.; Rahmayani, F.; Prapto, H.; Yudha, T.W. Seluang fish (Rasbora spp.) oil decreases inflammatory cytokines via increasing vitamin d level in systemic lupus erythematosus. Open Access Maced. J. Med. Sci. 2019, 7, 1418–1421. [Google Scholar]
- Salari Sharif, P.; Asalforoush, M.; Ameri, F.; Larijani, B.; Abdollahi, M. The effect of n-3 fatty acids on bone biomarkers in Iranian postmenopausal osteoporotic women: A randomized clinical trial. Age (Dordrecht, Netherlands) 2010, 32, 179–186. [Google Scholar]
- Khundmiri, S.J.; Murray, R.D.; Lederer, E. PTH and vitamin D. Compr. Physiol. 2016, 6, 561–601. [Google Scholar]
- Hutchins-Wiese, H.L.; Picho, K.; Watkins, B.A.; Li, Y.; Tannenbaum, S.; Claffey, K.; Kenny, A.M. High-Dose eicosapentaenoic acid and docosahexaenoic acid supplementation reduces bone resorption in postmenopausal breast cancer survivors on aromatase inhibitors: A pilot study. Nutr. Cancer 2014, 66, 68–76. [Google Scholar] [PubMed]
- Dou, Y.; Wang, Y.; Chen, Z.; Yu, X.; Ma, D. Effect of n-3 polyunsaturated fatty acid on bone health: A systematic review and meta-analysis of randomized controlled trials. Food Sci. Nutr. 2022, 10, 145–154. [Google Scholar] [PubMed]
- Ferreira, A.; Alho, I.; Casimiro, S.; Costa, L. Bone remodeling markers and bone metastases: From cancer research to clinical implications. Bonekey Rep. 2015, 4, 668. [Google Scholar] [PubMed]
- Bhattacharya, A.; Rahman, M.; Sun, D.; Fernandes, G. Effect of fish oil on bone mineral density in aging C57BL/6 female mice. J. Nutr. Biochem. 2007, 18, 372–379. [Google Scholar]
- Vanlint, S.J.; Ried, K. Efficacy and tolerability of calcium, vitamin D and a plant-based omega-3 oil for osteopenia: A pilot RCT. Maturitas 2012, 71, 44–48. [Google Scholar]
| Baseline (Randomized) | 6 Weeks | 12 Weeks | ||||
|---|---|---|---|---|---|---|
| Variables | ω-3VDCa (n = 19) | VDCa (n = 17) | ω-3VDCa (n = 12) | VDCa (n = 7) | ω-3VDCa (n = 12) | VDCa (n = 7) |
| Sex | ||||||
| Female n (%) | 10 (52.6) | 6 (35.3) | 5 (41.7) | 3 (42.9) | 5 (41.7) | 3 (42.9) |
| Age at study entry (years) | 9.0 (6.6,13.8) | 10.0 (7.1,16.0) | 7.0 (5.7,9.0) | 10 (7.1,15.1) | 7 (5.7, 9.0) | 10 (7.1, 15.1) |
| Nutritional status | ||||||
| Weight (kg) | 30.7 (21.6,48.6) | 42.9 (23.4,51.9) | 23.7 (20.3,29.5) | 46.5 (25.4,50.2) | 24.7 (20.9,29.3) | 47.6 (25.0,50.3) |
| Height (cm) | 127.1 (116,151.9) | 139.6 (123.1,162.6) | 119.3 (114.5,135.4) | 142.6 (127.5,162) | 119.8 (116.2,135.9) | 143.6 (128.5,166.8) |
| Eutrophic (BMI pc >5 to pc <85) | 14 (73.7) | 9 (52.9) | 10 (83.3) | 6 (85.7) | 9 (81.8) | 6 (85.7) |
| Overweight (BMI pc >85 to pc <95) | 4 (21.1) | 4 (23.5) | - | - | - | - |
| Obesity (BMI pc >95 | 1 (5.3) | 4 (23.5) | 1 (8.3) | 1 (14.3) | 1 (9.1) | 1 (14.3) |
| Fat (%) | 28.7 (24.5,35.2) | 25.1 (20.5,37.1) | 26.2 (23.3,28.6) | 22.8 (18.0,29.1) | 26.0 (21.8,28.6) | 20.9 (17.3,23.1) |
| Risk stratification | ||||||
| Habitual/intermediate risk, n (%) | 6 (31.6) | 8 (47.1) | 5 (41.7) | 3 (42.9) | 5 (41.7) | 3 (42.9) |
| High risk, n (%) | 13 (68.4) | 9 (52.9) | 7 (58.3) | 4 (57.1) | 7 (58.3) | 4 (57.1) |
| BMD L1–L4 Z-Score | Total Sample (n = 36) | Deficiency/Insufficiency (n = 33) | Sufficiency (n = 3) |
|---|---|---|---|
| >−1 SD Normal, n (%) | 21 (58) | 21 (64) | - |
| −1 SD and −2 SD Osteopenia, n (%) | 11 (31) | 10 (30) | 1 (33) |
| ≤2 SD Osteoporosis, n (%) | 4 (11) | 2 (6) | 2 (67) |
| ICTP | ||
|---|---|---|
| β | p | |
| DHA (%) | −0.630 | 0.012 |
| 25(OH)D (ng/mL) | −0.339 | 0.146 |
| Model | R2 = 0.341 | 0.036 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbosa-Cortés, L.; Morales-Montes, S.B.; Maldonado-Alvarado, M.; Martin-Trejo, J.A.; Atilano-Miguel, S.; Jiménez-Aguayo, E.; Martínez-Becerril, F.I.; Cortés-Beltrán, V.M.; Hernández-Barbosa, A.V.; Solís-Labastida, K.A.; et al. Impact of Omega-3 and Vitamin D Supplementation on Bone Turnover Markers in Children with Leukemia: Follow-Up During and After Supplementation. Nutrients 2025, 17, 2526. https://doi.org/10.3390/nu17152526
Barbosa-Cortés L, Morales-Montes SB, Maldonado-Alvarado M, Martin-Trejo JA, Atilano-Miguel S, Jiménez-Aguayo E, Martínez-Becerril FI, Cortés-Beltrán VM, Hernández-Barbosa AV, Solís-Labastida KA, et al. Impact of Omega-3 and Vitamin D Supplementation on Bone Turnover Markers in Children with Leukemia: Follow-Up During and After Supplementation. Nutrients. 2025; 17(15):2526. https://doi.org/10.3390/nu17152526
Chicago/Turabian StyleBarbosa-Cortés, Lourdes, Sharon B. Morales-Montes, Michelle Maldonado-Alvarado, Jorge A. Martin-Trejo, Salvador Atilano-Miguel, Emmanuel Jiménez-Aguayo, Fabián I. Martínez-Becerril, Víctor M. Cortés-Beltrán, Atzin V. Hernández-Barbosa, Karina A. Solís-Labastida, and et al. 2025. "Impact of Omega-3 and Vitamin D Supplementation on Bone Turnover Markers in Children with Leukemia: Follow-Up During and After Supplementation" Nutrients 17, no. 15: 2526. https://doi.org/10.3390/nu17152526
APA StyleBarbosa-Cortés, L., Morales-Montes, S. B., Maldonado-Alvarado, M., Martin-Trejo, J. A., Atilano-Miguel, S., Jiménez-Aguayo, E., Martínez-Becerril, F. I., Cortés-Beltrán, V. M., Hernández-Barbosa, A. V., Solís-Labastida, K. A., Maldonado-Hernández, J., Bautista-Martínez, B. A., Juárez-Moya, A., Hernández-Piñón, Z., Domínguez-Salgado, J. M., Villa-Morales, J., & Domínguez-Calderón, I. (2025). Impact of Omega-3 and Vitamin D Supplementation on Bone Turnover Markers in Children with Leukemia: Follow-Up During and After Supplementation. Nutrients, 17(15), 2526. https://doi.org/10.3390/nu17152526








