Decreased Memory Suppression Ability in Restrained Eaters on Food Information—Evidence from ERP Experiment
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Materials
2.2.1. Self-Measurements
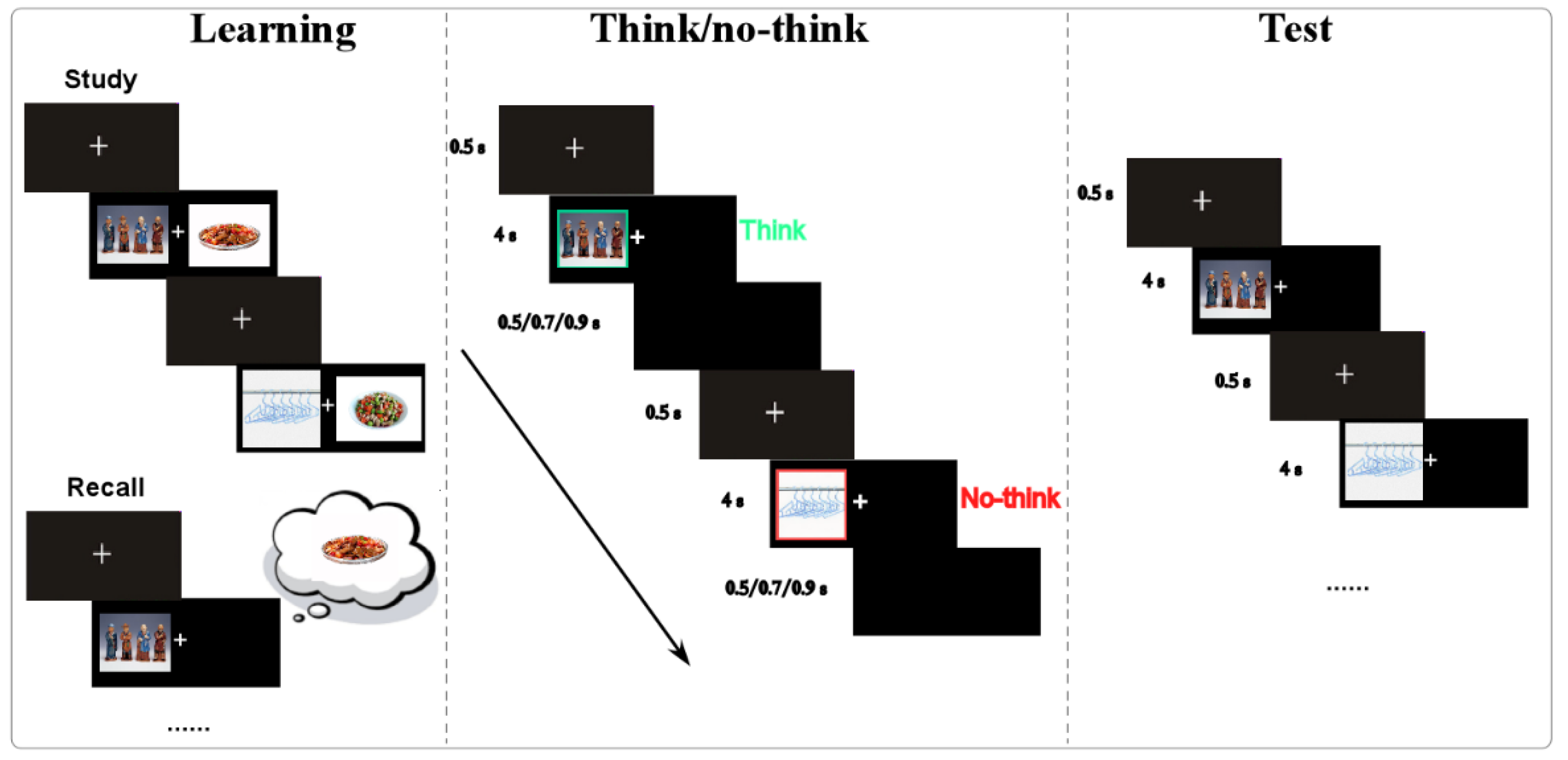
2.2.2. Experimental Procedure
- (1)
- Learning phase
- (2)
- Think/No-think phase
- (3)
- Test phase
2.2.3. Data Recording and Analysis
- (1)
- Behavior analyses
- (2)
- EEG Recording and analyses
3. Results
3.1. Self-Reported Results
3.2. EEG Results
- (1)
- ERP results
- (2)
- TFA results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Herman, C.P.; Mack, D. Restrained and unrestrained eating. J. Personal. 1975, 43, 647–660. [Google Scholar] [CrossRef] [PubMed]
- Polivy, J.; Herman, C.P.; Mills, J.S. What is restrained eating and how do we identify it? Appetite 2020, 155, 104820. [Google Scholar] [CrossRef] [PubMed]
- Yong, C.; Liu, H.; Yang, Q.; Luo, J.; Ouyang, Y.; Sun, M.; Xi, Y.; Xiang, C.; Lin, Q. The relationship between restrained eating, body image, and dietary intake among university students in China: A cross-sectional study. Nutrients 2021, 13, 990. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.-J.; Jackson, T.; Stice, E.; Chen, H. Effectiveness of an internet dissonance-based eating disorder prevention intervention among body-dissatisfied young Chinese women. Behav. Ther. 2021, 52, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Miyake, A.; Friedman, N.P. The nature and organization of individual differences in executive functions: Four general conclusions. Curr. Dir. Psychol. 2012, 21, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Diamond, A. Executive functions. Annu. Rev. Neurosci. 2013, 64, 135–168. [Google Scholar] [CrossRef] [PubMed]
- Xie, P.; Sang, H.-B.; Huang, C.-Z.; Zhou, A.-B. Effect of body-related information on food attentional bias in women with body weight dissatisfaction. Sci. Rep. 2023, 13, 16736. [Google Scholar] [CrossRef]
- Ramachandran, V.S.; Rogers-Ramachandran, D.; Stewart, M. Perceptual correlates of massive cortical reorganization. Science 1992, 258, 1159–1160. [Google Scholar] [CrossRef] [PubMed]
- Polivy, J.; Herman, C.P. Overeating in restrained and unrestrained eaters. Front. Nutr. 2020, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Bi, T.; Gong, G.; Jiang, Q.; Chen, H. Why do most restrained eaters fail in losing weight?: Evidence from an fMRI study. Psychol. Res. Behav. Manag. 2019, 12, 1127–1136. [Google Scholar] [CrossRef] [PubMed]
- Jansen, A.; Nederkoorn, C.; van Baak, L.; Keirse, C.; Guerrieri, R.; Havermans, R. High-restrained eaters only overeat when they are also impulsive. Behav. Res. Ther. 2009, 47, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Fishbach, A.; Shah, J.Y. Self-control in action: Implicit dispositions toward goals and away from temptations. J. Personal. Soc. Psychol. 2006, 90, 820–832. [Google Scholar] [CrossRef] [PubMed]
- Coppin, G.; Nolan-Poupart, S.; Jones-Gotman, M.; Small, D.M. Working memory and reward association learning impairments in obesity. Neuropsychologia 2014, 65, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Shields, G.S.; Wu, Q.; Liu, Y.; Chen, H.; Guo, C. The association between obesity and lower working memory is mediated by inflammation: Findings from a nationally representative dataset of U.S. adults. Brain Behav. Immun. 2020, 84, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Wegner, D.M.; Schneider, D.J.; Carter, S.R.; White, T.L. Paradoxical effects of tought suppression. J. Personal. Soc. Psychol. 1987, 53, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.C.; Green, C. Suppressing unwanted memories by executive control. Nature 2001, 410, 366–369. [Google Scholar] [CrossRef]
- Anderson, M.C.; Bjork, R.A.; Bjork, E.L. Remembering can cause forgetting: Retrieval dynamics in long-term memory. J. Exp. Psychol. Learn. Mem. Cogn. 1994, 20, 1063–1087. [Google Scholar] [CrossRef] [PubMed]
- Erskine, J.A.K.; Georgiou, G.J. Effects of thought suppression on eating behaviour in restrained and non-restrained eaters. Appetite 2010, 54, 499–503. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Yang, X.; Yang, R.; Xu, Z.; Sui, N.; Gao, X. Wanting to eat matters: Negative affect and emotional eating were associated with impaired memory suppression of food cues. Appetite 2020, 150, 104660. [Google Scholar] [CrossRef] [PubMed]
- Cui, K.; Zhao, J.; Li, R.; Gao, Y.; Gao, X. Higher visceral adipose tissue is associated with decreased memory suppression ability on food-related thoughts: A 1-year prospective ERP study. Appetite 2023, 191, 107048. [Google Scholar] [CrossRef] [PubMed]
- Bian, Z.; Yang, R.; Yang, X.; Liu, Y.; Gao, X.; Chen, H. Influence of negative mood on restrained eaters’ memory suppression of food cues: An event-related potentials study. Appetite 2021, 164, 105269. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Zhao, J.; Sun, J.; Yan, Y.; Ma, M.; Chen, Q.; Qiu, J.; Yang, W. Everyday creativity is associated with increased frontal electroencephalography alpha activity during creative ideation. Neuroscience 2022, 503, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Valentini, E.; Zhang, Z.; Liang, M.; Iannetti, G. The primary somatosensory cortex contributes to the latest part of the cortical response elicited by nociceptive somatosensory stimuli in humans. Neuroimage 2014, 84, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Mohan, U.R.; Zhang, H.; Ermentrout, B.; Jacobs, J. The direction of theta and alpha travelling waves modulates human memory processing. Nat. Hum. Behav. 2024, 8, 1124–1135. [Google Scholar] [CrossRef] [PubMed]
- Klimesch, W. EEG alpha and theta oscillations reflect cognitive and memory performance: A review and analysis. Brain Res. Rev. 1999, 29, 169–195. [Google Scholar] [CrossRef] [PubMed]
- Nyhus, E. Brain networks related to beta oscillatory activity during episodic memory retrieval. J. Cogn. Neurosci. 2018, 30, 174–187. [Google Scholar] [CrossRef] [PubMed]
- Lian, J.; Guo, J.; Dai, X.; Deng, X.; Liu, Y.; Zhao, J.; Lei, X. Decoding the impact of negative physical self-perception on inhibitory control ability from theta and beta rhythms. Cereb. Cortex 2025, 35, bhaf056. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, K.; Yamamoto, Y. Single-trial EEG power and phase dynamics associated with voluntary response inhibition. J. Cogn. Neurosci. 2010, 22, 714–727. [Google Scholar] [CrossRef] [PubMed]
- Crespo-García, M.; Wang, Y.; Jiang, M.; Anderson, M.C.; Lei, X. Anterior cingulate cortex signals the need to control intrusive thoughts during motivated forgetting. J. Neurosci. 2022, 42, 4342. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gao, X.; Zhao, J.; Zhang, L.; Chen, H. Neurocognitive correlates of food-related response inhibition in overweight/obese adults. Brain Topogr. 2020, 33, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, S.; Gilbert, S.; Serpell, L. Systematic review: Are overweight and obese individuals impaired on behavioural tasks of executive functioning? Neuropsychol. Rev. 2013, 23, 138–156. [Google Scholar] [CrossRef] [PubMed]
- Weng, C.Y.; Chen, H.; Zhu, L. Attentional biases toward food-related information among restrained eaters based on the goal conflict model of eating. Acta Psychol. Sin. 2012, 44, 680–689. [Google Scholar] [CrossRef]
- van Strien, T.; Frijters, J.E.R.; Van Staveren, W.A.; Defares, P.B.; Deurenberg, P. The predictive validity of the Dutch Restrained Eating Scale. Int. J. Eat. Disord. 1986, 5, 747–755. [Google Scholar] [CrossRef]
- Bohrer, B.K.; Forbush, K.T.; Hunt, T.K. Are common measures of dietary restraint and disinhibited eating reliable and valid in obese persons? Appetite 2015, 87, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhao, J.; Zhang, X.; Gao, X.; Xu, W.; Chen, H. Overweight adults are more impulsive than normal weight adults: Evidence from ERPs during a chocolate-related delayed discounting task. Neuropsychologia 2019, 133, 107181. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Wan, X.; Lian, J.; Ma, H.; Dong, D.; Liu, Y.; Zhao, J. Electrophysiological characteristics of inhibitive control for adults with different physiological or psychological obesity. Nutrients 2024, 16, 1252. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Pan, Y.; Han, Y.; Liang, Q.; Yang, X.; Meng, X.; Gao, X. Chinese food image database for eating and appetite studies. Nutrients 2022, 14, 2916. [Google Scholar] [CrossRef] [PubMed]
- Delorme, A.; Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, Y.; Qin, Y.; Liu, Y.; Chen, Q.; Zhao, K.; Long, Z. Electroencephalographic oscillations of alpha and beta rhythms during phrase-guessing procedure. Cogn. Neurodyn. 2023, 17, 1345–1355. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Zhang, Y.; Zhang, J.; Liu, Y.; Long, Z.; Ran, H.; Li, J.; Qin, Y.; Zhao, J. Neural responses to rapidly selecting color names with different novelty. Cogn. Neurodyn. 2021, 15, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.G.; Hu, L.; Hung, Y.S.; Mouraux, A.; Iannetti, G.D. Gamma-band oscillations in the primary somatosensory cortex--a direct and obligatory correlate of subjective pain intensity. J. Neurosci. 2012, 32, 7429–7438. [Google Scholar] [CrossRef] [PubMed]
- Klimesch, W. EEG-alpha rhythms and memory processes. Int. J. Psychophysiol. 1997, 26, 319–340. [Google Scholar] [CrossRef] [PubMed]
- Palva, S.; Palva, J.M. New vistas for alpha-frequency band oscillations. Trends Neurosci. 2007, 30, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Bagherzadeh, Y.; Baldauf, D.; Pantazis, D.; Desimone, R. Alpha synchrony and the neurofeedback control of spatial attention. Neuron 2020, 105, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Alamia, A.; Terral, L.; D’ambra, M.R.; VanRullen, R. Distinct roles of forward and backward alpha-band waves in spatial visual attention. eLife 2023, 12, e85035. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.; Ruiz, M.H.; Kilavik, B.E.; Lundqvist, M.; Starr, P.A.; Aron, A.R. Beta oscillations in working memory, executive control of movement and thought, and sensorimotor function. J. Neurosci. 2019, 39, 8231–8238. [Google Scholar] [CrossRef] [PubMed]


| Variable | Normal Group (M ± SD) N = 21 | RE Group (M ± SD) N = 21 | p |
|---|---|---|---|
| Age * | 19.00 (1.10) | 19.95 (2.16) | 0.079 |
| DEBQ-RS *** | 2.36 (0.54) | 3.53 (0.40) | <0.001 |
| BMI | 21.41 (4.57) | 21.32 (1.29) | 0.936 |
| Experimental Conditions | Normal Group (M ± SD) N = 21 | RE Group (M ± SD) N = 21 | |
|---|---|---|---|
| Pre-test | Think–Low-calorie food | 0.853 (0.024) | 0.842 (0.024) |
| Think–High-calorie food | 0.815 (0.026) | 0.803 (0.026) | |
| No-think–Low-calorie food | 0.858 (0.029) | 0.843 (0.029) | |
| No-think–High-calorie food | 0.765 (0.037) | 0.680 (0.037) | |
| Post-test | Think–Low-calorie food | 0.842 (0.025) | 0.863 (0.025) |
| Think–High-calorie food | 0.790 (0.026) | 0.835 (0.026) | |
| No-think–Low-calorie food | 0.828 (0.036) | 0.850 (0.036) | |
| No-think–High-calorie food | 0.720 (0.043) | 0.714 (0.043) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, Q.; Cui, K.; Luo, L.; Liu, Y.; Zhao, J. Decreased Memory Suppression Ability in Restrained Eaters on Food Information—Evidence from ERP Experiment. Nutrients 2025, 17, 2523. https://doi.org/10.3390/nu17152523
Qi Q, Cui K, Luo L, Liu Y, Zhao J. Decreased Memory Suppression Ability in Restrained Eaters on Food Information—Evidence from ERP Experiment. Nutrients. 2025; 17(15):2523. https://doi.org/10.3390/nu17152523
Chicago/Turabian StyleQi, Qi, Ke Cui, Li Luo, Yong Liu, and Jia Zhao. 2025. "Decreased Memory Suppression Ability in Restrained Eaters on Food Information—Evidence from ERP Experiment" Nutrients 17, no. 15: 2523. https://doi.org/10.3390/nu17152523
APA StyleQi, Q., Cui, K., Luo, L., Liu, Y., & Zhao, J. (2025). Decreased Memory Suppression Ability in Restrained Eaters on Food Information—Evidence from ERP Experiment. Nutrients, 17(15), 2523. https://doi.org/10.3390/nu17152523









