Effect of Probiotics on Uric Acid Levels: Meta-Analysis with Subgroup Analysis and Meta-Regression
Abstract
1. Introduction
2. Materials and Methods
2.1. Electronics Searches
2.2. Eligibility (Inclusion and Exclusion) Criteria
2.3. Primary Outcome Measure
2.4. Data Collection and Analysis
2.5. Evaluation of Effect Size
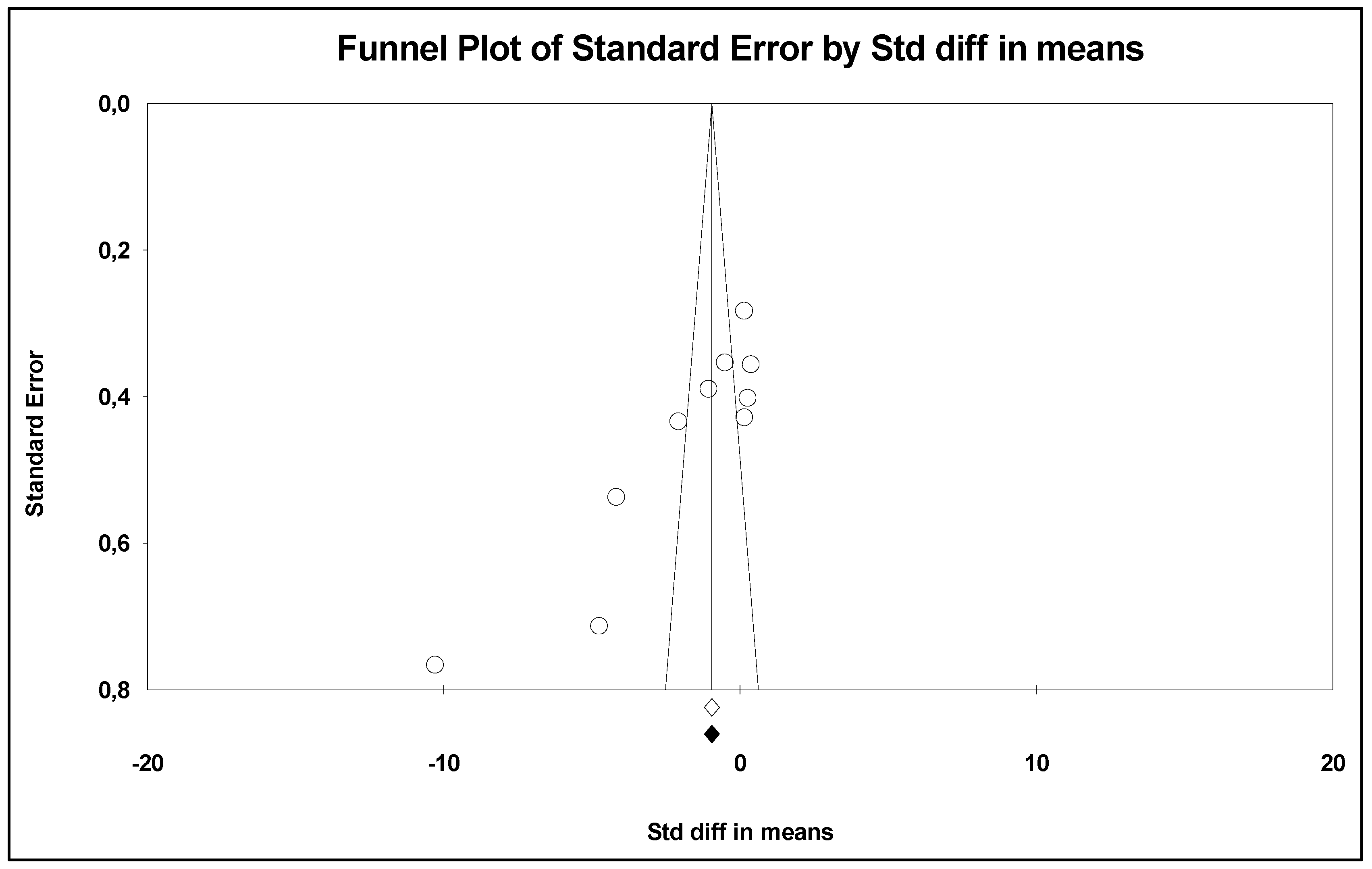
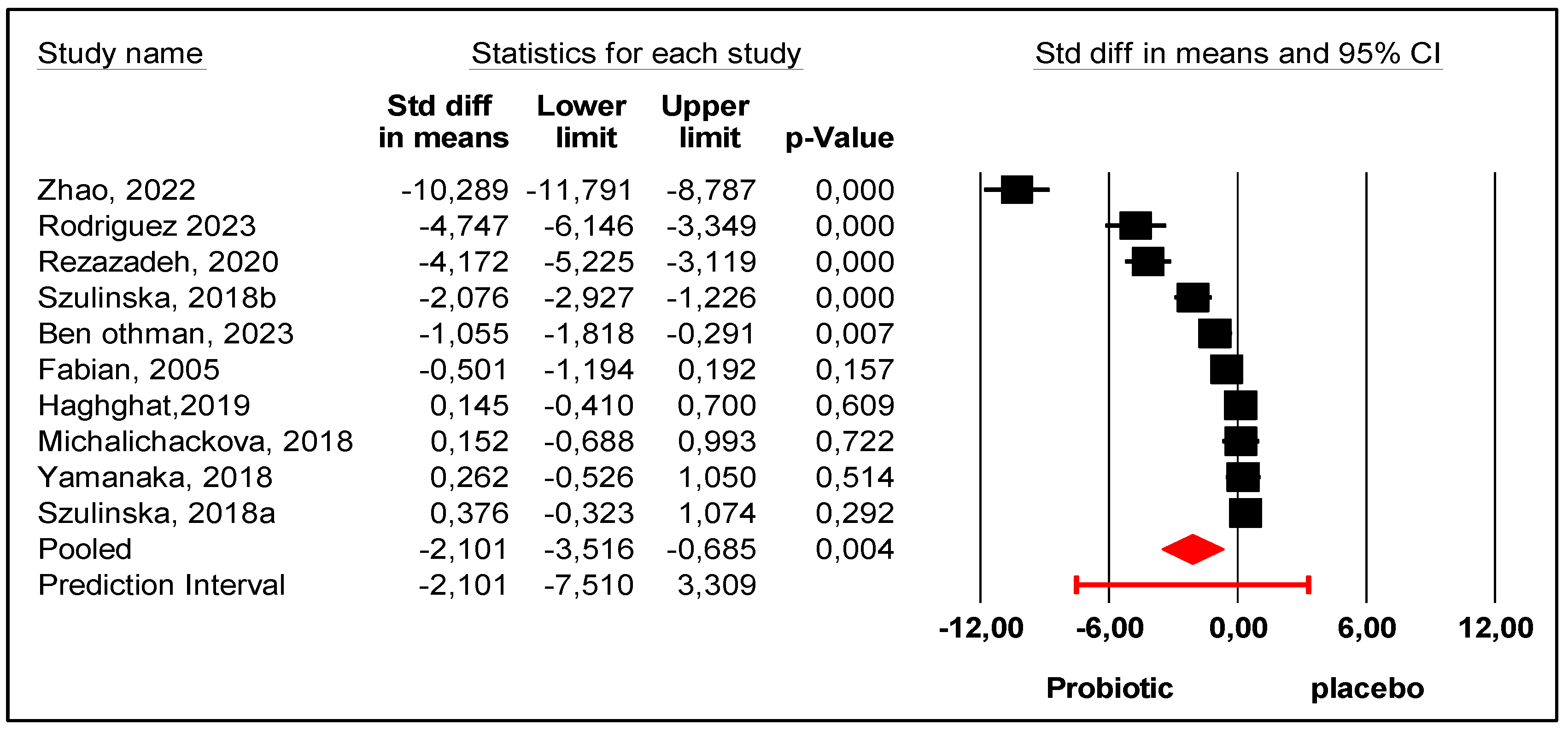
3. Results
Retrieved Studies
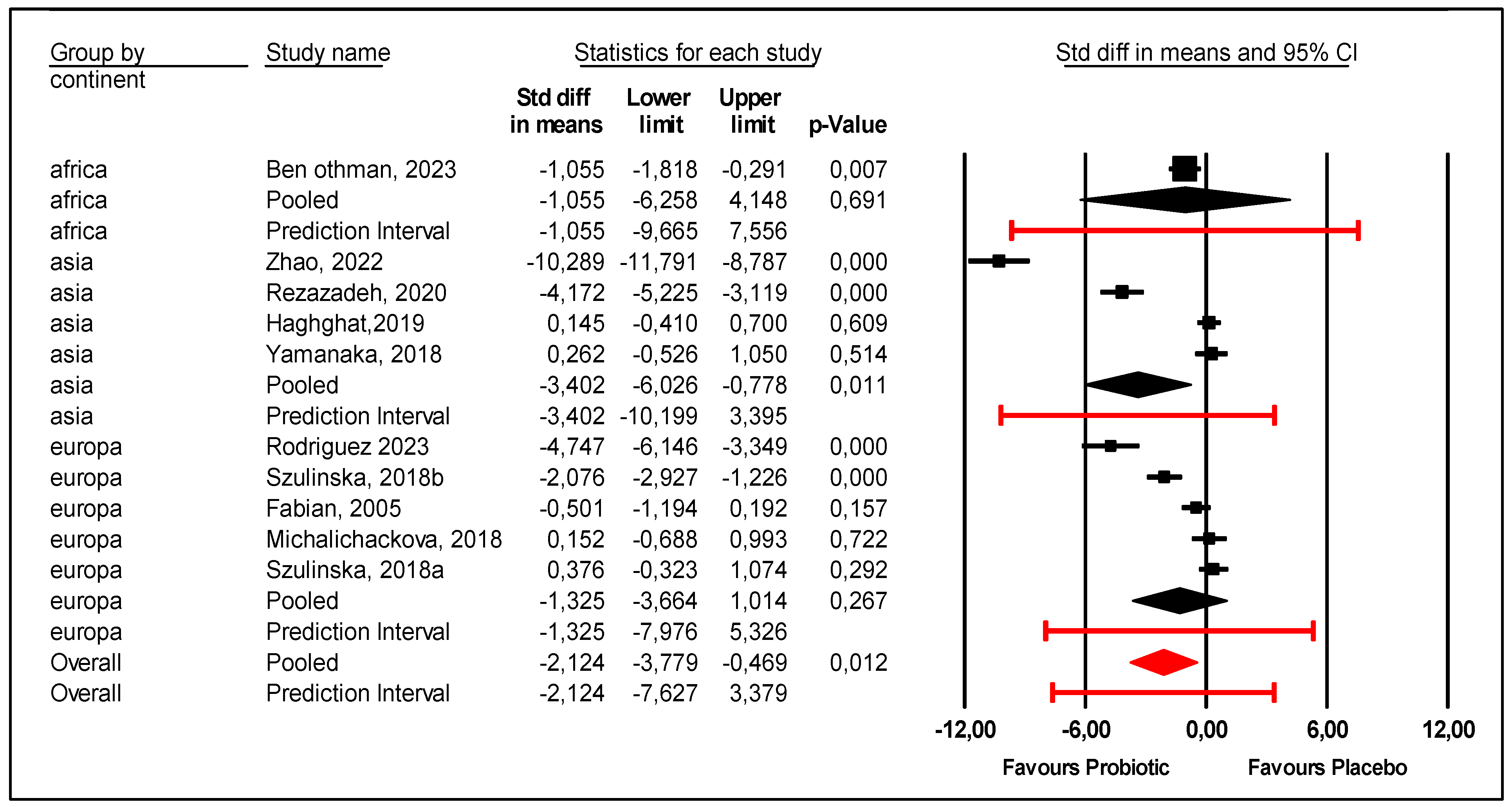
- An explanation of this heterogeneity is presented as follows.
- Regarding the continent, the subgroup of Asian patients were more likely to experience a reduction in uric acid levels with probiotics compared with placebo. The mean effect size was −3.402 with 95% CI (−6.026 to −0.778) (p = 0.011) (Figure 4). However, the prediction interval was large (−10.199 to +3.395), and heterogeneity among Asian patients was not explained. On the other hand, probiotics were not efficient for African (p = 0.691) or European patients (p = 0.267).
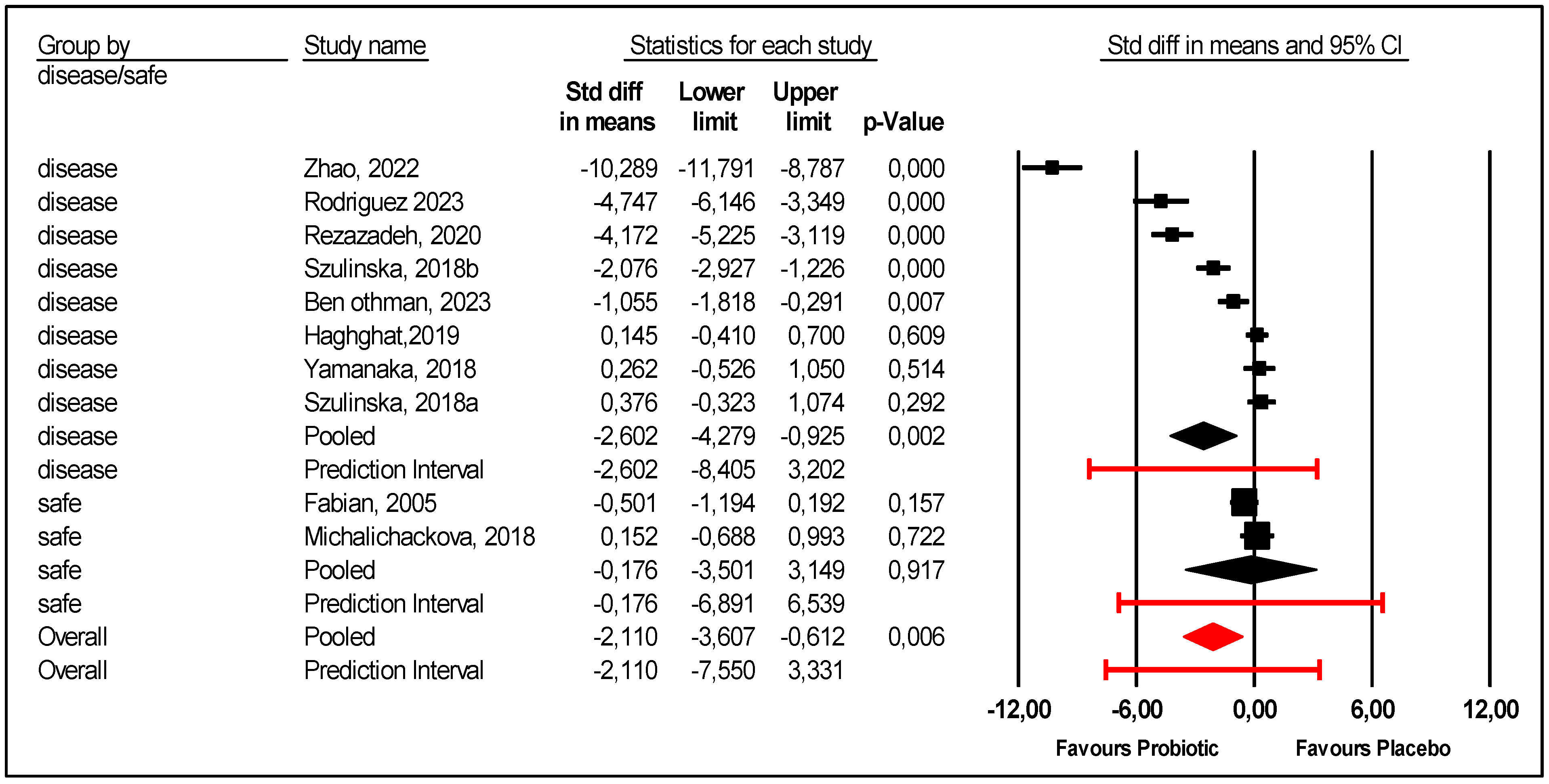
- Patients health status at inclusion was considered. There were two studies that included healthy adults: elite athletes and young healthy women [23,28]. In these two studies, there was no difference between the probiotic and placebo groups. In the subgroup of diseased patients, included patients had obesity [21,26], metabolic syndrome [22], hemodialysis [24], and HUA and/or gout [9,25,27]. Probiotics were more efficient for diseased patients than placebo (Figure 5); the mean effect size was −2.602 with 95% CI (−4.279 to −0.925) (p = 0.002). However, there was still unexplained heterogeneity: 95% PI (−8.405 to +3.202) for this subgroup of diseased patients (Figure 5).
- Single strains were assessed versus mixed multiple stains. Several strains were used with different doses. Mono-strain probiotics significantly decreased uric acid compared with placebo; mean effect size −3.682 with 95% CI (−6.046 to −1.319) (p = 0.002). However, there was an unexplained heterogeneity. In contrast, multi-strains probiotics were not efficient (Figure 6).
- Regarding gender, this meta-regression indicates that men are more sensitive to the effect of probiotics (Figure 7). There was a statically significant association between the proportion of male participants and the mean effect size (p = 0.007).
- Regarding age, BMI, and median follow-up, the meta-regression showed no statistical significance: age (p = 0.954), BMI (p = 0.73), median follow-up (p = 0.775).
4. Discussion
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| RCT | Randomized controlled trial |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Anlyses |
| HUA | Hyperuricemia |
| CVD | Cardiovascular disease |
| USA | United States of America |
| CKD | Chronic kidney disease |
| XOD | Xanthine oxidase |
| PB | Probiotic |
| CCT | Controlled clinical trial |
| BMI | Body mass index |
| IQR | Interquartile range |
| SD | Standard deviation |
| PI | Prediction interval |
| CI | Confidence interval |
| IL1β | Interleukin-1 beta |
| LPS | Lipopolysaccharide |
| ESRD | End-stage renal disease |
| CFU | Colony-forming unit |
References
- Chen-Xu, M.; Yokose, C.; Rai, S.K.; Pillinger, M.H.; Choi, H.K. Contemporary Prevalence of Gout and Hyperuricemia in the United States and Decadal Trends: The National Health and Nutrition Examination Survey, 2007–2016. Arthritis Rheumatol. 2019, 71, 991–999. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, T.; Liu, Y.; Chang, Q.; Zhao, Y.; Guo, C.; Xia, Y. Prevalence of Diabetes in Patients with Hyperuricemia and Gout: A Systematic Review and Meta-Analysis. Curr. Diabetes Rep. 2023, 23, 103–117. [Google Scholar] [CrossRef]
- Schwotzer, N.; Auberson, M.; Livio, F.; So, A.; Bonny, O. Hyperuricémie et maladie rénale: Prise en charge. Rev. Med. Suisse 2022, 771, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Kojima, S.; Matsui, K.; Hiramitsu, S.; Hisatome, I.; Waki, M.; Uchiyama, K.; Yokota, N.; Tokutake, E.; Wakasa, Y.; Jinnouchi, H.; et al. Febuxostat for cerebral and cardiorenovascular events prevention study. Eur. Heart J. 2019, 40, 1778–1786A. [Google Scholar] [CrossRef] [PubMed]
- Yokota, T.; Fukushima, A.; Kinugawa, S.; Okumura, T.; Murohara, T.; Tsutsui, H. Randomized trial of effect of urate-lowering agent febuxostat in chronic heart failure patients with hyperuricemia (LEAF-CHF) study design. Int. Heart J. 2018, 59, 976–982. [Google Scholar] [CrossRef]
- Mahomoodally, M.F.; Coodian, K.; Hosenally, M.; Zengin, G.; Shariati, M.A.; Abdalla, A.N.; Alhazmi, H.A.; Khuwaja, G.; Mohan, S.; Khalid, A. Herbal remedies in the management of hyperuricemia and gout: A review of in vitro, in vivo and clinical evidences. Phytother. Res. 2024, 38, 3370–3400. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.A.; Cleveland, J.D. Hypersensitivity reactions with allopurinol and febuxostat: A study using the Medicare claims data. Ann. Rheum. Dis. 2020, 79, 529–535. [Google Scholar] [CrossRef]
- Roddy, E.; Bajpai, R.; Forrester, H.; Partington, R.J.; Mallen, C.D.; Clarson, L.E.; Padmanabhan, N.; Whittle, R.; Muller, S. Safety of colchicine and NSAID prophylaxis when initiating urate-lowering therapy for gout: Propensity score-matched cohort studies in the UK Clinical Practice Research Datalink. Ann. Rheum. Dis. 2023, 82, 1618–1625. [Google Scholar] [CrossRef]
- Zhao, S.; Feng, P.; Hu, X.; Cao, W.; Liu, P.; Han, H.; Jin, W.; Li, X. Probiotic Limosilactobacillus fermentum GR-3 ameliorates human hyperuricemia via degrading and promoting excretion of uric acid. iScience 2022, 25, 105198. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, J.; Xin, J.; Sun, N.; Zhao, Z.; Gan, B.; Jiang, Y.; Gong, X.; Li, H.; Ma, H.; et al. Preventive effect of Lactobacillus johnsonii YH1136 against uric acid accumulation and renal damages. Front. Microbiol. 2024, 15, 1364857. [Google Scholar] [CrossRef]
- Chen, T.; Qiu, S. Recent Status of Probiotics in the Prevention and Treatment of Hyperuricemia (HUA). MedScien 2024, 1. [Google Scholar] [CrossRef]
- Lin, J.-H.; Lin, C.-H.; Kuo, Y.-W.; Liao, C.-A.; Chen, J.-F.; Tsai, S.-Y.; Li, C.-M.; Hsu, Y.-C.; Huang, Y.-Y.; Hsia, K.-C.; et al. Probiotic Lactobacillus fermentum TSF331, Lactobacillus reuteri TSR332, and Lactobacillus plantarum TSP05 improved liver function and uric acid management-A pilot study. PLoS ONE 2024, 19, e0307181. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef] [PubMed]
- Slim, K.; Nini, E.; Forestier, D.; Kwiatkowski, F.; Panis, Y.; Chipponi, J. Methodological index for non-randomized studies (minors): Development and validation of a new instrument. ANZ J. Surg. 2003, 73, 712–716. [Google Scholar] [CrossRef] [PubMed]
- Obtaining standard deviations from standard errors and confidence intervals for group means. Available online: https://handbook-5-1.cochrane.org/chapter_7/7_7_3_2_obtaining_standard_deviations_from_standard_errors_and.htm (accessed on 7 May 2025).
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.T.; Rothstein, H.R. Prediction Intervals. In Introduction to Meta-Analysis, 2nd Edition ed; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2021; Chapter 17; pp. 119–125. [Google Scholar]
- Borenstein, M. Common Mistakes in Meta-Analysis and How to Avoid Them, the Prediction Interval; Biostat, Inc.: Englewood, NJ, USA, 2019; Chapter 17; pp. 85–93. [Google Scholar]
- Dziri, C.; Fingerhut, A. Up-to-date composition and critical appraisal of meta-analyses of comparative studies. Ann. Laparosc. Endosc. Surg. 2025, 10, 4. [Google Scholar] [CrossRef]
- Thompson, S.G.; Higgins, J.P.T. How should meta-regression analyses be undertaken and interpreted? Stat. Med. 2002, 21, 1559–1573. [Google Scholar] [CrossRef]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.T.; Rothstein, H.R. Comprehensive Meta-Analysis, Version 4.0, Logiciel; Biostat: Englewood, NJ, USA, 2005.
- Ben Othman, R.; Ben Amor, N.; Mahjoub, F.; Berriche, O.; El Ghali, C.; Gamoudi, A.; Jamoussi, H. A clinical trial about effects of prebiotic and probiotic supplementation on weight loss, psychological profile and metabolic parameters in obese subjects. Endocrinol. Diabetes Metab. 2023, 6, e402. [Google Scholar] [CrossRef]
- Rezazadeh, L.; Alipour, B.; Jafarabadi, M.A.; Behrooz, M.; Gargari, B.P. Daily consumption effects of probiotic yogurt containing Lactobacillus acidophilus La5 and Bifidobacterium lactis Bb12 on oxidative stress in metabolic syndrome patients. Clin. Nutr. ESPEN 2021, 41, 136–142. [Google Scholar] [CrossRef]
- Michalickova, D.; Kotur-Stevuljevic, J.; Miljkovic, M.; Dikic, N.; Kostic-Vucicevic, M.; Andjelkovic, M.; Koricanac, V.; Djordjevic, B. Effects of Probiotic Supplementation on Selected Parameters of Blood Prooxidant-Antioxidant Balance in Elite Athletes: A Double-Blind Randomized Placebo-Controlled Study. J. Hum. Kinet. 2018, 64, 111–122. [Google Scholar] [CrossRef]
- Haghighat, N.; Mohammadshahi, M.; Shayanpour, S.; Haghighizadeh, M.H. Effect of Synbiotic and Probiotic Supplementation on Serum Levels of Endothelial Cell Adhesion Molecules in Hemodialysis Patients: A Randomized Control Study. Probiotics Antimicrob. Proteins 2019, 11, 1210–1218. [Google Scholar] [CrossRef]
- Rodríguez, J.M.; Garranzo, M.; Segura, J.; Orgaz, B.; Arroyo, R.; Alba, C.; Beltrán, D.; Fernández, L. A randomized pilot trial assessing the reduction of gout episodes in hyperuricemic patients by oral administration of Ligilactobacillus salivarius CECT 30632, a strain with the ability to degrade purines. Front. Microbiol. 2023, 14, 1111652. [Google Scholar] [CrossRef] [PubMed]
- Szulińska, M.; Łoniewski, I.; van Hemert, S.; Sobieska, M.; Bogdański, P. Dose-Dependent Effects of Multispecies Probiotic Supplementation on the Lipopolysaccharide (LPS) Level and Cardiometabolic Profile in Obese Postmenopausal Women: A 12-Week Randomized Clinical Trial. Nutrients 2018, 10, 773. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, H.; Taniguchi, A.; Tsuboi, H.; Kano, H.; Asami, Y. Hypouricaemic effects of yoghurt containing Lactobacillus gasseri PA-3 in patients with hyperuricaemia and/or gout: A randomised, double-blind, placebo-controlled study. Mod. Rheumatol. 2019, 29, 146–150. [Google Scholar] [CrossRef]
- Fabian, E.; Elmadfa, I. The effect of daily consumption of probiotic and conventional yoghurt on oxidant and anti-oxidant parameters in plasma of young healthy women. Int. J. Vitam. Nutr. Res. 2007, 77, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Wang, T.; Liu, Y.; Zhou, W.; Hao, H.; Liu, Q.; Yin, B.; Yi, H. Lactobacillus fermentum F40-4 ameliorates hyperuricemia by modulating the gut microbiota and alleviating inflammation in mice. Food Funct. 2023, 14, 3259–3268. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, J.; Lin, G.; Gao, K.; Yu, Y.; Chen, S.; Chen, L.; Chen, Z.; Li, L. Probiotic effects of Lacticaseibacillus rhamnosus 1155 and Limosilactobacillus fermentum 2644 on hyperuricemic rats. Front. Nutr. 2022, 9, 993951. [Google Scholar] [CrossRef]
- Zeng, L.; Deng, Y.; He, Q.; Yang, K.; Li, J.; Xiang, W.; Liu, H.; Zhu, X.; Chen, H. Safety and efficacy of probiotic supplementation in 8 types of inflammatory arthritis: A systematic review and meta-analysis of 34 randomized controlled trials. Front. Immunol. 2022, 13, 961325. [Google Scholar] [CrossRef]
- Peng, X.; Li, X.; Xie, B.; Lai, Y.; Sosnik, A.; Boucetta, H.; Chen, Z.; He, W. Gout therapeutics and drug delivery. J. Control. Release 2023, 362, 728–754. [Google Scholar] [CrossRef]
- Begg, A. Allopurinol. Pract. Diabetes 2023, 40, 42–43. [Google Scholar] [CrossRef]
- McInnes, G.T.; Lawson, D.H.; Jick, H. Acute adverse reactions attributed to allopurinol in hospitalised patients. Ann. Rheum. Dis. 1981, 40, 245–249. [Google Scholar] [CrossRef]
- Calin, A. Allopurinol Toxicity Masquerading as Malignancy. JAMA 1978, 239, 497. [Google Scholar] [CrossRef] [PubMed]
- Fagugli, R.M.; Gentile, G.; Ferrara, G.; Brugnano, R. Acute renal and hepatic failure associated with allopurinol treatment. Clin. Nephrol. 2008, 70, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Razavi, A.C.; Potts, K.S.; Kelly, T.N.; Bazzano, L.A. Sex, gut microbiome, and cardiovascular disease risk. Biol. Sex. Differ. 2019, 10, 29. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, S.; Li, X.; Wang, M.; Huang, B.; Feng, K.; Cui, J. Association between prebiotic, probiotic consumption and hyperuricemia in U.S. adults: A cross-sectional study from NHANES 2011-2018. Front. Nutr. 2025, 12, 1492708. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Fogacci, F.; Cincione, R.I.; Tocci, G.; Borghi, C. Clinical Effects of Xanthine Oxidase Inhibitors in Hyperuricemic Patients. Med. Princ. Pract. 2021, 30, 122–130. [Google Scholar] [CrossRef]
- Halperin Kuhns, V.L.; Woodward, O.M. Sex Differences in Urate Handling. Int. J. Mol. Sci. 2020, 21, 4269. [Google Scholar] [CrossRef]
- Sumino, H.; Ichikawa, S.; Kanda, T.; Nakamura, T.; Sakamaki, T. Reduction of serum uric acid by hormone replacement therapy in postmenopausal women with hyperuricaemia. Lancet 1999, 354, 650. [Google Scholar] [CrossRef]
- Chakrabarti, A.; Geurts, L.; Hoyles, L.; Iozzo, P.; Kraneveld, A.D.; La Fata, G.; Miani, M.; Patterson, E.; Pot, B.; Shortt, C.; et al. The microbiota-gut-brain axis: Pathways to better brain health. Perspectives on what we know, what we need to investigate and how to put knowledge into practice. Cell Mol. Life Sci. CMLS 2022, 79, 80. [Google Scholar] [CrossRef]
- Ichida, K. Uric Acid Metabolism, Uric Acid Transporters and Dysuricemia. Yakugaku Zasshi 2024, 144, 659–674. [Google Scholar] [CrossRef]
- Rhee, S.J.; Lee, J.-E.; Lee, C.-H. Importance of lactic acid bacteria in Asian fermented foods. Microb. Cell Factories 2011, 10 (Suppl. S1), S5. [Google Scholar] [CrossRef]
- Swain, M.R.; Anandharaj, M.; Ray, R.C.; Rani, R.P. Fermented fruits and vegetables of Asia: A potential source of probiotics. Biotechnol. Res. Int. 2014, 2014, 19. [Google Scholar] [CrossRef]
- Cooper, T.E.; Khalid, R.; Chan, S.; Craig, J.C.; Hawley, C.M.; Howell, M.; Johnson, D.W.; Jaure, A.; Teixeira-Pinto, A.; Wong, G. Synbiotics, prebiotics and probiotics for people with chronic kidney disease—Cooper, TE—2023|Cochrane Library. Cochrane Database Syst. Rev. 2023, 10, CD013631. [Google Scholar] [PubMed]
- Nourizadeh, R.; Sepehri, B.; Abbasi, A.; Sayyed, R.Z.; Khalili, L. Impact of Probiotics in Modulation of Gut Microbiome. In Microbiome-Gut-Brain Axis Implic. Health; Sayyed, R.Z., Khan, M., Eds.; Springer Nature: Singapore, 2022; pp. 401–409. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Y.; Zhong, H.; Chen, F.; Regenstein, J.; Hu, X.; Cai, L.; Feng, F. The gut microbiota as a target to control hyperuricemia pathogenesis: Potential mechanisms and therapeutic strategies. Crit. Rev. Food Sci. Nutr. 2022, 62, 3979–3989. [Google Scholar] [CrossRef] [PubMed]
- Lim, X.; Ooi, L.; Ding, U.; Wu, H.H.L.; Chinnadurai, R. Gut Microbiota in Patients Receiving Dialysis: A Review. Pathogens 2024, 13, 801. [Google Scholar] [CrossRef] [PubMed]
- Kuznetzova, A.B.; Prazdnova, E.V.; Chistyakov, V.A.; Kutsevalova, O.Y.; Batiushin, M.M. Are Probiotics Needed In Nephrology? Nephrol. St.-Petersburg 2022, 26, 18–30. [Google Scholar] [CrossRef]
- Prasad, C.; Iqbal, U.; Westfall, S.; Prakash, S. Management of hyperuricemia and gout by prebiotics and probiotics: Potentials and limitations. Int. J. Probiotics Prebiotics 2017, 12, 5–15. [Google Scholar]
- Wang, Q.; Liang, J.; Zou, Q.; Wang, W.; Yan, G.; Guo, R.; Yuan, T.; Wang, Y.; Liu, X.; Liu, Z. Tryptophan Metabolism-Regulating Probiotics Alleviate Hyperuricemia by Protecting the Gut Barrier Integrity and Enhancing Colonic Uric Acid Excretion. J. Agric. Food Chem. 2024, 72, 26746–26761. [Google Scholar] [CrossRef]
- Liang, L.; Meng, Z.; Zhang, F.; Jianguo, Z.; Fang, S.; Hu, Q.; Tang, X.; Li, Y. Lactobacillus gasseri LG08 and Leuconostoc mesenteroides LM58 exert preventive effect on the development of hyperuricemia by repairing antioxidant system and intestinal flora balance. Front. Microbiol. 2023, 14, 1211831. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, Y.; Yang, T.; Song, L.; Xiao, Y.; Chen, Y.; Chen, M.; Li, M.; Ren, Z. Lactococcus cremoris D2022 alleviates hyperuricemia and suppresses renal inflammation via potential gut-kidney axis. Food Funct. 2024, 15, 6015–6027. [Google Scholar] [CrossRef]
- Wu, J.; Aga, L.; Tang, L.; Li, H.; Wang, N.; Yang, L.; Zhang, N.; Wang, X.; Wang, X. Lacticaseibacillus paracasei JS-3 Isolated from “Jiangshui” Ameliorates Hyperuricemia by Regulating Gut Microbiota and iTS Metabolism. Foods 2024, 13, 1371. [Google Scholar] [CrossRef]
- Hussain, A.; Rui, B.; Ullah, H.; Dai, P.; Ahmad, K.; Yuan, J.; Liu, Y.; Li, M. Limosilactobacillus reuteri HCS02-001 Attenuates Hyperuricemia through Gut Microbiota-Dependent Regulation of Uric Acid Biosynthesis and Excretion. Microorganisms 2024, 12, 637. [Google Scholar] [CrossRef] [PubMed]
- Chapman, C.M.C.; Gibson, G.R.; Rowland, I. In vitro evaluation of single- and multi-strain probiotics: Inter-species inhibition between probiotic strains, and inhibition of pathogens. Anaerobe 2012, 18, 405–413. [Google Scholar] [CrossRef]
- Buiatte, V.; Schultheis, M.; Lorenzoni, A.G. Deconstruction of a multi-strain Bacillus-based probiotic used for poultry: An in vitro assessment of its individual components against C. perfringens. BMC Res. Notes 2023, 16, 117. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Luo, X.-D.; Li, J.-Z.; Mo, Q.-Y.; Wang, X.; Zhao, Y.; Zhang, Y.-M.; Luo, H.-T.; Xia, D.-Y.; Ma, W.-Q.; et al. Host-derived Lactobacillus plantarum alleviates hyperuricemia by improving gut microbial community and hydrolase-mediated degradation of purine nucleosides. eLife 2024, 13, e100068. [Google Scholar] [CrossRef] [PubMed]
- Sanders, M.E.; Benson, A.; Lebeer, S.; Merenstein, D.J.; Klaenhammer, T.R. Shared mechanisms among probiotic taxa: Implications for general probiotic claims. Curr. Opin. Biotechnol. 2018, 49, 207–216. [Google Scholar] [CrossRef]
- Forssten, S.; Ouwehand, A.C. Dose-Response Recovery of Probiotic Strains in Simulated Gastro-Intestinal Passage. Microorganisms 2020, 8, 112. [Google Scholar] [CrossRef]
- Pavan, M. Influence of prebiotic and probiotic supplementation on the progression of chronic kidney disease. Minerva Urol. Nephrol. 2016, 68, 222–226. [Google Scholar]
- Buford, T.W. (Dis)Trust your gut: The gut microbiome in age-related inflammation, health, and disease. Microbiome 2017, 5, 80. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.; Liao, W.; Huang, J.; Liu, Y.; Li, Z.; Tang, J. Gut microbiota remodeling: A promising therapeutic strategy to confront hyperuricemia and gout. Front. Cell. Infect. Microbiol. 2022, 12, 935723. [Google Scholar] [CrossRef]
- Shi, W.; Cai, Z.; Ren, X.; Wang, J.; Zhou, H.; Chen, Z. The relationship between serum uric acid and accelerated aging in middle-aged and older adults: A prospective cohort study based on CHARLS. J. Nutr. Health Aging 2025, 29, 100488. [Google Scholar] [CrossRef]
- Chenhuichen, C.; Cabello-Olmo, M.; Barajas, M.; Izquierdo, M.; Ramírez-Vélez, R.; Zambom-Ferraresi, F.; Martínez-Velilla, N. Impact of probiotics and prebiotics in the modulation of the major events of the aging process: A systematic review of randomized controlled trials. Exp. Gerontol. 2022, 164, 111809. [Google Scholar] [CrossRef]
- Rasaei, N.; Heidari, M.; Esmaeili, F.; Khosravi, S.; Baeeri, M.; Tabatabaei-Malazy, O.; Emamgholipour, S. The effects of prebiotic, probiotic or synbiotic supplementation on overweight/obesity indicators: An umbrella review of the trials’ meta-analyses. Front. Endocrinol. 2024, 15, 1277921. [Google Scholar] [CrossRef] [PubMed]
- Yarahmadi, A.; Afkhami, H.; Javadi, A.; Kashfi, M. Understanding the complex function of gut microbiota: Its impact on the pathogenesis of obesity and beyond: A comprehensive review. Diabetol. Metab. Syndr. 2024, 16, 308. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Kim, N.; Werlinger, P.; Suh, D.-A.; Lee, H.; Cho, J.-H.; Cheng, J. Probiotic Characterization of Lactobacillus brevis MJM60390 and In Vivo Assessment of Its Antihyperuricemic Activity. J. Med. Food 2022, 25, 367–380. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, N.; Ranganathan, P.; Friedman, E.A.; Joseph, A.; Delano, B.; Goldfarb, D.S.; Tam, P.; Rao, A.V.; Anteyi, E.; Musso, C.G. Pilot study of probiotic dietary supplementation for promoting healthy kidney function in patients with chronic kidney disease. Adv. Ther. 2010, 27, 634–647. [Google Scholar] [CrossRef]
- Zhao, F.; Tie, N.; Kwok, L.-Y.; Ma, T.; Wang, J.; Man, D.; Yuan, X.; Li, H.; Pang, L.; Shi, H.; et al. Baseline gut microbiome as a predictive biomarker of response to probiotic adjuvant treatment in gout management. Pharmacol. Res. 2024, 209, 107445. [Google Scholar] [CrossRef]


| First Author | Country of Origin | Year of Publication | Type of Disease | Number of Patients (Probiotics/ Comparator) | Sex Ratio (M/W) | Mean Ages (Years) | Mean BMI (kg/m2) | Intervention | Dosage | Comparator | Follow-Up (Months) | Uric Acid Before Intervention (mg/dL) | Uric Acid After Intervention (mg/dL) | Method of Uric Acid Dosage | JADAD /5 or MINORS/24 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ben Othman [21] | Africa | 2023 | Obesity | 30 (15/15) | - | 49 | - | one tab of: B. longum L. helveticus Lactococcal latis S. thermophilus | 10 × 109 UFC/capsule | Low-calorie diet | 1 | 4.97 ± 1.7 | 4.78 ± 1.34 | NA | 18 * |
| Haghighat [24] | Asia | 2019 | Hemodialysis | 50 (25/25) | 1.2 | 47 | 23 | 5 g of probiotic powder: B. bifidum B. lactis B. longum | 2.7 × 107 CFU/g each | Placebo: 20 g of maltodextrin powder | 3 | 6.99 ± 2.06 | 6.98 ± 1.81 | Clauss technique and the uricase enzymatic test | 5 |
| Michalichova [23] | Europa | 2018 | Elite athletes | 22 (10/12) | 22 | 22 | 23 | 1 capsule of L. helveticus | 2 × 1010 | Placebo | 3.5 | 4.02 ± 0.77 | 3.95 ± 1.41 | NA | 4 |
| Rezazadeh [22] | Asia | 2020 | Metabolic syndrome | 44 (22/22) | 1 | 44 | 32 | 300 g of yogurt containing L. bulgaricus, S. thermophile, B. lactis, L. acidophilus | 3.55 × 106 CFU/g of B. lactis, 4.41 × 106 CFU/g of L. acidophilus/d | Conventional yogurt: L. bulgaricus, S. thermophile | 2 | 6.33 ± 1.4 | 4.65 ± 0.72 | enzymatic method | 5 |
| Rodriguez [25] | Europa | 2023 | Hyperuricemia (>7 mg/dL), a history of recurrent gout episodes (≥3 episodes/year) | 30 (15/15) | - | 54 | 32 | L. Salivarus CECT 30632 | 1010 CFU | Allopurinol | 6 | 9.04 ± 0.25 | 9.03 ± 0.25 | HPLC | 5 |
| Szulinska (a) [26] | Europa | 2018 | Obese post-menopausal | 36 (24/12) | 0 | 57 | 36 | Sachets containing 2 g of probiotic divided in 2 equal doses: B. bifidum B. lactis w51 L. acidophilus L. brevis L. casei L. salivarius L. lactis w19 L. lactis w58 | 1 × 1010 CFU/d | Placebo | 3 | 5.26 ± 1.04 | 5.28 ± 1.09 | Dimension EXL with LM Integrated Chemistry System Analyzer | 5 |
| Szulinska (b) [26] | Europa | 2018 | Obese post-menopausal | 35 (23/12) | 0 | 58 | 36 | 2.5 × 109 CFU/d | Placebo | 3 | 6.02 ± 0.71 | 5.35 ± 0.91 | 5 | ||
| Yamanaka [27] | Asia | 2018 | Hyperuricemia and/or gout | 17 (9/8) | 17 | 63 | 25 | 100 g of yogurt with L. bulgaricus, S. thermophile, L. delbruecki | 8.5 × 107 CFU/g | Conventional yogurt: L. bulgaricus, S. thermophile | 2 | 8.7 ± 1 | 8.7 ± 1.2 | Uricase-POD method | 3 |
| Zhao [9] | Asia | 2022 | UA > 7 mg/dL | 97 (52/45) | 2.87 | 39 | 28.6 | Yogurt containing L. bulgaricus, S. thermophiles, Limosi L. fermentum GR-3 | 2.0 × 109 CFU/g of each bacterial strain/d | Conventional yogurt: L. bulgaricus, S. thermophile | 2 | 9.65 ± 0.76 | 7.12 ± 0.22 | NA | 5 |
| Fabian [28] | Europa | 2007 | Healthy women | 33 (17/16) | 0/33 | 24 | 21 | Yogurt containing L. bulgaricus, S. thermophiles, L. paracasei subsp. Paracasei | 3.6 × 108 CFU/g | Conventional yogurt: L. bulgaricus, S. thermophile | 1 | 2.45 ± 0.42 | 2.2 ± 0.48 | Enzymatic method | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Othman, R.B.; Sassi, M.B.; Hammamia, S.B.; Dziri, C.; Zanina, Y.; Salem, K.B.; Jamoussi, H. Effect of Probiotics on Uric Acid Levels: Meta-Analysis with Subgroup Analysis and Meta-Regression. Nutrients 2025, 17, 2467. https://doi.org/10.3390/nu17152467
Othman RB, Sassi MB, Hammamia SB, Dziri C, Zanina Y, Salem KB, Jamoussi H. Effect of Probiotics on Uric Acid Levels: Meta-Analysis with Subgroup Analysis and Meta-Regression. Nutrients. 2025; 17(15):2467. https://doi.org/10.3390/nu17152467
Chicago/Turabian StyleOthman, Rym Ben, Mouna Ben Sassi, Syrine Ben Hammamia, Chadli Dziri, Youssef Zanina, Kamel Ben Salem, and Henda Jamoussi. 2025. "Effect of Probiotics on Uric Acid Levels: Meta-Analysis with Subgroup Analysis and Meta-Regression" Nutrients 17, no. 15: 2467. https://doi.org/10.3390/nu17152467
APA StyleOthman, R. B., Sassi, M. B., Hammamia, S. B., Dziri, C., Zanina, Y., Salem, K. B., & Jamoussi, H. (2025). Effect of Probiotics on Uric Acid Levels: Meta-Analysis with Subgroup Analysis and Meta-Regression. Nutrients, 17(15), 2467. https://doi.org/10.3390/nu17152467