A Prospective Interventional Study on the Beneficial Effect of Fish Oil-Enriched High-Protein Oral Nutritional Supplement (FOHP-ONS) on Malnourished Older Cancer Patients
Abstract
1. Introduction
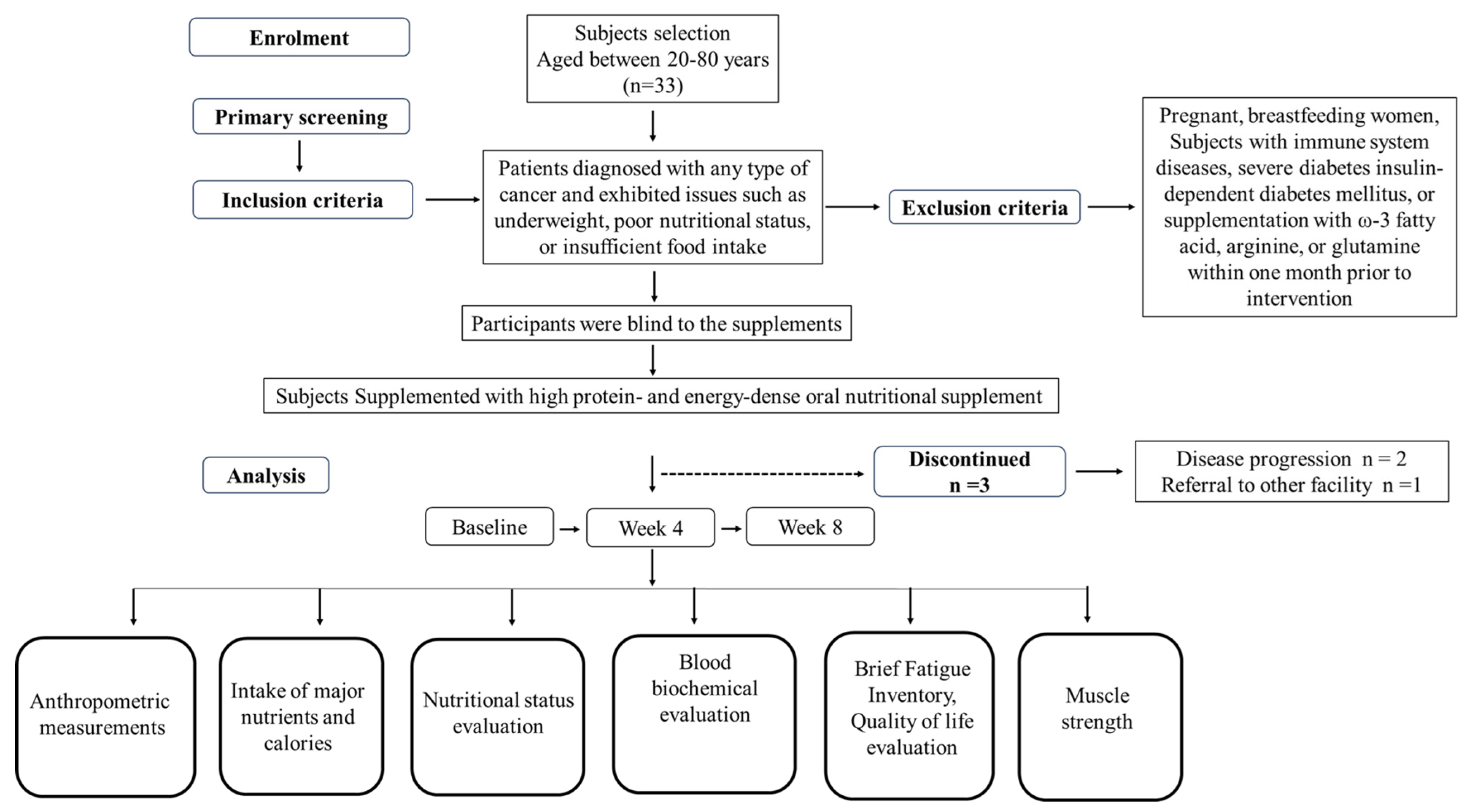
2. Materials and Methods
2.1. Human Trial Study
2.2. Intervention
2.3. Measurement
2.4. Statistical Analysis
3. Results
3.1. Compliance and Tolerance
3.2. Nutritional Intake and Energy Balance
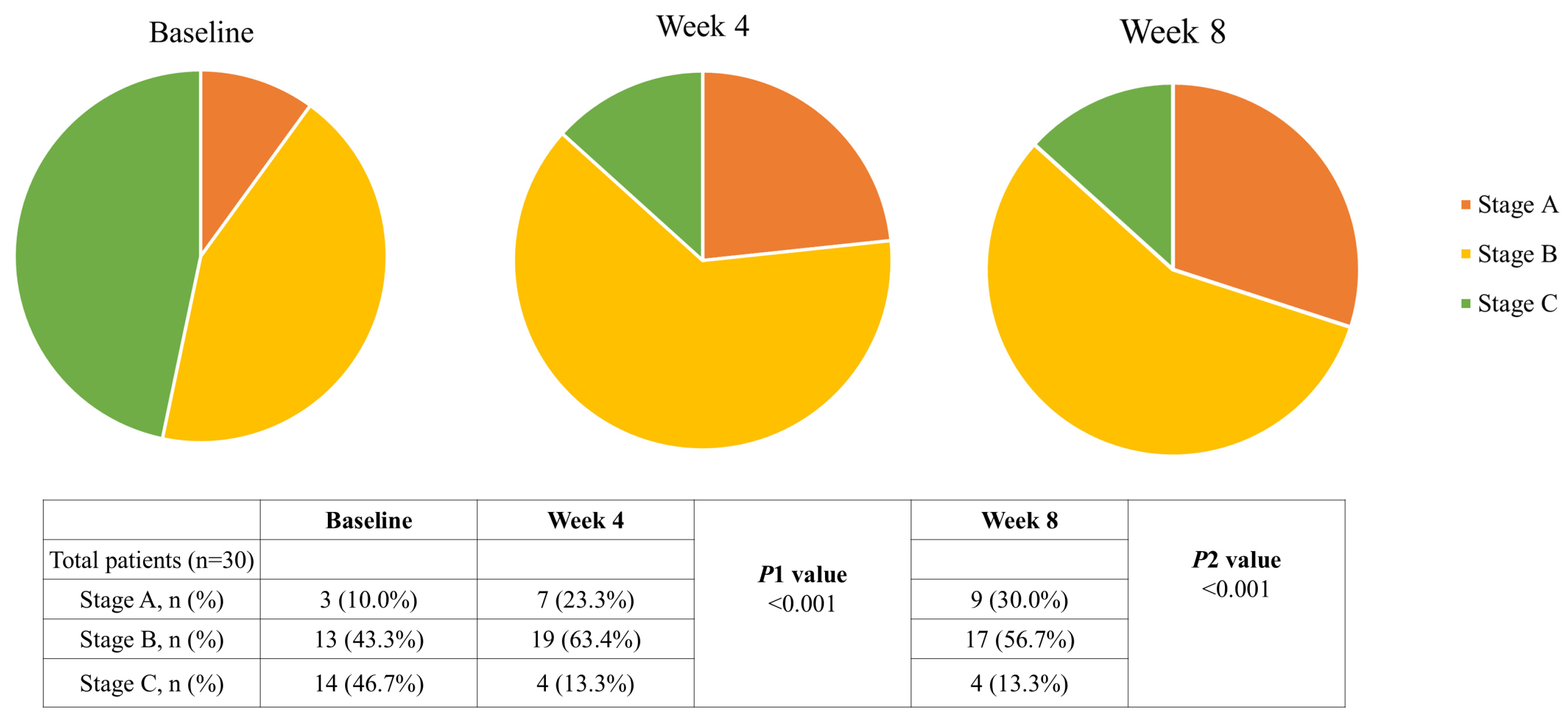
3.3. Nutritional Status
3.4. Biochemical Parameters
3.5. Cancer-Related Fatigue
3.6. Quality of Life
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Arends, J. Malnutrition in Cancer Patients: Causes, Consequences and Treatment Options. Eur. J. Surg. Oncol. 2024, 50, 107074. [Google Scholar] [CrossRef] [PubMed]
- Muscaritoli, M.; Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN Practical Guideline: Clinical Nutrition in Cancer. Clin. Nutr. 2021, 40, 2898–2913. [Google Scholar] [CrossRef]
- Muscaritoli, M.; Corsaro, E.; Molfino, A. Awareness of Cancer-Related Malnutrition and Its Management: Analysis of the Results From a Survey Conducted Among Medical Oncologists. Front. Oncol. 2021, 11, 682999. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, J.L.; Lu, J.; Song, Y.; Kwak, K.S.; Jiao, Q.; Rosenfeld, R.; Chen, Q.; Boone, T.; Simonet, W.S.; et al. Reversal of Cancer Cachexia and Muscle Wasting by Act RIIB Antagonism Leads to Prolonged Survival. Cell 2010, 142, 531–543. [Google Scholar] [CrossRef]
- Gorjao, R.; Dos Santos, C.M.M.; Serdan, T.D.A.; Diniz, V.L.S.; Alba-Loureiro, T.C.; Cury-Boaventura, M.F.; Hatanaka, E.; Levada-Pires, A.C.; Sato, F.T.; Pithon-Curi, T.C.; et al. New Insights on the Regulation of Cancer Cachexia by N-3 Polyunsaturated Fatty Acids. Pharmacol. Ther. 2019, 196, 117–134. [Google Scholar] [CrossRef]
- Yeom, E.; Yu, K. Understanding the Molecular Basis of Anorexia and Tissue Wasting in Cancer Cachexia. Exp. Mol. Med. 2022, 54, 426–432. [Google Scholar] [CrossRef]
- Milla, S.P.; Luna, P.P.G.; Casariego, A.V.; González, F.V.; Folgueras, T.M.; Jáuregui, O.I.; Rey, S.G.; Fernández, A.C.; Plaza, B.L.; Quintana, T.C.; et al. Adherence and impact of an oral nutritional supplement enriched in leucine, EVOO, EPA and DHA, and beta-glucans on the coverage of energy and protein requirements in patients with cancer and malnutrition: Alisenoc study. Nutrition 2024, 120, 112355. [Google Scholar] [CrossRef]
- de van der Schueren, M.A.E.; Laviano, A.; Blanchard, H.; Jourdan, M.; Arends, J.; Baracos, V.E. Systematic review and meta-analysis of the evidence for oral nutritional intervention on nutritional and clinical outcomes during chemo(radio)therapy: Current evidence and guidance for design of future trials. Ann. Oncol. 2018, 29, 1141–1153. [Google Scholar] [CrossRef]
- Habibi, S.; Talebi, S.; Khosravinia, D.; Mohammadi, H. Oral nutritional supplementation in cancer patients: A systematic review and dose-response meta-analysis. Clin. Nutr. 2025, 47, 28–39. [Google Scholar] [CrossRef]
- Fatigue (PDQ®)–Health Professional Version. National Cancer Institute. Available online: https://www.cancer.gov/about-cancer/treatment/side-effects/fatigue (accessed on 30 January 2025).
- Bower, J.E. Cancer-Related Fatigue—Mechanisms, Risk Factors, and Treatments. Nat. Rev. Clin. Oncol. 2014, 11, 597–609. [Google Scholar] [CrossRef] [PubMed]
- Thong, M.S.Y.; van Noorden, C.J.F.; Steindorf, K.; Arndt, V. Cancer-Related Fatigue: Causes and Current Treatment Options. Curr. Treat. Options Oncol. 2020, 21, 17. [Google Scholar] [CrossRef] [PubMed]
- Fabi, A.; Bhargava, R.; Fatigoni, S.; Guglielmo, M.; Horneber, M.; Roila, F.; Weis, J.; Jordan, K.; Ripamonti, C.I.; ESMO Guidelines Committee. Cancer-Related Fatigue: ESMO Clinical Practice Guidelines for Diagnosis and Treatment. Ann. Oncol. 2020, 31, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Inglis, J.E.; Lin, P.J.; Kerns, S.L.; Kleckner, I.R.; Kleckner, A.S.; Castillo, D.A.; Mustian, K.M.; Peppone, L.J. Nutritional Interventions for Treating Cancer-Related Fatigue: A Qualitative Review. Nutr. Cancer 2019, 71, 21–40. [Google Scholar] [CrossRef]
- Mustian, K.M.; Alfano, C.M.; Heckler, C.; Kleckner, A.S.; Kleckner, I.R.; Leach, C.R.; Mohr, D.; Palesh, O.G.; Peppone, L.J.; Piper, B.F.; et al. Comparison of Pharmaceutical, Psychological, and Exercise Treatments for Cancer-Related Fatigue: A Meta-Analysis. JAMA Oncol. 2017, 3, 961–968. [Google Scholar] [CrossRef]
- Gramignano, G.; Lusso, M.R.; Madeddu, C.; Massa, E.; Serpe, R.; Deiana, L.; Lamonica, G.; Dessì, M.; Spiga, C.; Astara, G.; et al. Efficacy of l-carnitine administration on fatigue, nutritional status, oxidative stress, and related quality of life in 12 advanced cancer patients undergoing anticancer therapy. Nutrition 2006, 22, 136–145. [Google Scholar] [CrossRef]
- Stobaus, N.; Muller, M.J.; Kupferling, S.; Schulzke, J.D.; Norman, K. Low Recent Protein Intake Predicts Cancer-Related Fatigue and Increased Mortality in Patients with Advanced Tumor Disease Undergoing Chemotherapy. Nutr. Cancer 2015, 67, 818–824. [Google Scholar] [CrossRef]
- Fayers, P.M.; Aaronson, N.K.; Bjordal, K.; Groenvold, M.; Curran, D.; Bottomley, A.; on behalf of the EORTC Quality of Life Group. The EORTC QLQ-C30 Scoring Manual, 3rd ed.; European Organisation for Research and Treatment of Cancer: Brussels, Belgium, 2021. [Google Scholar]
- Lin, C.C.; Chang, A.P.; Chen, M.L.; Cleeland, C.S.; Mendoza, T.R.; Wang, X.S. Validation of the Taiwanese Version of the Brief Fatigue Inventory. J. Pain Symptom Manage. 2006, 32, 52–59. [Google Scholar] [CrossRef]
- Kim, J.M.; Sung, M.K. The efficacy of oral nutritional intervention in malnourished cancer patients: A systemic review. Clin. Nutr. Res. 2016, 5, 219–236. [Google Scholar] [CrossRef]
- Arends, J.; Baracos, V.; Bertz, H.; Bozzetti, F.; Calder, P.C.; Deutz, N.E.P.; Erickson, N.; Laviano, A.; Lisanti, M.P.; Lobo, D.N.; et al. ESPEN Expert Group Recommendations for Action Against Cancer-Related Malnutrition. Clin. Nutr. 2017, 36, 1187–1196. [Google Scholar] [CrossRef]
- Lee, J.L.C.; Leong, L.P.; Lim, S.L. Nutrition Intervention Approaches to Reduce Malnutrition in Oncology Patients: A Systematic Review. Support Care Cancer 2016, 24, 469–480. [Google Scholar] [CrossRef]
- Bossi, P.; De Luca, R.; Ciani, O.; D’Angelo, E.; Caccialanza, R. Malnutrition Management in Oncology: An Expert View on Controversial Issues and Future Perspectives. Front. Oncol. 2022, 12, 910770. [Google Scholar] [CrossRef]
- Arends, J.; Strasser, F.; Gonella, S.; Solheim, T.S.; Madeddu, C.; Ravasco, P.; Buonaccorso, L.; de van der Schueren, M.A.E.; Baldwin, C.; Chasen, M.; et al. Cancer Cachexia in Adult Patients: ESMO Clinical Practice Guidelines. ESMO Open 2021, 6, 100092. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Li, X.; Sun, C.R. Predictive Value of Serum Albumin Levels on Cancer Survival: A Prospective Cohort Study. Front. Oncol. 2024, 14, 1323192. [Google Scholar] [CrossRef] [PubMed]
- Banh, L. Serum proteins as markers of nutrition: What are we treating? Pract. Gastroenterol. 2006, 30, 46–64. [Google Scholar]
- Hao, J.; Chen, C.; Wan, F.; Zhu, Y.; Jin, H.; Zhou, J.; Chen, N.; Yang, J.; Pu, Q. Prognostic value of pre-treatment prognostic nutritional index in esophageal cancer: A systematic review and meta-analysis. Front. Oncol. 2020, 10, 797. [Google Scholar] [CrossRef]
- Jeon, C.H.; Park, K.B.; Jung, Y.J.; Seo, H.S.; Park, C.H.; Song, K.Y.; Lee, H.H. Modified controlling nutritional status score: A refined prognostic indicator depending on the stage of gastric cancer. Surg. Oncol. 2020, 34, 261–269. [Google Scholar] [CrossRef]
- Bullock, A.F.; Greenley, S.L.; McKenzie, G.A.G.; Paton, L.W.; Johnson, M.J. Relationship between markers of malnutrition and clinical outcomes in older adults with cancer: Systematic review, narrative synthesis and meta-analysis. Eur. J. Clin. Nutr. 2020, 74, 1519–1535. [Google Scholar] [CrossRef]
- Ryan, A.M.; Power, D.G.; Daly, L.; Cushen, S.J.; Ní Bhuachalla, Ē.; Prado, C.M. Cancer-Associated Malnutrition, Cachexia and Sarcopenia: The Skeleton in the Hospital Closet 40 Years Later. Proc. Nutr. Soc. 2016, 75, 199–211. [Google Scholar] [CrossRef]
- Prado, C.M.; Purcell, S.A.; Laviano, A. Nutrition Interventions to Treat Low Muscle Mass in Cancer. J. Cachexia Sarcopenia Muscle 2020, 11, 366–380. [Google Scholar] [CrossRef]
- Prado, C.M.; Sawyer, M.B.; Ghosh, S.; Lieffers, J.R.; Esfandiari, N.; Antoun, S.; Baracos, V.E. Central Tenet of Cancer Cachexia Therapy: Do Patients with Advanced Cancer Have Exploitable Anabolic Potential? Am. J. Clin. Nutr. 2013, 98, 1012–1019. [Google Scholar] [CrossRef] [PubMed]
- Zanetti, M.; Gortan Cappellari, G.; Barazzoni, R.; Sanson, G. The Impact of Protein Supplementation Targeted at Improving Muscle Mass on Strength in Cancer Patients: A Scoping Review. Nutrients 2020, 12, 2099. [Google Scholar] [CrossRef] [PubMed]
- Engelen, M.P.K.J.; Safar, A.M.; Bartter, T.; Koeman, F.; Deutz, N.E.P. High Anabolic Potential of Essential Amino Acid Mixtures in Advanced Nonsmall Cell Lung Cancer. Ann. Oncol. 2015, 26, 1960–1966. [Google Scholar] [CrossRef]
- Rehman, S.U.; Ali, R.; Zhang, H.; Zafar, M.H.; Wang, M. Research Progress in the Role and Mechanism of Leucine in Regulating Animal Growth and Development. Front. Physiol. 2023, 14, 1252089. [Google Scholar] [CrossRef] [PubMed]
- Soares, J.D.P.; Siqueira, J.M.; Brito, F.D.S.B.; Pimentel, G.D. A Randomized Controlled Trial on the Effects of Leucine-Supplement Combined with Nutritional Counseling on Body Composition in Mix Cancer Older Men. Nutrients 2024, 16, 210. [Google Scholar] [CrossRef]
- van Vliet, S.; Burd, N.A.; van Loon, L.J. The Skeletal Muscle Anabolic Response to Plant- versus Animal-Based Protein Consumption. J. Nutr. 2015, 145, 1981–1991. [Google Scholar] [CrossRef]
- Nabavi, S.F.; Bilotto, S.; Russo, G.L.; Orhan, I.E.; Habtemariam, S.; Daglia, M.; Devi, K.P.; Loizzo, M.R.; Tundis, R.; Nabavi, S.M. Omega-3 Polyunsaturated Fatty Acids and Cancer: Lessons Learned from Clinical Trials. Cancer Metastasis Rev. 2015, 34, 359–380. [Google Scholar] [CrossRef]
- Malta, F.A.P.S.; Estadella, D.; Gonçalves, D.C. The Role of Omega 3 Fatty Acids in Suppressing Muscle Protein Catabolism: A Possible Therapeutic Strategy to Reverse Cancer Cachexia? J. Funct. Foods 2019, 54, 1–12. [Google Scholar] [CrossRef]
- Sánchez-Lara, K.; Turcott, J.G.; Juárez-Hernández, E.; Nuñez-Valencia, C.; Villanueva, G.; Guevara, P.; De la Torre-Vallejo, M.; Mohar, A.; Arrieta, O. Effects of an Oral Nutritional Supplement Containing Eicosapentaenoic Acid on Nutritional and Clinical Outcomes in Patients with Advanced Non-Small Cell Lung Cancer: Randomised Trial. Clin. Nutr. 2014, 33, 1017–1023. [Google Scholar] [CrossRef]
- de Aguiar Pastore Silva, J.; Emilia de Souza Fabre, M.; Waitzberg, D.L. Omega-3 Supplements for Patients in Chemotherapy and/or Radiotherapy: A Systematic Review. Clin. Nutr. 2015, 34, 359–366. [Google Scholar] [CrossRef]
- Wei, L.; Wu, Z.; Chen, Y.Q. Multi-Targeted Therapy of Cancer by Omega-3 Fatty Acids—An Update. Cancer Lett. 2022, 526, 193–204. [Google Scholar] [CrossRef]
- Bornfeldt, K.E. Triglyceride Lowering by Omega-3 Fatty Acids: A Mechanism Mediated by N-Acyl Taurines. J. Clin. Investig. 2021, 131, e147558. [Google Scholar] [CrossRef]
- Alexander, D.D.; Miller, P.E.; Van Elswyk, M.E.; Kuratko, C.N.; Bylsma, L.C. A Meta-Analysis of Randomized Controlled Trials and Prospective Cohort Studies of Eicosapentaenoic and Docosahexaenoic Long-Chain Omega-3 Fatty Acids and Coronary Heart Disease Risk. Mayo Clin. Proc. 2017, 92, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Skulas-Ray, A.C.; Wilson, P.W.F.; Harris, W.S.; Brinton, E.A.; Kris-Etherton, P.M.; Richter, C.K.; Jacobson, T.A.; Engler, M.B.; Miller, M.; Robinson, J.G.; et al. Omega-3 Fatty Acids for the Management of Hypertriglyceridemia: A Science Advisory from the American Heart Association. Circulation 2019, 140, e673–e691. [Google Scholar] [CrossRef] [PubMed]
- O’Mahoney, L.L.; Matu, J.; Price, O.J.; Birch, K.M.; Ajjan, R.A.; Farrar, D.; Tapp, R.; West, D.J.; Deighton, K.; Campbell, M.D. Omega-3 Polyunsaturated Fatty Acids Favourably Modulate Cardiometabolic Biomarkers in Type 2 Diabetes: A Meta-Analysis and Meta-Regression of Randomized Controlled Trials. Cardiovasc. Diabetol. 2018, 17, 98. [Google Scholar] [CrossRef] [PubMed]
- de Jesus, M.; Mohammed, T.; Singh, M.; Tiu, J.G.; Kim, A.S. Etiology and Management of Dyslipidemia in Patients with Cancer. Front. Cardiovasc. Med. 2022, 9, 892335. [Google Scholar] [CrossRef]
- Bhatnagar, R.; Dixit, N.M.; Yang, E.H.; Sallam, T. Cancer Therapy’s Impact on Lipid Metabolism: Mechanisms and Future Avenues. Front. Cardiovasc. Med. 2022, 9, 925816. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. 5. Facilitating Positive Health Behaviors and Well-Being to Improve Health Outcomes: Standards of Care in Diabetes—2025. Diabetes Care 2025, 48 (Suppl. S1), S86–S127. [Google Scholar] [CrossRef]
- Xu, J.; Li, Q.; Gao, Z.; Ji, P.; Ji, Q.; Song, M.; Chen, Y.; Sun, H.; Wang, X.; Zhang, L.; et al. Impact of Cancer-Related Fatigue on Quality of Life in Patients with Cancer: Multiple Mediating Roles of Psychological Coherence and Stigma. BMC Cancer 2025, 25, 64. [Google Scholar] [CrossRef]
- Sharour, L.A. Cancer-Related Fatigue, Laboratory Markers as Indicators for Nutritional Status among Patients with Colorectal Cancer. Nutr. Cancer 2020, 72, 903–908. [Google Scholar] [CrossRef]
- Wang, X.S. Pathophysiology of Cancer-Related Fatigue. Clin. J. Oncol. Nurs. 2008, 12 (Suppl. S5), 11–20. [Google Scholar] [CrossRef]
- Kleckner, A.S.; Culakova, E.; Kleckner, I.R.; Belcher, E.K.; Demark-Wahnefried, W.; Parker, E.A.; Padula, G.D.A.; Ontko, M.; Janelsins, M.C.; Mustian, K.M.; et al. Nutritional Status Predicts Fatty Acid Uptake from Fish and Soybean Oil Supplements for Treatment of Cancer-Related Fatigue: Results from a Phase II Nationwide Study. Nutrients 2021, 14, 184. [Google Scholar] [CrossRef]
- Zick, S.M.; Colacino, J.; Cornellier, M.; Khabir, T.; Surnow, K.; Djuric, Z. Fatigue Reduction Diet in Breast Cancer Survivors: A Pilot Randomized Clinical Trial. Breast Cancer Res. Treat. 2017, 161, 299–310. [Google Scholar] [CrossRef]
- Alfano, C.M.; Imayama, I.; Neuhouser, M.L.; Kiecolt-Glaser, J.K.; Smith, A.W.; Meeske, K.; McTiernan, A.; Bernstein, L.; Baumgartner, K.B.; Ulrich, C.M.; et al. Fatigue, Inflammation, and ω-3 and ω-6 Fatty Acid Intake among Breast Cancer Survivors. J. Clin. Oncol. 2012, 30, 1280–1287. [Google Scholar] [CrossRef]
- Rau, K.M.; Shun, S.C.; Hung, S.H.; Chou, H.L.; Ho, C.L.; Chao, T.C.; Liu, C.Y.; Lien, C.T.; Hong, M.Y.; Wu, C.J.; et al. Management of Cancer-Related Fatigue in Taiwan: An Evidence-Based Consensus for Screening, Assessment and Treatment. Jpn. J. Clin. Oncol. 2023, 53, 46–56. [Google Scholar] [CrossRef]
| Formula Description | FOHP-ONS (237 mL/can) |
|---|---|
| Energy (kcal/100 mL) | 150 |
| Energy-dense (kcal/cc) | 1.5 |
| Macronutrients | |
| Total protein (g/100 mL) | 9.5 |
| Total carbohydrate (g/100 mL) | 13.7 |
| Dietary fiber (g/100 mL) | 1.1 |
| Total lipid (g/100 mL) | 6.6 |
| ω-3 fatty acid (g/100 mL) | 0.9 |
| EPA (g/100 mL) | 0.5 |
| DHA (g/100 mL) | 0.1 |
| Description | Intervention Group (n = 30) |
|---|---|
| Age, y | 67.90 ± 8.86 |
| Female, n (%) | 9 (30%) |
| Current Treatment, n | |
| Chemotherapy | 7 |
| Surgery | 9 |
| Radiotherapy | 2 |
| Surgery + radiotherapy | 6 |
| Surgery + radiotherapy + chemotherapy | 1 |
| None | 5 |
| Nutritional Status | |
| BMI (kg/m2) | 19.98 ± 2.96 |
| BMI < 18.5, n (%) | 10 (33.3%) |
| Albumin (g/dl) | 3.51 ± 0.44 |
| PG-SGA, n (%) | |
| Stage A (well-nourished) | 3 (10.0%) |
| Stage B (moderate/suspected malnutrition) | 13 (43.3%) |
| Stage C (severely malnourished) | 14 (46.6%) |
| Insufficient Food Intake, n (%) | |
| Energy intake < 25 kcal/BW kg/d | 12 (40.0%) |
| Protein intake < 1.2 g/BW kg/d | 25 (83.3%) |
| Intervention Group (n = 30) | ||
|---|---|---|
| Energy goal (30 kcal/kg BW/d) # | 1716.93 ± 160.44 | |
| Protein goal (1.2 g/kg BW/d) # | 86.00 ± 8.46 | |
| Actual intake | Baseline | Week 8 |
| Energy (kcal) | 1458.03 ± 138.65 *b | 1713.58 ± 132.27 a |
| Energy (kcal/kg IBW) | 25.64 ± 2.92 | 29.99 ± 0.84 |
| Protein (g/d) | 60.81 ± 10.80 *b | 86.23 ± 5.70 a |
| Protein (g/kg IBW) | 1.06 ± 0.18 | 1.50 ± 0.07 |
| Carbohydrate (g/d) | 193.69 ± 22.10 | 199.82 ± 21.74 |
| Fat (g/d) | 45.50 ± 9.83 b | 61.82 ± 4.60 a |
| Baseline | Week 4 | Week 8 | |
|---|---|---|---|
| Body Composition | |||
| Body weight (kg) | 51.81 ± 8.28 c | 52.04 ± 8.23 b | 52.29 ± 8.08 a |
| BMI (kg/m2) | 19.98 ± 2.96 c | 20.06 ± 2.95 b | 20.17 ± 2.97 a |
| MAC (cm) | 24.59 ± 3.34 b | 24.64 ± 3.34 b | 24.80 ± 3.18 a |
| MAMC (cm) | 20.43 ± 2.82 a | 20.46 ± 2.82 a | 20.57 ± 2.74 a |
| Grip strength (kg) | 14.08 ± 8.39 | 15.43 ± 9.08 * | 15.98 ± 9.56 * |
| Biochemical Parameters | |||
| Albumin (g/dL) | 3.51 ± 0.44 b | 3.59 ± 0.30 ab | 3.66 ± 0.35 a |
| hs-CRP (mg/dL) | 0.86 ± 1.43 a | 0.72 ± 1.35 a | 0.71 ± 1.02 a |
| Fasting blood sugar (mg/dL) | 95.70 ± 16.38 a | 93.57 ± 14.44 ab | 91.40 ± 13.12 b |
| HbA1c (% of Hb) | 5.70 ± 0.48 a | 5.70 ± 0.55 a | 5.70 ± 0.47 a |
| HOMA-IR index | 2.25 ± 3.79 a | 2.16 ± 2.26 a | 1.97 ± 1.59 a |
| Triglyceride (mg/dL) | 99.63 ± 44.00 a | 85.40 ± 33.30 b | 84.23 ± 33.82 b |
| Total Cholesterol (mg/dL) | 142.00 ± 34.13 a | 139.77 ± 31.98 a | 138.57 ± 25.79 a |
| HDL-c (mg/dL) | 39.77 ± 7.56 a | 40.17 ± 5.97 a | 40.67 ± 7.46 a |
| LDL-c (mg/dL) | 85.30 ± 31.34 a | 84.03 ± 29.55 a | 81.47 ± 23.86 a |
| Baseline | Week 4 | Week 8 | |
|---|---|---|---|
| Fatigue Severity | |||
| Fatigue right now | 5.93 ± 1.96 | 5.61 ±1.50 * | 5.07 ± 1.68 * |
| Usual fatigue in the last 24 h | 5.23 ± 2.46 | 4.93 ±1.98 * | 4.43 ± 1.85 * |
| The worst fatigue in the last 24 h | 6.87 ± 1.78 | 6.71 ±1.54 * | 6.07 ± 1.74 * |
| Functional Scales | |||
| General activities | 4.93 ± 2.30 | 4.82 ± 1.96 * | 4.32 ± 1.72 * |
| Mood | 3.80 ± 2.38 | 3.54 ± 2.17 | 3.00 ± 1.76 * |
| Walking | 3.73 ± 2.55 | 3.57 ± 2.20 | 3.04 ± 1.82 * |
| Normal work | 6.07 ± 2.12 | 5.96 ± 1.71 * | 5.32 ± 1.85 * |
| Relations with other people | 3.47 ± 2.69 | 3.43 ± 2.59 * | 2.89 ± 2.10 * |
| Enjoyment of life | 5.63 ± 2.37 | 5.61 ± 1.85 * | 4.79 ± 1.62 * |
| Overall fatigue score | 5.07 ± 2.01 | 4.59 ± 2.02 * | 4.04 ± 1.76 * |
| Baseline | Week 4 | Week 8 | |
|---|---|---|---|
| Global Health Status/QoL | |||
| Global health status/QoL | 44.72 ± 14.60 | 49.72 ± 12.66 * | 51.94 ± 12.12 * |
| Functional Scales | |||
| Physical functioning | 25.78 ± 22.74 | 38.00 ± 19.11 * | 50.44 ± 20.39 * |
| Role functioning | 27.22 ± 28.19 | 32.78 ± 26.07 * | 47.22 ± 30.98 * |
| Cognitive functioning | 56.11 ± 21.66 | 72.22 ± 24.11 * | 76.11 ± 26.87 * |
| Emotional functioning | 60.00 ± 19.38 | 80.00 ± 23.43 * | 81.11 ± 24.07 * |
| Social functioning | 37.78 ± 22.29 | 37.22 ± 19.42 | 51.11 ± 23.13 * |
| Symptom Scales | |||
| Fatigue | 65.93 ± 17.25 | 45.56 ± 20.91 * | 34.81 ± 29.29 * |
| Pain | 43.89 ± 19.81 | 25.00 ± 27.60 * | 25.56 ± 27.93 * |
| Nausea and vomiting | 18.33 ± 18.23 | 16.11 ± 18.30 | 16.11 ± 18.30 |
| Dyspnoea | 28.89 ± 25.87 | 20.00 ± 25.67 * | 16.67 ± 25.89 * |
| Insomnia | 30.00 ± 23.73 | 15.56 ± 20.96 * | 16.67 ± 20.99 * |
| Appetite loss | 27.78 ± 21.59 | 15.56 ± 16.91 * | 16.67 ± 16.95 * |
| Constipation | 32.22 ± 16.34 | 16.67 ± 19.08 * | 13.33 ± 18.77 * |
| Diarrhea | 17.78 ± 16.91 | 14.44 ± 16.80 | 15.56 ± 19.04 |
| Financial difficulties | 48.89 ± 19.04 | 58.89 ± 16.80 * | 43.33 ± 17.83 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiu, H.-F.; Zhuang, S.R.; Shen, Y.-C.; Thangaleela, S.; Wang, C.-K. A Prospective Interventional Study on the Beneficial Effect of Fish Oil-Enriched High-Protein Oral Nutritional Supplement (FOHP-ONS) on Malnourished Older Cancer Patients. Nutrients 2025, 17, 2433. https://doi.org/10.3390/nu17152433
Chiu H-F, Zhuang SR, Shen Y-C, Thangaleela S, Wang C-K. A Prospective Interventional Study on the Beneficial Effect of Fish Oil-Enriched High-Protein Oral Nutritional Supplement (FOHP-ONS) on Malnourished Older Cancer Patients. Nutrients. 2025; 17(15):2433. https://doi.org/10.3390/nu17152433
Chicago/Turabian StyleChiu, Hui-Fang, Shu Ru Zhuang, You-Cheng Shen, Subramanian Thangaleela, and Chin-Kun Wang. 2025. "A Prospective Interventional Study on the Beneficial Effect of Fish Oil-Enriched High-Protein Oral Nutritional Supplement (FOHP-ONS) on Malnourished Older Cancer Patients" Nutrients 17, no. 15: 2433. https://doi.org/10.3390/nu17152433
APA StyleChiu, H.-F., Zhuang, S. R., Shen, Y.-C., Thangaleela, S., & Wang, C.-K. (2025). A Prospective Interventional Study on the Beneficial Effect of Fish Oil-Enriched High-Protein Oral Nutritional Supplement (FOHP-ONS) on Malnourished Older Cancer Patients. Nutrients, 17(15), 2433. https://doi.org/10.3390/nu17152433







