Bone Health in Children and Adolescents with Type 1 Diabetes: Optimizing Bone Accrual and Preventing Fractures
Abstract
1. Introduction
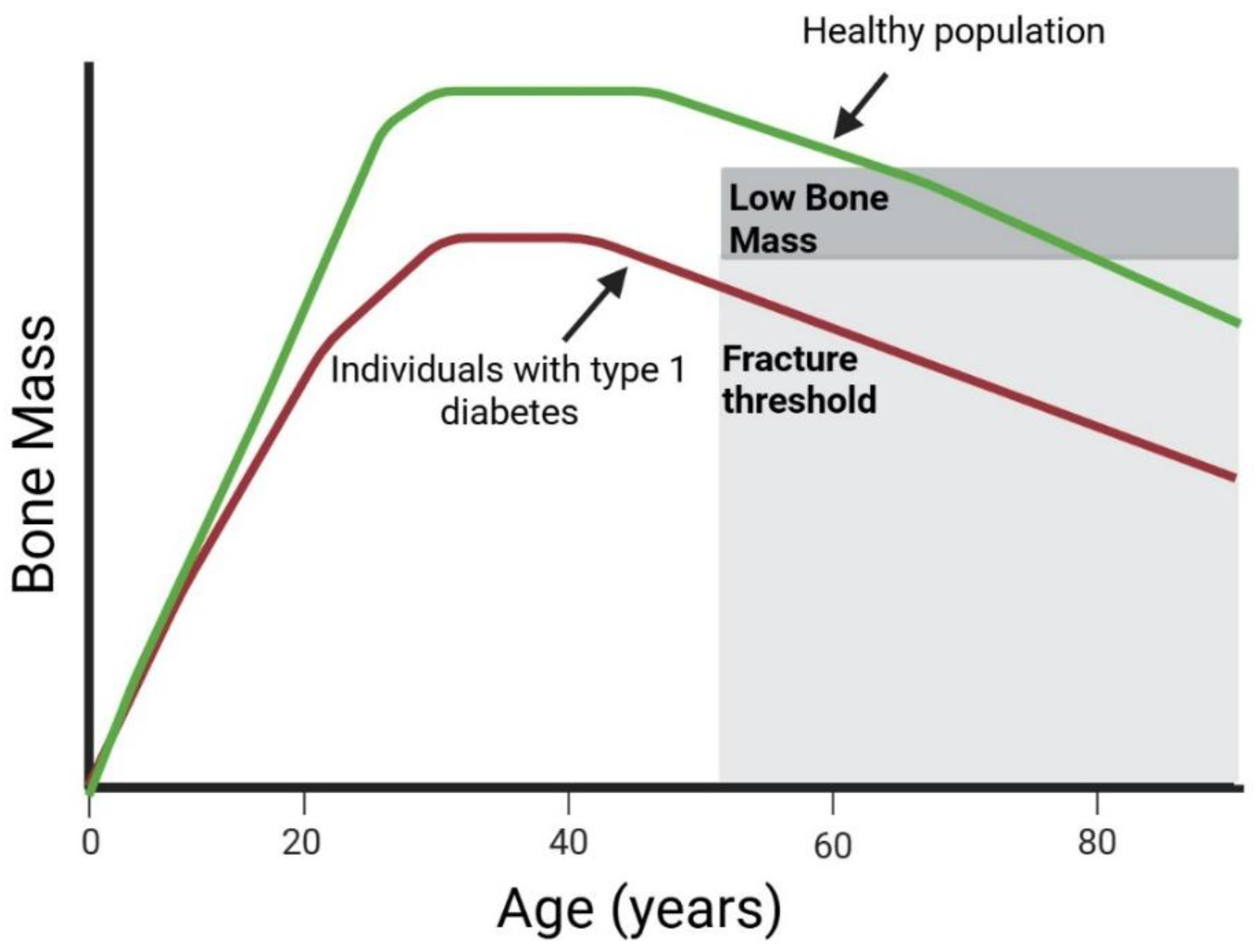
1.1. Mechanism of Bone Fragility in T1D
1.2. Bone Mineral Density in Pediatric Patients with T1D
1.3. Bone Microarchitecture
1.4. Bone Turnover Markers
1.5. Fracture Risk
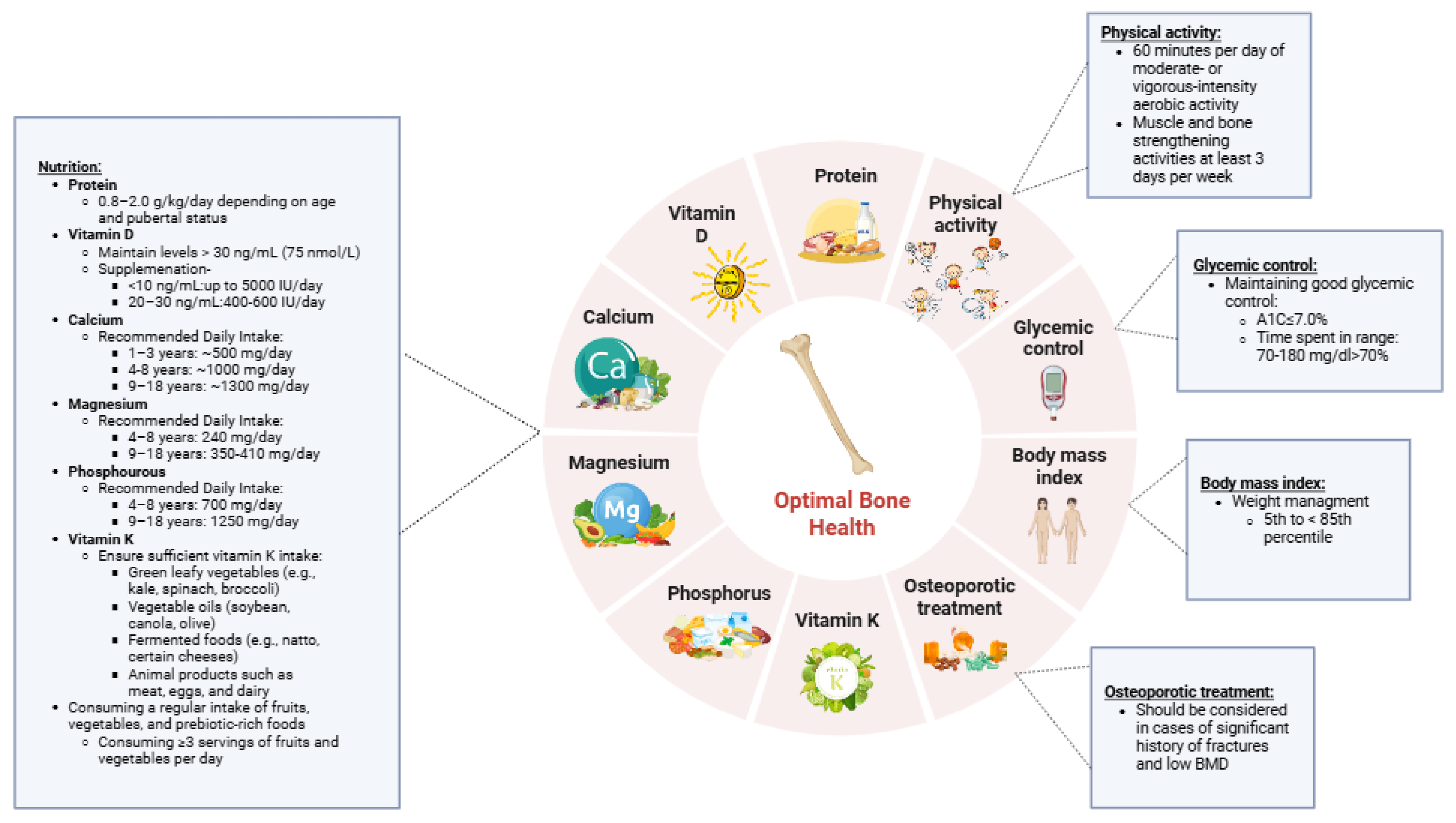
2. Prevention
3. Glycemic Control
4. Nutrition
5. Protein
6. Calcium
7. Vitamin D
8. Magnesium
9. Phosphorus
10. Vitamin K
11. Fruits, Vegetables, and Prebiotics
12. Physical Activity
13. Body Mass Index (BMI)
14. Monitoring BMD and Antiresorptive Medications
15. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Frohlich-Reiterer, E.; Elbarbary, N.S.; Simmons, K.; Buckingham, B.; Humayun, K.N.; Johannsen, J.; Holl, R.W.; Betz, S.; Mahmud, F.H. ISPAD Clinical Practice Consensus Guidelines 2022: Other complications and associated conditions in children and adolescents with type 1 diabetes. Pediatr. Diabetes 2022, 23, 1451–1467. [Google Scholar] [CrossRef] [PubMed]
- Loxton, P.; Narayan, K.; Munns, C.F.; Craig, M.E. Bone Mineral Density and Type 1 Diabetes in Children and Adolescents: A Meta-analysis. Diabetes Care 2021, 44, 1898–1905. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zheng, Y.; Rostami Haji Abadi, M.; Ghafouri, Z.; Meira Goes, S.; Johnston, J.J.D.; Nour, M.; Kontulainen, S. Bone deficits in children and youth with type 1 diabetes: A systematic review and meta-analysis. Bone 2022, 163, 116509. [Google Scholar] [CrossRef] [PubMed]
- Madsen, J.O.B.; Herskin, C.W.; Zerahn, B.; Jorgensen, N.R.; Olsen, B.S.; Pociot, F.; Johannesen, J. Decreased markers of bone turnover in children and adolescents with type 1 diabetes. Pediatr. Diabetes 2020, 21, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.R.; Gordon, R.J.; Kelley, J.C.; Leonard, M.B.; Willi, S.M.; Hatch-Stein, J.; Kelly, A.; Kosacci, O.; Kucheruk, O.; Kaafarani, M.; et al. Poor Glycemic Control Is Associated With Impaired Bone Accrual in the Year Following a Diagnosis of Type 1 Diabetes. J. Clin. Endocrinol. Metab. 2019, 104, 4511–4520. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Eckert, A.J.; Semler, O.; Schnabel, D.; Kostner, K.; Wurm, D.; Bechtold-Dalla Pozza, S.; Schaaf, K.; Hortenhuber, T.; Hammersen, J.; Holl, R.W. Bone Fractures in Children and Young Adults With Type 1 Diabetes: Age Distribution, Fracture Location, and the Role of Glycemic Control. J. Bone Miner. Res. 2021, 36, 2371–2380. [Google Scholar] [CrossRef] [PubMed]
- Stumpf, U.; Hadji, P.; van den Boom, L.; Bocker, W.; Kostev, K. Incidence of fractures in patients with type 1 diabetes mellitus-a retrospective study with 4420 patients. Osteoporos. Int. 2020, 31, 1315–1322. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.R.; Haynes, K.; Leonard, M.B.; Willi, S.M.; Denburg, M.R. Type 1 diabetes is associated with an increased risk of fracture across the life span: A population-based cohort study using The Health Improvement Network (THIN). Diabetes Care. 2015, 38, 1913–1920. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Weaver, C.M.; Gordon, C.M.; Janz, K.F.; Kalkwarf, H.J.; Lappe, J.M.; Lewis, R.; O’karma, M.; Wallace, T.C.; Zemel, B.S. The National Osteoporosis Foundation’s position statement on peak bone mass development and lifestyle factors: A systematic review and implementation recommendations. Osteoporos Int. 2016, 27, 1281–1386. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sheu, A.; White, C.P.; Center, J.R. Bone metabolism in diabetes: A clinician’s guide to understanding the bone-glucose interplay. Diabetologia 2024, 67, 1493–1506. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brunetti, G.; D’Amato, G.; De Santis, S.; Grano, M.; Faienza, M.F. Mechanisms of altered bone remodeling in children with type 1 diabetes. World J. Diabetes 2021, 12, 997–1009. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Napoli, N.; Chandran, M.; Pierroz, D.D.; Abrahamsen, B.; Schwartz, A.V.; Ferrari, S.L.; IOF Bone and Diabetes Working Group. Mechanisms of diabetes mellitus-induced bone fragility. Nat. Rev. Endocrinol. 2017, 13, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Torres, H.M.; Arnold, K.M.; Oviedo, M.; Westendorf, J.J.; Weaver, S.R. Inflammatory Processes Affecting Bone Health and Repair. Curr. Osteoporos. Rep. 2023, 21, 842–853. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, A.; Midura, R.J.; Vasanji, A.; Wang, A.J.; Hascall, V.C. Hyperglycemia diverts dividing osteoblastic precursor cells to an adipogenic pathway and induces synthesis of a hyaluronan matrix that is adhesive for monocytes. J. Biol. Chem. 2014, 289, 11410–11420. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Eckhardt, B.A.; Rowsey, J.L.; Thicke, B.S.; Fraser, D.G.; O’Grady, K.L.; Bondar, O.P.; Hines, J.M.; Singh, R.J.; Thoreson, A.R.; Rakshit, K.; et al. Accelerated osteocyte senescence and skeletal fragility in mice with type 2 diabetes. JCI Insight 2020, 5, e135236. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Asadipooya, K.; Uy, E.M. Advanced Glycation End Products (AGEs), Receptor for AGEs, Diabetes, and Bone: Review of the Literature. J. Endocr. Soc. 2019, 3, 1799–1818. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hofbauer, L.C.; Busse, B.; Eastell, R.; Ferrari, S.; Frost, M.; Muller, R.; Burden, A.M.; Rivadeneira, F.; Napoli, N.; Rauner, M. Bone fragility in diabetes: Novel concepts and clinical implications. Lancet Diabetes Endocrinol. 2022, 10, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, L.; He, X.; Farmer, P.; Boden, S.; Kozlowski, M.; Rubin, J.; Nanes, M.S. Inhibition of osteoblast differentiation by tumor necrosis factor-alpha. Endocrinology 2000, 141, 3956–3964. [Google Scholar] [CrossRef] [PubMed]
- Glantschnig, H.; Fisher, J.E.; Wesolowski, G.; Rodan, G.A.; Reszka, A.A. M-CSF, TNFalpha and RANK ligand promote osteoclast survival by signaling through mTOR/S6 kinase. Cell Death Differ. 2003, 10, 1165–1177. [Google Scholar] [CrossRef] [PubMed]
- Raisingani, M.; Preneet, B.; Kohn, B.; Yakar, S. Skeletal growth and bone mineral acquisition in type 1 diabetic children; abnormalities of the GH/IGF-1 axis. Growth Horm IGF Res. 2017, 34, 13–21. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hamada, Y.; Fujii, H.; Fukagawa, M. Role of oxidative stress in diabetic bone disorder. Bone 2009, 45 (Suppl. S1), S35–S38. [Google Scholar] [CrossRef] [PubMed]
- Morrison, L. Bone development in diabetic children; roentgen study. Am. J. Med. Sci. 1927, 274, 313–319. [Google Scholar] [CrossRef]
- Broy, S.B.; Cauley, J.A.; Lewiecki, M.E.; Schousboe, J.T.; Shepherd, J.A.; Leslie, W.D. Fracture Risk Prediction by Non-BMD DXA Measures: The 2015 ISCD Official Positions Part 1: Hip Geometry. J. Clin. Densitom. 2015, 18, 287–308. [Google Scholar] [CrossRef] [PubMed]
- Silva, B.C.; Broy, S.B.; Boutroy, S.; Schousboe, J.T.; Shepherd, J.A.; Leslie, W.D. Fracture Risk Prediction by Non-BMD DXA Measures: The 2015 ISCD Official Positions Part 2: Trabecular Bone Score. J. Clin. Densitom. 2015, 18, 309–330. [Google Scholar] [CrossRef] [PubMed]
- Mikolajewicz, N.; Bishop, N.; Burghardt, A.J.; Folkestad, L.; Hall, A.; Kozloff, K.M.; Lukey, P.T.; Molloy-Bland, M.; Morin, S.N.; Offiah, A.C.; et al. HR-pQCT Measures of Bone Microarchitecture Predict Fracture: Systematic Review and Meta-Analysis. J. Bone Miner. Res. 2020, 35, 446–459. [Google Scholar] [CrossRef] [PubMed]
- Cheung, W.H.; Hung, V.W.Y.; Cheuk, K.Y.; Chau, W.W.; Tsoi, K.K.F.; Wong, R.M.Y.; Chow, S.K.H.; Lam, T.P.; Yung, P.S.H.; Law, S.W.; et al. Best Performance Parameters of HR-pQCT to Predict Fragility Fracture: Systematic Review and Meta-Analysis. J. Bone Miner. Res. 2021, 36, 2381–2398. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Diemar, S.S.; Lylloff, L.; Ronne, M.S.; Mollehave, L.T.; Heidemann, M.; Thuesen, B.H.; Johannesen, J.; Schou, A.J.; Husby, S.; Wedderkopp, N.; et al. Reference intervals in Danish children and adolescents for bone turnover markers carboxy-terminal cross-linked telopeptide of type I collagen (beta-CTX), pro-collagen type I N-terminal propeptide (PINP), osteocalcin (OC) and bone-specific alkaline phosphatase (bone ALP). Bone 2021, 146, 115879. [Google Scholar] [CrossRef] [PubMed]
- Schini, M.; Vilaca, T.; Gossiel, F.; Salam, S.; Eastell, R. Bone Turnover Markers: Basic Biology to Clinical Applications. Endocr. Rev. 2023, 44, 417–473. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ladang, A.; Rauch, F.; Delvin, E.; Cavalier, E. Bone Turnover Markers in Children: From Laboratory Challenges to Clinical Interpretation. Calcif. Tissue Int. 2023, 112, 218–232. [Google Scholar] [CrossRef] [PubMed]
- Bhattoa, H.P.; Vasikaran, S.; Trifonidi, I.; Kapoula, G.; Lombardi, G.; Jorgensen, N.R.; Pikner, R.; Miura, M.; Chapurlat, R.; Hiligsmann, M.; et al. Update on the role of bone turnover markers in the diagnosis and management of osteoporosis: A consensus paper from The European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO), International Osteoporosis Foundation (IOF), and International Federation of Clinical Chemistry and Laboratory Medicine (IFCC). Osteoporos Int. 2025, 36, 579–608. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Madsen, J.O.B.; Jorgensen, N.R.; Pociot, F.; Johannesen, J. Bone turnover markers in children and adolescents with type 1 diabetes-A systematic review. Pediatr. Diabetes 2019, 20, 510–522. [Google Scholar] [CrossRef] [PubMed]
- Thong, E.P.; Herath, M.; Weber, D.R.; Ranasinha, S.; Ebeling, P.R.; Milat, F.; Teede, H. Fracture risk in young and middle-aged adults with type 1 diabetes mellitus: A systematic review and meta-analysis. Clin. Endocrinol. 2018, 89, 314–323. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Thayakaran, R.; Perrins, M.; Gokhale, K.M.; Kumaran, S.; Narendran, P.; Price, M.J.; Nirantharakumar, K.; Toulis, K.A. Impact of glycaemic control on fracture risk in 5368 people with newly diagnosed Type 1 diabetes: A time-dependent analysis. Diabet. Med. 2019, 36, 1013–1019. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.H.; Vestergaard, P. Hypoglycaemia and type 1 diabetes are associated with an increased risk of fractures. Osteoporos. Int. 2019, 30, 1663–1670. [Google Scholar] [CrossRef] [PubMed]
- Leanza, G.; Maddaloni, E.; Pitocco, D.; Conte, C.; Palermo, A.; Maurizi, A.R.; Pantano, A.L.; Suraci, C.; Altomare, M.; Strollo, R.; et al. Risk factors for fragility fractures in type 1 diabetes. Bone 2019, 125, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Loureiro, M.B.; Ururahy, M.A.; Freire-Neto, F.P.; Oliveira, G.H.; Duarte, V.M.; Luchessi, A.D.; Brandao-Neto, J.; Hirata, R.D.C.; Hirata, M.H.; Maciel-Neto, J.J.; et al. Low bone mineral density is associated to poor glycemic control and increased OPG expression in children and adolescents with type 1 diabetes. Diabetes Res. Clin. Pract. 2014, 103, 452–457. [Google Scholar] [CrossRef] [PubMed]
- Wierzbicka, E.; Swiercz, A.; Pludowski, P.; Jaworski, M.; Szalecki, M. Skeletal Status, Body Composition, and Glycaemic Control in Adolescents with Type 1 Diabetes Mellitus. J. Diabetes Res. 2018, 2018, 8121634. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fuusager, G.B.; Christesen, H.T.; Milandt, N.; Schou, A.J. Glycemic control and bone mineral density in children and adolescents with type 1 diabetes. Pediatr. Diabetes 2019, 20, 629–636. [Google Scholar] [CrossRef] [PubMed]
- de Bock, M.; Agwu, J.C.; Deabreu, M.; Dovc, K.; Maahs, D.M.; Marcovecchio, M.L.; Mahmud, F.H.; Novoa-Medina, Y.; Priyambada, L.; Smart, C.E.; et al. International Society for Pediatric and Adolescent Diabetes Clinical Practice Consensus Guidelines 2024: Glycemic Targets. Horm. Res. Paediatr. 2024, 97, 546–554. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Norris, S.A.; Frongillo, E.A.; Black, M.M.; Dong, Y.; Fall, C.; Lampl, M.; Liese, A.D.; Naguib, M.; Prentice, A.; Rochat, T.; et al. Nutrition in adolescent growth and development. Lancet 2022, 399, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Rizzoli, R.; Biver, E.; Brennan-Speranza, T.C. Nutritional intake and bone health. Lancet Diabetes Endocrinol. 2021, 9, 606–621. [Google Scholar] [CrossRef] [PubMed]
- Annan, S.F.; Higgins, L.A.; Jelleryd, E.; Hannon, T.; Rose, S.; Salis, S.; Baptista, J.; Chinchilla, P.; Marcovecchio, M.L. ISPAD Clinical Practice Consensus Guidelines 2022: Nutritional management in children and adolescents with diabetes. Pediatr. Diabetes 2022, 23, 1297–1321. [Google Scholar] [CrossRef] [PubMed]
- Maahs, D.M.; Daniels, S.R.; de Ferranti, S.D.; Dichek, H.L.; Flynn, J.; Goldstein, B.I.; Kelly, A.S.; Nadeau, K.J.; Martyn-Nemeth, P.; Osganian, S.K.; et al. Cardiovascular disease risk factors in youth with diabetes mellitus: A scientific statement from the American Heart Association. Circulation 2014, 130, 1532–1558. [Google Scholar] [CrossRef] [PubMed]
- Bercaw, H.; Reid, L.A.; Mendoza, J.A.; Frongillo, E.A.; Sauder, K.A.; Reboussin, B.A.; Mayer-Davis, E.J.; Dabelea, D.; Marcovina, S.M.; Mercado, C.; et al. Food Insecurity and Adequacy of Dietary Intake in Youth and Young Adults With Youth-Onset Type 1 and Type 2 Diabetes. J. Acad. Nutr. Diet. 2023, 123, 1162–1172.e1. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guo, C.; Li, K.; Zheng, M.; Chen, X.; Yin, Y.; Chen, S. Association between dietary protein intake and bone mineral density in adolescents: A cross-sectional study. Arch. Osteoporos. 2025, 20, 41. [Google Scholar] [CrossRef] [PubMed]
- Alexy, U.; Remer, T.; Manz, F.; Neu, C.M.; Schoenau, E. Long-term protein intake and dietary potential renal acid load are associated with bone modeling and remodeling at the proximal radius in healthy children. Am. J. Clin. Nutr. 2005, 82, 1107–1114. [Google Scholar] [CrossRef] [PubMed]
- Elango, R.; Humayun, M.A.; Ball, R.O.; Pencharz, P.B. Protein requirement of healthy school-age children determined by the indicator amino acid oxidation method. Am. J. Clin. Nutr. 2011, 94, 1545–1552. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Narumi-Hyakutake, A.; Kakutani, Y.; Tsuji, M.; Hatamoto, Y.; Higaki, Y.; Sasaki, S. Evaluation of protein requirements using the indicator amino acid oxidation method: A scoping review. J. Nutr. 2023, 153, 3472–3489. [Google Scholar] [CrossRef] [PubMed]
- Thongpaeng, S.; Sorncharoen, P.; Preechasuk, L.; Santiprabhob, J. Dietary Intake and Physical Activity of Thai Children and Adolescents with Type 1 Diabetes Mellitus. Nutrients 2022, 14, 5169. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Leao, A.A.P.; Fritz, C.K.; Dias, M.R.M.G.; Carvalho, J.A.R.; Mascarenhas, L.P.G.; Cat, M.N.L.; Radominski, R.; Nesi-Franca, S. Bone mass and dietary intake in children and adolescents with type 1 diabetes mellitus. J. Diabetes Complicat. 2020, 34, 107573. [Google Scholar] [CrossRef] [PubMed]
- Saunders, R.K.; Kilroe, K.M.; Joseph, T.V.; Caksa, S.; Bouxsein, M.L.; Misra, M.; Mitchell, D.M. Total Calcium Intake Is Associated With Trabecular Bone Density in Adolescent Girls With Type 1 Diabetes. JBMR Plus 2023, 7, e10813. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rahmani, R.; Stevens, E.; Rackovsky, N.; O’Brien, K.O.; Schwartz, G.J.; Weber, D.R. Female Sex and Obesity Are Risk Factors for Inadequate Calcium Intake in Youth With Type 1 Diabetes. Front. Clin. Diabetes Healthc. 2021, 2, 723855. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Weber, D.R.; O’Brien, K.O.; Ballester, L.; Rackovsky, N.; Graulich, B.; Schwartz, G.J. Greater Urinary Calcium Excretion Is Associated With Diminished Bone Accrual in Youth With Type 1 Diabetes. J. Clin. Endocrinol. Metab. 2025, 110, e1802–e1810. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wynn, E.; Krieg, M.A.; Aeschlimann, J.M.; Burckhardt, P. Alkaline mineral water lowers bone resorption even in calcium sufficiency: Alkaline mineral water and bone metabolism. Bone 2009, 44, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Straub, D.A. Calcium supplementation in clinical practice: A review of forms, doses, and indications. Nutr. Clin. Pract. 2007, 22, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Khadilkar, A.; Oza, C.; Antani, M.; Shah, N.; Lohiya, N.; Khadilkar, V.; Bhor, S.; Kajale, N.; Gondhalekar, K.; More, C.; et al. Effect of Calcium and Vitamin D Supplementation (Dairy vs. Pharmacological) on Bone Health of Underprivileged Indian Children and Youth with Type-1 Diabetes: A Randomized Controlled Trial. J. Clin. Densitom. 2024, 27, 101468. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D. Vitamin D and bone. Curr. Osteoporos. Rep. 2012, 10, 151–159. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, X.; Chai, M.; Lin, M. Proportion of vitamin D deficiency in children/adolescents with type 1 diabetes: A systematic review and meta-analysis. BMC Pediatr. 2024, 24, 192. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mondkar, S.; Oza, C.; Dange, N.; Soren, P.; Kajale, N.; Kardile, M.; Yewale, S.; Gondhalekar, K.; Khadilkar, V.; Khadilkar, A. Assessment of Vitamin D Status, its Determinants and Relationship with Bone Health in Indian Children and Young Adults with Type-1 Diabetes. Indian. J. Endocrinol. Metab. 2024, 28, 405–412. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Demay, M.B.; Pittas, A.G.; Bikle, D.D.; Diab, D.L.; Kiely, M.E.; Lazaretti-Castro, M.; Lips, P.; Mitchell, D.M.; Murad, M.H.; Powers, S.; et al. Vitamin D for the Prevention of Disease: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2024, 109, 1907–1947. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Luo, W.; Wang, B.; Yi, Z.; Gong, P.; Xiong, Y. 1alpha,25-Dihydroxyvitamin D3 ameliorates diabetes-induced bone loss by attenuating FoxO1-mediated autophagy. J. Biol. Chem. 2021, 296, 100287. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chevalley, T.; Brandi, M.L.; Cashman, K.D.; Cavalier, E.; Harvey, N.C.; Maggi, S.; Cooper, C.; Al-Daghri, N.; Bock, O.; Bruyere, O.; et al. Role of vitamin D supplementation in the management of musculoskeletal diseases: Update from an European Society of Clinical and Economical Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO) working group. Aging Clin. Exp. Res. 2022, 34, 2603–2623. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Munns, C.F.; Shaw, N.; Kiely, M.; Specker, B.L.; Thacher, T.D.; Ozono, K.; Michigami, T.; Tiosano, D.; Mughal, M.Z.; Makitie, O.; et al. Global Consensus Recommendations on Prevention and Management of Nutritional Rickets. J. Clin. Endocrinol. Metab. 2016, 101, 394–415. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ciosek, Z.; Kot, K.; Kosik-Bogacka, D.; Lanocha-Arendarczyk, N.; Rotter, I. The Effects of Calcium, Magnesium, Phosphorus, Fluoride, and Lead on Bone Tissue. Biomolecules 2021, 11, 506. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Van Eyck, A.; Ledeganck, K.J.; Vermeiren, E.; De Lamper, A.; Eysackers, M.; Mortier, J.; Van Vliet, M.P.; Broere, P.; Roebersen, M.; France, A.; et al. Body composition helps to elucidate the different origins of low serum magnesium in children with obesity compared to children with type 1 diabetes. Eur. J. Pediatr. 2023, 182, 3743–3753. [Google Scholar] [CrossRef] [PubMed]
- Shahbah, D.; El Naga, A.A.; Hassan, T.; Zakaria, M.; Beshir, M.; Al Morshedy, S.; Abdalhady, M.; Kamel, E.; Rahman, D.A.; Kamel, L.; et al. Status of serum magnesium in Egyptian children with type 1 diabetes and its correlation to glycemic control and lipid profile. Medicine 2016, 95, e5166. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Miyamoto, K.I.; Oh, J.; Razzaque, M.S. Common Dietary Sources of Natural and Artificial Phosphate in Food. Adv. Exp. Med. Biol. 2022, 1362, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride; The National Academies Collection: Reports funded by National Institutes of Health; National Academies Press: Washington, DC, USA, 1997.
- Tsugawa, N.; Shiraki, M. Vitamin K Nutrition and Bone Health. Nutrients 2020, 12, 1909. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Berezovska, O.; Yildirim, G.; Budell, W.C.; Yagerman, S.; Pidhaynyy, B.; Bastien, C.; van der Meulen, M.C.H.; Dowd, T.L. Osteocalcin affects bone mineral and mechanical properties in female mice. Bone 2019, 128, 115031. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Salma; Ahmad, S.S.; Karim, S.; Ibrahim, I.M.; Alkreathy, H.M.; Alsieni, M.; Khan, M.A. Effect of Vitamin K on Bone Mineral Density and Fracture Risk in Adults: Systematic Review and Meta-Analysis. Biomedicines 2022, 10, 1048. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Koziol-Kozakowska, A.; Maresz, K. The Impact of Vitamin K2 (Menaquionones) in Children’s Health and Diseases: A Review of the Literature. Children 2022, 9, 78. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Martiniakova, M.; Babikova, M.; Mondockova, V.; Blahova, J.; Kovacova, V.; Omelka, R. The Role of Macronutrients, Micronutrients and Flavonoid Polyphenols in the Prevention and Treatment of Osteoporosis. Nutrients 2022, 14, 523. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brondani, J.E.; Comim, F.V.; Flores, L.M.; Martini, L.A.; Premaor, M.O. Fruit and vegetable intake and bones: A systematic review and meta-analysis. PLoS ONE 2019, 14, e0217223. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rizzoli, R.; Chevalley, T. Bone health: Biology and nutrition. Curr. Opin. Clin. Nutr. Metab. Care 2024, 27, 24–30. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liang, A.; Leonard, W.; Beasley, J.T.; Fang, Z.; Zhang, P.; Ranadheera, C.S. Anthocyanins-gut microbiota-health axis: A review. Crit. Rev. Food Sci. Nutr. 2024, 64, 7563–7588. [Google Scholar] [CrossRef] [PubMed]
- Whisner, C.M.; Martin, B.R.; Schoterman, M.H.C.; Nakatsu, C.H.; McCabe, L.D.; McCabe, G.P.; Wastney, M.E.; van den Heuvel, E.G.H.M.; Weaver, C.M. Galacto-oligosaccharides increase calcium absorption and gut bifidobacteria in young girls: A double-blind cross-over trial. Br. J. Nutr. 2013, 110, 1292–1303. [Google Scholar] [CrossRef] [PubMed]
- Abrams, S.A.; Griffin, I.J.; Hawthorne, K.M.; Liang, L.; Gunn, S.K.; Darlington, G.; Ellis, K.J. A combination of prebiotic short- and long-chain inulin-type fructans enhances calcium absorption and bone mineralization in young adolescents. Am. J. Clin. Nutr. 2005, 82, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Abate, V.; Vergatti, A.; Altavilla, N.; Garofano, F.; Salcuni, A.S.; Rendina, D.; De Filippo, G.; Vescini, F.; D’Elia, L. Potassium Intake and Bone Health: A Narrative Review. Nutrients 2024, 16, 3016. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Demigne, C.; Sabboh, H.; Remesy, C.; Meneton, P. Protective effects of high dietary potassium: Nutritional and metabolic aspects. J. Nutr. 2004, 134, 2903–2906. [Google Scholar] [CrossRef] [PubMed]
- Frassetto, L.; Banerjee, T.; Powe, N.; Sebastian, A. Acid Balance, Dietary Acid Load, and Bone Effects-A Controversial Subject. Nutrients 2018, 10, 517. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- New, S.A. Intake of fruit and vegetables: Implications for bone health. Proc. Nutr. Soc. 2003, 62, 889–899. [Google Scholar] [CrossRef] [PubMed]
- Granchi, D.; Torreggiani, E.; Massa, A.; Caudarella, R.; Di Pompo, G.; Baldini, N. Potassium citrate prevents increased osteoclastogenesis resulting from acidic conditions: Implication for the treatment of postmenopausal bone loss. PLoS ONE 2017, 12, e0181230. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tylavsky, F.A.; Holliday, K.; Danish, R.; Womack, C.; Norwood, J.; Carbone, L. Fruit and vegetable intakes are an independent predictor of bone size in early pubertal children. Am. J. Clin. Nutr. 2004, 79, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Byberg, L.; Bellavia, A.; Orsini, N.; Wolk, A.; Michaelsson, K. Fruit and vegetable intake and risk of hip fracture: A cohort study of Swedish men and women. J. Bone Miner. Res. 2015, 30, 976–984. [Google Scholar] [CrossRef] [PubMed]
- Huerta-Uribe, N.; Hormazabal-Aguayo, I.A.; Izquierdo, M.; Garcia-Hermoso, A. Youth with type 1 diabetes mellitus are more inactive and sedentary than apparently healthy peers: A systematic review and meta-analysis. Diabetes Res. Clin. Pract. 2023, 200, 110697. [Google Scholar] [CrossRef] [PubMed]
- Anneli Heikkila, E.; Pokka, T.M.; Korpelainen, R.; Tossavainen, P.H. Level, types and determinants of physical activity in children with type 1 diabetes and their parents: A cross-sectional study. Diabet. Med. 2024, 41, e15149. [Google Scholar] [CrossRef] [PubMed]
- Livny, R.; Said, W.; Shilo, S.; Bar-Yoseph, R.; Gal, S.; Oren, M.; Levy, M.; Weiss, R.; Shehadeh, N.; Zuckerman-Levin, N.; et al. Identifying sources of support and barriers to physical activity in pediatric type 1 diabetes. Pediatr. Diabetes 2020, 21, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Maggio, A.B.; Rizzoli, R.R.; Marchand, L.M.; Ferrari, S.; Beghetti, M.; Farpour-Lambert, N.J. Physical activity increases bone mineral density in children with type 1 diabetes. Med. Sci. Sports Exerc. 2012, 44, 1206–1211. [Google Scholar] [CrossRef] [PubMed]
- Gil-Diaz, M.C.; Raynor, J.; O’Brien, K.O.; Schwartz, G.J.; Weber, D.R. Systematic review: Associations of calcium intake, vitamin D intake, and physical activity with skeletal outcomes in people with Type 1 diabetes mellitus. Acta Diabetol. 2019, 56, 1091–1102. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zheng, Y.; Nour, M.A.; Lanovaz, J.; Johnston, J.J.D.; Kontulainen, S. Bone and muscle differences in children and adolescents with type 1 diabetes: The mediating role of physical activity. Bone 2024, 187, 117206. [Google Scholar] [CrossRef] [PubMed]
- West, S.L.; Furman, M.; Moineddin, R.; Sochett, E. Association of daily physical activity and bone microarchitecture in young adults with type 1 diabetes—A pilot exploratory study. Bone Rep. 2024, 23, 101813. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- American Diabetes Association Professional Practice C. 14. Children and Adolescents: Standards of Care in Diabetes-2025. Diabetes Care 2025, 48 (Suppl. S1), S283–S305. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- American Diabetes Association Professional Practice C. 4. Comprehensive Medical Evaluation and Assessment of Comorbidities: Standards of Care in Diabetes-2025. Diabetes Care 2025, 48 (Suppl. S1), S59–S85. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bishop, N.; Arundel, P.; Clark, E.; Dimitri, P.; Farr, J.; Jones, G.; Makitie, O.; Munns, C.F.; Shaw, N.; International Society of Clinical Densitometry. Fracture prediction and the definition of osteoporosis in children and adolescents: The ISCD 2013 Pediatric Official Positions. J. Clin. Densitom. 2014, 17, 275–280. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Levran, N.; Shalev-Goldman, E.; Levy-Shraga, Y. Bone Health in Children and Adolescents with Type 1 Diabetes: Optimizing Bone Accrual and Preventing Fractures. Nutrients 2025, 17, 2400. https://doi.org/10.3390/nu17152400
Levran N, Shalev-Goldman E, Levy-Shraga Y. Bone Health in Children and Adolescents with Type 1 Diabetes: Optimizing Bone Accrual and Preventing Fractures. Nutrients. 2025; 17(15):2400. https://doi.org/10.3390/nu17152400
Chicago/Turabian StyleLevran, Neriya, Einat Shalev-Goldman, and Yael Levy-Shraga. 2025. "Bone Health in Children and Adolescents with Type 1 Diabetes: Optimizing Bone Accrual and Preventing Fractures" Nutrients 17, no. 15: 2400. https://doi.org/10.3390/nu17152400
APA StyleLevran, N., Shalev-Goldman, E., & Levy-Shraga, Y. (2025). Bone Health in Children and Adolescents with Type 1 Diabetes: Optimizing Bone Accrual and Preventing Fractures. Nutrients, 17(15), 2400. https://doi.org/10.3390/nu17152400




