The Visceral Adiposity Index and Its Usefulness in the Prediction of Cardiometabolic Disorders
Abstract
1. Introduction
1.1. Obesity and Its Phenotypes
1.2. Metabolic Syndrome
1.3. Diabetes Mellitus and Cardiovascular Disease
1.4. Purpose of This Paper
2. Adipose Tissue Dysfunction and Cardiometabolic Disease
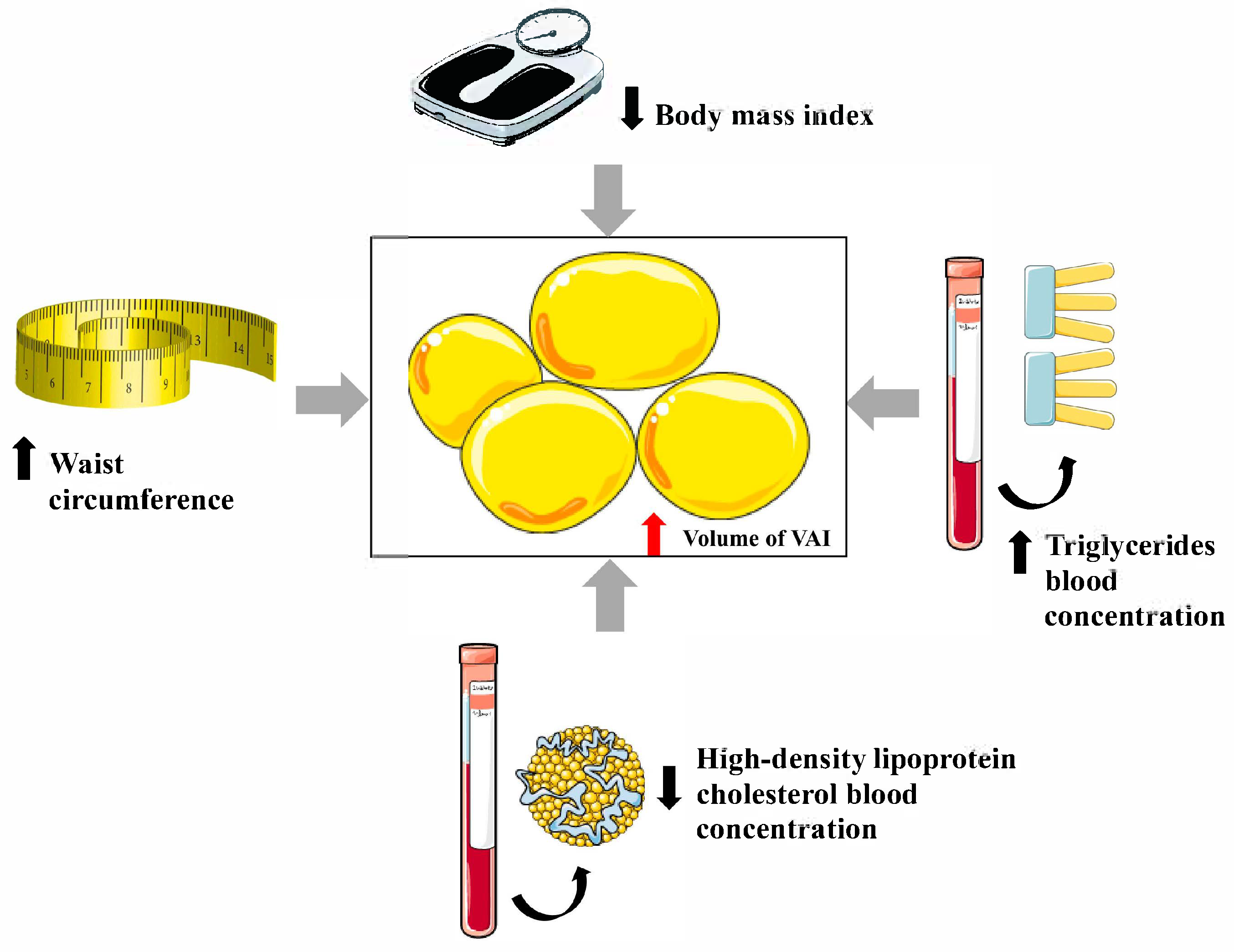
3. Visceral Adiposity Index
4. Visceral Adiposity Index and Metabolic Disorders
4.1. Prediabetes
4.2. Diabetes
4.3. Metabolic Syndrome
4.4. Insulin Resistance
4.5. Fatty Liver Disease
5. Visceral Adiposity Index and Cardiovascular Diseases
5.1. Cerebrovascular Disease
5.2. Coronary Heart Disease
5.3. Heart Failure
5.4. Peripheral Arterial Disease
5.5. Atrial Fibrillation
6. Visceral Adiposity Index and Diabetic Kidney Disease
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rosengren, A. Obesity and cardiovascular health: The size of the problem. Eur. Heart J. 2021, 42, 3404–3406. [Google Scholar] [CrossRef]
- Wiechert, M.; Holzapfel, C. Nutrition concepts for the treatment of obesity in adults. Nutrients 2021, 14, 169. [Google Scholar] [CrossRef] [PubMed]
- Jakubiak, G.K.; Osadnik, K.; Lejawa, M.; Kasperczyk, S.; Osadnik, T.; Pawlas, N. Oxidative stress in association with metabolic health and obesity in young adults. Oxid. Med. Cell. Longev. 2021, 2021, 9987352. [Google Scholar] [CrossRef] [PubMed]
- Jakubiak, G.K.; Osadnik, K.; Lejawa, M.; Osadnik, T.; Goławski, M.; Lewandowski, P.; Pawlas, N. “Obesity and insulin resistance” is the component of the metabolic syndrome most strongly associated with oxidative stress. Antioxidants 2021, 11, 79. [Google Scholar] [CrossRef] [PubMed]
- Yumuk, V.; Tsigos, C.; Fried, M.; Schindler, K.; Busetto, L.; Micic, D.; Toplak, H. Obesity management task force of the European Association for the Study of Obesity. European guidelines for obesity management in adults. Obes. Facts. 2015, 8, 402–424. [Google Scholar] [CrossRef]
- Czapla, M.; Surma, S.; Kwaśny, A.; Lewandowski, Ł. Association of body mass index with outcomes in patients with heart failure with reduced ejection fraction (HFrEF). Nutrients 2024, 16, 2473. [Google Scholar] [CrossRef]
- Bergman, R.N.; Stefanovski, D.; Buchanan, T.A.; Sumner, A.E.; Reynolds, J.C.; Sebring, N.G.; Xiang, A.H.; Watanabe, R.M. A better index of body adiposity. Obesity 2011, 19, 1083–1089. [Google Scholar] [CrossRef]
- Thomas, D.M.; Bredlau, C.; Bosy-Westphal, A.; Mueller, M.; Shen, W.; Gallagher, D.; Maeda, Y.; McDougall, A.; Peterson, C.M.; Ravussin, E.; et al. Relationships between body roundness with body fat and visceral adipose tissue emerging from a new geometrical model. Obesity 2013, 21, 2264–2271. [Google Scholar] [CrossRef]
- Krakauer, N.Y.; Krakauer, J.C. A new body shape index predicts mortality hazard independently of body mass index. PLoS ONE 2012, 7, e39504. [Google Scholar] [CrossRef]
- Belarmino, G.; Torrinhas, R.S.; Sala, P.; Horie, L.M.; Damiani, L.; Lopes, N.C.; Heymsfield, S.B.; Waitzberg, D.L. A new anthropometric index for body fat estimation in patients with severe obesity. BMC Obes. 2018, 5, 25. [Google Scholar] [CrossRef]
- Amato, M.C.; Giordano, C.; Galia, M.; Criscimanna, A.; Vitabile, S.; Midiri, M.; Galluzzo, A.; AlkaMeSy Study Group. Visceral Adiposity Index: A reliable indicator of visceral fat function associated with cardiometabolic risk. Diabetes Care 2010, 33, 920–922. [Google Scholar] [CrossRef]
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.; Loria, C.M.; Smith, S.C., Jr.; et al. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef]
- Kim, T.; Kang, J. Relationship between obstructive sleep apnea, insulin resistance, and metabolic syndrome: A nationwide population-based survey. Endocr. J. 2023, 70, 107–119. [Google Scholar] [CrossRef]
- Bowden, R.G.; Richardson, K.A.; Richardson, L.T. Uric acid and metabolic syndrome: Findings from national health and nutrition examination survey. Front. Med. 2022, 9, 1039230. [Google Scholar] [CrossRef] [PubMed]
- Seravalle, G.; Vanoli, J.; Molisano, C.; Merati, V.; Grassi, G. Heart rate thresholds for cardiovascular risk and sympathetic activation in the metabolic syndrome. Acta Diabetol. 2022, 59, 1429–1435. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi-Roshan, M.; Shoaibinobarian, N.; Noormohammadi, M.; Fakhr Mousavi, A.; Savar Rakhsh, A.; Salari, A.; Ghorbani, Z. Inflammatory markers and atherogenic coefficient: Early markers of metabolic syndrome. Int. J. Endocrinol. Metab. 2022, 20, e127445. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.E.; Kim, H.; Sung, J.; Kim, D.K.; Lee, M.S.; Han, S.W.; Kim, H.J.; Kim, S.H.; Ryu, K.H. The association between metabolic syndrome and heart failure in middle-aged men and women: Population-based study of 2 million individuals. Epidemiol. Health 2022, 44, e2022078. [Google Scholar] [CrossRef]
- Zhou, Y.; Fu, L.; Sun, J.; Zhu, Z.; Xing, Z.; Zhou, S.; Tai, S.; Wang, Y. Association between metabolic syndrome and an increased risk of hospitalization for heart failure in population of HFpEF. Front. Cardiovasc. Med. 2021, 8, 698117. [Google Scholar] [CrossRef]
- Fanaei, S.M.; Mehran, L.; Amouzegar, A.; Masoumi, S.; Amouzegar, A.; Azizi, F. The impact of metabolic syndrome on chronic kidney disease development. Insights from a big prospective study. Eur. J. Clin. Investig. 2022, 53, e13945. [Google Scholar] [CrossRef]
- Malnick, S.D.H.; Ohayon Michael, S. The intestinal microbiome and the metabolic syndrome-how its manipulation may affect metabolic-associated fatty liver disease (MAFLD). Curr. Issues Mol. Biol. 2023, 45, 7197–7211. [Google Scholar] [CrossRef]
- Romero-García, T.; Vázquez-Jiménez, J.G.; Sánchez-Hernández, R.; Olivares-Reyes, J.A.; Rueda, A. Insulin resistance, Ca2+ signaling alterations and vascular dysfunction in prediabetes and metabolic syndrome. Front. Physiol. 2025, 16, 1535153. [Google Scholar] [CrossRef]
- Rashid, N.; Nigam, A.; Kauser, S.; Prakash, P.; Jain, S.K.; Wajid, S. Assessment of insulin resistance and metabolic syndrome in young reproductive aged women with polycystic ovarian syndrome: Analogy of surrogate indices. Arch. Physiol. Biochem. 2022, 128, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Jakubiak, G.K. Cardiac troponin serum concentration measurement is useful not only in the diagnosis of acute cardiovascular events. J. Pers. Med. 2024, 14, 230. [Google Scholar] [CrossRef] [PubMed]
- Starzak, M.; Stanek, A.; Jakubiak, G.K.; Cholewka, A.; Cieślar, G. Arterial stiffness assessment by pulse wave velocity in patients with metabolic syndrome and its components: Is it a useful tool in clinical practice? Int. J. Environ. Res. Public Health 2022, 19, 10368. [Google Scholar] [CrossRef] [PubMed]
- Mućka, S.; Miodońska, M.; Jakubiak, G.K.; Starzak, M.; Cieślar, G.; Stanek, A. Endothelial function assessment by flow-mediated dilation method: A valuable tool in the evaluation of the cardiovascular system. Int. J. Environ. Res. Public Health 2022, 19, 11242. [Google Scholar] [CrossRef]
- Yun, J.S.; Ko, S.H. Current trends in epidemiology of cardiovascular disease and cardiovascular risk management in type 2 diabetes. Metabolism 2021, 123, 154838. [Google Scholar] [CrossRef]
- American Diabetes Association. Economic costs of diabetes in the U.S. in 2017. Diabetes Care 2018, 41, 917–928. [Google Scholar] [CrossRef]
- Banach, M.; Surma, S.; Guzik, T.J.; Penson, P.E.; Blaha, M.J.; Pinto, F.J.; Sperling, L.S. Upfront lipid-lowering combination therapy in high cardiovascular risk patients: A route to effective atherosclerotic cardiovascular disease prevention. Cardiovasc. Res. 2025, 121, 851–859. [Google Scholar] [CrossRef]
- Jakubiak, G.K.; Pawlas, N.; Cieślar, G.; Stanek, A. Chronic lower extremity ischemia and its association with the frailty syndrome in patients with diabetes. Int. J. Environ. Res. Public Health 2020, 17, 9339. [Google Scholar] [CrossRef]
- Jakubiak, G.K.; Pawlas, N.; Cieślar, G.; Stanek, A. Pathogenesis and clinical significance of in-stent restenosis in patients with diabetes. Int. J. Environ. Res. Public Health 2021, 18, 11970. [Google Scholar] [CrossRef]
- Morais, J.B.S.; Dias, T.M.D.S.; Cardoso, B.E.P.; de Paiva Sousa, M.; Sousa, T.G.V.; Araújo, D.S.C.; Marreiro, D.D.N. Adipose tissue dysfunction: Impact on metabolic changes? Horm. Metab. Res. 2022, 54, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Parasiliti-Caprino, M.; Bollati, M.; Merlo, F.D.; Ghigo, E.; Maccario, M.; Bo, S. Adipose tissue dysfunction in obesity: Role of mineralocorticoid receptor. Nutrients 2022, 14, 4735. [Google Scholar] [CrossRef]
- van Greevenbroek, M.M.; Schalkwijk, C.G.; Stehouwer, C.D. Dysfunctional adipose tissue and low-grade inflammation in the management of the metabolic syndrome: Current practices and future advances. F1000Res 2016, 5, 2515. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Alam, R.; Ahsan, H.; Khan, S. Role of adipokines (omentin and visfatin) in coronary artery disease. Nutr. Metab. Cardiovasc. Dis. 2022, 33, 483–493. [Google Scholar] [CrossRef] [PubMed]
- You, M.; Liu, Y.; Wang, B.; Li, L.; Zhang, H.; He, H.; Zhou, Q.; Cao, T.; Wang, L.; Zhao, Z.; et al. Asprosin induces vascular endothelial-to-mesenchymal transition in diabetic lower extremity peripheral artery disease. Cardiovasc. Diabetol. 2022, 21, 25. [Google Scholar] [CrossRef]
- Miricescu, D.; Balan, D.G.; Tulin, A.; Stiru, O.; Vacaroiu, I.A.; Mihai, D.A.; Popa, C.C.; Enyedi, M.; Nedelea, A.S.; Nica, A.E.; et al. Impact of adipose tissue in chronic kidney disease development (Review). Exp. Ther. Med. 2021, 21, 539. [Google Scholar] [CrossRef]
- Wang, X.; Rao, H.; Liu, F.; Wei, L.; Li, H.; Wu, C. Recent advances in adipose tissue dysfunction and its role in the pathogenesis of non-alcoholic fatty liver disease. Cells 2021, 10, 3300. [Google Scholar] [CrossRef]
- Stanek, A.; Brożyna-Tkaczyk, K.; Myśliński, W. The role of obesity-induced perivascular adipose tissue (PVAT) dysfunction in vascular homeostasis. Nutrients 2021, 13, 3843. [Google Scholar] [CrossRef]
- Patel, V.; Patel, J. Cellular cross talk between epicardial fat and cardiovascular risk. J. Basic Clin. Physiol. Pharmacol. 2022, 33, 683–694. [Google Scholar] [CrossRef]
- Farkhondeh, T.; Llorens, S.; Pourbagher-Shahri, A.M.; Ashrafizadeh, M.; Talebi, M.; Shakibaei, M.; Samarghandian, S. An overview of the role of adipokines in cardiometabolic diseases. Molecules 2020, 25, 5218. [Google Scholar] [CrossRef]
- Aljafary, M.A.; Al-Suhaimi, E.A. Adiponectin system (rescue hormone): The missing link between metabolic and cardiovascular diseases. Pharmaceutics 2022, 14, 1430. [Google Scholar] [CrossRef] [PubMed]
- Recinella, L.; Orlando, G.; Ferrante, C.; Chiavaroli, A.; Brunetti, L.; Leone, S. Adipokines: New potential therapeutic target for obesity and metabolic, rheumatic, and cardiovascular diseases. Front. Physiol. 2020, 11, 578966. [Google Scholar] [CrossRef] [PubMed]
- Kirichenko, T.V.; Markina, Y.V.; Bogatyreva, A.I.; Tolstik, T.V.; Varaeva, Y.R.; Starodubova, A.V. The role of adipokines in inflammatory mechanisms of obesity. Int. J. Mol. Sci. 2022, 23, 14982. [Google Scholar] [CrossRef] [PubMed]
- Clemente-Suárez, V.J.; Redondo-Flórez, L.; Beltrán-Velasco, A.I.; Martín-Rodríguez, A.; Martínez-Guardado, I.; Navarro-Jiménez, E.; Laborde-Cárdenas, C.C.; Tornero-Aguilera, J.F. The role of adipokines in health and disease. Biomedicines 2023, 11, 1290. [Google Scholar] [CrossRef]
- Datta, S.; Koka, S.; Boini, K.M. Understanding the role of adipokines in cardiometabolic dysfunction: A review of current knowledge. Biomolecules 2025, 15, 612. [Google Scholar] [CrossRef]
- Xia, M.F.; Chen, Y.; Lin, H.D.; Ma, H.; Li, X.M.; Aleteng, Q.; Li, Q.; Wang, D.; Hu, Y.; Pan, B.S.; et al. A indicator of visceral adipose dysfunction to evaluate metabolic health in adult Chinese. Sci. Rep. 2016, 6, 38214. [Google Scholar] [CrossRef]
- Li, H.H.; Wang, J.M.; Ji, Y.X.; Lin, L.; Li, S.W.; Cai, D.; Huang, S.; Hua, F.; Liu, X.Z. Association of visceral adiposity surrogates with impaired fasting glucose in nonobese individuals. Metab. Syndr. Relat. Disord. 2020, 18, 128–133. [Google Scholar] [CrossRef]
- Gu, D.; Ding, Y.; Zhao, Y.; Qu, Q. Visceral adiposity index was a useful predictor of prediabetes. Exp. Clin. Endocrinol. Diabetes 2018, 126, 596–603. [Google Scholar] [CrossRef]
- Ramdas Nayak, V.K.; Nayak, K.R.; Vidyasagar, S.; Rekha, P. Predictive performance of traditional and novel lipid combined anthropometric indices to identify prediabetes. Diabetes Metab. Syndr. 2020, 14, 1265–1272. [Google Scholar] [CrossRef]
- Ramírez-Vélez, R.; Pérez-Sousa, M.Á.; González-Ruíz, K.; Cano-Gutierrez, C.A.; Schmidt-RioValle, J.; Correa-Rodríguez, M.; Izquierdo, M.; Romero-García, J.A.; Campos-Rodríguez, A.Y.; Triana-Reina, H.R.; et al. Obesity- and lipid-related parameters in the identification of older adults with a high risk of prediabetes according to the American Diabetes Association: An analysis of the 2015 Health, Well-Being, and Aging Study. Nutrients 2019, 11, 2654. [Google Scholar] [CrossRef]
- Wang, D.; Fang, R.; Han, H.; Zhang, J.; Chen, K.; Fu, X.; He, Q.; Yang, Y. Association between visceral adiposity index and risk of prediabetes: A meta-analysis of observational studies. J. Diabetes Investig. 2022, 13, 543–551. [Google Scholar] [CrossRef]
- Huang, L.; Liao, J.; Lu, C.; Yin, Y.; Ma, Y.; Wen, Y. The non-linear relationship between the visceral adiposity index and the risk of prediabetes and diabetes. Front. Endocrinol. 2025, 16, 1407873. [Google Scholar] [CrossRef]
- Park, M.J.; Kang, M.; Jang, S.Y.; Jang, A.; Song, E.; Choi, K.M.; Baik, S.H.; Yoo, H.J. Sex- and age-specific body composition indices as predictors of new-onset type 2 diabetes mellitus in Koreans: A nationwide cohort study. BMJ Open 2025, 15, e093598. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Chen, L. Perspective from NHANES data: Synergistic effects of visceral adiposity index and lipid accumulation products on diabetes risk. Sci. Rep. 2025, 15, 258. [Google Scholar] [CrossRef] [PubMed]
- Benbaibeche, H.; Bounihi, A.; Saidi, H.; Koceir, E.A.; Khan, N.A. Cardiometabolic markers in Algerian obese subjects with and without type 2 diabetes: Adipocytokine imbalance as a risk factor. J. Clin. Med. 2025, 14, 1770. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Ortega, J.L.; Caballero-Vidal, J.; Yupari-Azabache, I.L.; Sevilla, J.M.A.; Conde-Parada, N.E. Predictive capacity of different indicators of adiposity for metabolic syndrome in adults in the city of Trujillo, Peru. Medicina 2025, 61, 419. [Google Scholar] [CrossRef]
- Ferreira, J.R.S.; Libardi, M.C.; do Prado, C.B.; Zandonade, E.; Bezerra, O.M.P.A.; Salaroli, L.B. Predicting metabolic syndrome by lipid accumulation product, visceral adiposity index and body roundness index in Brazilian rural workers. BMC Public Health 2025, 25, 544. [Google Scholar] [CrossRef]
- Li, J.; Qiao, J.; Li, Y.; Qin, G.; Xu, Y.; Lao, K.; Wang, Y.; Fan, Y.; Tang, P.; Han, L. Metabolic disorders in polycystic ovary syndrome: From gut microbiota biodiversity to clinical intervention. Front. Endocrinol. 2025, 16, 1526468. [Google Scholar] [CrossRef]
- Keyif, B.; Yavuzcan, A. Visceral and dysfunctional adiposity indices as predictors of insulin resistance and metabolic syndrome in women with polycystic ovary syndrome: A cross-sectional study. Medicina 2025, 61, 424. [Google Scholar] [CrossRef]
- Jafari, A.; Ilaghi, M.; Najafipour, H.; Shadkam, M. Assessing three novel composite anthropometric-metabolic indices for predicting 10-year incidence of metabolic syndrome: Findings from the kerman coronary artery disease risk factors study (KERCADRS). Hormones 2025, 1–9. [Google Scholar] [CrossRef]
- Jiang, K.; Luan, H.; Pu, X.; Wang, M.; Yin, J.; Gong, R. Association between visceral adiposity index and insulin resistance: A cross-sectional study based on US adults. Front. Endocrinol. 2022, 13, 921067. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.H.; Park, C.E.; Gi, M.Y.; Cha, J.A.; Moon, A.E.; Kang, J.K.; Seong, J.M.; Lee, J.H.; Yoon, H. The association of the visceral adiposity index with insulin resistance and beta-cell function in Korean adults with and without type 2 diabetes mellitus. Endocr. J. 2020, 67, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Štěpánek, L.; Horáková, D.; Cibičková, Ľ.; Vaverková, H.; Karásek, D.; Nakládalová, M.; Zapletalová, J. Can visceral adiposity index serve as a simple tool for identifying individuals with insulin resistance in daily clinical practice? Medicina 2019, 55, 545. [Google Scholar] [CrossRef] [PubMed]
- Habibullah, M.; Jemmieh, K.; Ouda, A.; Haider, M.Z.; Malki, M.I.; Elzouki, A.N. Metabolic-associated fatty liver disease: A selective review of pathogenesis, diagnostic approaches, and therapeutic strategies. Front. Med. 2024, 11, 1291501. [Google Scholar] [CrossRef]
- Eslam, M.; Sanyal, A.J.; George, J.; International Consensus Panel. MAFLD: A consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology 2020, 158, 1999–2014.e1. [Google Scholar] [CrossRef]
- Wang, Y.; Fu, C.; Jin, H.; Yang, Y.; Li, X.; Liu, K. Lipid metabolism indicators provide tools for the diagnosis of non-alcoholic fatty liver disease: Results of a nationwide survey. Front. Endocrinol. 2025, 15, 1468228. [Google Scholar] [CrossRef]
- Li, Q.; Wang, L.; Wu, J.; Wang, J.; Wang, Y.; Zeng, X. Role of age, gender and ethnicity in the association between visceral adiposity index and non-alcoholic fatty liver disease among US adults (NHANES 2003-2018): Cross-sectional study. BMJ Open 2022, 12, e058517. [Google Scholar] [CrossRef]
- Yi, X.; Zhu, S.; Zhu, L. Diagnostic accuracy of the visceral adiposity index in patients with metabolic-associated fatty liver disease: A meta-analysis. Lipids Health Dis. 2022, 21, 28. [Google Scholar] [CrossRef]
- Lajeunesse-Trempe, F.; Dugas, S.; Maltais-Payette, I.; Tremblay, È.J.; Piché, M.E.; Dimitriadis, G.K.; Lafortune, A.; Marceau, S.; Biertho, L.; Tchernof, A. Anthropometric indices and metabolic dysfunction-associated fatty liver disease in males and females living with severe obesity. Can. J. Gastroenterol. Hepatol. 2025, 2025, 5545227. [Google Scholar] [CrossRef]
- Dereziński, T.; Zozulińska-Ziółkiewicz, D.; Uruska, A.; Dąbrowski, M. Visceral adiposity index as a useful tool for the assessment of cardiometabolic disease risk in women aged 65 to 74. Diabetes Metab. Res. Rev. 2018, 34, e3052. [Google Scholar] [CrossRef]
- Tawfik, M.Y.; Mohamed, S.F.; Elotla, S.F. Association of novel visceral obesity indices with 10-year risk of major cardiovascular events in patients with type 2 diabetes mellitus. J. Egypt. Public Health Assoc. 2025, 100, 12. [Google Scholar] [CrossRef]
- Zhao, W.; Ma, X.; Ju, J.; Zhao, Y.; Wang, X.; Li, S.; Sui, Y.; Sun, Q. Association of visceral adiposity index with asymptomatic intracranial arterial stenosis: A population-based study in Shandong, China. Lipids Health Dis. 2023, 22, 64. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, Z.; Luo, N.; Qi, Y. Elevated visceral adiposity index is associated with increased stroke prevalence and earlier age at first stroke onset: Based on a national cross-sectional study. Front. Endocrinol. 2023, 13, 1086936. [Google Scholar] [CrossRef]
- Cui, C.; He, C.; Sun, Q.; Xu, Z.; Li, Q.; Yue, S.; Liu, J.; Wang, L.; Wang, H. Association between visceral adiposity index and incident stroke: Data from the China Health and Retirement Longitudinal Study. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 1202–1209. [Google Scholar] [CrossRef]
- Chang, Y.; Zhang, L.; Li, Y.; Wang, D.; Fang, Q.; Tang, X. Derivation and validation of a new visceral adiposity index for predicting short-term mortality of patients with acute ischemic stroke in a Chinese population. Brain Sci. 2023, 13, 297. [Google Scholar] [CrossRef]
- Zhang, X.; Shu, X.O.; Li, H.; Yang, G.; Xiang, Y.B.; Cai, Q.; Ji, B.T.; Gao, Y.T.; Zheng, W. Visceral adiposity and risk of coronary heart disease in relatively lean Chinese adults. Int. J. Cardiol. 2013, 168, 2141–2145. [Google Scholar] [CrossRef]
- Park, H.J.; Kim, J.; Park, S.E.; Park, C.Y.; Lee, W.Y.; Oh, K.W.; Park, S.W.; Rhee, E.J. Increased risk of subclinical atherosclerosis associated with high visceral adiposity index in apparently healthy Korean adults: The Kangbuk Samsung Health Study. Ann. Med. 2016, 48, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Fu, K.L.; Zhao, J.; Wang, Z.H.; Tang, M.X.; Wang, J.; Wang, H.; Zhang, Y.; Zhang, W.; Zhong, M. Visceral adiposity index score indicated the severity of coronary heart disease in Chinese adults. Diabetol. Metab. Syndr. 2014, 6, 143. [Google Scholar] [CrossRef] [PubMed]
- Candemir, M.; Kiziltunç, E.; Candemir, B.; Nurkoç, S.; Şahinarslan, A. Visceral adiposity index is associated with the increased Syntax score in patients with type 2 diabetes mellitus. Metab. Syndr. Relat. Disord. 2022, 20, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Biswas, E.; Choudhury, A.K.; Amin, M.R.; Khalequzzaman, M.; Chowdhury, S.; Kabir, F.I.; Sakib, M.M.; Mahabub, E.E.; Singha, C.K. Visceral adiposity index score is the better predictor of clinical and coronary angiographic severity assessment than other adiposity indices in patients with acute coronary syndrome. Mymensingh. Med. J. 2019, 28, 382–388. [Google Scholar]
- Doganay, B.; Celebi, O.O. Association between the visceral adiposity index and the coronary artery calcification score and atherosclerotic plaque morphology. Kardiol. Pol. 2023, 81, 716–725. [Google Scholar] [CrossRef]
- Bagyura, Z.; Kiss, L.; Lux, Á.; Csobay-Novák, C.; Jermendy, Á.L.; Polgár, L.; Szelid, Z.; Soós, P.; Merkely, B. Association between coronary atherosclerosis and visceral adiposity index. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Cai, B.; Jin, W. Association between two novel visceral obesity indicators and heart failure among US adults: A cross-sectional study. Metab. Syndr. Relat. Disord. 2025, 23, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Guo, Y.; Zhang, S.; Lai, Y.; Huang, M.; Zhan, R.; Liu, M.; Xiong, Z.; Huang, Y.; Huang, R.; et al. Visceral adiposity index and the risk of heart failure, late-life cardiac structure, and function in ARIC study. Eur. J. Prev. Cardiol. 2023, 30, 1182–1192. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Lin, S.; Liu, Z.; Yuan, J.; Ren, H.; Tan, H.; Guo, Y.; Jiang, X. Metabolic syndrome, left ventricular diastolic dysfunction and heart failure with preserved ejective fraction. Front. Endocrinol. 2025, 16, 1544908. [Google Scholar] [CrossRef]
- Lockwood, F.; Lachaux, M.; Harouki, N.; Soulié, M.; Nicol, L.; Renet, S.; Dumesnil, A.; Vercauteren, M.; Bellien, J.; Iglarz, M.; et al. Dual ETA-ETB receptor antagonism improves metabolic syndrome-induced heart failure with preserved ejection fraction. Fundam. Clin. Pharmacol. 2025, 39, e70006. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, C.; Yang, C.; Qiao, W.; Liu, Y.; Liu, S.; Dong, G. A rat model of metabolic syndrome-related heart failure with preserved ejection fraction phenotype: Pathological alterations and possible molecular mechanisms. Front. Cardiovasc. Med. 2023, 10, 1208370. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, Y.; Li, Y.; Wang, C.; Wang, Y.; Dong, M.; Xiao, J.; Lin, Z.; Lu, H.; Ji, X. Association between visceral adiposity index and heart failure: A cross-sectional study. Clin. Cardiol. 2023, 46, 310–319. [Google Scholar] [CrossRef]
- Wu, M.; Lai, W.; Huo, X.; Wang, Q.; Zhou, Y.; Gao, D. Association of visceral adiposity index (VAI) with prognosis in patients with metabolic syndrome and heart failure with reduced ejection fraction. BMC Cardiovasc. Disord. 2025, 25, 160. [Google Scholar] [CrossRef]
- Vogel, P.; Stein, A.; Marcadenti, A. Visceral adiposity index and prognosis among patients with ischemic heart failure. Sao Paulo Med. J. 2016, 134, 211–218. [Google Scholar] [CrossRef]
- Yurista, S.R.; Eder, R.A.; Butsch, W.S.; Luptak, I. Do weight loss interventions challenge the obesity paradox in heart failure? Trends Endocrinol. Metab. 2025, 36, 295–297. [Google Scholar] [CrossRef]
- Merkel, E.D.; Behon, A.; Masszi, R.; Schwertner, W.R.; Kuthi, L.; Veres, B.; Osztheimer, I.; Papp, R.; Molnár, L.; Zima, E.; et al. Obesity paradox in patients with reduced ejection fraction eligible for device implantation—An observational study. ESC Heart Fail. 2024, 11, 3616–3625. [Google Scholar] [CrossRef]
- Miura, Y.; Higuchi, S.; Kohno, T.; Shiraishi, Y.; Kitamura, M.; Nagatomo, Y.; Kawakubo Ichihara, Y.; Mizuno, A.; Nakano, S.; Soejima, K.; et al. Cachectic biomarkers as confounders behind the obesity paradox in patients with acute decompensated heart failure. Int. J. Obes. 2025, 49, 888–895. [Google Scholar] [CrossRef]
- Shi, Y.; Yu, C.; Hu, L.; Li, M.; Zhou, W.; Wang, T.; Zhu, L.; Bao, H.; Li, P.; Cheng, X. Visceral adiposity index and sex differences in relation to peripheral artery disease in normal-weight adults with hypertension. Biol. Sex Differ. 2022, 13, 22. [Google Scholar] [CrossRef]
- Zierfuss, B.; Höbaus, C.; Herz, C.T.; Pesau, G.; Koppensteiner, R.; Schernthaner, G.H. Predictive power of novel and established obesity indices for outcome in PAD during a five-year follow-up. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 1179–1187. [Google Scholar] [CrossRef]
- Ozkan, U.; Gurdogan, M. Novel predictor of the AF development in patients with OSAS: Importance of visceral adipose index. Medeni Med. J. 2023, 38, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Engin, M.; Ozsin, K.K.; Savran, M.; Guvenc, O.; Yavuz, S.; Ozyazicioglu, A.F. Visceral adiposity index and prognostic nutritional index in predicting atrial fibrillation after on-pump coronary artery bypass operations: A prospective study. Braz. J. Cardiovasc. Surg. 2021, 36, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jin, X.; Chen, K.; Yan, W.; Wang, A.; Zhu, B.; Wang, W.; Gao, Z.; Tang, X.; Yan, L.; et al. Visceral adiposity index is closely associated with urinary albumin-creatinine ratio in the Chinese population with prediabetes. Diabetes Metab. Res. Rev. 2021, 37, e3424. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Yu, S.; Kang, X.; Liu, Y.; Xu, Z.; Li, Z.; Wang, J.; Miao, X.; Liu, X.; Li, X.; et al. Association of visceral adiposity index with incident nephropathy and retinopathy: A cohort study in the diabetic population. Cardiovasc. Diabetol. 2022, 21, 32. [Google Scholar] [CrossRef]
- Zhao, X.; Sun, J.; Xin, S.; Zhang, X. Study on the association between visceral adiposity index and diabetic kidney disease in hospitalized patients with type 2 diabetes mellitus in China. Front. Endocrinol. 2025, 16, 1549954. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, Y.; Yang, S.; He, P.; Wu, Q.; Ye, Z.; Liu, M.; Zhang, Y.; Li, R.; Liu, C.; et al. Associations between visceral adiposity index and incident nephropathy outcomes in diabetic patients: Insights from the ACCORD trial. Diabetes Metab. Res. Rev. 2022, 39, e3602. [Google Scholar] [CrossRef]
| Parameter | Definition |
|---|---|
| BAI | |
| BRI | |
| BSI | |
| BeWI |
| Pro-Inflammatory Adipokines | Anti-Inflammatory Adipokines |
|---|---|
| leptin, visfatin, chemerin, resistin, osteopontin, retinol-binding protein 4 (RBP-4), angiopoetin-like protein (ANGPTL), tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6) | adiponectin, omentin, apelin, nesfatin-1, isthmin-1, secreted frizzled-related proteins (SFRPs), C1q/TNF-related protein (CTRP) family, myeloid-derived growth factor (MYDGF) |
| Subject Matter | The Most Important Conclusions |
|---|---|
| Relationship between the visceral adiposity index (VAI) and the risk of metabolic disorders | The studies conducted so far do not provide convincing evidence of a relationship between the VAI and the risk of prediabetes [47,48,49,50,51]. |
| There is a clear positive association between the VAI and the risk of diabetes mellitus [52,53,54]. | |
| The VAI is valuable in assessing the risk of metabolic syndrome [55,56,57,59]. | |
| Relationship between the VAI and cardiovascular diseases | A higher VAI indicates an increased risk of asymptomatic intracranial arterial stenosis [72], risk of stroke [73,74], and a worse prognosis [75]. |
| According to most studies, the VAI correlates well with the incidence of coronary heart disease (CHD) and the advancement of atherosclerotic lesions [76,77,78,79,80,81,82]. | |
| An elevated VAI indicates an increased risk of heart failure, especially with preserved ejection fraction [83,84,88]. The prognostic value is ambiguous [89,90]. | |
| The VAI has no evidence of utility in assessing the risk of peripheral arterial disease (PAD), nor is there a significant prognostic value of the VAI in patients already diagnosed with PAD [94,95]. | |
| The VAI correlates with risk of atrial fibrillation in patients with obstructive sleep apnea [96] and in patients undergoing surgical myocardial revascularization [97]. | |
| Relationship between the VAI and diabetic kidney disease | The VAI is closely related to the risk of diabetic kidney disease [98,99,100,101]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jakubiak, G.K.; Badicu, G.; Surma, S.; Waluga-Kozłowska, E.; Chwalba, A.; Pawlas, N. The Visceral Adiposity Index and Its Usefulness in the Prediction of Cardiometabolic Disorders. Nutrients 2025, 17, 2374. https://doi.org/10.3390/nu17142374
Jakubiak GK, Badicu G, Surma S, Waluga-Kozłowska E, Chwalba A, Pawlas N. The Visceral Adiposity Index and Its Usefulness in the Prediction of Cardiometabolic Disorders. Nutrients. 2025; 17(14):2374. https://doi.org/10.3390/nu17142374
Chicago/Turabian StyleJakubiak, Grzegorz K., Georgian Badicu, Stanisław Surma, Ewa Waluga-Kozłowska, Artur Chwalba, and Natalia Pawlas. 2025. "The Visceral Adiposity Index and Its Usefulness in the Prediction of Cardiometabolic Disorders" Nutrients 17, no. 14: 2374. https://doi.org/10.3390/nu17142374
APA StyleJakubiak, G. K., Badicu, G., Surma, S., Waluga-Kozłowska, E., Chwalba, A., & Pawlas, N. (2025). The Visceral Adiposity Index and Its Usefulness in the Prediction of Cardiometabolic Disorders. Nutrients, 17(14), 2374. https://doi.org/10.3390/nu17142374









