Gut Microbiota Shifts After a Weight Loss Program in Adults with Obesity: The WLM3P Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Trial Design
2.2. Participants
2.3. Dietary Interventions
2.3.1. WLM3P (Intervention Group)
2.3.2. LCD (Active Control Group)
2.4. Body Measurements and Composition
2.4.1. Biochemical Measurements
2.4.2. Fecal Sample Collection and Sequencing of Gut Microbiota
2.5. Statistical Analysis
3. Results
3.1. Baseline Data and Effect of the WLMP3P Intervention Compared with Control Group on the Main Anthropometric and Biochemical Variables After 6 Months: Mixed-Effects Model Results
3.2. Comparison of Gut Microbiota Composition Between WLM3P and LCD Groups
3.3. Analysis of the Differential Microbiota Abundances at Genus Level Between WLM3P and LCD Groups After 6 Months of Intervention
3.4. Association Analysis Between Gut Microbiota and Anthropometric and Biochemical Changes After 6 Months in WLM3P and LCD Groups
3.5. Linear Regression Analysis for the Relationship Between Gut Microbiota Composition and Body Composition Variables in Each Group of Study
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ng, M.; Gakidou, E.; Lo, J.; Abate, Y.H.; Abbafati, C.; Abbas, N.; Abbasian, M.; Abd ElHafeez, S.; Abdel-Rahman, W.M.; Abd-Elsalam, S.; et al. Global, Regional, and National Prevalence of Adult Overweight and Obesity, 1990–2021, with Forecasts to 2050: A Forecasting Study for the Global Burden of Disease Study 2021. Lancet 2025, 405, 813–838. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.D.; Ryan, D.H.; Apovian, C.M.; Ard, J.D.; Comuzzie, A.G.; Donato, K.A.; Hu, F.B.; Hubbard, V.S.; Jakicic, J.M.; Kushner, R.F.; et al. 2013 AHA/ACC/TOS Guideline for the Management of Overweight and Obesity in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Obesity Society. Circulation 2014, 129, S102–S138. [Google Scholar] [CrossRef] [PubMed]
- Durrer Schutz, D.; Busetto, L.; Dicker, D.; Farpour-Lambert, N.; Pryke, R.; Toplak, H.; Widmer, D.; Yumuk, V.; Schutz, Y. European Practical and Patient-Centred Guidelines for Adult Obesity Management in Primary Care. Obes. Facts 2019, 12, 40–66. [Google Scholar] [CrossRef] [PubMed]
- Zsálig, D.; Berta, A.; Tóth, V.; Szabó, Z.; Simon, K.; Figler, M.; Pusztafalvi, H.; Polyák, É. A Review of the Relationship between Gut Microbiome and Obesity. Appl. Sci. 2023, 13, 610. [Google Scholar] [CrossRef]
- Purdel, C.; Margină, D.; Adam-Dima, I.; Ungurianu, A. The Beneficial Effects of Dietary Interventions on Gut Microbiota-An Up-to-Date Critical Review and Future Perspectives. Nutrients 2023, 15, 5005. [Google Scholar] [CrossRef] [PubMed]
- Lou, X.; Li, P.; Luo, X.; Lei, Z.; Liu, X.; Liu, Y.; Gao, L.; Xu, W.; Liu, X. Dietary Patterns Interfere with Gut Microbiota to Combat Obesity. Front. Nutr. 2024, 11, 1387394. [Google Scholar] [CrossRef] [PubMed]
- Maioli, T.U.; Borras-Nogues, E.; Torres, L.; Barbosa, S.C.; Martins, V.D.; Langella, P.; Azevedo, V.A.; Chatel, J.M. Possible Benefits of Faecalibacterium Prausnitzii for Obesity-Associated Gut Disorders. Front. Pharmacol. 2021, 12, 740636. [Google Scholar] [CrossRef] [PubMed]
- Jian, C.; Silvestre, M.P.; Middleton, D.; Korpela, K.; Jalo, E.; Broderick, D.; de Vos, W.M.; Fogelholm, M.; Taylor, M.W.; Raben, A.; et al. Gut Microbiota Predicts Body Fat Change Following a Low-Energy Diet: A PREVIEW Intervention Study. Genome Med. 2022, 14, 54. [Google Scholar] [CrossRef] [PubMed]
- Dong, T.S.; Luu, K.; Lagishetty, V.; Sedighian, F.; Woo, S.L.; Dreskin, B.W.; Katzka, W.; Chang, C.; Zhou, Y.; Arias-Jayo, N.; et al. A High Protein Calorie Restriction Diet Alters the Gut Microbiome in Obesity. Nutrients 2020, 12, 3221. [Google Scholar] [CrossRef] [PubMed]
- Kahleova, H.; Rembert, E.; Alwarith, J.; Yonas, W.N.; Tura, A.; Holubkov, R.; Agnello, M.; Chutkan, R.; Barnard, N.D. Effects of a Low-Fat Vegan Diet on Gut Microbiota in Overweight Individuals and Relationships with Body Weight, Body Composition, and Insulin Sensitivity. A Randomized Clinical Trial. Nutrients 2020, 12, 2917. [Google Scholar] [CrossRef] [PubMed]
- Ang, W.S.; Law, J.W.F.; Letchumanan, V.; Hong, K.W.; Wong, S.H.; Ab Mutalib, N.S.; Chan, K.G.; Lee, L.H.; Tan, L.T.H. A Keystone Gut Bacterium Christensenella Minuta—A Potential Biotherapeutic Agent for Obesity and Associated Metabolic Diseases. Foods 2023, 12, 2485. [Google Scholar] [CrossRef] [PubMed]
- Koutoukidis, D.A.; Jebb, S.A.; Zimmerman, M.; Otunla, A.; Henry, J.A.; Ferrey, A.; Schofield, E.; Kinton, J.; Aveyard, P.; Marchesi, J.R. The Association of Weight Loss with Changes in the Gut Microbiota Diversity, Composition, and Intestinal Permeability: A Systematic Review and Meta-Analysis. Gut Microbes 2022, 14, e2020068. [Google Scholar] [CrossRef] [PubMed]
- Stanislawski, M.A.; Frank, D.N.; Borengasser, S.J.; Ostendorf, D.M.; Ir, D.; Jambal, P.; Bing, K.; Wayland, L.; Siebert, J.C.; Bessesen, D.H.; et al. The Gut Microbiota during a Behavioral Weight Loss Intervention. Nutrients 2021, 13, 3248. [Google Scholar] [CrossRef] [PubMed]
- Kumbhare, S.V.; Pedroso, I.; Joshi, B.; Muthukumar, K.M.; Saravanan, S.K.; Irudayanathan, C.; Kochhar, G.S.; Dulai, P.S.; Sinha, R.; Almonacid, D.E. Longitudinal Gut Microbial Signals Are Associated with Weight Loss: Insights from a Digital Therapeutics Program. Front. Nutr. 2024, 11, 1363079. [Google Scholar] [CrossRef] [PubMed]
- Scott, K.P.; Gratz, S.W.; Sheridan, P.O.; Flint, H.J.; Duncan, S.H. The Influence of Diet on the Gut Microbiota. Pharmacol. Res. 2013, 69, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Deehan, E.C.; Yang, C.; Perez-Muñoz, M.E.; Nguyen, N.K.; Cheng, C.C.; Triador, L.; Zhang, Z.; Bakal, J.A.; Walter, J. Precision Microbiome Modulation with Discrete Dietary Fiber Structures Directs Short-Chain Fatty Acid Production. Cell Host Microbe 2020, 27, 389–404.e6. [Google Scholar] [CrossRef] [PubMed]
- Duncan, S.H.; Belenguer, A.; Holtrop, G.; Johnstone, A.M.; Flint, H.J.; Lobley, G.E. Reduced Dietary Intake of Carbohydrates by Obese Subjects Results in Decreased Concentrations of Butyrate and Butyrate-Producing Bacteria in Feces. Appl. Environ. Microbiol. 2007, 73, 1073–1078. [Google Scholar] [CrossRef] [PubMed]
- Ang, Q.Y.; Alexander, M.; Newman, J.C.; Tian, Y.; Cai, J.; Upadhyay, V.; Turnbaugh, J.A.; Verdin, E.; Hall, K.D.; Leibel, R.L.; et al. Ketogenic Diets Alter the Gut Microbiome Resulting in Decreased Intestinal Th17 Cells. Cell 2020, 181, 1263–1275.e16. [Google Scholar] [CrossRef] [PubMed]
- Russell, W.R.; Gratz, S.W.; Duncan, S.H.; Holtrop, G.; Ince, J.; Scobbie, L.; Duncan, G.; Johnstone, A.M.; Lobley, G.E.; Wallace, R.J.; et al. High-Protein, Reduced-Carbohydrate Weight-Loss Diets Promote Metabolite Profiles Likely to Be Detrimental to Colonic Health. Am. J. Clin. Nutr. 2011, 93, 1062–1072. [Google Scholar] [CrossRef] [PubMed]
- Paukkonen, I.; Törrönen, E.N.; Lok, J.; Schwab, U.; El-Nezami, H. The Impact of Intermittent Fasting on Gut Microbiota: A Systematic Review of Human Studies. Front. Nutr. 2024, 11, 1342787. [Google Scholar] [CrossRef] [PubMed]
- Pieczyńska-Zając, J.M.; Malinowska, A.; Łagowska, K.; Leciejewska, N.; Bajerska, J. The Effects of Time-Restricted Eating and Ramadan Fasting on Gut Microbiota Composition: A Systematic Review of Human and Animal Studies. Nutr. Rev. 2024, 82, 777–793. [Google Scholar] [CrossRef] [PubMed]
- Pereira, V.; Barreiros-Mota, I.; Cortez, F.; Castela, I.; Teixeira, D.; Calhau, C.; Camila Dias, C.; Moreira-Rosário, A.; Silvestre, M.P. A Randomized Controlled Trial of a Weight Loss Maintenance Program in Adults with Obesity: The WLM3P Study. Eur. J. Clin. Nutr. 2024, 78, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, S.S.P.; Franchini, B.M.; De Pinho, I.S.M.; Graça, A.P.S.R. The Portuguese Mediterranean Diet Wheel: Development Considerations. Br. J. Nutr. 2022, 128, 1315–1321. [Google Scholar] [CrossRef] [PubMed]
- WHO. Obesity: Preventig and Managing the Global Pandemic. In WHO Technical Report Series; World Health Organization: Geneva, Switzerland, 2000; Volume 894. [Google Scholar]
- Direção-Geral da Saúde. Avaliação Antropométrica No Adulto. Orientação no 017/2013 de 05/12/2013, 2013, 1–9. Available online: https://www.lexpoint.pt/Default.aspx?Tag=CONTENT&ContentId=52824 (accessed on 16 March 2025).
- Feng, Y.; Zhu, J.; Wang, Q.; Cao, H.; He, F.; Guan, Y.; Li, D.; Yan, J.; Yang, J.; Xia, Y.; et al. White Common Bean Extract Remodels the Gut Microbiota and Ameliorates Type 2 Diabetes and Its Complications: A Randomized Double-Blinded Placebo-Controlled Trial. Front. Endocrinol. 2022, 13, 999715. [Google Scholar] [CrossRef] [PubMed]
- Santamarina, A.B.; Filho, V.N.; de Freitas, J.A.; Franco, L.A.M.; Martins, R.C.; Fonseca, J.V.; Orellana Turri, J.A.; Hufnagel, M.T.; Demarque, D.P.; da Silva, B.F.R.B.; et al. Nutraceutical Blends Promote Weight Loss, Inflammation Reduction, and Better Sleep: The Role of Faecalibacterium Prausnitzii in Overweight Adults-A Double-Blind Trial. Mol. Nutr. Food Res. 2025, e202400806. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Irajizad, E.; Hoffman, K.L.; Fahrmann, J.F.; Li, F.; Seo, Y.D.; Browman, G.J.; Dennison, J.B.; Vykoukal, J.; Luna, P.N.; et al. Modulating a Prebiotic Food Source Influences Inflammation and Immune-Regulating Gut Microbes and Metabolites: Insights from the BE GONE Trial. EBioMedicine 2023, 98, 104873. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Wang, J.H.; Shin, J.H.; Lee, D.; Lee, S.N.; Seo, J.G.; Shin, J.H.; Nam, Y.D.; Kim, H.; Sun, X. Pharmaceutical Efficacy of Novel Human-Origin Faecalibacterium Prausnitzii Strains on High-Fat-Diet-Induced Obesity and Associated Metabolic Disorders in Mice. Front. Endocrinol. 2023, 14, 1220044. [Google Scholar] [CrossRef] [PubMed]
- Mohr, A.E.; Sweazea, K.L.; Bowes, D.A.; Jasbi, P.; Whisner, C.M.; Sears, D.D.; Krajmalnik-Brown, R.; Jin, Y.; Gu, H.; Klein-Seetharaman, J.; et al. Gut Microbiome Remodeling and Metabolomic Profile Improves in Response to Protein Pacing with Intermittent Fasting versus Continuous Caloric Restriction. Nat. Commun. 2024, 15, 4155. [Google Scholar] [CrossRef] [PubMed]
- Hibberd, A.A.; Yde, C.C.; Ziegler, M.L.; Honoré, A.H.; Saarinen, M.T.; Lahtinen, S.; Stahl, B.; Jensen, H.M.; Stenman, L.K. Probiotic or Synbiotic Alters the Gut Microbiota and Metabolism in a Randomised Controlled Trial of Weight Management in Overweight Adults. Benef. Microbes 2019, 10, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Chansa, O.; Shantavasinkul, P.C.; Monsuwan, W.; Sirivarasai, J. Association between Gut Microbiota Profiles, Dietary Intake, and Inflammatory Markers in Overweight and Obese Women. Foods 2024, 13, 2592. [Google Scholar] [CrossRef] [PubMed]
- Muñiz Pedrogo, D.A.; Jensen, M.D.; Van Dyke, C.T.; Murray, J.A.; Woods, J.A.; Chen, J.; Kashyap, P.C.; Nehra, V. Gut Microbial Carbohydrate Metabolism Hinders Weight Loss in Overweight Adults Undergoing Lifestyle Intervention With a Volumetric Diet. Mayo Clin. Proc. 2018, 93, 1104–1110. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Li, D.; He, Y.; Li, Y.; Yang, Z.; Zhao, X.; Liu, Y.; Wang, Y.; Sun, J.; Feng, X.; et al. Discrepant Gut Microbiota Markers for the Classification of Obesity-Related Metabolic Abnormalities. Sci. Rep. 2019, 9, 13424. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.A.; Karavetian, M.; Moubareck, C.A.; Wazz, G.; Mahdy, T.; Venema, K. Association of the Gut Microbiota with Clinical Variables in Obese and Lean Emirati Subjects. Front. Microbiol. 2023, 14, 1182460. [Google Scholar] [CrossRef] [PubMed]
- Langley, G.; Hao, Y.; Pondo, T.; Miller, L.; Petit, S.; Thomas, A.; Lindegren, M.L.; Farley, M.M.; Dumyati, G.; Como-Sabetti, K.; et al. The Impact of Obesity and Diabetes on the Risk of Disease and Death Due to Invasive Group A Streptococcus Infections in Adults. Clin. Infect. Dis. 2016, 62, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Chanda, W.; Jiang, H.; Liu, S.-J. The Ambiguous Correlation of Blautia with Obesity: A Systematic Review. Microorganisms 2024, 12, 1768. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, M.; Miyai, N.; Zhang, Y.; Sakamoto, Y.; Terada, K.; Utsumi, M.; Takeshita, T.; Arita, M. The Gut Microbiota Genus Blautia Is Associated with Skeletal Muscle Mass Reduction in Community-Dwelling Older Japanese Adults: The Wakayama Study. Eur. Geriatr. Med. 2024, 16, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, L.; Yang, F.; Hu, Q.; Xiong, D. Correlation between Gut Bacteria Phascolarctobacterium and Exogenous Metabolite α-Linolenic Acid in T2DM: A Case-Control Study. Ann. Transl. Med. 2022, 10, 1056. [Google Scholar] [CrossRef] [PubMed]
- Precup, G.; Vodnar, D.C. Gut Prevotella as a Possible Biomarker of Diet and Its Eubiotic versus Dysbiotic Roles: A Comprehensive Literature Review. Br. J. Nutr. 2019, 122, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Abuqwider, J.N.; Mauriello, G.; Altamimi, M. Akkermansia Muciniphila, a New Generation of Beneficial Microbiota in Modulating Obesity: A Systematic Review. Microorganisms 2021, 9, 1098. [Google Scholar] [CrossRef] [PubMed]
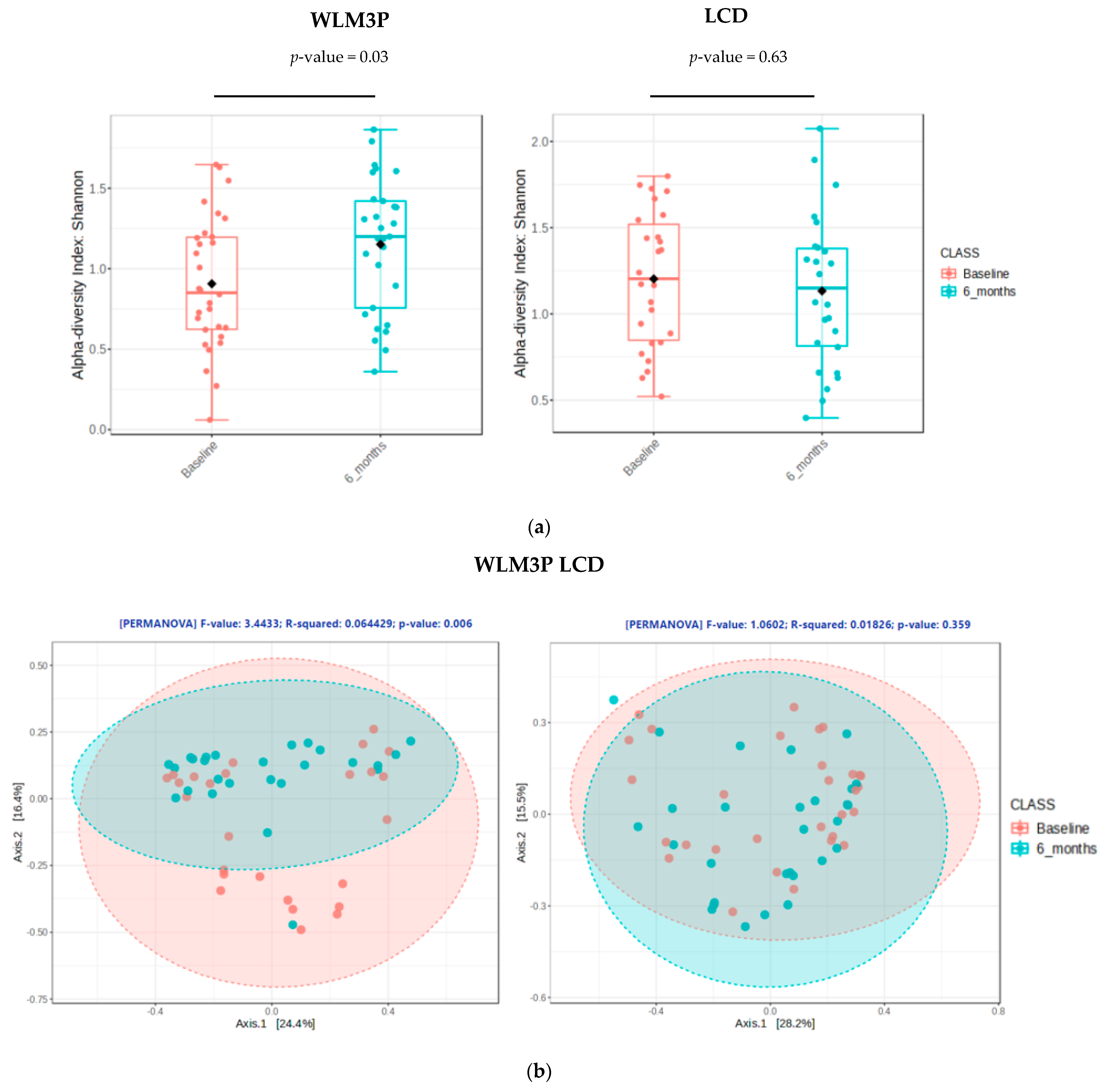
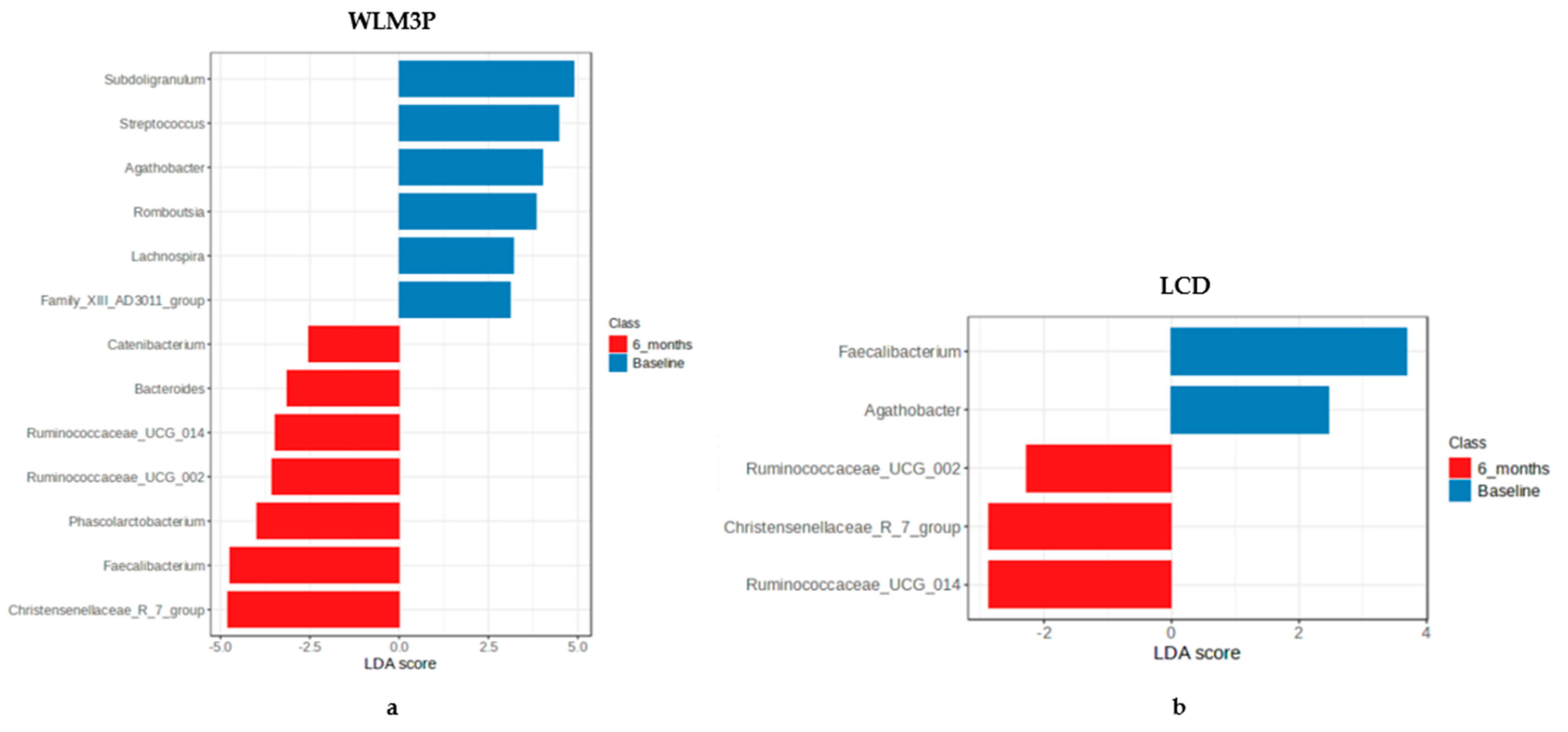
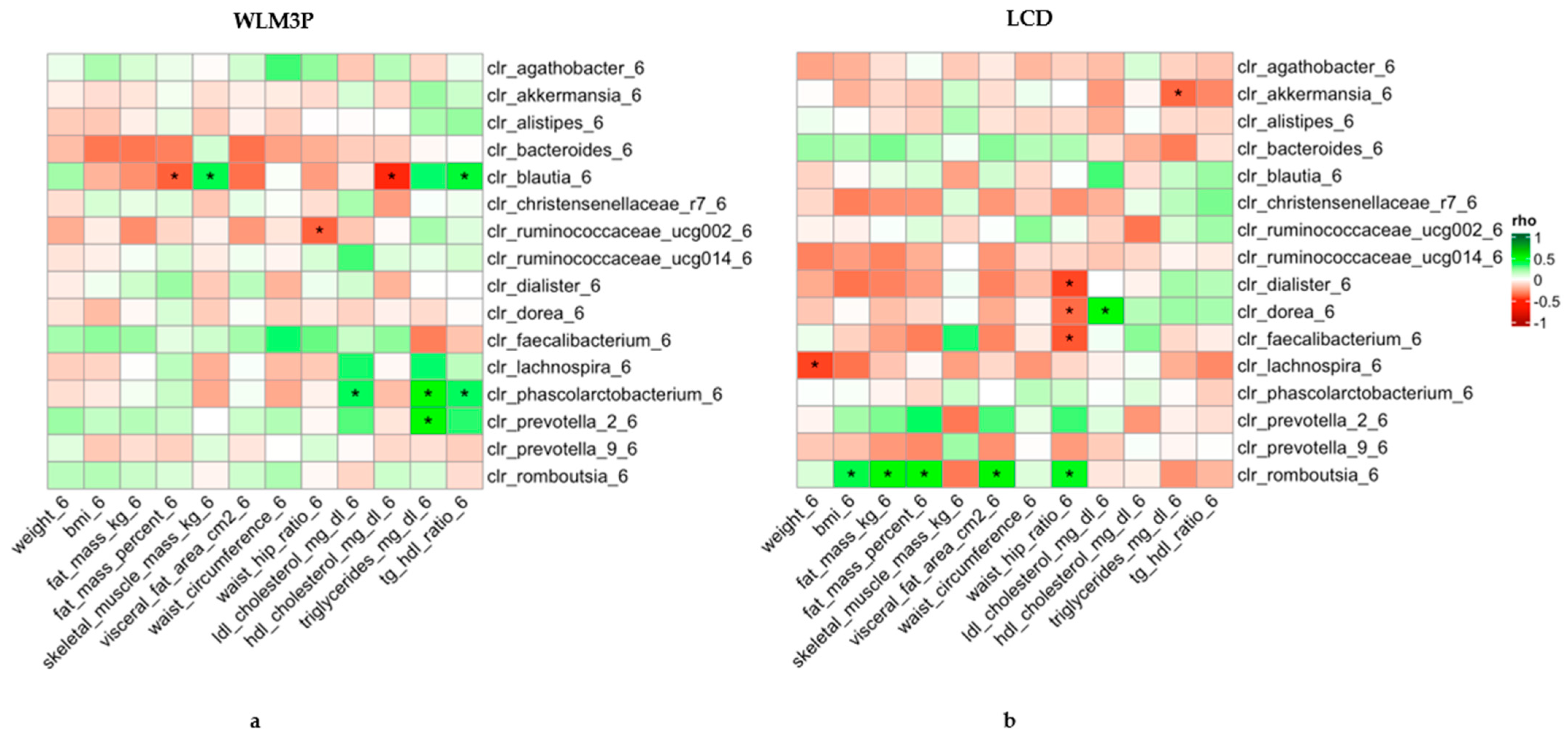
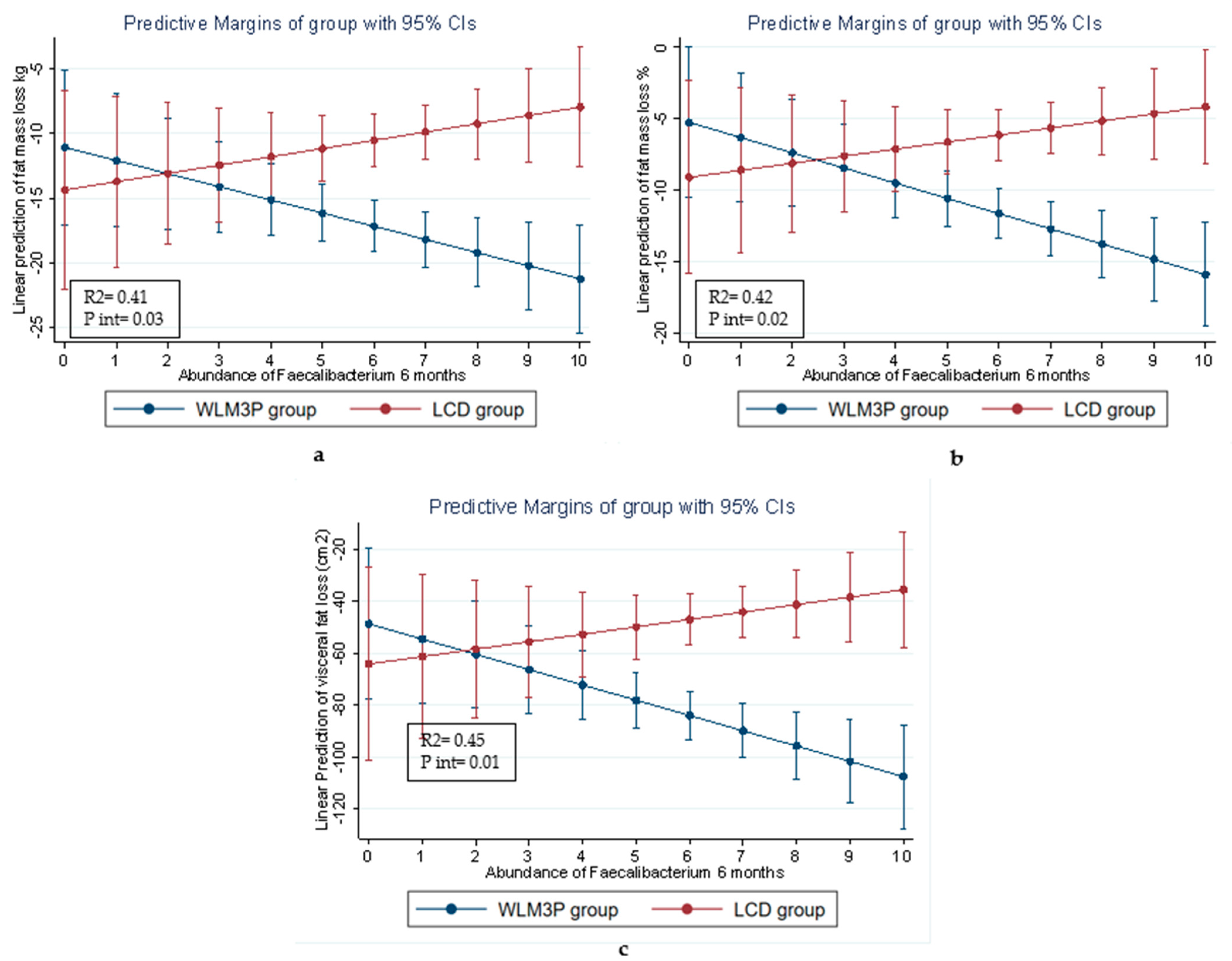
| Variables | WLM3P Group (CI) | LCD Group (CI) | Δ Groups (CI) | p-Value | Intervention Time |
|---|---|---|---|---|---|
| Weight (kg) | |||||
| Baseline | 97.1 (93.3; 100.9) | 96.4 (92.6; 100.2) | 0.73 (−4.70; 6.15) | 0.79 | <0.001 |
| 6 months | 76.5 (73.1; 80.8) | 83.4 (79.6; 87.3) | −6.50 (−11.93; −1.07) | 0.01 | |
| Δ visit | −20.6 (−17.9; −22.4) | −12.9 (−10.7; −15.2) | |||
| BMI (Kg/m2) | |||||
| Baseline | 34.1 (33.3; 34.9) | 34.2 (33.4; 34.9) | −0.12 (−1.20; −0.96) | 0.82 | <0.001 |
| 6 months | 27.0 (26.3; 27.8) | 29.6 (28.8; 30.4) | −2.61 (−3.69; −1.52) | <0.001 | |
| Δ visit | −7.1 (−6.3; −7.8) | −4.6 (−3.9; −5.3) | |||
| Fat mass (Kg) | |||||
| Baseline | 41.5(39.1; 44.0) | 41.1 (38.6; 43.5) | 0.46 (−3.02; 3.94) | 0.79 | <0.001 |
| 6 months | 24.5 (22.0; 26.9) | 30.6 (28.1; 33.0) | −6.09 (−9.57; −2.61) | <0.001 | |
| Δ visit | −17.0 (−14.9; −19.1) | −10.5 (−8.4; −12.5) | |||
| Fat mass (%) | |||||
| Baseline | 43.3 (40.7; 45.8) | 42.9 (40.3; 45.5) | |||
| 6 months | 31.7 (29.1; 34.3) | 36.8 (34.2; 39.3) | 0.39 (−3.25; 4.03) | 0.79 | <0.001 |
| Δ visit | −11.6 (−9.7; −13.4) | −6.11 (−4.3; −7.9) | −5.06 (−8.70; −1.42) | 0.07 | |
| Skeletal muscle mass (kg) | |||||
| Baseline | 30.9 (28.8; 33.0) | 30.9 (28.8; 33.0) | |||
| 6 months | 28.8 (26.8; 31.0) | 29.3 (27.2; 31.4) | −0.03 (−3.00; 2.94) | 0.98 | 0.14 |
| Δ visit | −2.0 (1.6; −2.4) | −1.6 (1.2; −2.0) | −0.42 (−3.39; 2.56) | 0.78 | |
| Visceral fat (cm2) | |||||
| Baseline | 200.3 (186.2; 213.8) | 193.8 (180.3; 207.3) | |||
| 6 months | 116.6 (103.1; 130.1) | 147.3 (133.8; 160.8) | 6.46 (−12.62; 25.55) | 0.51 | <0.001 |
| Δ visit | −87.7(−73.9; −93.5) | −46.5 (−36.7; −56.3) | −30.70 (−49.78; −11.61) | <0.001 | |
| Waist (cm) | |||||
| Baseline | 100.3 (97.2; 103.4) | 103.7 (100.6; 106.8) | −3.38 (−7.78; 1.02) | ||
| 6 months | 83.6 (80.7; 87.0) | 92.8 (89.6; 95.9) | −8.93 (−13.34; −4.52) | 0.13 | <0.001 |
| Δ visit | −16.5 (−14.5; −18.4) | −10.9 (−8.9; −12.9) | <0.001 | ||
| Waist-to-hip ratio | |||||
| Baseline | 1.04 (0.99; 1.04) | 0.99 (0.97; 1.01) | 0.03 (−0.01; 0.06) | 0.11 | <0.001 |
| 6 months | 0.91 (0.88; 0.93) | 0.94 (0.92; 0.96) | −0.04 (−0.07; −0.01) | 0.02 | |
| Δ visit | −0.1 (−0.1; −0.1) | −0.1 (−0.0; −0.1) | |||
| LDL cholesterol (mg/dL) | |||||
| Baseline | 114.0 (101.6; 126.5) | 123.6 (111.2; 136.1) | −9.60 (−27.20; 8.00) | 0.28 | 0.41 |
| 6 months | 114.0 (101.5; 126.4) | 118.3 (105.9; 130.8) | −4.37 (−21.97; 13.23) | 0.62 | |
| Δ visit | 0.03 (−8.94; 9.00) | −5.27 (−14.24; 3.70) | |||
| HDL cholesterol (mg/dL) | |||||
| Baseline | 47.9 (43.6; 52.2) | 52.4 (48.1; 56.6) | 4.47 (10.51; 1.58) | 0.15 | 0.16 |
| 6 months | 56.3 (52.1; 60.6) | 58.0 (53.7; 62.3) | 1.70 (−4.34; 7.74) | 0.58 | |
| Δ visit | 8.4 (11.14; 5.66) | 5.6 (8.4; 2.9) | |||
| Triglycerides (mg/dL) | |||||
| Baseline | 129.1 (106.2; 151.9) | 130.4 (107.5; 153.2) | −1.30 (−33.62; 31.02) | 0.93 | 0.71 |
| 6 months | 81.6 (58.8; 104.5) | 89.3 (66.4; 112.1) | −7.63 (−39.96; 24.69) | 0.64 | |
| Δ visit | −47.4 (−71.6; 23.2) | −41.1 (−65.3; 16.9) | |||
| Tg/HDL ratio | |||||
| Baseline | 3.0 (2.3; 3.7) | 2.7 (2.0; 3.4) | 0.28 (−0.72; 1.28) | 0.58 | 0.57 |
| 6 months | 1.6 (0.9; 2.3) | 1.6 (0.9; 2.4) | −0.03 (−1.03; 0.97) | 0.95 | |
| Δ visit | −1.4 (−2.2; 0.6) | −1.1 (−1.9; 0.3) |
| Name | log2FC | p-Value | FDR |
|---|---|---|---|
| Romboutsia | −5.01 | 2.62 × 10−8 | 5.25 × 10−7 |
| Family XIII AD3011 group | −4.29 | 2.60 × 10−7 | 2.63 × 10−6 |
| Subdoligranulum | −4.33 | 5.85 × 10−5 | 3.90 × 10−4 |
| Phascolarctobacterium | 3.89 | 1.31 × 10−4 | 5.78 × 10−4 |
| Streptococcus | −3.88 | 1.44 × 10−4 | 5.77 × 10−4 |
| Christensenellaceae_R_7_group | 2.66 | 6.92 × 10−4 | 0.002 |
| Ruminococcaceae UCG_005 | −3.46 | 0.002 | 0.006 |
| Agathobacter | −2.28 | 0.005 | 0.011 |
| Ruminococcus 1 | 2.58 | 0.005 | 0.011 |
| Dialister | −2.87 | 0.009 | 0.019 |
| Name | log2FC | p-Value | FDR |
|---|---|---|---|
| Bacteroides | 38.234 | 1.03 × 10−4 | 0.001 |
| Lachnospira | 28.216 | 4.57 × 10−4 | 0.003 |
| Phascolarctobacterium | 29.346 | 5.42 × 10−4 | 0.003 |
| Ruminococcaceae_UCG_014 | 27.338 | 0.003 | 0.013 |
| Ruminococcaceae_UCG_005 | 27.072 | 0.005 | 0.017 |
| Ruminococcaceae_UCG_002 | 1.984 | 0.002 | 0.048 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, V.; Cuevas-Sierra, A.; de la O, V.; Salvado, R.; Barreiros-Mota, I.; Castela, I.; Camelo, A.; Brandão, I.; Santo, C.E.; Faria, A.; et al. Gut Microbiota Shifts After a Weight Loss Program in Adults with Obesity: The WLM3P Study. Nutrients 2025, 17, 2360. https://doi.org/10.3390/nu17142360
Pereira V, Cuevas-Sierra A, de la O V, Salvado R, Barreiros-Mota I, Castela I, Camelo A, Brandão I, Santo CE, Faria A, et al. Gut Microbiota Shifts After a Weight Loss Program in Adults with Obesity: The WLM3P Study. Nutrients. 2025; 17(14):2360. https://doi.org/10.3390/nu17142360
Chicago/Turabian StylePereira, Vanessa, Amanda Cuevas-Sierra, Victor de la O, Rita Salvado, Inês Barreiros-Mota, Inês Castela, Alexandra Camelo, Inês Brandão, Christophe Espírito Santo, Ana Faria, and et al. 2025. "Gut Microbiota Shifts After a Weight Loss Program in Adults with Obesity: The WLM3P Study" Nutrients 17, no. 14: 2360. https://doi.org/10.3390/nu17142360
APA StylePereira, V., Cuevas-Sierra, A., de la O, V., Salvado, R., Barreiros-Mota, I., Castela, I., Camelo, A., Brandão, I., Santo, C. E., Faria, A., Calhau, C., Silvestre, M. P., & Moreira-Rosário, A. (2025). Gut Microbiota Shifts After a Weight Loss Program in Adults with Obesity: The WLM3P Study. Nutrients, 17(14), 2360. https://doi.org/10.3390/nu17142360









