Probiotic Potentials and Protective Effects of Ligilactobacillus animalis LA-1 Against High-Fat Diet-Induced Obesity in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparations of L. animalis LA-1 and Pathogenic Strains
2.2. Evaluation of the Probiotic Potential of L. animalis LA-1
2.2.1. Simulated Gastrointestinal Tolerance
2.2.2. Surface Hydrophobicity
2.2.3. Antibiotic Susceptibility
2.2.4. Hemolytic Activity
2.2.5. Antibacterial Activity
2.2.6. Cholesterol-Reducing Rate
2.2.7. The Activity of Bile Salt Hydrolase (BSH)
2.2.8. The Inhibition Activity of α-Glucosidase
2.2.9. Fatty Acid Absorption
2.3. Animals Experiment Design
2.4. Sample Collection
2.5. Biochemical Assay of Serum and Liver Tissues
2.6. Histological Evaluation
2.6.1. Oil Red O Staining of Liver Tissue
2.6.2. Hematoxylin and Eosin (HE) Staining of Liver and Adipose Tissues
2.7. Gut Microbiota Analysis: 16S rRNA Gene Sequencing
2.8. Determination of Short-Chain Fatty Acids (SCFAs)
2.9. Untargeted Metabolomics Analysis of Liver
2.10. Statistical Analysis
3. Results
3.1. Probiotic Properties of L. animalis LA-1 In Vitro
3.1.1. Simulated Gastrointestinal Tolerance
3.1.2. Surface Hydrophobicity
3.1.3. Antibiotic Susceptibility
3.1.4. Hemolytic Activity
3.1.5. Antibacterial Activity
3.1.6. Cholesterol-Reducing Rate
3.1.7. The Activity of Bile Salt Hydrolase (BSH)
3.1.8. The Inhibition Activity of α-Glucosidase
3.1.9. Fatty Acid Absorption
3.2. L. animalis LA-1 Alleviated HFD-Induced Obesity in Mice
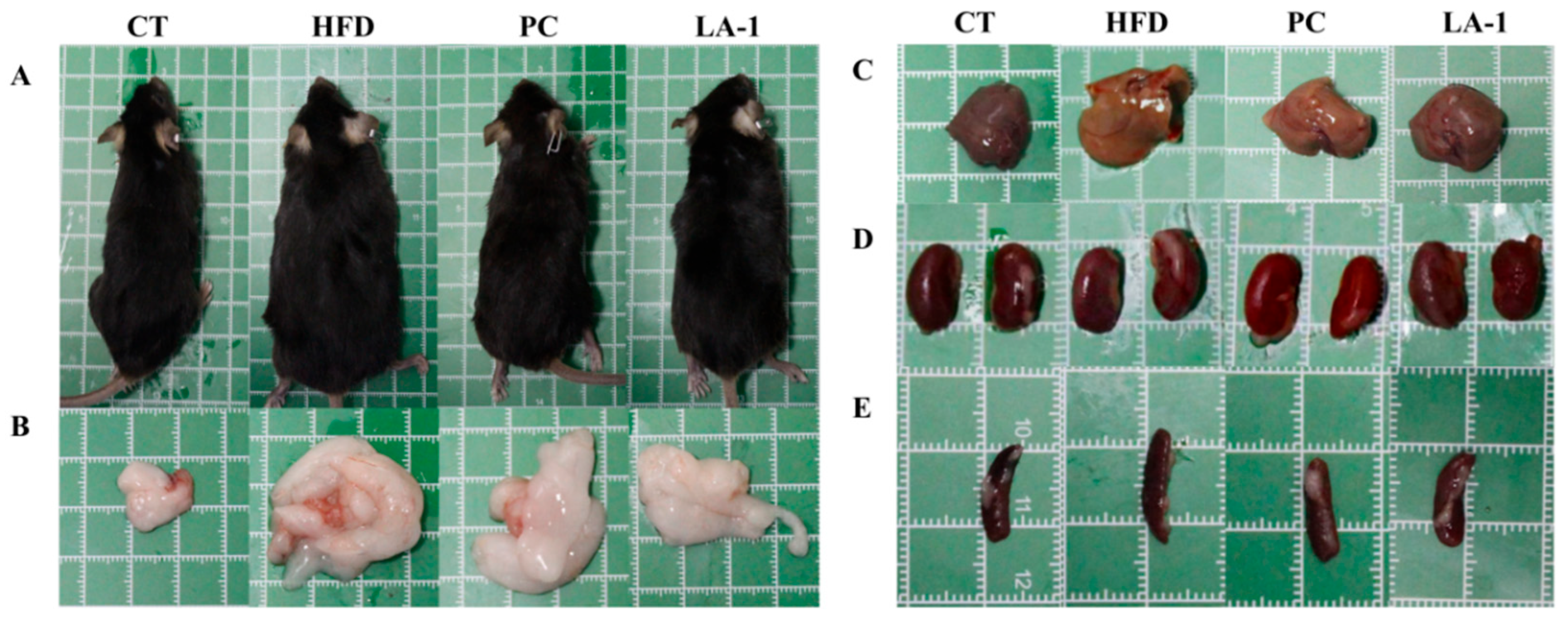
3.2.1. L. animalis LA-1 Reduced Body Weight Gain and Organ Enlargement in Obese Mice
3.2.2. L. animalis LA-1 Prevented Lipid Accumulation in Serum and Liver of Obese Mice
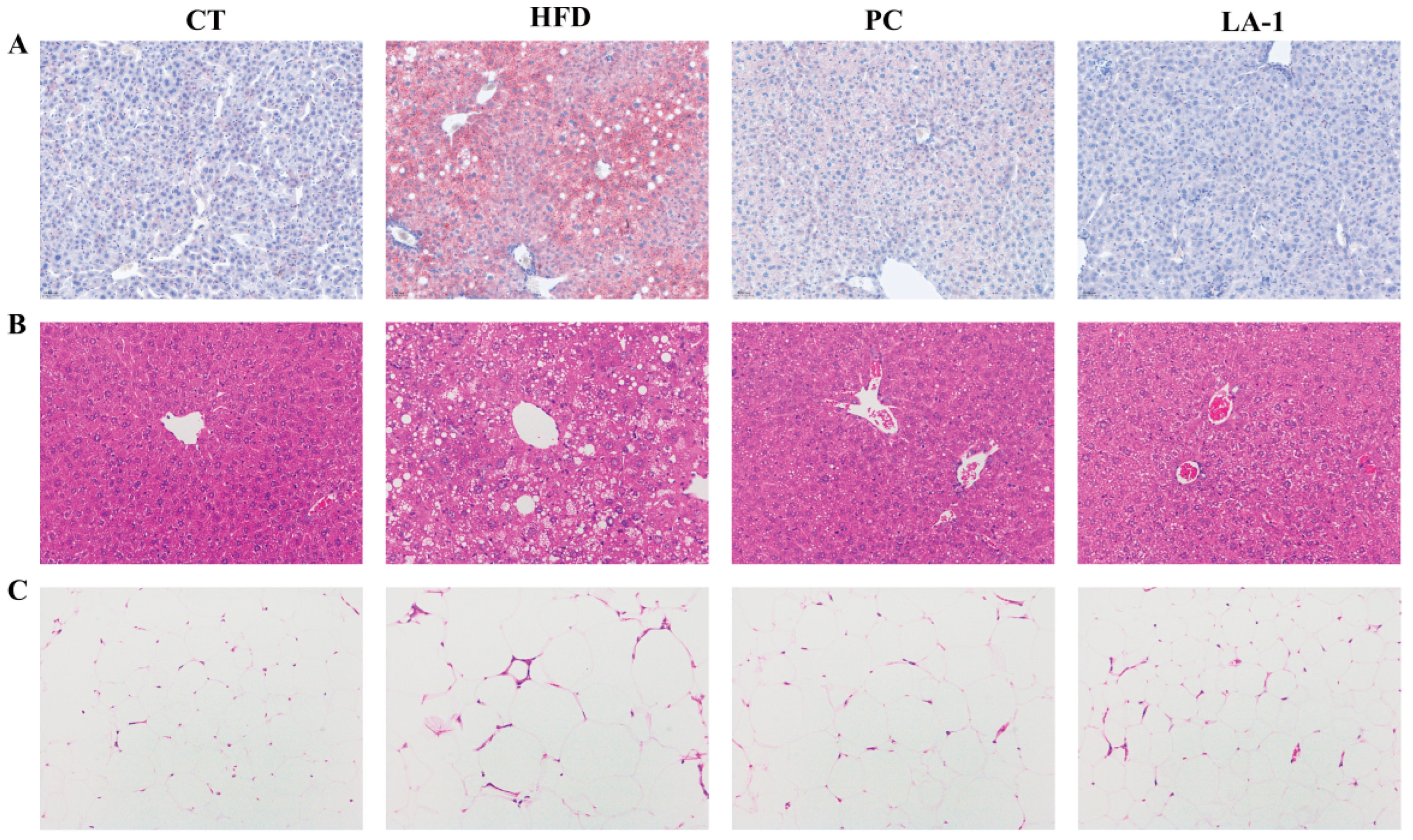
3.2.3. L. animalis LA-1 Prevented Liver and WAT Injury in Obese Mice
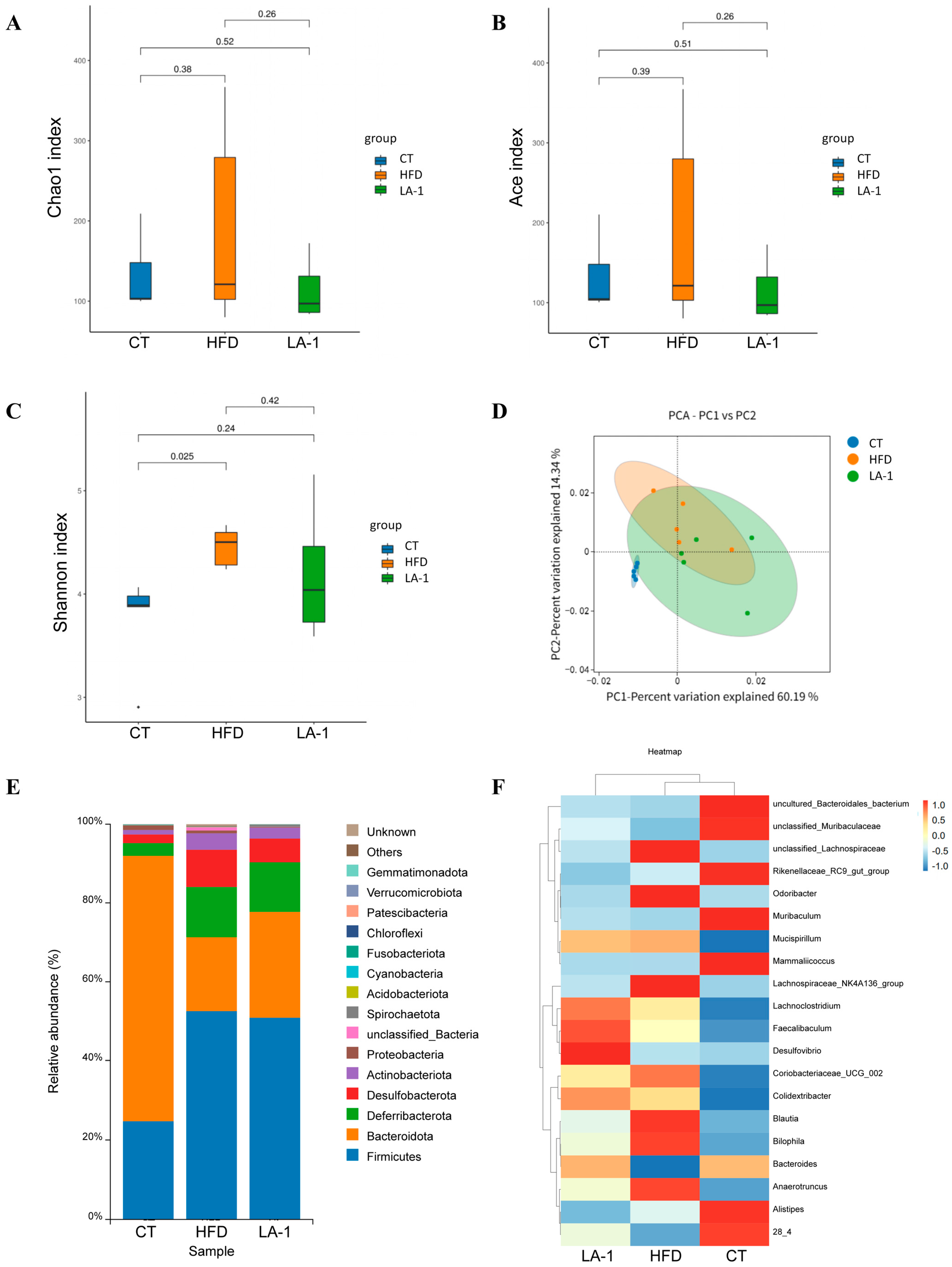
3.2.4. L. animalis LA-1 Modulated the Structure and Composition of the Gut Microbiota in Obese Mice
3.2.5. L. animalis LA-1 Promoted SCFAs Accumulation in the Cecal Contents of Obese Mice
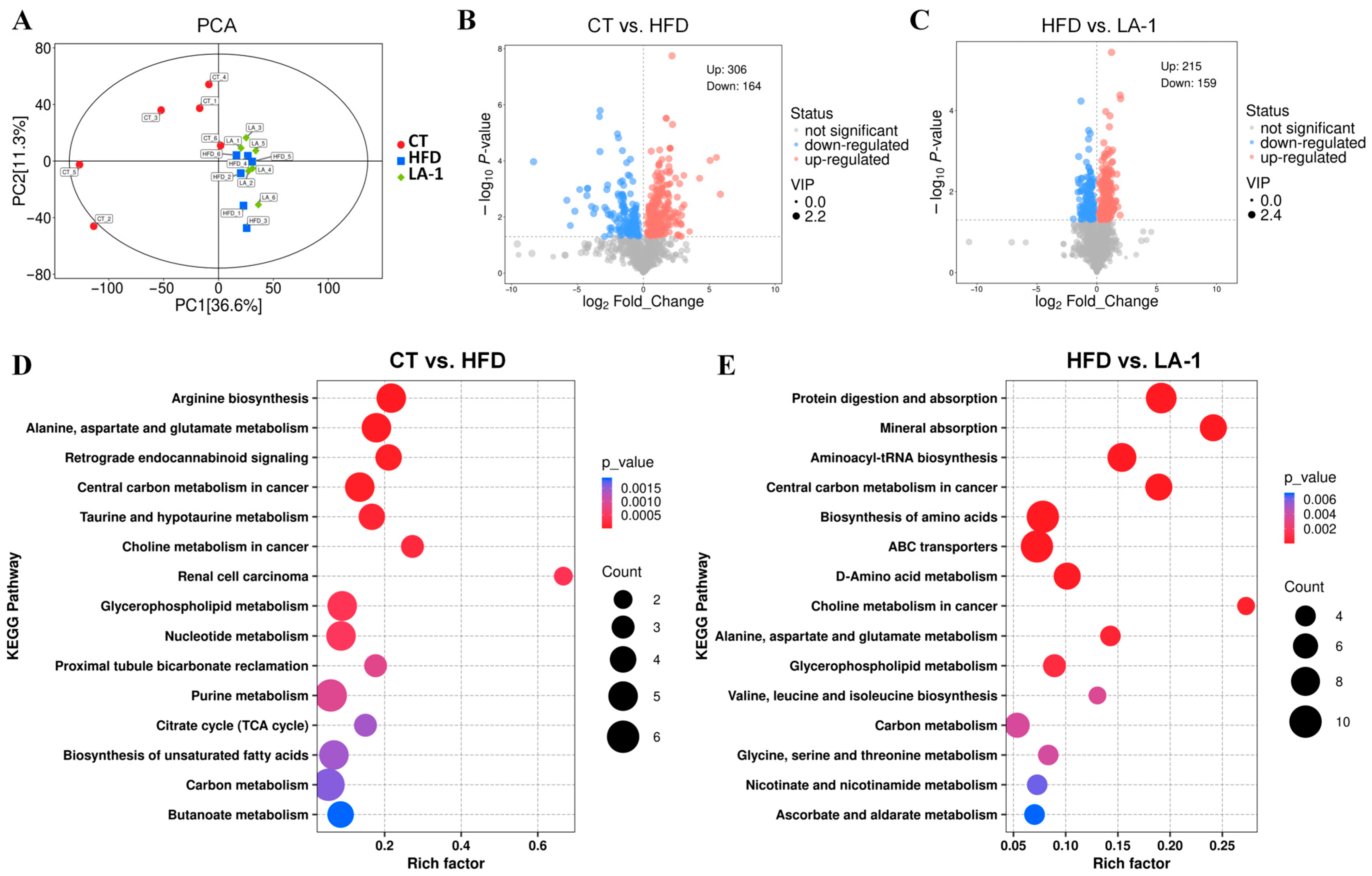
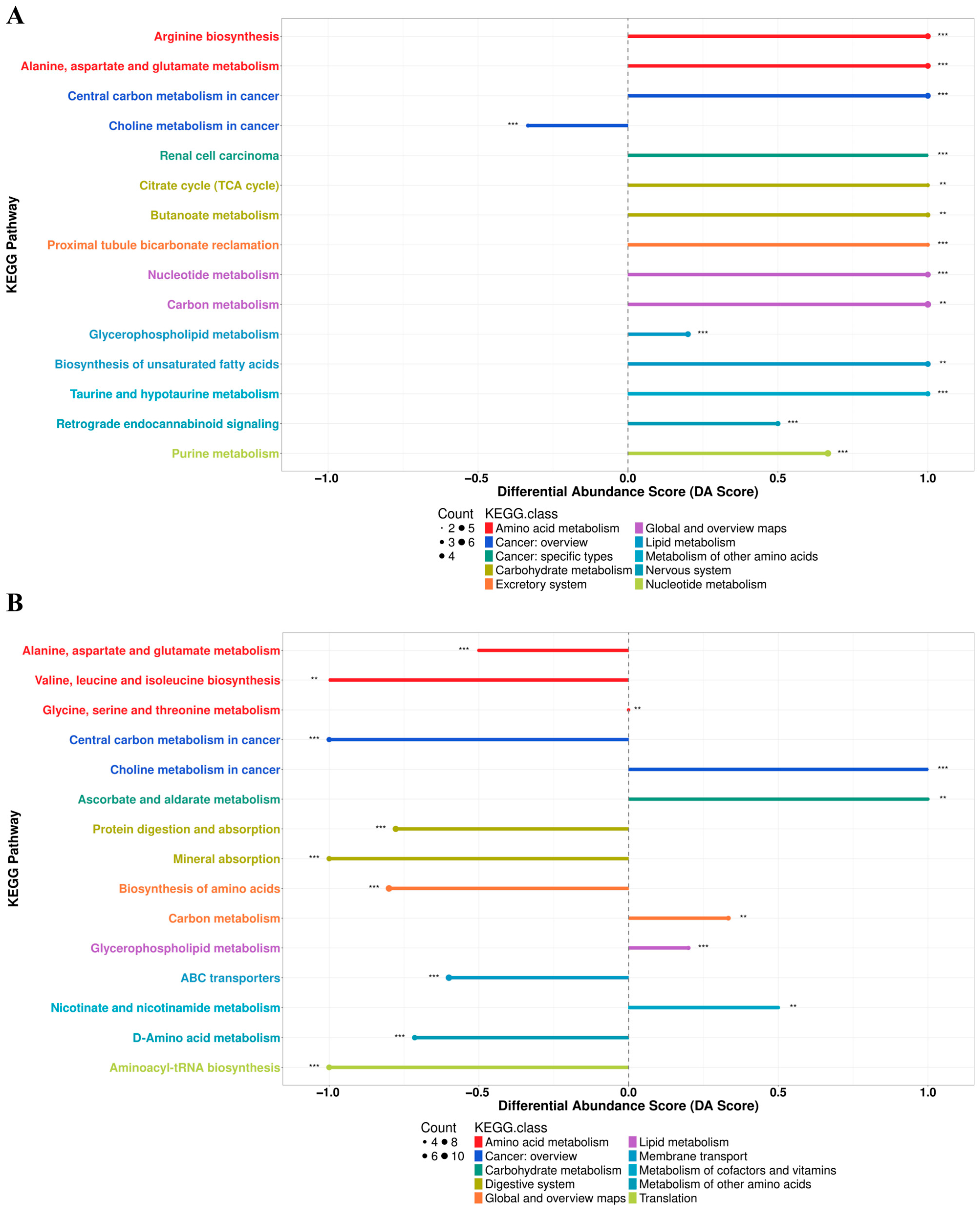
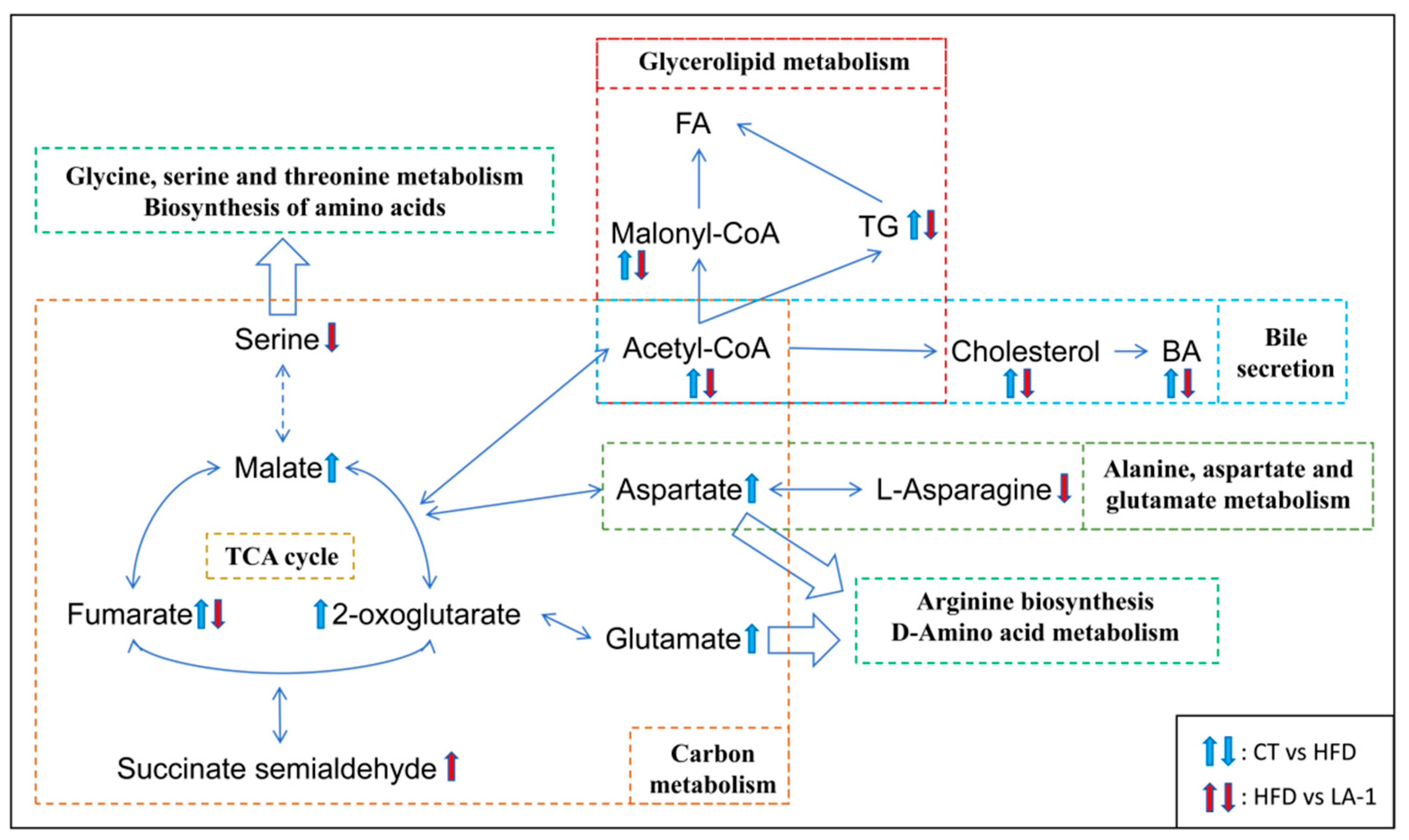
3.2.6. L. animalis LA-1 Improved Hepatic Lipid and Amino Acid Metabolism in Obese Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Simati, S.; Kokkinos, A.; Dalamaga, M.; Argyrakopoulou, G. Obesity paradox: Fact or fiction? Curr. Obes. Rep. 2023, 12, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Boutari, C.; Mantzoros, C.S. A 2022 update on the epidemiology of obesity and a call to action: As its twin COVID-19 pandemic appears to be receding, the obesity and dysmetabolism pandemic continues to rage on. Metabolism 2022, 133, 155217. [Google Scholar] [CrossRef] [PubMed]
- Blüher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Lin, X.; Jian, S.; Wen, J.; Jian, X.; He, S.; Wen, C.; Liu, T.; Qi, X.; Yin, Y.; et al. Changes in gut microbiota and short-chain fatty acids are involved in the process of canine obesity after neutering. J. Anim. Sci. 2023, 101, skad283. [Google Scholar] [CrossRef] [PubMed]
- German, A.J. The growing problem of obesity in dogs and cats. J. Nutr. 2006, 136, 1940S–1946S. [Google Scholar] [CrossRef] [PubMed]
- Wallis, N.J.; McClellan, A.; Mörseburg, A.; Kentistou, K.A.; Jamaluddin, A.; Dowsett, G.K.C.; Schofield, E.; Morros-Nuevo, A.; Saeed, S.; Lam, B.Y.H.; et al. Canine genome-wide association study identifies DENND1B as an obesity gene in dogs and humans. Science 2025, 387, eads2145. [Google Scholar] [CrossRef] [PubMed]
- Marchi, P.H.; Vendramini, T.H.A.; Perini, M.P.; Zafalon, R.V.A.; Amaral, A.R.; Ochamotto, V.A.; Da Silveira, J.C.; Dagli, M.L.Z.; Brunetto, M.A. Obesity, inflammation, and cancer in dogs: Review and perspectives. Front. Vet. Sci. 2022, 9, 1004122. [Google Scholar] [CrossRef] [PubMed]
- Summers, J.F.; O’Neill, D.G.; Church, D.; Collins, L.; Sargan, D.; Brodbelt, D.C. Health-related welfare prioritisation of canine disorders using electronic health records in primary care practice in the UK. BMC Vet. Res. 2019, 15, 163. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Xiao, X.; Li, Y.; Lu, S.; Zi, J.; Sun, X.; Xu, J.; Liu, H.-Y.; Li, X.; Song, T.; et al. Insights into the interplay between gut microbiota and lipid metabolism in the obesity management of canines and felines. J. Anim. Sci. Biotechnol. 2024, 15, 114. [Google Scholar] [CrossRef] [PubMed]
- Proietto, J. Medicines for long-term obesity management. Aust. Prescr. 2022, 45, 38–40. [Google Scholar] [CrossRef] [PubMed]
- Chao, A.M.; Taylor, S.; Moore, M.; Amaro, A.; Wadden, T.A. Evolving approaches for pharmacological therapy of obesity. Annu. Rev. Pharmacol. Toxicol. 2025, 65, 169–189. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Yang, F.; Zhong, H.; Hong, J.; Lin, H.; Zong, M.; Ren, H.; Zhao, S.; Chen, Y.; Shi, Z.; et al. Obesity-enriched gut microbe degrades myo-inositol and promotes lipid absorption. Cell Host Microbe 2024, 32, 1301–1314.e9. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Guan, L.; Wu, C.; Gong, Y.; Wu, L.; Zhang, M.; Cao, Z.; Chen, Y.; Yang, C.; Wang, B.; et al. Gut microbiota-butyrate-PPARγ axis modulates adipose regulatory T cell population. Adv. Sci. 2025, 12, e2411086. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wang, Q.; Li, D.; Chen, S.; Chen, J.; Zhu, X.; Bai, F. Gut microbiome composition and metabolic activity in metabolic-associated fatty liver disease. Virulence 2025, 16, 2482158. [Google Scholar] [CrossRef] [PubMed]
- Legg, K. Gut microbial metabolites protect against obesity. Nat. Rev. Endocrinol. 2025, 21, 328. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, T.; Kubota, T.; Nakanishi, Y.; Tsugawa, H.; Suda, W.; Kwon, A.T.-J.; Yazaki, J.; Ikeda, K.; Nemoto, S.; Mochizuki, Y.; et al. Gut microbial carbohydrate metabolism contributes to insulin resistance. Nature 2023, 621, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Cai, X.; Fei, W.; Ye, Y.; Zhao, M.; Zheng, C. The role of short-chain fatty acids in immunity, inflammation and metabolism. Crit. Rev. Food Sci. Nutr. 2022, 62, 1–12. [Google Scholar] [CrossRef] [PubMed]
- You, H.; Tan, Y.; Yu, D.; Qiu, S.; Bai, Y.; He, J.; Cao, H.; Che, Q.; Guo, J.; Su, Z. The therapeutic effect of SCFA-mediated regulation of the intestinal environment on obesity. Front. Nutr. 2022, 9, 886902. [Google Scholar] [CrossRef] [PubMed]
- Sanders, M.E.; Merenstein, D.J.; Reid, G.; Gibson, G.R.; Rastall, R.A. Probiotics and prebiotics in intestinal health and disease: From biology to the clinic. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, T.L.; Eckstrom, K.; Lile, K.H.; Caldwell, S.; Heney, E.R.; Lahue, K.G.; D’Alessandro, A.; Wargo, M.J.; Krementsov, D.N. Lactobacillus reuteri tryptophan metabolism promotes host susceptibility to CNS autoimmunity. Microbiome 2022, 10, 198. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Pan, T.; Li, L.; Wang, H.; Zhu, J.; Zhang, H.; Zhao, J.; Chen, W.; Lu, W. Bifidobacterium longum mediated tryptophan metabolism to improve atopic dermatitis via the gut-skin axis. Gut Microbes 2022, 14, 2044723. [Google Scholar] [CrossRef] [PubMed]
- Arellano-García, L.; Trepiana, J.; Martínez, J.A.; Portillo, M.P.; Milton-Laskibar, I. Beneficial effects of viable and heat-inactivated Lactobacillus rhamnosus GG administration on oxidative stress and inflammation in diet-induced NAFLD in rats. Antioxidants 2023, 12, 717. [Google Scholar] [CrossRef] [PubMed]
- Jing, N.; Liu, F.; Wang, R.; Zhang, Y.; Yang, J.; Hou, Y.; Zhang, H.; Xie, Y.; Liu, H.; Ge, S.; et al. Both live and heat-killed Bifidobacterium animalis J-12 alleviated oral ulcers in LVG golden Syrian hamsters by gavage by directly intervening in the intestinal flora structure. Food Funct. 2023, 14, 2045–2058. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Meng, K.; Liu, G.; Wen, Z.; Han, Y.; Liu, W.; Xu, X.; Song, L.; Cai, H.; Yang, P. Lactiplantibacillus plantarum FRT4 protects against fatty liver hemorrhage syndrome: Regulating gut microbiota and FoxO/TLR-4/NF-κB signaling pathway in laying hens. Microbiome 2025, 13, 88. [Google Scholar] [CrossRef] [PubMed]
- Vitetta, L.; Coulson, S.; Thomsen, M.; Nguyen, T.; Hall, S. Probiotics, D-Lactic acidosis, oxidative stress and strain specificity. Gut Microbes 2017, 8, 311–322. [Google Scholar] [CrossRef] [PubMed]
- McFarland, L.V.; Evans, C.T.; Goldstein, E.J.C. Strain-specificity and disease-specificity of probiotic efficacy: A systematic review and meta-analysis. Front. Med. 2018, 5, 124. [Google Scholar] [CrossRef] [PubMed]
- Bazireh, H.; Shariati, P.; Azimzadeh Jamalkandi, S.; Ahmadi, A.; Boroumand, M.A. Isolation of novel probiotic Lactobacillus and Enterococcus strains from human salivary and fecal sources. Front. Microbiol. 2020, 11, 597946. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Peng, Q.; Zhang, Y.; Tian, D.; Zhang, P.; Huang, Y.; Ma, L.; Dia, V.P.; Qiao, Y.; Shi, B. Antibacterial potential of a novel Lactobacillus casei strain isolated from Chinese northeast sauerkraut and the antibiofilm activity of its exopolysaccharides. Food Funct. 2020, 11, 4697–4706. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Li, X.; Li, D.; Liu, W.; Han, Y.; Xu, X.; Yang, P.; Meng, K. Optimization of gamma-aminobutyric acid production by Lactiplantibacillus plantarum FRT7 from Chinese Paocai. Foods 2023, 12, 3034. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-T.; Kim, J.-W.; Kim, S.-I.; Kim, S.; Nguyen, T.H.; Kang, C.-H. Antioxidant and anti-inflammatory effect and probiotic properties of lactic acid bacteria isolated from canine and feline feces. Microorganisms 2021, 9, 1971. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Zhang, Y.; Li, Y.; Li, G. Developing gut-healthy strains for pets: Probiotic potential and genomic insights of canine-derived Lactobacillus acidophilus GLA09. Microorganisms 2025, 13, 350. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, J.; Zheng, H.; Xin, J.; Zhong, Z.; Liu, H.; Fu, H.; Zhou, Z.; Qiu, X.; Peng, G. Multi-functional properties of lactic acid bacteria strains derived from canine feces. Front. Vet. Sci. 2024, 11, 1404580. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-J.; Lee, J.-B.; Park, S.-Y.; Choi, I.-S.; Lee, S.-W. Antimicrobial activity of Ligilactobacillus animalis SWLA-1 and its cell-free supernatant against multidrug-resistant bacteria and its potential use as an alternative to antimicrobial agents. Microorganisms 2023, 11, 182. [Google Scholar] [CrossRef] [PubMed]
- Boll, E.J.; Winther, K.D.; Knudsen, T.T.M.; Copani, G.; Cappellozza, B.I. Ligilactobacillus animalis 506 protects the intestinal barrier from the damaging effects of enteric pathogens and deoxynivalenol. Animals 2024, 14, 269. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Wang, Q.; Han, X.; Zhang, H.; Wang, N.; Huang, Y.; Yang, P.; Zhang, R.; Meng, K. In Vitro Evaluation of Probiotic Activities and Anti-Obesity Effects of Enterococcus faecalis EF-1 in Mice Fed a High-Fat Diet. Foods 2024, 13, 4095. [Google Scholar] [CrossRef] [PubMed]
- Dianawati, D.; Shah, N.P. Survival, acid and bile tolerance, and surface hydrophobicity of microencapsulated B. animalis ssp. lactis Bb12 during storage at room temperature. J. Food Sci. 2011, 76, M592–M599. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.N.; Afrin, S.; Humayun, S.; Ahmed, M.M.; Saha, B.K. Identification and growth characterization of a novel strain of Saccharomyces boulardii isolated from soya paste. Front. Nutr. 2020, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Argyri, A.A.; Zoumpopoulou, G.; Karatzas, K.-A.G.; Tsakalidou, E.; Nychas, G.-J.E.; Panagou, E.Z.; Tassou, C.C. Selection of potential probiotic lactic acid bacteria from fermented olives by in vitro tests. Food Microbiol. 2013, 33, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Du Toit, M.; Franz, C.M.; Dicks, L.M.; Schillinger, U.; Haberer, P.; Warlies, B.; Ahrens, F.; Holzapfel, W.H. Characterisation and selection of probiotic lactobacilli for a preliminary minipig feeding trial and their effect on serum cholesterol levels, faeces pH and faeces moisture content. Int. J. Food Microbiol. 1998, 40, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Apostolidis, E.; Kwon, Y.-I.; Shetty, K. Inhibitory potential of herb, fruit, and fungal-enriched cheese against key enzymes linked to type 2 diabetes and hypertension. Innov. Food Sci. Emerg. Technol. 2007, 8, 46–54. [Google Scholar] [CrossRef]
- Lee, N.-R.; Kwon, T.-J.; Chung, E.-C.; Bae, J.; Soung, S.-H.; Tak, H.-J.; Choi, J.-Y.; Lee, Y.-E.; Won Hwang, N.; Lee, J.S.; et al. Combination of Lacticaseibacillus paracasei BEPC22 and Lactiplantibacillus plantarum BELP53 attenuates fat accumulation and alters the metabolome and gut microbiota in mice with high-fat diet-induced obesity. Food Funct. 2024, 15, 647–662. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Wen, Z.; Zhao, L.; Yu, D.; Meng, K.; Yang, P. Lactobacillus plantarum FRT4 alleviated obesity by modulating gut microbiota and liver metabolome in high-fat diet-induced obese mice. Food Nutr. Res. 2022, 66. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.-L.; Li, S.-Z.; Xiao, P.-T.; Cai, Y.-Y.; Chu, C.; Chen, B.-Z.; Li, P.; Li, J.; Liu, E.-H. Citrus polymethoxyflavones attenuate metabolic syndrome by regulating gut microbiome and amino acid metabolism. Sci. Adv. 2020, 6, eaax6208. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Shakirova, L.; Grube, M.; Gavare, M.; Auzina, L.; Zikmanis, P. Lactobacillus acidophilus La5 and Bifidobacterium lactis Bb12 cell surface hydrophobicity and survival of the cells under adverse environmental conditions. J. Ind. Microbiol. Biotechnol. 2013, 40, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Oh, N.S.; Joung, J.Y.; Lee, J.Y.; Kim, Y. Probiotic and anti-inflammatory potential of Lactobacillus rhamnosus 4B15 and Lactobacillus gasseri 4M13 isolated from infant feces. PLoS ONE 2018, 13, e0192021. [Google Scholar] [CrossRef] [PubMed]
- She, J.; Wong, C.C.; Yu, J. Targeted prebiotics alter the obese gut microbiome in humans. Signal Transduct. Target. Ther. 2021, 6, 363. [Google Scholar] [CrossRef] [PubMed]
- Di Giacomo, S.; Toussaint, F.; Ledesma-García, L.; Knoops, A.; Vande Capelle, F.; Fremaux, C.; Horvath, P.; Ladrière, J.-M.; Ait-Abderrahim, H.; Hols, P.; et al. Expanding natural transformation to improve beneficial lactic acid bacteria. FEMS Microbiol. Rev. 2022, 46, fuac014. [Google Scholar] [CrossRef] [PubMed]
- Green, D.L.; Keenan, K.; Fredricks, K.J.; Huque, S.I.; Mushi, M.F.; Kansiime, C.; Asiimwe, B.; Kiiru, J.; Mshana, S.E.; Neema, S.; et al. The role of multidimensional poverty in antibiotic misuse: A mixed-methods study of self-medication and non-adherence in Kenya, Tanzania, and Uganda. Lancet Glob. Health 2023, 11, e59–e68. [Google Scholar] [CrossRef] [PubMed]
- Machowska, A.; Stålsby Lundborg, C. Drivers of irrational use of antibiotics in Europe. Int. J. Environ. Res. Public Health 2018, 16, 27. [Google Scholar] [CrossRef] [PubMed]
- Klaver, F.A.; van der Meer, R. The assumed assimilation of cholesterol by Lactobacilli and Bifidobacterium bifidum is due to their bile salt-deconjugating activity. Appl. Environ. Microbiol. 1993, 59, 1120–1124. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.I.A.; Gibson, G.R. Cholesterol assimilation by lactic acid bacteria and bifidobacteria isolated from the human gut. Appl. Environ. Microbiol. 2002, 68, 4689–4693. [Google Scholar] [CrossRef] [PubMed]
- Foley, M.H.; O’Flaherty, S.; Allen, G.; Rivera, A.J.; Stewart, A.K.; Barrangou, R.; Theriot, C.M. Lactobacillus bile salt hydrolase substrate specificity governs bacterial fitness and host colonization. Proc. Natl. Acad. Sci. USA 2021, 118, e2017709118. [Google Scholar] [CrossRef] [PubMed]
- Horáčková, Š.; Plocková, M.; Demnerová, K. Importance of microbial defence systems to bile salts and mechanisms of serum cholesterol reduction. Biotechnol. Adv. 2018, 36, 682–690. [Google Scholar] [CrossRef] [PubMed]
- Nishimoto, S.; Debarbat, A.; Ikeda, Y.; Arikawa, E.; Odagaki, Y.; Yano, H.; Qiao, Y.; Ito, M.; Kimura, T.; Takita, T.; et al. Expression of recombinant human α-glucosidase in HEK293 cells. J. Agric. Food Chem. 2025, 73, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.; Tang, B.; Stewart, A.J.; Tao, Y.; Shao, Y.; Cui, Y.; Yue, H.; Pei, J.; Liu, Z.; Mei, L.; et al. Erythritol attenuates postprandial blood glucose by inhibiting α-glucosidase. J. Agric. Food Chem. 2018, 66, 1401–1407. [Google Scholar] [CrossRef] [PubMed]
- Sethi, J.K.; Vidal-Puig, A.J. Thematic review series: Adipocyte biology. Adipose tissue function and plasticity orchestrate nutritional adaptation. J. Lipid Res. 2007, 48, 1253–1262. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Luo, K.; Yuan, J.; Gao, J.; Cui, B.; Yu, Z.; Lu, Z. Hepatic DDAH1 mitigates hepatic steatosis and insulin resistance in obese mice: Involvement of reduced S100A11 expression. Acta Pharm. Sin. B 2023, 13, 3352–3364. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.P.; Wang, B.; Jain, S.; Ding, J.; Rejeski, J.; Furdui, C.M.; Kitzman, D.W.; Taraphder, S.; Brechot, C.; Kumar, A.; et al. A mechanism by which gut microbiota elevates permeability and inflammation in obese/diabetic mice and human gut. Gut 2023, 72, 1848–1865. [Google Scholar] [CrossRef] [PubMed]
- Seganfredo, F.B.; Blume, C.A.; Moehlecke, M.; Giongo, A.; Casagrande, D.S.; Spolidoro, J.V.N.; Padoin, A.V.; Schaan, B.D.; Mottin, C.C. Weight-loss interventions and gut microbiota changes in overweight and obese patients: A systematic review. Obes. Rev. 2017, 18, 832–851. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Yang, Z.; Wang, F.; Li, D.; Liu, Y.; Wang, D.; Zhao, X.; Li, Y.; Wang, Y.; Feng, X.; et al. Association between metabolic status and gut microbiome in obese populations. Microb. Genom. 2021, 7, 000639. [Google Scholar] [CrossRef] [PubMed]
- Vallianou, N.; Stratigou, T.; Christodoulatos, G.S.; Dalamaga, M. Understanding the role of the gut microbiome and microbial metabolites in obesity and obesity-associated metabolic disorders: Current evidence and perspectives. Curr. Obes. Rep. 2019, 8, 317–332. [Google Scholar] [CrossRef] [PubMed]
- Zafar, H.; Saier, M.H., Jr. Gut Bacteroides species in health and disease. Gut Microbes 2021, 13, 1848158. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Chen, B.; Zhang, X.; Akbar, M.T.; Wu, T.; Zhang, Y.; Zhi, L.; Shen, Q. Exploration of the Muribaculaceae family in the gut microbiota: Diversity, metabolism, and function. Nutrients 2024, 16, 2660. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Lee, S.; Park, S.-C.; Kim, N.-E.; Shin, C.; Lee, S.K.; Jung, Y.; Yoon, D.; Kim, H.; Kim, S.; et al. Role of an unclassified Lachnospiraceae in the pathogenesis of type 2 diabetes: A longitudinal study of the urine microbiome and metabolites. Exp. Mol. Med. 2022, 54, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Herp, S.; Durai Raj, A.C.; Salvado Silva, M.; Woelfel, S.; Stecher, B. The human symbiont Mucispirillum schaedleri: Causality in health and disease. Med. Microbiol. Immunol. 2021, 210, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Huang, H.; Xu, H.; Xia, H.; Zhang, C.; Ye, D.; Bi, F. Endogenous Coriobacteriaceae enriched by a high-fat diet promotes colorectal tumorigenesis through the CPT1A-ERK axis. NPJ Biofilms Microbiomes 2024, 10, 5. [Google Scholar] [CrossRef] [PubMed]
- Natividad, J.M.; Lamas, B.; Pham, H.P.; Michel, M.-L.; Rainteau, D.; Bridonneau, C.; da Costa, G.; van Hylckama Vlieg, J.; Sovran, B.; Chamignon, C.; et al. Bilophila wadsworthia aggravates high fat diet induced metabolic dysfunctions in mice. Nat. Commun. 2018, 9, 2802. [Google Scholar] [CrossRef] [PubMed]
- Martin-Gallausiaux, C.; Marinelli, L.; Blottière, H.M.; Larraufie, P.; Lapaque, N. SCFA: Mechanisms and functional importance in the gut. Proc. Nutr. Soc. 2021, 80, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Dilixiati, Y.; Xiao, L.; Yang, H.; Zhang, Z. Different short-chain fatty acids unequally modulate intestinal homeostasis and reverse obesity-related symptoms in lead-exposed high-Fat diet mice. J. Agric. Food Chem. 2024, 72, 18971–18985. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Yao, Q.; Meng, L.; Wang, J.; Zheng, N. Double-side role of short chain fatty acids on host health via the gut-organ axes. Anim. Nutr. 2024, 18, 322–339. [Google Scholar] [CrossRef] [PubMed]
- Kondo, T.; Kishi, M.; Fushimi, T.; Kaga, T. Acetic acid upregulates the expression of genes for fatty acid oxidation enzymes in liver to suppress body fat accumulation. J. Agric. Food Chem. 2009, 57, 5982–5986. [Google Scholar] [CrossRef] [PubMed]
- Zhuge, A.; Li, S.; Han, S.; Yuan, Y.; Shen, J.; Wu, W.; Wang, K.; Xia, J.; Wang, Q.; Gu, Y.; et al. Akkermansia muciniphila-derived acetate activates the hepatic AMPK/SIRT1/PGC-1α axis to alleviate ferroptosis in metabolic-associated fatty liver disease. Acta Pharm. Sin. B 2025, 15, 151–167. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Zhang, R.; Mu, Y.; Song, Y.; Hao, N.; Wei, Y.; Wang, Q.; Mackay, C.R. Propionate ameliorates alcohol-induced liver injury in mice via the gut-liver axis: Focus on the improvement of intestinal permeability. J. Agric. Food Chem. 2022, 70, 6084–6096. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D. Is colonic propionate delivery a novel solution to improve metabolism and inflammation in overweight or obese subjects? Gut 2019, 68, 1352–1353. [Google Scholar] [CrossRef] [PubMed]
- Jiao, A.; Yu, B.; He, J.; Yu, J.; Zheng, P.; Luo, Y.; Luo, J.; Yan, H.; Wang, Q.; Wang, H.; et al. Sodium acetate, propionate, and butyrate reduce fat accumulation in mice via modulating appetite and relevant genes. Nutrition 2021, 87–88, 111198. [Google Scholar] [CrossRef] [PubMed]
- Sivaprakasam, S.; Prasad, P.D.; Singh, N. Benefits of short-chain fatty acids and their receptors in inflammation and carcinogenesis. Pharmacol. Ther. 2016, 164, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Scheppach, W. Effects of short chain fatty acids on gut morphology and function. Gut 1994, 35, S35–S38. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Wei, F.; Chen, Y.; Chen, Z.; Luo, Y.; Fan, J.; Xu, Y.; Hu, M.; Li, P.; Gu, Q. Lactiplantibacillus plantarum ZJ316 alleviates Helicobacter pylori-induced intestinal inflammation by sustaining intestinal homeostasis. Probiotics Antimicrob. Proteins 2025. [Google Scholar] [CrossRef] [PubMed]
- Kimura, I.; Inoue, D.; Maeda, T.; Hara, T.; Ichimura, A.; Miyauchi, S.; Kobayashi, M.; Hirasawa, A.; Tsujimoto, G. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41). Proc. Natl. Acad. Sci. USA 2011, 108, 8030–8035. [Google Scholar] [CrossRef] [PubMed]
- Tomita, T.; Hosoda, K.; Fujikura, J.; Inagaki, N.; Nakao, K. The G-protein-coupled long-chain fatty acid receptor GPR40 and glucose metabolism. Front. Endocrinol. 2014, 5, 152. [Google Scholar] [CrossRef] [PubMed]
- Samuel, B.S.; Shaito, A.; Motoike, T.; Rey, F.E.; Backhed, F.; Manchester, J.K.; Hammer, R.E.; Williams, S.C.; Crowley, J.; Yanagisawa, M.; et al. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc. Natl. Acad. Sci. USA 2008, 105, 16767–16772. [Google Scholar] [CrossRef] [PubMed]
- Tolhurst, G.; Heffron, H.; Lam, Y.S.; Parker, H.E.; Habib, A.M.; Diakogiannaki, E.; Cameron, J.; Grosse, J.; Reimann, F.; Gribble, F.M. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes 2012, 61, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Karin, M. Obesity, inflammation, and liver cancer. J. Hepatol. 2012, 56, 704–713. [Google Scholar] [CrossRef] [PubMed]
- Vinolo, M.A.R.; Rodrigues, H.G.; Nachbar, R.T.; Curi, R. Regulation of inflammation by short chain fatty acids. Nutrients 2011, 3, 858–876. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; van Esch, B.C.A.M.; Wagenaar, G.T.M.; Garssen, J.; Folkerts, G.; Henricks, P.A.J. Pro- and anti-inflammatory effects of short chain fatty acids on immune and endothelial cells. Eur. J. Pharmacol. 2018, 831, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Han, J.B.; Wang, Y.G. mTORC1 signaling in hepatic lipid metabolism. Protein Cell 2018, 9, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Bhat, N.; Mani, A. Dysregulation of lipid and glucose metabolism in nonalcoholic fatty liver disease. Nutrients 2023, 15, 2323. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Chen, Q.; Liu, L.; Huang, H.; Sun, J.; Bai, X.; Jin, C.; Li, H.; Sun, F.; Xiao, X.; et al. Amino acid is a major carbon source for hepatic lipogenesis. Cell Metab. 2024, 36, 2437–2448.e8. [Google Scholar] [CrossRef] [PubMed]
- Felix, J.B.; Cox, A.R.; Hartig, S.M. Acetyl-CoA and Metabolite Fluxes Regulate White Adipose Tissue Expansion. Trends Endocrinol. Metab. 2021, 32, 320–332. [Google Scholar] [CrossRef] [PubMed]
- Lackey, D.E.; Lynch, C.J.; Olson, K.C.; Mostaedi, R.; Ali, M.; Smith, W.H.; Karpe, F.; Humphreys, S.; Bedinger, D.H.; Dunn, T.N.; et al. Regulation of adipose branched-chain amino acid catabolism enzyme expression and cross-adipose amino acid flux in human obesity. Am. J. Physiol. Endocrinol. Metab. 2013, 304, E1175–E1187. [Google Scholar] [CrossRef] [PubMed]
- Sanders, F.W.B.; Griffin, J.L. De novo lipogenesis in the liver in health and disease: More than just a shunting yard for glucose. Biol. Rev. Camb. Philos. Soc. 2016, 91, 452–468. [Google Scholar] [CrossRef] [PubMed]
- Walther, T.C.; Farese, R.V. Lipid droplets and cellular lipid metabolism. Annu. Rev. Biochem. 2012, 81, 687–714. [Google Scholar] [CrossRef] [PubMed]
- Morita, S.-Y.; Ikeda, Y. Regulation of membrane phospholipid biosynthesis in mammalian cells. Biochem. Pharmacol. 2022, 206, 115296. [Google Scholar] [CrossRef] [PubMed]

| Items | CT | HFD | PC | LA-1 | p Value |
|---|---|---|---|---|---|
| Food intake (g/week) | 50.44 ± 0.37 a | 36.50 ± 0.32 b | 36.05 ± 0.09 b | 36.64 ± 1.62 b | <0.001 |
| Initial body weight (g/each) | 22.11 ± 0.03 a | 22.37 ± 0.02 a | 22.15 ± 0.15 a | 22.14 ± 0.56 a | 0.788 |
| Final body weight (g/each) | 26.95 ± 0.21 b | 34.61 ± 2.69 a | 33.29 ± 0.08 a | 31.93 ± 0.55 a | 0.019 |
| Body weight gain (g/each) | 4.84 ± 0.18 b | 12.23 ± 2.67 a | 11.15 ± 0.08 a | 9.79 ± 0.01 a | 0.018 |
| Liver index | 3.44 ± 0.13 b | 4.08 ± 0.04 a | 3.48 ± 0.19 b | 3.64 ± 0.17 b | 0.019 |
| WATs index | 1.71 ± 0.12 c | 6.45 ± 0.83 a | 4.66 ± 1.02 b | 3.91 ± 0.68 b | <0.001 |
| Kidney index | 1.30 ± 0.02 ab | 1.43 ± 0.10 a | 1.30 ± 0.08 ab | 1.22 ± 0.05 b | 0.023 |
| Spleen index | 0.25 ± 0.01 b | 0.38 ± 0.11 a | 0.25 ± 0.03 b | 0.25 ± 0.03 b | 0.014 |
| Items | CT | HFD | PC | LA-1 | p Value |
|---|---|---|---|---|---|
| TC (mmol/L) | 3.03 ± 0.14 b | 5.03 ± 0.35 a | 4.61 ± 0.29 a | 4.70 ± 0.16 a | <0.001 |
| TG (mmol/L) | 1.15 ± 0.04 c | 1.65 ± 0.09 a | 1.37 ± 0.17 b | 1.27 ± 0.10 bc | <0.001 |
| LDL-C (mmol/L) | 0.77 ± 0.07 b | 1.32 ± 0.08 a | 1.25 ± 0.05 a | 1.26 ± 0.03 a | <0.001 |
| GLU (mmol/L) | 6.00 ± 0.58 c | 11.18 ± 0.79 a | 8.98 ± 1.26 b | 6.10 ± 0.33 c | <0.001 |
| ALT (U/L) | 31.69 ± 1.07 b | 42.21 ± 3.58 a | 34.32 ± 2.28 b | 22.70 ± 1.82 c | <0.001 |
| AST (U/L) | 128.77 ± 7.65 ab | 165.44 ± 17.63 a | 146.83 ± 3.82 b | 116.15 ± 8.27 c | <0.001 |
| NEFA (mmol/L) | 2.40 ± 0.11 b | 2.88 ± 0.15 a | 2.41 ± 0.19 bc | 2.64 ± 0.26 b | 0.011 |
| Items | CT | HFD | PC | LA-1 | p Value |
|---|---|---|---|---|---|
| TC (µmol/g) | 43.22 ± 3.16 b | 53.27 ± 1.97 a | 39.54 ± 3.35 b | 38.11 ± 1.27 b | <0.001 |
| TG (µmol/g) | 102.37 ± 12.59 c | 204.84 ± 34.63 a | 150.18 ± 14.13 b | 132.95 ± 17.16 bc | <0.001 |
| HDL-C (µmol/g) | 8.83 ± 0.13 a | 6.06 ± 1.20 b | 9.76 ± 1.50 a | 9.58 ± 0.46 a | 0.029 |
| LDL-C (µmol/g) | 19.23 ± 1.07 c | 29.25 ± 1.23 a | 23.77 ± 1.83 b | 17.66 ± 3.69 c | <0.001 |
| GLU (mmol/g) | 1.32 ± 0.17 a | 1.83 ± 0.20 a | 1.34 ± 0.29 a | 1.49 ± 0.05 a | 0.168 |
| ALT (U/g) | 770.53 ± 57.90 c | 1265.76 ± 135.10 a | 1034.99 ± 65.23 b | 825.89 ± 94.91 c | <0.001 |
| AST (U/g) | 2368.14 ± 200.34 b | 3463.37 ± 397.84 a | 2754.15 ± 345.32 b | 2385.84 ± 131.85 b | 0.005 |
| Groups | CT | HFD | LA-1 |
|---|---|---|---|
| Firmicutes | 24.71% | 52.54% | 50.86% |
| Bacteroidetes | 67.24% | 18.77% | 26.86% |
| F/B ratio | 37% | 280% | 189% |
| Items | CT | HFD | LA-1 | p Value |
|---|---|---|---|---|
| Acetic acid (μg/mg) | 0.8355 ± 0.086 a | 0.501 ± 0.0.0506 b | 0.5074 ± 0.0299 b | 0.018 |
| Propionic acid (μg/mg) | 0.4446 ± 0.0078 a | 0.1687 ± 0.0013 b | 0.1801 ± 0.0366 b | 0.002 |
| Butyric acid (μg/mg) | 0.4929 ± 0.0785 a | 0.2600 ± 0.0037 b | 0.2609 ± 0.0527 b | 0.037 |
| Isobutyric acid (μg/mg) | 0.0367 ± 0.0021 a | 0.0347 ± 0.0012 a | 0.0484 ± 0.0100 a | 0.187 |
| Pentanoic acid (μg/mg) | 0.0514 ± 0.0012 a | 0.057 ± 0.025 a | 0.0681 ± 0.0182 a | 0.668 |
| Isovaleric acid (μg/mg) | 0.0320 ± 0.0019 a | 0.0315 ± 0.0001 a | 0.04632 ± 0.0087 a | 0.100 |
| Hexanoic acid (μg/mg) | 0.0045 ± 0.0001 a | 0.0024 ± 0.0000 a | 0.0044 ± 0.0012 a | 0.087 |
| Total SCFAs concentration (μg/mg) | 1.7942 ± 0.0217 a | 1.0282 ± 0.0273 c | 1.1157 ± 0.0213 b | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Huang, Y.; Meng, K.; Zhang, H.; Han, Y.; Zhang, R.; Han, X.; Liu, G.; Cai, H.; Yang, P. Probiotic Potentials and Protective Effects of Ligilactobacillus animalis LA-1 Against High-Fat Diet-Induced Obesity in Mice. Nutrients 2025, 17, 2346. https://doi.org/10.3390/nu17142346
Wang Q, Huang Y, Meng K, Zhang H, Han Y, Zhang R, Han X, Liu G, Cai H, Yang P. Probiotic Potentials and Protective Effects of Ligilactobacillus animalis LA-1 Against High-Fat Diet-Induced Obesity in Mice. Nutrients. 2025; 17(14):2346. https://doi.org/10.3390/nu17142346
Chicago/Turabian StyleWang, Qingya, Yuyin Huang, Kun Meng, Haiou Zhang, Yunsheng Han, Rui Zhang, Xiling Han, Guohua Liu, Hongying Cai, and Peilong Yang. 2025. "Probiotic Potentials and Protective Effects of Ligilactobacillus animalis LA-1 Against High-Fat Diet-Induced Obesity in Mice" Nutrients 17, no. 14: 2346. https://doi.org/10.3390/nu17142346
APA StyleWang, Q., Huang, Y., Meng, K., Zhang, H., Han, Y., Zhang, R., Han, X., Liu, G., Cai, H., & Yang, P. (2025). Probiotic Potentials and Protective Effects of Ligilactobacillus animalis LA-1 Against High-Fat Diet-Induced Obesity in Mice. Nutrients, 17(14), 2346. https://doi.org/10.3390/nu17142346








