Effects of Time-Restricted Eating (Early and Late) Combined with Energy Restriction vs. Energy Restriction Alone on the Gut Microbiome in Adults with Obesity
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Participants
2.3. Intervention
2.4. Anthropometric and Biochemical Measurements
2.5. Gut Microbiome Analysis
2.6. Statistical Analysis
3. Results
3.1. Characteristics of Study Participants at Baseline, During the Intervention and After Follow-Up
3.2. Impact of eTRE + ER, lTRE + ER, and ER Interventions on Gut Microbiome Diversity After a 3-Month Intervention and Follow-Up
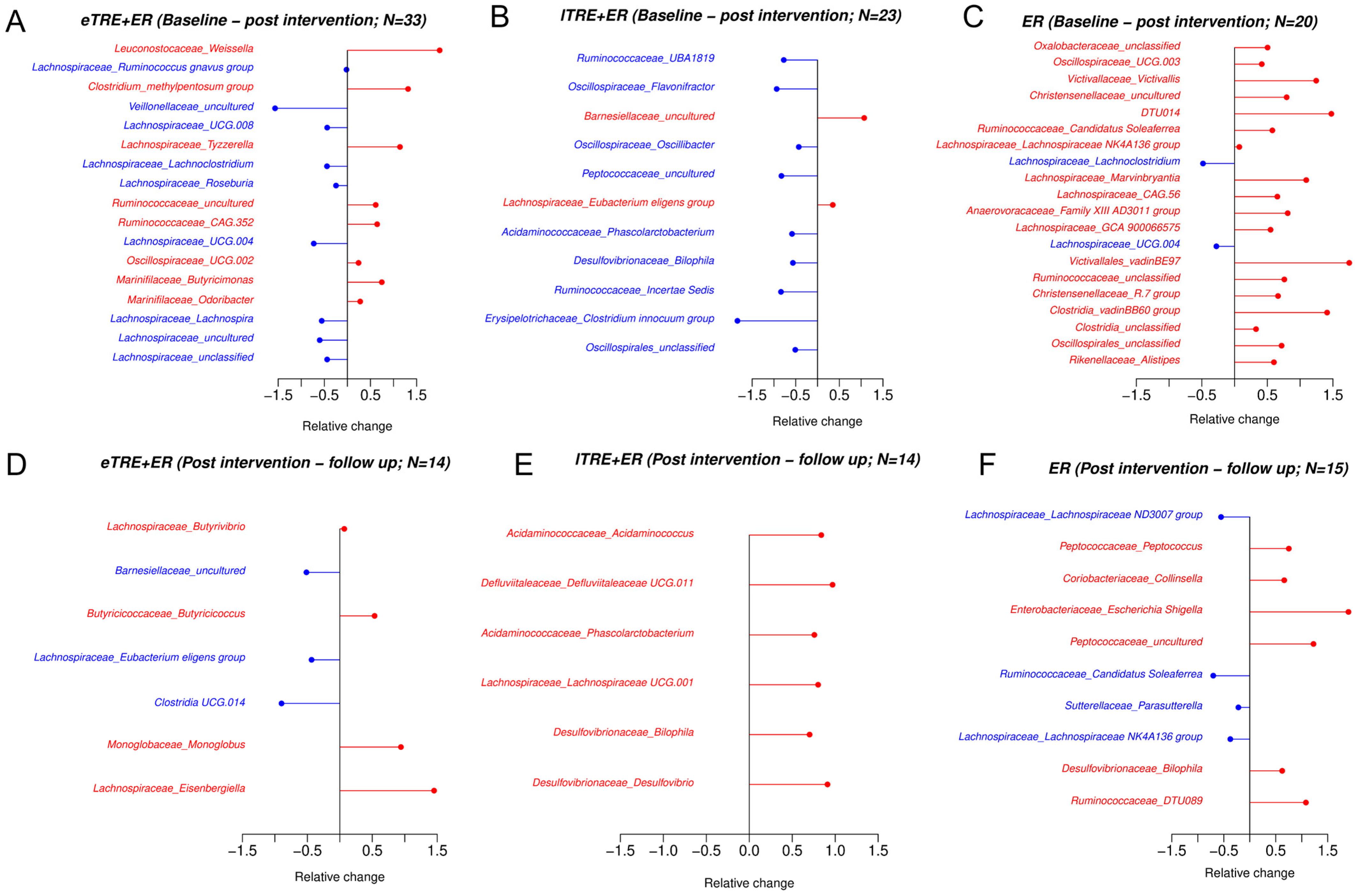
3.3. Impact of eTRE + ER, lTRE + ER and ER Interventions on Gut Microbiome Composition at the Phylum and Genus Level After a 3-Month Intervention and During Follow-Up
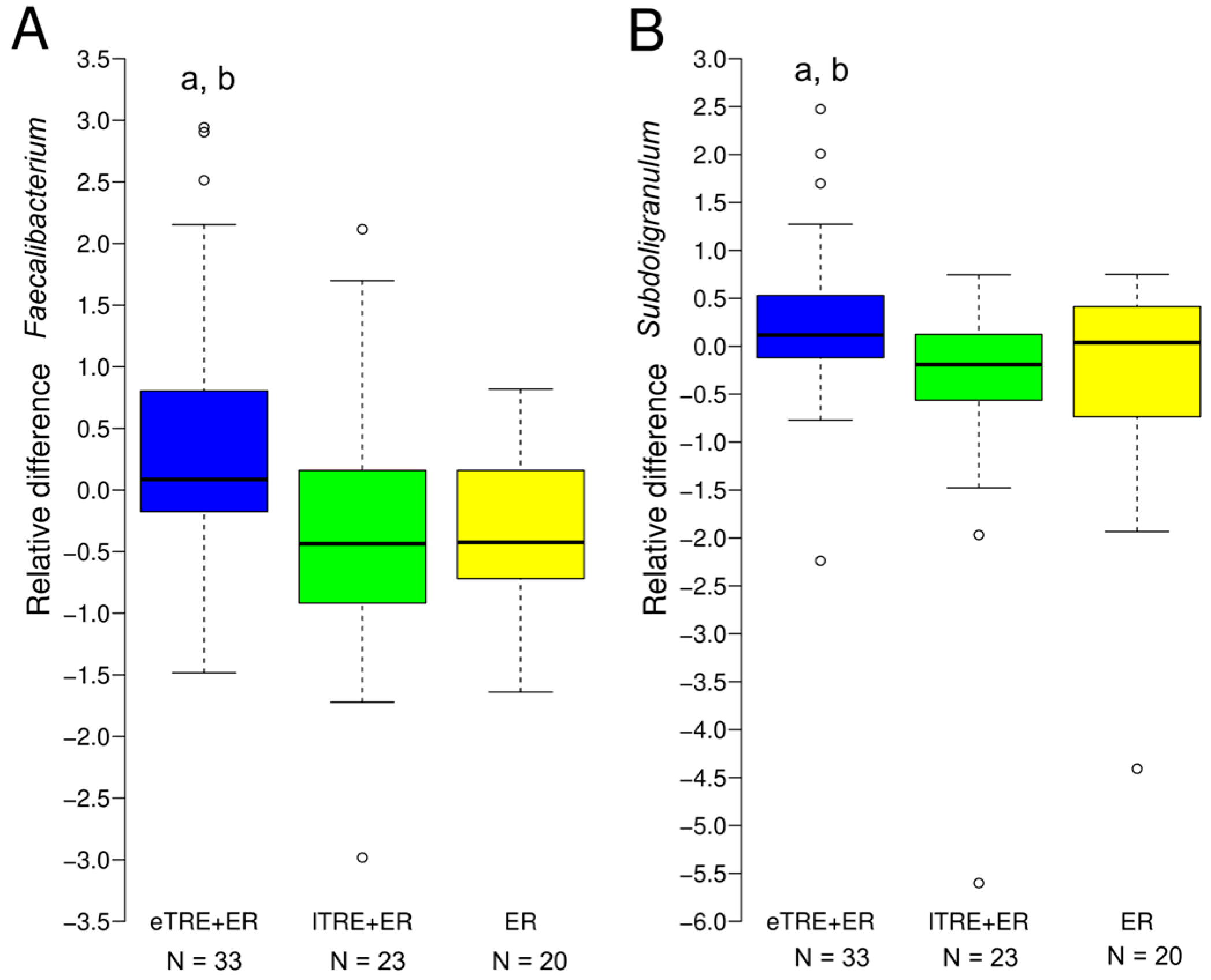
3.4. Associations of Taxonomic Alterations with Changes in Clinical Parameters Induced by Interventions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| eTRE | Early time-restricted eating |
| lTRE | Late time-restricted eating |
| HDL | High-density lipoprotein |
| LDL | Low-density lipoprotein |
| TG | Triacylglycerols |
| DBP | Diastolic blood pressure |
| TRE | Time-restricted eating |
| T2DM | Type 2 diabetes mellitus |
| SCFAs | Short-chain fatty acids |
| ER | Energy restriction |
| SD | Standard deviation |
References
- Blüher, M. Obesity: Global Epidemiology and Pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.D.; Ryan, D.H.; Apovian, C.M.; Ard, J.D.; Comuzzie, A.G.; Donato, K.A.; Hu, F.B.; Hubbard, V.S.; Jakicic, J.M.; Kushner, R.F.; et al. 2013 AHA/ACC/TOS Guideline for the Management of Overweight and Obesity in Adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J. Am. Coll. Cardiol. 2014, 63, 2985–3023. [Google Scholar] [CrossRef]
- Liu, D.; Huang, Y.; Huang, C.; Yang, S.; Wei, X.; Zhang, P.; Guo, D.; Lin, J.; Xu, B.; Li, C.; et al. Calorie Restriction with or without Time-Restricted Eating in Weight Loss. N. Engl. J. Med. 2022, 386, 1495–1504. [Google Scholar] [CrossRef]
- Črešnovar, T.; Habe, B.; Mohorko, N.; Kenig, S.; Jenko Pražnikar, Z.; Petelin, A. Early Time-Restricted Eating with Energy Restriction Has a Better Effect on Body Fat Mass, Diastolic Blood Pressure, Metabolic Age and Fasting Glucose Compared to Late Time-Restricted Eating with Energy Restriction and/or Energy Restriction Alone: A 3-Month Randomized Clinical Trial. Clin. Nutr. 2025, 49, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Chang, H.-W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of Diet on the Gut Microbiome and Implications for Human Health. J. Transl. Med. 2017, 15, 73. [Google Scholar] [CrossRef] [PubMed]
- Maruvada, P.; Leone, V.; Kaplan, L.M.; Chang, E.B. The Human Microbiome and Obesity: Moving beyond Associations. Cell Host Microbe 2017, 22, 589–599. [Google Scholar] [CrossRef]
- John, G.K.; Wang, L.; Nanavati, J.; Twose, C.; Singh, R.; Mullin, G. Dietary Alteration of the Gut Microbiome and Its Impact on Weight and Fat Mass: A Systematic Review and Meta-Analysis. Genes 2018, 9, 167. [Google Scholar] [CrossRef]
- Le Chatelier, E.; Nielsen, T.; Qin, J.; Prifti, E.; Hildebrand, F.; Falony, G.; Almeida, M.; Arumugam, M.; Batto, J.-M.; Kennedy, S.; et al. Richness of Human Gut Microbiome Correlates with Metabolic Markers. Nature 2013, 500, 541–546. [Google Scholar] [CrossRef]
- Vallianou, N.; Stratigou, T.; Christodoulatos, G.S.; Dalamaga, M. Understanding the Role of the Gut Microbiome and Microbial Metabolites in Obesity and Obesity-Associated Metabolic Disorders: Current Evidence and Perspectives. Curr. Obes. Rep. 2019, 8, 317–332. [Google Scholar] [CrossRef]
- Bezek, K.; Petelin, A.; Pražnikar, J.; Nova, E.; Redondo, N.; Marcos, A.; Jenko Pražnikar, Z. Obesity Measures and Dietary Parameters as Predictors of Gut Microbiota Phyla in Healthy Individuals. Nutrients 2020, 12, 2695. [Google Scholar] [CrossRef]
- Depommier, C.; Everard, A.; Druart, C.; Plovier, H.; Van Hul, M.; Vieira-Silva, S.; Falony, G.; Raes, J.; Maiter, D.; Delzenne, N.M.; et al. Supplementation with Akkermansia Muciniphila in Overweight and Obese Human Volunteers: A Proof-of-Concept Exploratory Study. Nat. Med. 2019, 25, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Šik Novak, K.; Bogataj Jontez, N.; Petelin, A.; Hladnik, M.; Baruca Arbeiter, A.; Bandelj, D.; Pražnikar, J.; Kenig, S.; Mohorko, N.; Jenko Pražnikar, Z. Could Gut Microbiota Composition Be a Useful Indicator of a Long-Term Dietary Pattern? Nutrients 2023, 15, 2196. [Google Scholar] [CrossRef]
- Zmora, N.; Suez, J.; Elinav, E. You Are What You Eat: Diet, Health and the Gut Microbiota. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 35–56. [Google Scholar] [CrossRef] [PubMed]
- Zarrinpar, A.; Chaix, A.; Yooseph, S.; Panda, S. Diet and Feeding Pattern Affect the Diurnal Dynamics of the Gut Microbiome. Cell Metab. 2014, 20, 1006–1017. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Xie, C.; Lu, S.; Nichols, R.G.; Tian, Y.; Li, L.; Patel, D.; Ma, Y.; Brocker, C.N.; Yan, T.; et al. Intermittent Fasting Promotes White Adipose Browning and Decreases Obesity by Shaping the Gut Microbiota. Cell Metab. 2017, 26, 672–685.e4. [Google Scholar] [CrossRef]
- Khan, M.N.; Khan, S.I.; Rana, M.I.; Ayyaz, A.; Khan, M.Y.; Imran, M. Intermittent Fasting Positively Modulates Human Gut Microbial Diversity and Ameliorates Blood Lipid Profile. Front. Microbiol. 2022, 13, 922727. [Google Scholar] [CrossRef]
- Zeb, F.; Wu, X.; Chen, L.; Fatima, S.; Haq, I.U.; Chen, A.; Majeed, F.; Feng, Q.; Li, M. Effect of Time-Restricted Feeding on Metabolic Risk and Circadian Rhythm Associated with Gut Microbiome in Healthy Males. Br. J. Nutr. 2020, 123, 1216–1226. [Google Scholar] [CrossRef]
- Ozkul, C.; Yalinay, M.; Karakan, T. Islamic Fasting Leads to an Increased Abundance of Akkermansia Muciniphila and Bacteroides Fragilis Group: A Preliminary Study on Intermittent Fasting. Turk. J. Gastroenterol. 2020, 30, 1030–1035. [Google Scholar] [CrossRef]
- Xie, Z.; Sun, Y.; Ye, Y.; Hu, D.; Zhang, H.; He, Z.; Zhao, H.; Yang, H.; Mao, Y. Randomized Controlled Trial for Time-Restricted Eating in Healthy Volunteers without Obesity. Nat. Commun. 2022, 13, 1003. [Google Scholar] [CrossRef]
- Zeb, F.; Wu, X.; Chen, L.; Fatima, S.; Haq, I.-U.; Chen, A.; Xu, C.; Jianglei, R.; Feng, Q.; Li, M. Time-Restricted Feeding Is Associated with Changes in Human Gut Microbiota Related to Nutrient Intake. Nutrition 2020, 78, 110797. [Google Scholar] [CrossRef]
- Chen, Y.; Li, X.; Yang, M.; Jia, C.; He, Z.; Zhou, S.; Ruan, P.; Wang, Y.; Tang, C.; Pan, W.; et al. Time-Restricted Eating Reveals a “Younger” Immune System and Reshapes the Intestinal Microbiome in Human. Redox Biol. 2024, 78, 103422. [Google Scholar] [CrossRef]
- Ferrocino, I.; Pellegrini, M.; D’Eusebio, C.; Goitre, I.; Ponzo, V.; Fadda, M.; Rosato, R.; Mengozzi, G.; Beccuti, G.; Merlo, F.D.; et al. The Effects of Time-Restricted Eating on Metabolism and Gut Microbiota: A Real-Life Study. Nutrients 2022, 14, 2569. [Google Scholar] [CrossRef]
- Gabel, K.; Marcell, J.; Cares, K.; Kalam, F.; Cienfuegos, S.; Ezpeleta, M.; Varady, K.A. Effect of Time Restricted Feeding on the Gut Microbiome in Adults with Obesity: A Pilot Study. Nutr. Health 2020, 26, 79–85. [Google Scholar] [CrossRef]
- Dawson, M.A.; Cheung, S.N.; La Frano, M.R.; Nagpal, R.; Berryman, C.E. Early Time-Restricted Eating Improves Markers of Cardiometabolic Health but Has No Impact on Intestinal Nutrient Absorption in Healthy Adults. Cell Rep. Med. 2024, 5, 101363. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Ye, Y.; Mao, Y.; Liao, W.; Xu, W. Time-Restricted Feeding during Childhood Has Persistent Effects on Mice Commensal Microbiota. Ann. Transl. Med. 2019, 7, 556. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Mao, Y.; Xu, G.; Liao, W.; Ren, J.; Yang, H.; Yang, J.; Sun, L.; Chen, H.; Wang, W.; et al. Time-Restricted Feeding Causes Irreversible Metabolic Disorders and Gut Microbiota Shift in Pediatric Mice. Pediatr. Res. 2019, 85, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.J.; Alvear, A.; Knights, D.; Chow, L.S.; Bantle, A.E. A Randomized Pilot Study of Time-Restricted Eating Shows Minimal Microbiome Changes. Nutrients 2025, 17, 185. [Google Scholar] [CrossRef]
- Huang, C.; Liu, D.; Yang, S.; Huang, Y.; Wei, X.; Zhang, P.; Lin, J.; Xu, B.; Liu, Y.; Guo, D.; et al. Effect of Time-Restricted Eating Regimen on Weight Loss Is Mediated by Gut Microbiome. iScience 2024, 27, 110202. [Google Scholar] [CrossRef]
- Vacca, M.; Celano, G.; Calabrese, F.M.; Portincasa, P.; Gobbetti, M.; De Angelis, M. The Controversial Role of Human Gut Lachnospiraceae. Microorganisms 2020, 8, 573. [Google Scholar] [CrossRef]
- Lippert, K.; Kedenko, L.; Antonielli, L.; Kedenko, I.; Gemeier, C.; Leitner, M.; Kautzky-Willer, A.; Paulweber, B.; Hackl, E. Gut Microbiota Dysbiosis Associated with Glucose Metabolism Disorders and the Metabolic Syndrome in Older Adults. Benef. Microbes 2017, 8, 545–556. [Google Scholar] [CrossRef]
- Chávez-Carbajal, A.; Nirmalkar, K.; Pérez-Lizaur, A.; Hernández-Quiroz, F.; Ramírez-del-Alto, S.; García-Mena, J.; Hernández-Guerrero, C. Gut Microbiota and Predicted Metabolic Pathways in a Sample of Mexican Women Affected by Obesity and Obesity Plus Metabolic Syndrome. Int. J. Mol. Sci. 2019, 20, 438. [Google Scholar] [CrossRef] [PubMed]
- Salonen, A.; Lahti, L.; Salojärvi, J.; Holtrop, G.; Korpela, K.; Duncan, S.H.; Date, P.; Farquharson, F.; Johnstone, A.M.; Lobley, G.E.; et al. Impact of Diet and Individual Variation on Intestinal Microbiota Composition and Fermentation Products in Obese Men. ISME J. 2014, 8, 2218–2230. [Google Scholar] [CrossRef] [PubMed]
- Frost, F.; Storck, L.J.; Kacprowski, T.; Gärtner, S.; Rühlemann, M.; Bang, C.; Franke, A.; Völker, U.; Aghdassi, A.A.; Steveling, A.; et al. A Structured Weight Loss Program Increases Gut Microbiota Phylogenetic Diversity and Reduces Levels of Collinsella in Obese Type 2 Diabetics: A Pilot Study. PLoS ONE 2019, 14, e0219489. [Google Scholar] [CrossRef]
- Heinsen, F.-A.; Fangmann, D.; Müller, N.; Schulte, D.M.; Rühlemann, M.C.; Türk, K.; Settgast, U.; Lieb, W.; Baines, J.F.; Schreiber, S.; et al. Beneficial Effects of a Dietary Weight Loss Intervention on Human Gut Microbiome Diversity and Metabolism Are Not Sustained during Weight Maintenance. Obes. Facts 2016, 9, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Kim, D.; Kim, K.-W.; Lee, S.-H.; Jang, A. Comparative Analysis of the Gut Microbiota of Mice Fed a Diet Supplemented with Raw and Cooked Beef Loin Powder. Sci. Rep. 2021, 11, 11489. [Google Scholar] [CrossRef]
- Wu, H.; Tremaroli, V.; Schmidt, C.; Lundqvist, A.; Olsson, L.M.; Krämer, M.; Gummesson, A.; Perkins, R.; Bergström, G.; Bäckhed, F. The Gut Microbiota in Prediabetes and Diabetes: A Population-Based Cross-Sectional Study. Cell Metab. 2020, 32, 379–390.e3. [Google Scholar] [CrossRef]
- Moreno-Navarrete, J.M.; Serino, M.; Blasco-Baque, V.; Azalbert, V.; Barton, R.H.; Cardellini, M.; Latorre, J.; Ortega, F.; Sabater-Masdeu, M.; Burcelin, R.; et al. Gut Microbiota Interacts with Markers of Adipose Tissue Browning, Insulin Action and Plasma Acetate in Morbid Obesity. Mol. Nutr. Food Res. 2018, 62, 1700721. [Google Scholar] [CrossRef]
- Tao, S.; Li, L.; Li, L.; Liu, Y.; Ren, Q.; Shi, M.; Liu, J.; Jiang, J.; Ma, H.; Huang, Z.; et al. Understanding the Gut–Kidney Axis among Biopsy-Proven Diabetic Nephropathy, Type 2 Diabetes Mellitus and Healthy Controls: An Analysis of the Gut Microbiota Composition. Acta Diabetol. 2019, 56, 581–592. [Google Scholar] [CrossRef]
- Zeng, Q.; Li, D.; He, Y.; Li, Y.; Yang, Z.; Zhao, X.; Liu, Y.; Wang, Y.; Sun, J.; Feng, X.; et al. Discrepant Gut Microbiota Markers for the Classification of Obesity-Related Metabolic Abnormalities. Sci. Rep. 2019, 9, 13424. [Google Scholar] [CrossRef]
- Asnicar, F.; Berry, S.E.; Valdes, A.M.; Nguyen, L.H.; Piccinno, G.; Drew, D.A.; Leeming, E.; Gibson, R.; Le Roy, C.; Khatib, H.A.; et al. Microbiome Connections with Host Metabolism and Habitual Diet from 1,098 Deeply Phenotyped Individuals. Nat. Med. 2021, 27, 321–332. [Google Scholar] [CrossRef]
- Su, J.; Wang, Y.; Zhang, X.; Ma, M.; Xie, Z.; Pan, Q.; Ma, Z.; Peppelenbosch, M.P. Remodeling of the Gut Microbiome during Ramadan-Associated Intermittent Fasting. Am. J. Clin. Nutr. 2021, 113, 1332–1342. [Google Scholar] [CrossRef]
- Zhu, Q.; Gao, R.; Zhang, Y.; Pan, D.; Zhu, Y.; Zhang, X.; Yang, R.; Jiang, R.; Xu, Y.; Qin, H. Dysbiosis Signatures of Gut Microbiota in Coronary Artery Disease. Physiol. Genomics 2018, 50, 893–903. [Google Scholar] [CrossRef] [PubMed]
- Masenga, S.K.; Hamooya, B.; Hangoma, J.; Hayumbu, V.; Ertuglu, L.A.; Ishimwe, J.; Rahman, S.; Saleem, M.; Laffer, C.L.; Elijovich, F.; et al. Recent Advances in Modulation of Cardiovascular Diseases by the Gut Microbiota. J. Hum. Hypertens. 2022, 36, 952–959. [Google Scholar] [CrossRef] [PubMed]
- Belcheva, A.; Irrazabal, T.; Robertson, S.J.; Streutker, C.; Maughan, H.; Rubino, S.; Moriyama, E.H.; Copeland, J.K.; Surendra, A.; Kumar, S.; et al. Gut Microbial Metabolism Drives Transformation of Msh2-Deficient Colon Epithelial Cells. Cell 2014, 158, 288–299. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Honda, T.; Inukai, Y.; Yokoyama, S.; Ito, T.; Imai, N.; Ishizu, Y.; Nakamura, M.; Kawashima, H. Identification of the Microbiome Associated with Prognosis in Patients with Chronic Liver Disease. Microorganisms 2024, 12, 610. [Google Scholar] [CrossRef]
- Mohr, A.E.; Sweazea, K.L.; Bowes, D.A.; Jasbi, P.; Whisner, C.M.; Sears, D.D.; Krajmalnik-Brown, R.; Jin, Y.; Gu, H.; Klein-Seetharaman, J.; et al. Gut Microbiome Remodeling and Metabolomic Profile Improves in Response to Protein Pacing with Intermittent Fasting versus Continuous Caloric Restriction. Nat. Commun. 2024, 15, 4155. [Google Scholar] [CrossRef]
- Poteres, E.; Hubert, N.; Poludasu, S.; Brigando, G.; Moore, J.; Keeler, K.; Isabelli, A.; Ibay, I.C.V.; Alt, L.; Pytynia, M.; et al. Selective Regional Alteration of the Gut Microbiota by Diet and Antibiotics. Front. Physiol. 2020, 11, 797. [Google Scholar] [CrossRef]
- An, R.; Wilms, E.; Gerritsen, J.; Kim, H.K.; Pérez, C.S.; Besseling-van der Vaart, I.; Jonkers, D.M.A.E.; Rijkers, G.T.; de Vos, W.M.; Masclee, A.A.M.; et al. Spatio-Temporal Dynamics of the Human Small Intestinal Microbiome and Its Response to a Synbiotic. Gut Microbes 2024, 16, 2350173. [Google Scholar] [CrossRef]
- Ugras, S. Evaluating of Altered Hydration Status on Effectiveness of Body Composition Analysis Using Bioelectric Impedance Analysis. Libyan J. Med. 2020, 15, 1741904. [Google Scholar] [CrossRef]


| Characteristics | eTRE + ER (n = 33, 14 #) | lTRE + ER (n = 23, 14 #) | ER (n = 20, 15 #) | p-Value |
|---|---|---|---|---|
| Sex (F/M) | 8/25, 5/9 # | 10/13, 5/9 # | 10/10, 7/8 # | 0.124/0.783 # |
| Age (years) | 44.3 ± 8.7 | 49.2 ± 8.7 | 48.4 ± 6.4 | 0.110 |
| Baseline body mass (kg) | 84.7 ± 12.7 | 87.6 ± 9.9 | 86.7 ± 15.6 | 0.517 |
| 1 Body mass change (kg) | −6.5 ± 2.5 *** | −6.0 ± 2.8 *** | −5.5 ± 2.9 *** | 0.478 |
| 2 Body mass follow-up change (kg) | 1.3 ± 2.8 | 1.2 ± 2.9 | 1.5 ± 2.3 * | 0.978 |
| Baseline BMI (kg/m2) | 29.0 ± 3.0 | 28.8 ± 2.0 | 29.1 ± 3.8 | 0.845 |
| 1 BMI change (kg/m2) | −2.2 ± 1.0 *** | −2.0 ± 1.0 *** | −1.8 ± 1.0 *** | 0.300 |
| 2 BMI follow-up change (kg/m2) | 0.4 ± 0.8 | 0.4 ± 0.9 | 0.5 ± 0.8 * | 0.995 |
| Baseline body fat mass (%) | 34.1 ± 6.4 | 31.8 ± 7.1 | 30.4 ± 6.9 | 0.096 |
| 1 Body fat mass change (%) | −3.5 ± 2.4 *** | −2.5 ± 1.7 *** | −2.7 ± 1.6 *** | 0.142 |
| 2 Body fat mass follow-up change (%) | 0.6 ± 2.8 | 0.6 ± 1.8 | 0.9 ± 1.5 * | 0.840 |
| Baseline muscle mass (kg) | 53.1 ± 10.7 | 57.0 ± 10.8 | 57.4 ± 11.6 | 0.328 |
| 1 Muscle mass change (kg) | −1.5 ± 1.1 *** | −1.9 ± 1.2 *** | −1.7 ± 1.4 *** | 0.382 |
| 2 Muscle mass follow-up change (kg) | 0.4 ± 1.3 | 0.1 ± 1.0 | 0.3 ± 1.1 | 0.793 |
| REE (kcal/day) | 1570 ± 370 | 1680 ± 360 | 1650 ± 360 | 0.510 |
| 1 REE change (kcal/day) | −160 ± 290 ** | −120 ± 230 * | −130 ± 200 ** | 0.844 |
| 2 REE follow-up change (kcal/day) | −60 ± 320 | −90 ± 230 | 100 ± 180 | 0.124 |
| Baseline SBP (mmHg) | 134 ± 16 | 133 ± 10 | 131 ± 13 | 0.662 |
| 1 SBP change (mmHg) | −11 ± 12 *** | −10 ± 14 ** | −9 ± 13 ** | 0.782 |
| 2 SBP follow-up change (mmHg) | 1 ± 13 | 2 ± 13 | 4 ± 11 | 0.654 |
| Baseline DBP (mmHg) | 88 ± 9 | 85 ± 9 | 83 ± 10 | 0.130 |
| 1 DBP change (mmHg) | −12 ± 8 *** | −10 ± 9 *** | −6 ± 6 *** | 0.031 b |
| 2 DBP follow-up change (mmHg) | 4 ± 7 * | 2 ± 9 | 1 ± 8 | 0.377 |
| Baseline fasting glucose (mmol/L) | 5.16 ± 0.55 | 5.41 ± 0.47 | 5.16 ± 0.69 | 0.249 |
| 1 Glucose change (mmol/L) | −0.41 ± 0.55 *** | −0.14 ± 0.30 | −0.10 ± 0.55 | 0.047 a,b |
| 2 Glucose follow-up change (mmol/L) | 0.19 ± 0.40 | 0.05 ± 0.37 | 0.22 ± 0.50 | 0.582 |
| Baseline total cholesterol (mmol/L) | 5.09 ± 1.03 | 5.86 ± 1.14 | 5.13 ± 0.85 | 0.019 a |
| 1 Total cholesterol change (mmol/L) | −0.21 ± 0.58 * | −0.24 ± 0.58 * | −0.24 ± 0.55 * | 0.938 |
| 2 Total cholesterol follow-up (mmol/L) change | 0.10 ± 0.56 | −0.05 ± 0.74 | −0.25 ± 0.65 | 0.369 |
| Baseline triacylglycerols (mmol/L) | 1.31 ± 1.45 | 1.67 ± 1.67 | 1.29 ± 0.63 | 0.209 |
| 1 Triacylglycerol change (mmol/L) | −0.24 ± 0.80 * | −0.27 ± 0.53 * | −0.08 ± 0.31 | 0.916 |
| 2 Triacylglycerol follow-up change (mmol/L) | 0.11 ± 0.39 | −0.22 ± 0.71 | −0.01 ± 0.40 | 0.236 |
| Baseline energy intake (kcal) | 2320 ± 480 | 2360 ± 910 | 2380 ± 530 | 0.638 |
| 1 Energy intake change (kcal) | −650 ± 480 *** | −580 ± 650 *** | −700 ± 300 *** | 0.378 |
| 2 Energy intake follow-up change (kcal) | 50 ± 380 | 20 ± 280 | 280 ± 430 * | 0.134 |
| Baseline eating window (h) | 12.9 ± 1.0 | 12.6 ± 1.1 | 12.7 ± 1.1 | 0.619 |
| 1 Eating window at week 12 (h) | 8.1 ± 0.5 *** | 8.0 ± 0.3 *** | 12.1 ± 0.2 | <0.001 b,c |
| 2 Eating window at follow-up (h) | 10.4 ± 1.9 ** | 8.6 ± 0.9 * | 12.1 ± 0.9 | 0.002 a,b,c |
| Gut Microbiota at the Phylum Level | eTRE + ER (n = 33, 14 #) | lTRE + ER (n = 23, 14 #) | ER (n = 20, 15 #) | p-Value |
|---|---|---|---|---|
| Baseline Bacteroidota (%) | 49.7 ± 20.8 | 43.5 ± 18.7 | 44.3 ± 23.2 | 0.413 |
| 1 Bacteroidota after 3-month change (%) | −4.4 ± 20.9 | 5.0 ± 18.6 | 0.6 ± 17.0 | 0.201 |
| 2 Bacteroidota follow-up change (%) | 10.2 ± 13.2 * | 1.0 ± 23.0 | 3.0 ± 16.8 | 0.377 |
| Baseline Bacillota (%) | 43.1 ± 19.9 | 51.4 ± 20.4 | 50.3 ± 24.0 | 0.241 |
| 1 Bacillota after 3-month change (%) | 4.7 ± 21.7 | −6.6 ± 20.9 | −1.0 ± 18.7 | 0.123 |
| 2 Bacillota follow-up change (%) | −8.5 ± 13.4 * | −1.9 ± 24.5 | −4.9 ± 14.6 | 0.632 |
| Baseline Desulfobacterota (%) | 0.4 ± 0.8 | 0.3 ± 0.5 | 0.2 ± 0.4 | 0.431 |
| 1 Desulfobacterota after 3-month change (%) | −0.1 ± 0.8 | −0.1 ± 0.3 | 0.0 ± 0.3 | 0.475 |
| 2 Desulfobacterota follow-up change (%) | 0.1 ± 0.2 * | 0.2 ± 0.4 * | 0.1 ± 0.3 * | 0.948 |
| Baseline Proteobacteria (%) | 4.9 ± 6.6 | 3.7 ± 5.1 | 3.8 ± 5.1 | 0.503 |
| 1 Proteobacteria after 3-month change (%) | −0.4 ± 6.7 | 0.6 ± 8.0 | −0.5 ± 5.1 | 0.595 |
| 2 Proteobacteria follow-up change (%) | −1.1 ± 5.2 | 1.3 ± 4.4 | 3.0 ± 6.8 | 0.433 |
| Baseline Cyanobacteria (%) | 0.5 ± 1.4 | 0.3 ± 0.7 | 0.5 ± 1.4 | 0.953 |
| 1 Cyanobacteria after 3-month change (%) | 0.5 ± 2.7 | 0.6 ± 3.0 | 0.5 ± 2.9 | 0.920 |
| 2 Cyanobacteria follow-up change (%) | −0.7 ± 2.5 | −0.6 ± 4.3 | −0.6 ± 2.7 | 0.556 |
| Baseline Actinobacteriota (%) | 0.4 ± 0.5 | 0.4 ± 0.7 | 0.4 ± 0.6 | 0.578 |
| 1 Actinobacteriota after 3-month change (%) | 0.3 ± 1.1 | −0.2 ± 0.8 | −0.1 ± 0.5 | 0.297 |
| 2 Actinobacteriota follow-up change (%) | −0.2 ± 0.6 | −0.1 ± 0.6 | 0.0 ± 0.4 | 0.459 |
| Baseline Verrucomicrobiota (%) | 1.0 ± 2.4 | 0.3 ± 0.5 | 0.2 ± 0.4 | 0.072 |
| 1 Verrucomicrobiota after 3-month change (%) | −0.4 ± 2.3 | 0.6 ± 2.2 | 0.5 ± 1.3 * | 0.541 |
| 2 Verrucomicrobiota follow-up change (%) | 0.3 ± 1.8 | 0.0 ± 0.5 | −0.4 ± 1.3 | 0.966 |
| Baseline Bacillota/Bacteroidota ratio | 1.5 ± 1.6 | 3.4 ± 8.4 | 2.1 ± 2.3 | 0.336 |
| 1 Bacillota/Bacteroidota ratio after 3-month change | 0.2 ± 1.5 | −2.0 ± 7.6 | −0.7 ± 1.9 | 0.188 |
| 2 Bacillota/Bacteroidota ratio follow-up change | −1.1 ± 1.8 * | −0.1 ± 1.0 | −0.1 ± 0.8 | 0.071 |
| Changes in Relative Abundances—Different Between Groups (Genera) | Changes in Metabolic and Anthropometric Parameters—Different Between Groups | |
|---|---|---|
| ∆ Glucose (mmol/L) | ∆ DBP (mmHg) | |
| ∆ Faecalibacterium | −0.226 * | −0.029 |
| ∆ Subdoligranulum | 0.056 | −0.238 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Habe, B.; Črešnovar, T.; Hladnik, M.; Pražnikar, J.; Kenig, S.; Bandelj, D.; Mohorko, N.; Petelin, A.; Jenko Pražnikar, Z. Effects of Time-Restricted Eating (Early and Late) Combined with Energy Restriction vs. Energy Restriction Alone on the Gut Microbiome in Adults with Obesity. Nutrients 2025, 17, 2284. https://doi.org/10.3390/nu17142284
Habe B, Črešnovar T, Hladnik M, Pražnikar J, Kenig S, Bandelj D, Mohorko N, Petelin A, Jenko Pražnikar Z. Effects of Time-Restricted Eating (Early and Late) Combined with Energy Restriction vs. Energy Restriction Alone on the Gut Microbiome in Adults with Obesity. Nutrients. 2025; 17(14):2284. https://doi.org/10.3390/nu17142284
Chicago/Turabian StyleHabe, Bernarda, Tanja Črešnovar, Matjaž Hladnik, Jure Pražnikar, Saša Kenig, Dunja Bandelj, Nina Mohorko, Ana Petelin, and Zala Jenko Pražnikar. 2025. "Effects of Time-Restricted Eating (Early and Late) Combined with Energy Restriction vs. Energy Restriction Alone on the Gut Microbiome in Adults with Obesity" Nutrients 17, no. 14: 2284. https://doi.org/10.3390/nu17142284
APA StyleHabe, B., Črešnovar, T., Hladnik, M., Pražnikar, J., Kenig, S., Bandelj, D., Mohorko, N., Petelin, A., & Jenko Pražnikar, Z. (2025). Effects of Time-Restricted Eating (Early and Late) Combined with Energy Restriction vs. Energy Restriction Alone on the Gut Microbiome in Adults with Obesity. Nutrients, 17(14), 2284. https://doi.org/10.3390/nu17142284







