Effect of Creatine Monohydrate on Spatial Working Memory, Body Weight, and Food Intake in Male and Female Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Housing and Care
2.2. Habituation Period
2.3. Supplement Administration
2.4. Morris Water Maze Test
2.5. Statistical Analysis
3. Results
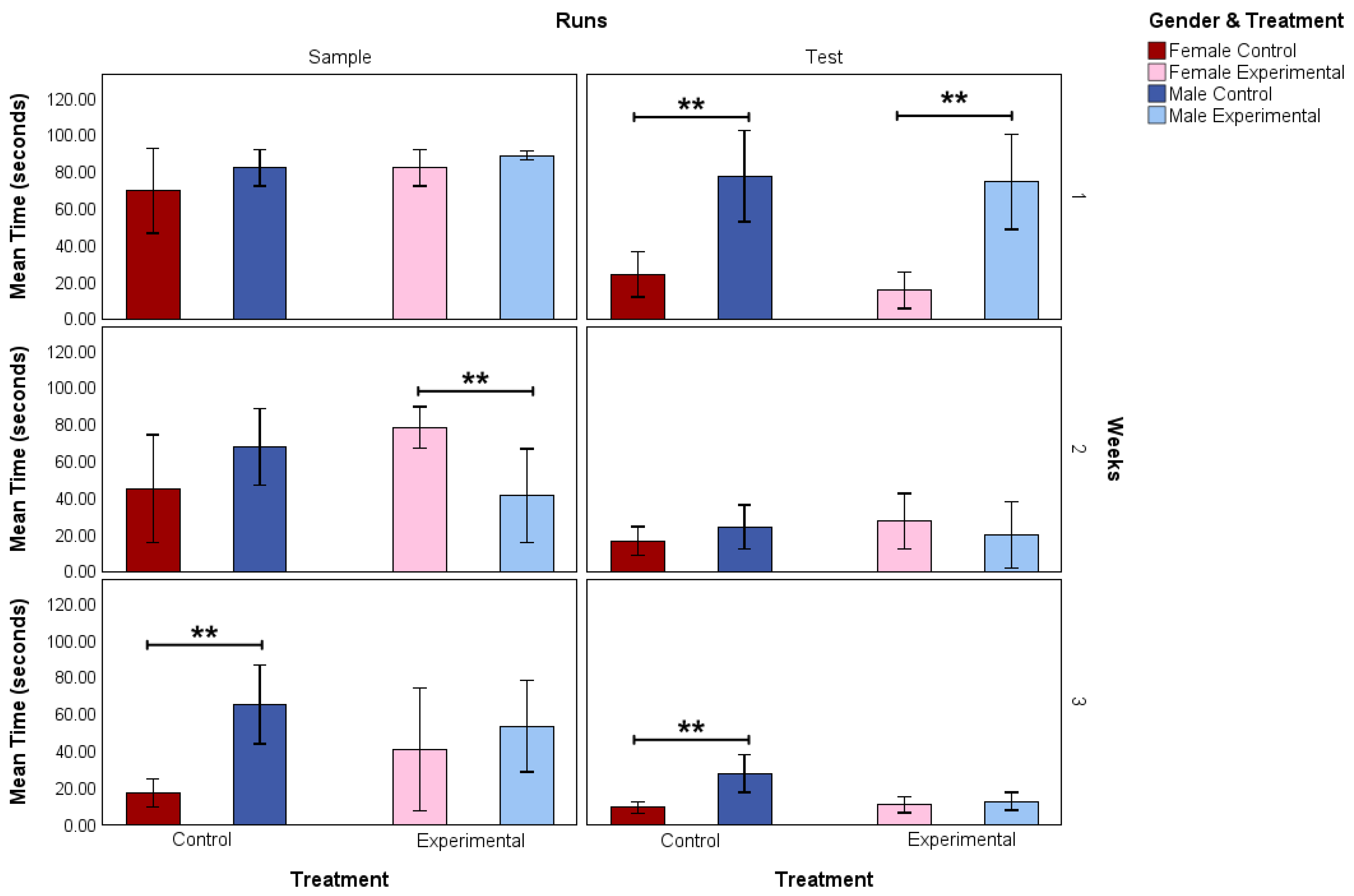
3.1. Morris Water Maze
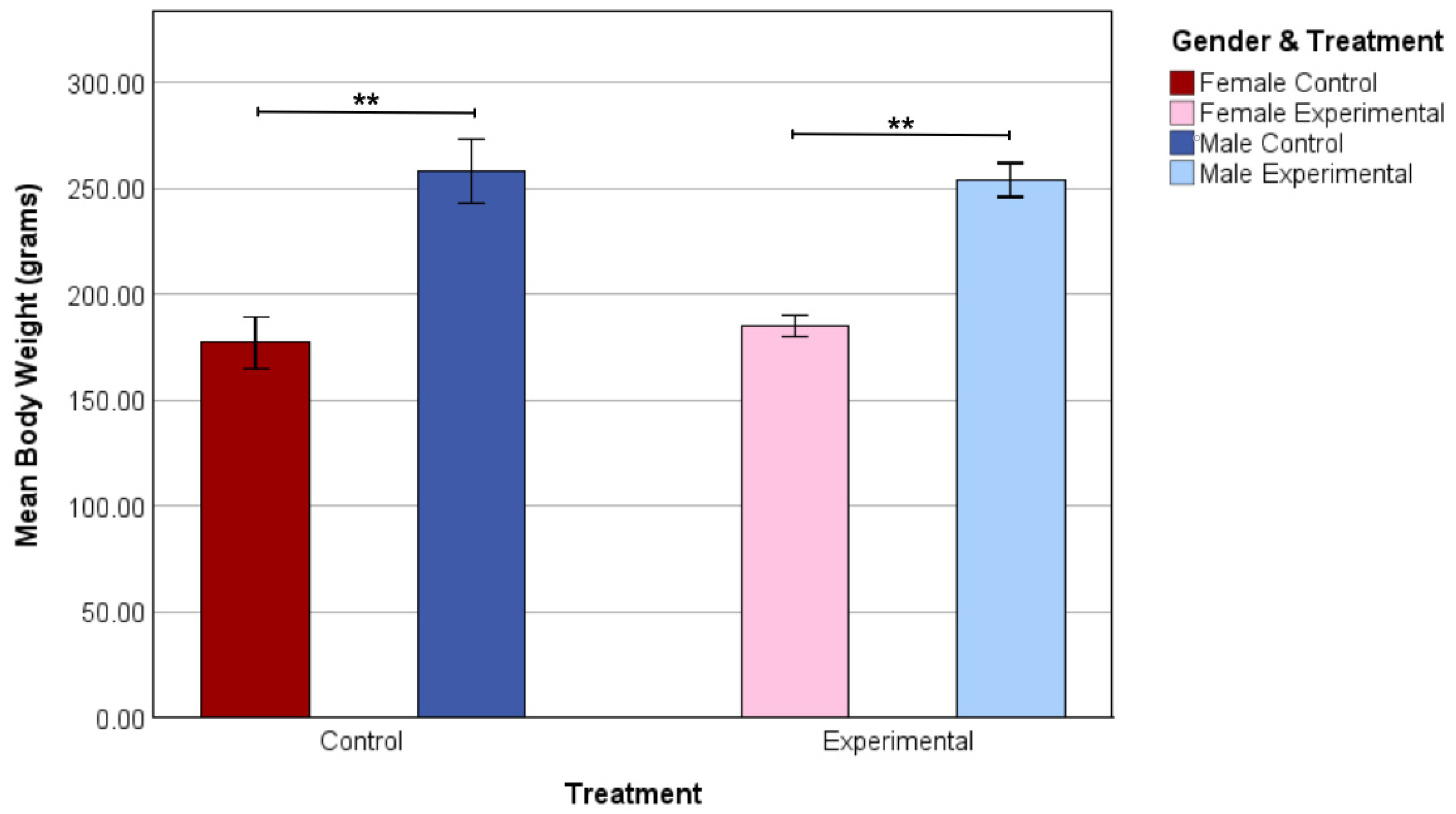
3.2. Body Weight
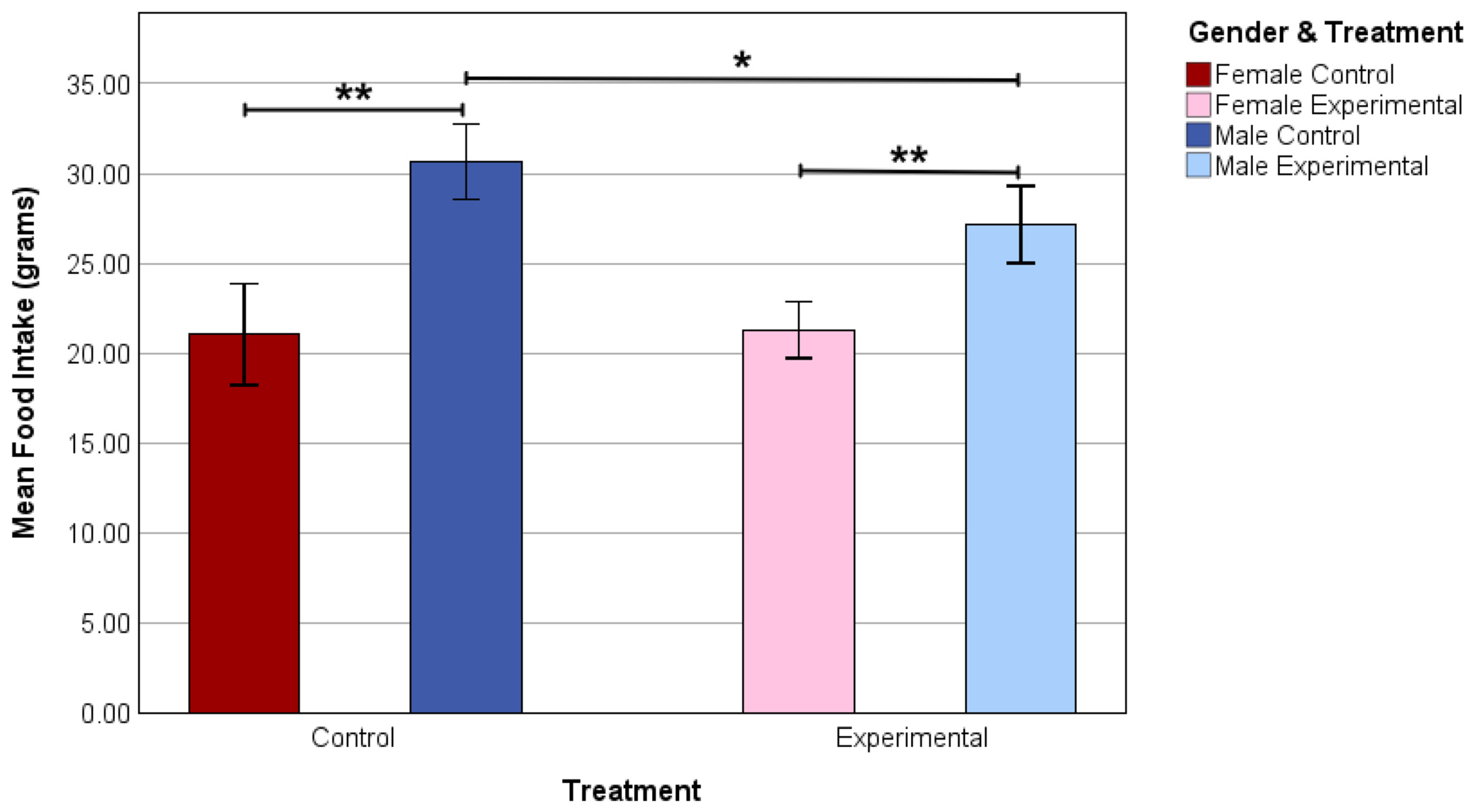
3.3. Food Intake
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Avgerinos, K.I.; Spyrou, N.; Bougioukas, K.I.; Kapogiannis, D. Effects of creatine supplementation on cognitive function of healthy individuals: A systematic review of randomized controlled trials. Exp. Gerontol. 2018, 108, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Ostojic, S.M. Creatine as a food supplement for the general public. J. Funct. Foods 2021, 83, 104568. [Google Scholar] [CrossRef]
- Hultman, E.; Soderlund, K.; Timmons, J.A.; Cedarblad, G.; Greenhaff, P.L. Muscle creatine loading in men. J. Appl. Physiol. 1996, 81, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Jäger, R.; Purpura, M.; Shao, A.; Inoue, T.; Kreider, R.B. Analysis of the efficacy, safety, and regulatory status of novel forms of creatine. Amino Acids 2011, 40, 1369–1383. [Google Scholar] [CrossRef]
- Roschel, H.; Gualano, B.; Ostojic, S.M.; Rawson, E.S. Creatine supplementation and brain health. Nutrients 2021, 13, 586. [Google Scholar] [CrossRef]
- Turner, C.E.; Gant, N.; Byblow, W.D. Creatine supplementation enhances corticomotor excitability and cognitive performance during oxygen deprivation. J. Neurosci. 2015, 35, 1773–1780. [Google Scholar] [CrossRef]
- McMorris, T.; Harris, R.C.; Howard, A.N.; Langridge, G.; Hall, B.; Corbett, J.; Dicks, M.; Hodgson, C. Creatine supplementation, sleep deprivation, cortisol, melatonin and behavior. Physiol. Behav. 2007, 90, 21–28. [Google Scholar] [CrossRef]
- Yildiz, A.; Ozdemir, E.; Gulturk, S.; Erdal, S. The effects of creatine long-term supplementation on muscle morphology and swimming performance in rats. JSSM 2009, 8, 516–522. [Google Scholar]
- Brannon, T.A.; Adams, G.R.; Conniff, C.L.; Baldwin, K.M. Effects of creatine loading and training on running performance and biochemical properties of rat skeletal muscle. Med. Sci. Sports Exerc. 1997, 29, 489–495. [Google Scholar] [CrossRef]
- Seidel dos Santos, F.; Augusto da Silva, L.; Pochapski, J.A.; Raczenski, A.; Claudio da Silva, W.; Grassiolli, S.; Malfatti, C.R.M. Effects of l-arginine and creatine administration on spatial memory in rats subjected to a chronic variable stress model. Pharm. Biol. 2014, 52, 1033–1038. [Google Scholar] [CrossRef]
- Robinson, J.L.; McBreairty, L.E.; Ryan, R.A.; Randunu, R.; Walsh, C.J.; Martin, G.M.; Brunton, J.A.; Bertolo, R.F. Effects of supplemental creatine and guanidinoacetic acid on spatial memory and the brain of weaned Yucatan miniature pigs. PLoS ONE 2020, 15, e0226806. [Google Scholar] [CrossRef] [PubMed]
- Vorhees, C.V.; Williams, M.T. Morris water maze: Procedures for assessing spatial and related forms of learning and memory. Nat. Protoc. 2006, 1, 848–858. [Google Scholar] [CrossRef]
- Axmacher, N.; Elger, C.E.; Fell, J. Working memory-related hippocampal deactivation interferes with long-term memory formation. J. Neurosci. 2009, 29, 1052–1060. [Google Scholar] [CrossRef] [PubMed]
- Gallbraith, R.A.; Furukawa, M.; Li, M. Possible role of creatine concentrations in the brain in regulating appetite and weight. Brain Res. 2006, 1101, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Dunham, T.; Murphy, J.; Colonna, K.; Fajardo, V.; MacPherson, R.; Ward, W.; Roy, B. The effect of creatine monohydrate supplementation on tissue creatine concentrations in male and female rats. FASEB J. 2020, 34, 1. [Google Scholar] [CrossRef]
- Young, R.E.; Young, J.C. The effect of creatine supplementation on mass and performance of rat skeletal muscle. Life Sci. 2007, 81, 710–716. [Google Scholar] [CrossRef]
- Alraddadi, E.A.; Lillico, R.; Vennerstrom, J.L.; Lakowski, T.M.; Miller, D.W. Absolute oral bioavailability of creatine monohydrate in rats: Debunking a myth. Pharmaceutics 2018, 10, 31. [Google Scholar] [CrossRef]
- Murphy, H.M.; Ekstrand, D.; Tarchick, M.; Wideman, C.H. Modafinil as a cognitive enhancer of spatial working memory in rats. Physiol. Behav. 2015, 142, 126–130. [Google Scholar] [CrossRef]
- Rae, C.; Digney, A.L.; Mcewan, S.R.; Bates, T.C. Oral creatine monohydrate supplementation improves brain performance: A double-blind, placebo-controlled, cross-over trial. Proc. R. Soc. B Biol. Sci. 2003, 1529, 2147–2150. [Google Scholar] [CrossRef]
- McMorris, T.; Mielcarz, G.; Harris, R.C.; Swain, J.P.; Howard, A. Creatine supplementation and cognitive performance in elderly individuals. Aging Neuropsychol. Cogn. 2007, 14, 517–528. [Google Scholar] [CrossRef]
- Rawson, E.S.; Lieberman, H.R.; Walsh, T.M.; Zuber, S.M.; Harhart, J.M.; Matthews, T.C. Creatine supplementation does not improve cognitive function in young adults. Physiol. Behav. 2008, 95, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Allen, P.J.; DeBold, J.F.; Rios, M.; Kanarek, R.B. Chronic high-dose creatine has opposing effects on depression-related gene expression and behavior in intact and sex hormone-treated gonadecatomized male and female rats. Pharmacol. Biochem. Behav. 2015, 130, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Krolick, K.K.; Cao, J.; Gulla, E.M.; Bhardwaj, M.; Marshall, S.J.; Zhou, E.Y.; Kiss, A.J.; Choueiry, F.; Zhu, J.; Shi, H. Subregion-specific transcriptomic profiling of rat brain reveals sex-distinct gene expression impacted by adolescent stress. Neuroscience 2024, 553, 19–39. [Google Scholar] [CrossRef] [PubMed]
- Stoccoro, A. Epigenetic mechanisms underlying sex differences in neurodegenerative diseases. Biology 2025, 14, 98. [Google Scholar] [CrossRef]
- Wu, Y.; Lin, Z.; Li, C.; Lin, X.; Shan, S.; Guo, B.; Zheng, M.; Li, F.; Yuan, L.; Li, Z. Epigenetic regulation in metabolic mechanisms and advances in clinical study. Signal Transduct. Target. Ther. 2023, 8, 98. [Google Scholar] [CrossRef]
- Magali, L.; Poortmans, J.R.; Francaux, M.; Berré, J.; Boisseau, N. No effect of creatine supplementation on human myofibrillar and sarcoplasmic protein synthesis after resistance exercise. Am. J. Phys. 2003, 285, E1089–E1094. [Google Scholar] [CrossRef]
- Delpino, F.M.; Figueiredo, L.M.; Forbes, S.C.; Candow, D.G.; Santos, H.O. Influence of age, sex, and type of exercise on the efficacy of creatine supplementation on lean body mass: A systematic review and meta-analysis of randomized clinical trials. Nutrition 2022, 103–104, 111791. [Google Scholar] [CrossRef]
- Cognuck, S.Q.; Reis, W.L.; Silva, M.; Debarba, L.K.; Mecawi, A.S.; De Paula, F.J.A.; Franci, C.R.; Elias, L.L.K.; Antunes-Rodrigues, J. Sex differences in body composition, metabolism-related hormones, and energy homeostasis during aging in Wistar rats. Physiol. Rep. 2020, 8, e14597. [Google Scholar] [CrossRef]
- De Moraes, S.M.F.; Brogio, T.A.; Zanoni, J.N.; Zapater, M.C.V.U.; Peres, S.B.; Hernandes, L. Creatine supplementation in trained rats causes changes in myenteric neurons and intestinal wall morphometry. Biocell 2013, 37, 37–43. [Google Scholar] [CrossRef]
- Snow, W.M.; Cadonic, C.; Cortes-Perez, C.; Adlimoghaddam, A.; Chowdhury, S.K.R.; Thomson, E.; Anozie, A.; Bernstein, M.J.; Gough, K.; Fernyhough, P.; et al. Sex-specific effects of chronic creatine supplementation on hippocampal-mediated spatial cognition in the 3xTg mouse model of Alzheimer’s disease. Nutrients 2020, 12, 3589. [Google Scholar] [CrossRef]
- Alrabadi, N.; Al-Rabadi, G.J.; Maraqa, R.; Sarayrah, H.; Alzoubi, K.; Alqudah, M.; Al-u’datt, D.G. Androgen effect on body weight and behaviour of male and female rats: Novel insight on the clinical value. Andrologia 2020, 52, e13730. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, A.; Hagiwara, H.; Fujioka, H.; Kimura, F.; Akema, T.; Funabashi, T. Sex differences in feeding behavior in rats: The relationship with neuronal activation in the hypothalamus. Front. Neurosci. 2015, 9, 88. [Google Scholar] [CrossRef] [PubMed]
- Nunez, A.A.; Grundman, M. Testosterone affects food intake and body weight of weanling male rats. Pharmacol. Biochem. Behav. 1982, 16, 933–936. [Google Scholar] [CrossRef] [PubMed]
- Ellery, S.J.; Walker, D.W.; Dickinson, H. Creatine for women: A review of the relationship between creatine and the reproductive cycle and female-specific benefits of creatine therapy. Amino Acids 2016, 48, 1807–1817. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wideman, C.; Iemma, A.; Janolo, O.; Kalinina, A.; Murphy, H. Effect of Creatine Monohydrate on Spatial Working Memory, Body Weight, and Food Intake in Male and Female Rats. Nutrients 2025, 17, 2218. https://doi.org/10.3390/nu17132218
Wideman C, Iemma A, Janolo O, Kalinina A, Murphy H. Effect of Creatine Monohydrate on Spatial Working Memory, Body Weight, and Food Intake in Male and Female Rats. Nutrients. 2025; 17(13):2218. https://doi.org/10.3390/nu17132218
Chicago/Turabian StyleWideman, Cyrilla, Alexandria Iemma, Olivia Janolo, Anastasiya Kalinina, and Helen Murphy. 2025. "Effect of Creatine Monohydrate on Spatial Working Memory, Body Weight, and Food Intake in Male and Female Rats" Nutrients 17, no. 13: 2218. https://doi.org/10.3390/nu17132218
APA StyleWideman, C., Iemma, A., Janolo, O., Kalinina, A., & Murphy, H. (2025). Effect of Creatine Monohydrate on Spatial Working Memory, Body Weight, and Food Intake in Male and Female Rats. Nutrients, 17(13), 2218. https://doi.org/10.3390/nu17132218



