Nano-Encapsulated Phytosterols Ameliorate Hypercholesterolemia in Mice via Dual Modulation of Cholesterol Metabolism Pathways
Abstract
1. Introduction
2. Materials and Methods
2.1. PN Preparation
2.2. Diet Preparation, Experimental Design, and Sample Collection
2.3. Biochemical and Histological Assessments
2.3.1. Plasma Lipid Profile
2.3.2. Hepatic Lipid Content
2.3.3. Fecal Sterol and Cholesterol Analysis
2.3.4. Liver Histology
2.3.5. Gene Expression Analysis (RT-qPCR)
2.3.6. Protein Expression Analysis (Western Blot)
2.4. Data Calculations and Statistical Analysis
3. Results
3.1. Average Body Weight and Daily Feed Intake
3.2. Serum and Liver Lipid Profile
3.3. Liver Histomorphology
3.4. Fecal Cholesterol and Phytosterol Excretion
3.5. Expression of Genes and Proteins Involved in Lipid Metabolism
4. Discussion
4.1. Dual Modulation of Cholesterol Metabolism: Synthesis Suppression and Catabolism Enhancement
4.2. Intestinal Cholesterol Absorption Blockade and Fecal Sterol Excretion
4.3. Liver Protection and Histological Improvements
4.4. Comparative Effectiveness and Advantages over Simvastatin
4.5. Role of LDLR and LXR-α: Feedback Regulation and Complexity
4.6. Potential Microbiota–Bile Acid–Liver Axis Mediated Effects of Phytosterols
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Frampton, J.E. Inclisiran: A Review in Hypercholesterolemia. Am. J. Cardiovasc. Drugs 2023, 23, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Mormone, A.; Tortorella, G.; Esposito, F.; Caturano, A.; Marrone, A.; Cozzolino, D.; Galiero, R.; Marfella, R.; Sasso, F.C.; Rinaldi, L. Advances in Pharmacological Approaches for Managing Hypercholesterolemia: A Comprehensive Overview of Novel Treatments. Biomedicines 2024, 12, 432. [Google Scholar] [CrossRef] [PubMed]
- Pećin, I.; Reiner, Ž. Novel experimental agents for the treatment of hypercholesterolemia. J. Exp. Pharmacol. 2021, 13, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Turini, E.; Sarsale, M.; Petri, D.; Totaro, M.; Lucenteforte, E.; Tavoschi, L.; Baggiani, A. Efficacy of Plant Sterol-Enriched Food for Primary Prevention and Treatment of Hypercholesterolemia: A Systematic Literature Review. Foods 2022, 11, 839. [Google Scholar] [CrossRef]
- Feng, S.; Wang, L.; Shao, P.; Sun, P.; Yang, C.S. A review on chemical and physical modifications of phytosterols and their influence on bioavailability and safety. Crit Rev Food Sci Nutr. 2022, 62, 5638–5657. [Google Scholar] [CrossRef]
- Li, X.; Xin, Y.; Mo, Y.; Marozik, P.; He, T.; Guo, H. The bioavailability and biological activities of phytosterols as modulators of cholesterol metabolism. Molecules 2022, 27, 523. [Google Scholar] [CrossRef]
- Pandak, W.M.; Schwarz, C.; Hylemon, P.B.; Mallonee, D.; Valerie, K.; Heuman, D.M.; Fisher, R.A.; Redford, K.; Vlahcevic, Z.R. Effects of CYP7A1 overexpression on cholesterol and bile acid homeostasis. Am. J. Physiol.—Gastrointest. Liver Physiol. 2001, 281, G878–G889. [Google Scholar] [CrossRef]
- Zhang, Z.S.; Hou, D.M.; He, W.; Wang, J.Y. Effect of phytosterol on blood lipid in hyperlipidemia rats. Food Sci. 2011, 32, 306–309. [Google Scholar] [CrossRef]
- Sharpe, L.J.; Brown, A.J. Controlling cholesterol synthesis beyond 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR). J. Biol. Chem. 2013, 288, 18707–18715. [Google Scholar] [CrossRef]
- Li, A.; Zhu, A.; Kong, D.; Wang, C.; Liu, S.; Zhou, L.; Cheng, M. Water-dispersible phytosterol nanoparticles: Preparation, characterization, and in vitro digestion. Front. Nutr. 2022, 8, 793009. [Google Scholar] [CrossRef]
- Zhang, J.; Pan, W.; Wang, Y.; Zhang, C.; Wang, C.; Li, S.; Chen, F.; Zhu, A. Enhancing vaccine efficacy: Evaluating the superiority of cationic liposome-embedded squalene adjuvant against PCV2 infection. Virology 2024, 600, 110251. [Google Scholar] [CrossRef] [PubMed]
- Kuang, M.; Yu, H.; Qiao, S.; Huang, T.; Zhang, J.; Sun, M.; Shi, X.; Chen, H. A Novel Nano-Antimicrobial Polymer Engineered with Chitosan Nanoparticles and Bioactive Peptides as Promising Food Biopreservative Effective against Foodborne Pathogen E. coli O157-Caused Epithelial Barrier Dysfunction and Inflammatory Responses. Int. J. Mol. Sci. 2021, 22, 13580. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.T.; Zhang, J.Q.; Sun, M.C.; Zhao, Y.; Wang, L.; Chen, X. Polymeric Nanohybrids Engineered by Chitosan Nanoparticles and Antimicrobial Peptides as Novel Antimicrobials in Food Biopreservatives: Risk Assessment and Anti-Foodborne Pathogen. J. Agric. Food Chem. 2022, 70, 12535–12549. [Google Scholar] [CrossRef] [PubMed]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and Validation of a Histological Scoring System for Nonalcoholic Fatty Liver Disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef]
- Hayat, A.; Aal, A.E.; Lamiaa, A.; El-Mahdy, M.M. Combination of Carvacrol with Simvastatin Improves the Lipid-Lowering Efficacy and Alleviates Simvastatin Side Effects. J. Biochem. Mol. Toxicol. 2017, 31, e21981. [Google Scholar] [CrossRef]
- Hu, N.; Chen, C.; Wang, J.; Huang, J.; Yao, D.; Li, C. Atorvastatin Ester Regulates Lipid Metabolism in Hyperlipidemia Rats via the PPAR-signaling Pathway and HMGCR Expression in the Liver. Int. J. Mol. Sci. 2021, 22, 11107. [Google Scholar] [CrossRef]
- Horton, J.D.; Goldstein, J.L.; Brown, M.S. SREBPs: Activators of the complete program of cholesterol and fatty acid synthesis in the liver. J. Clin. Investig. 2002, 109, 1125–1131. [Google Scholar] [CrossRef]
- Benatzy, Y.; Palmer, M.A.; Lütjohann, D.; Ohno, R.; Kampschulte, N.; Schebb, N.H.; Fuhrmann, D.C.; Snodgrass, R.G.; Brüne, B. ALOX15B controls macrophage cholesterol homeostasis via lipid peroxidation, ERK1/2 and SREBP2. Redox. Biol. 2024, 72, 103149. [Google Scholar] [CrossRef]
- Xu, M.; Jiang, S.; Tang, S.; Zhu, M.; Hu, Y.; Li, J.; Yan, J.; Qin, C.; Tan, D.; An, Y. Nuclear SREBP2 condensates regulate the transcriptional activation of lipogenic genes and cholesterol homeostasis. Nat. Metab. 2025, 7, 1034–1051. [Google Scholar] [CrossRef]
- De Fabiani, E.; Mitro, N.; Anzulovich, A.C.; Pinelli, A.; Galli, G.; Crestani, M. The negative effects of bile acids and tumor necrosis factor-α on the transcription of cholesterol 7α-hydroxylase gene (CYP7A1) converge to hepatic nuclear factor-4: A novel mechanism of feedback regulation of bile acid synthesis mediated by nuclear receptors. J. Biol. Chem. 2001, 276, 30708–30716. [Google Scholar] [CrossRef]
- Tempel, W.; Grabovec, I.; MacKenzie, F.; Dichenko, Y.V.; Usanov, S.A.; Gilep, A.A.; Park, H.; Strushkevich, N. Structural characterization of human cholesterol 7α-hydroxylase. J. Lipid Res. 2014, 55, 1925–1932. [Google Scholar] [CrossRef] [PubMed]
- Song, K.H.; Li, T.; Owsley, E.; Chiang, J. A putative role of micro RNA in regulation of cholesterol 7α-hydroxylase expression in human hepatocytes. J. Lipid Res. 2010, 51, 2223–2233. [Google Scholar] [CrossRef] [PubMed]
- Chun, K.-H. Molecular Targets and Signaling Pathways of microRNA-122 in Hepatocellular Carcinoma. Pharmaceutics 2022, 14, 1380. [Google Scholar] [CrossRef]
- Jang, S.; Lee, M.-S.; Kang, S.-A.; Kim, C.-T.; Kim, Y. Portulaca oleracea L. Extract Regulates Hepatic Cholesterol Metabolism via the AMPK/MicroRNA-33/34a Pathway in Rats Fed a High-Cholesterol Diet. Nutrients 2022, 14, 3330. [Google Scholar] [CrossRef]
- Wang, M.; Tan, Y.; Costa, R.H.; Holterman, A.X.L. In vivo regulation of murine CYP7A1 by HNF-6: A novel mechanism for diminished CYP7A1 expression in biliary obstruction. Hepatology 2004, 40, 600–608. [Google Scholar] [CrossRef]
- Chen, Z.; Ding, H.; Zhu, H.; Huang, S.; Yan, C.; Chen, Z. Additional mechanism for selective absorption of cholesterol and phytosterols. Food Chem. 2024, 458, 140300. [Google Scholar] [CrossRef]
- Stellaard, F.; Lütjohann, D. Phytosterol-Enriched Dietary Supplements for Lowering Plasma LDL-Cholesterol: Yes or No? Nutrients 2025, 17, 654. [Google Scholar] [CrossRef]
- Zhang, R.; Han, Y.; McClements, D.J.; Xu, D.; Chen, S. Production, characterization, delivery, and cholesterol-lowering mechanism of phytosterols: A review. J. Agric. Food Chem. 2022, 70, 2483–2494. [Google Scholar] [CrossRef]
- Eng, J.M.; Estall, J.L. Diet-induced models of non-alcoholic fatty liver disease: Food for thought on sugar, fat, and cholesterol. Cells 2021, 10, 1805. [Google Scholar] [CrossRef]
- Li, H.; Yu, X.; Ou, X.; Ouyang, X.; Tang, C. Hepatic cholesterol transport and its role in non-alcoholic fatty liver disease and atherosclerosis. Prog. Lipid Res. 2021, 83, 101109. [Google Scholar] [CrossRef]
- Poudel, P.; Petropoulos, S.A.; Di Gioia, F. Plant Tocopherols and Phytosterols and Their Bioactive Properties. In Natural Secondary Metabolites: From Nature, Through Science, to Industry; Springer International Publishing: Cham, Switzerland, 2023; pp. 285–319. [Google Scholar] [CrossRef]
- Shen, M.; Yuan, L.; Zhang, J.; Wang, X.; Zhang, M.; Li, H.; Jing, Y.; Zeng, F.; Xie, J. Phytosterols: Physiological Functions and Potential Application. Foods 2024, 13, 1754. [Google Scholar] [CrossRef] [PubMed]
- Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Alexiou, A.; Papadakis, M.; Alsayegh, A.A.; Almohmadi, N.H.; Saad, H.M.; Batiha, G.E.S. Pros and cons for statins use and risk of Parkinson’s disease: An updated perspective. Pharmacol. Res. Perspect. 2023, 11, e01063. [Google Scholar] [CrossRef] [PubMed]
- Sattar, N. Statins and diabetes: What are the connections? Best Pract. Res. Clin. Endocrinol. Metab. 2023, 37, 101749. [Google Scholar] [CrossRef] [PubMed]
- Micallef, M.A.; Garg, M.L. Beyond blood lipids: Phytosterols, statins and omega-3 polyunsaturated fatty acid therapy for hyperlipidemia. J. Nutr. Biochem. 2009, 20, 927–939. [Google Scholar] [CrossRef]
- Du, Y.; Xi, M.; Li, Y.; Zheng, R.; Ding, X.; Li, X.; Zhang, X.; Wang, L.; Xing, J.; Hong, B. Tilianin improves lipid profile and alleviates atherosclerosis in ApoE−/− mice through up-regulation of SREBP2-mediated LDLR expression. Phytomedicine 2023, 109, 154577. [Google Scholar] [CrossRef]
- Li, Y.; Song, Y.; Zhao, M.; Guo, Y.; Yu, C.; Chen, W.; Shao, S.; Xu, C.; Zhou, X.; Zhao, L. A novel role for CRTC2 in hepatic cholesterol synthesis through SREBP-2. Hepatology 2017, 66, 481–497. [Google Scholar] [CrossRef]
- Davis, H.R.; Zhu, L.; Hoos, L.M.; Tetzloff, G.; Maguire, M.; Liu, J.; Yao, X.R.; Iyer, S.P.; Lam, M.; Lund, E.G.; et al. Niemann-Pick C1 Like 1 (NPC1L1) is the intestinal phytosterol and cholesterol transporter and a key modulator of whole-body cholesterol homeostasis. J. Biol. Chem. 2004, 279, 33586–33592. [Google Scholar] [CrossRef]
- Lv, W.J.; Huang, J.Y.; Lin, J.; Wang, Z.; Fang, H.; Zhu, X.; Liu, J.; Cao, Y.; Wang, F.; Zhang, Y. Phytosterols Alleviate Hyperlipidemia by Regulating Gut Microbiota and Cholesterol Metabolism in Mice. Oxid. Med. Cell. Longev. 2023, 2023, 6409385. [Google Scholar] [CrossRef]
- Zhang, Y.; Gu, Y.; Jiang, J.; Shen, H.; Tian, L.; Liu, S.; Jiang, Y. Stigmasterol Attenuates Hepatic Steatosis in Rats by Strengthening the Intestinal Barrier and Improving Bile Acid Metabolism. NPJ Sci. Food 2022, 6, 38. [Google Scholar] [CrossRef]
- Qin, D.; Pan, P.; Lyu, B.; Zhang, R.; Yang, Y.; Su, J.; Pan, W.; Wei, L. Lupeol Improves Bile Acid Metabolism and Metabolic Dysfunction-Associated Steatotic Liver Disease in Mice via FXR Signaling Pathway and Gut-Liver Axis. Biomed. Pharmacother. 2024, 177, 116942. [Google Scholar] [CrossRef]
- Katafuchi, T.; Makishima, M. Molecular Basis of Bile Acid-FXR-FGF15/19 Signaling Axis. Int. J. Mol. Sci. 2022, 23, 6046. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Fang, W.; Li, X.; Chen, Y.; Lu, Q.; Zhou, Y.; Chi, S.; Tan, B.; Zhang, H.; Dong, X.; et al. Increased LDL Receptor by SREBP2 or SREBP2-Induced lncRNA LDLR-AS Promotes Triglyceride Accumulation in Fish. iScience 2022, 25, 104596. [Google Scholar] [CrossRef] [PubMed]

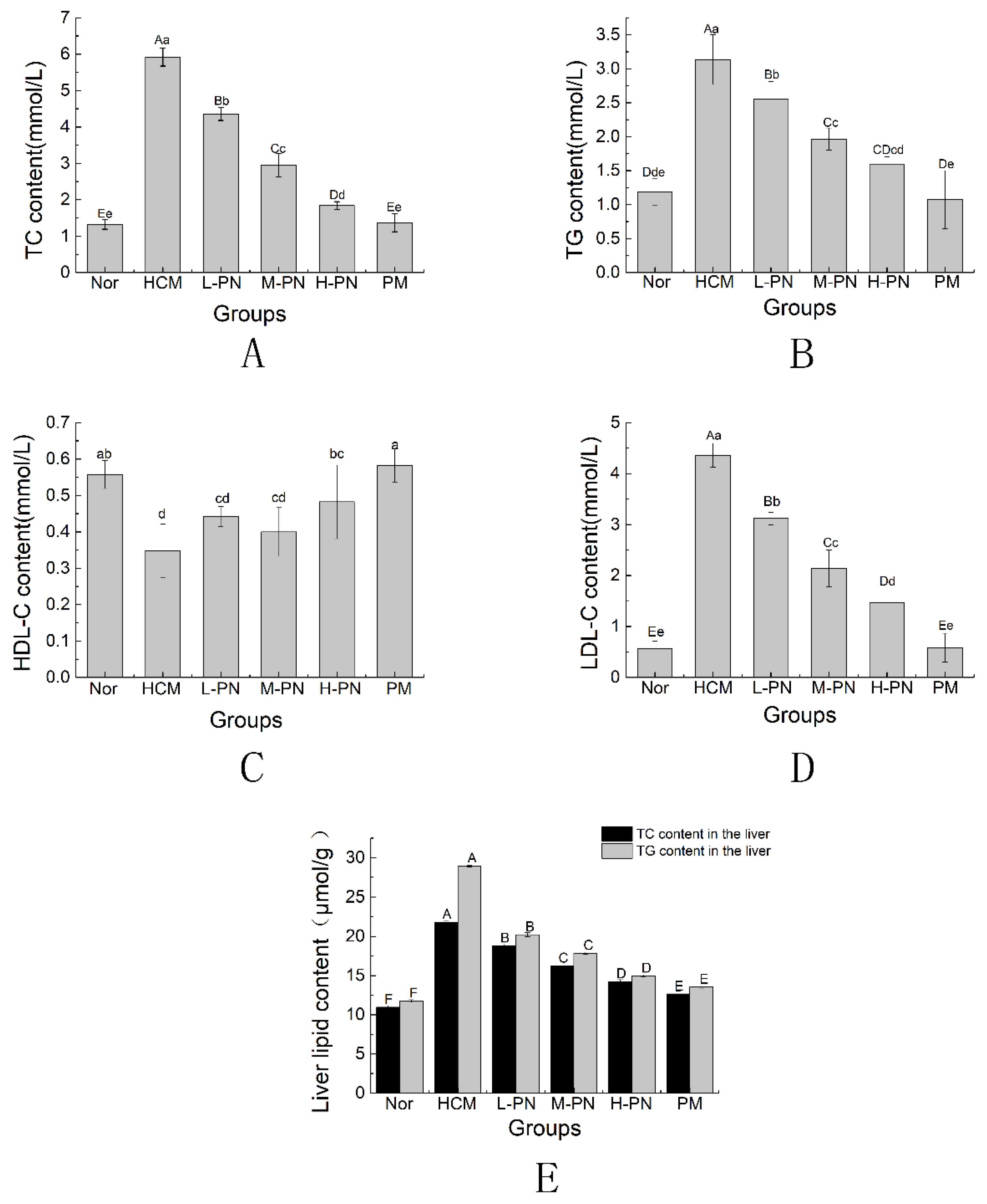
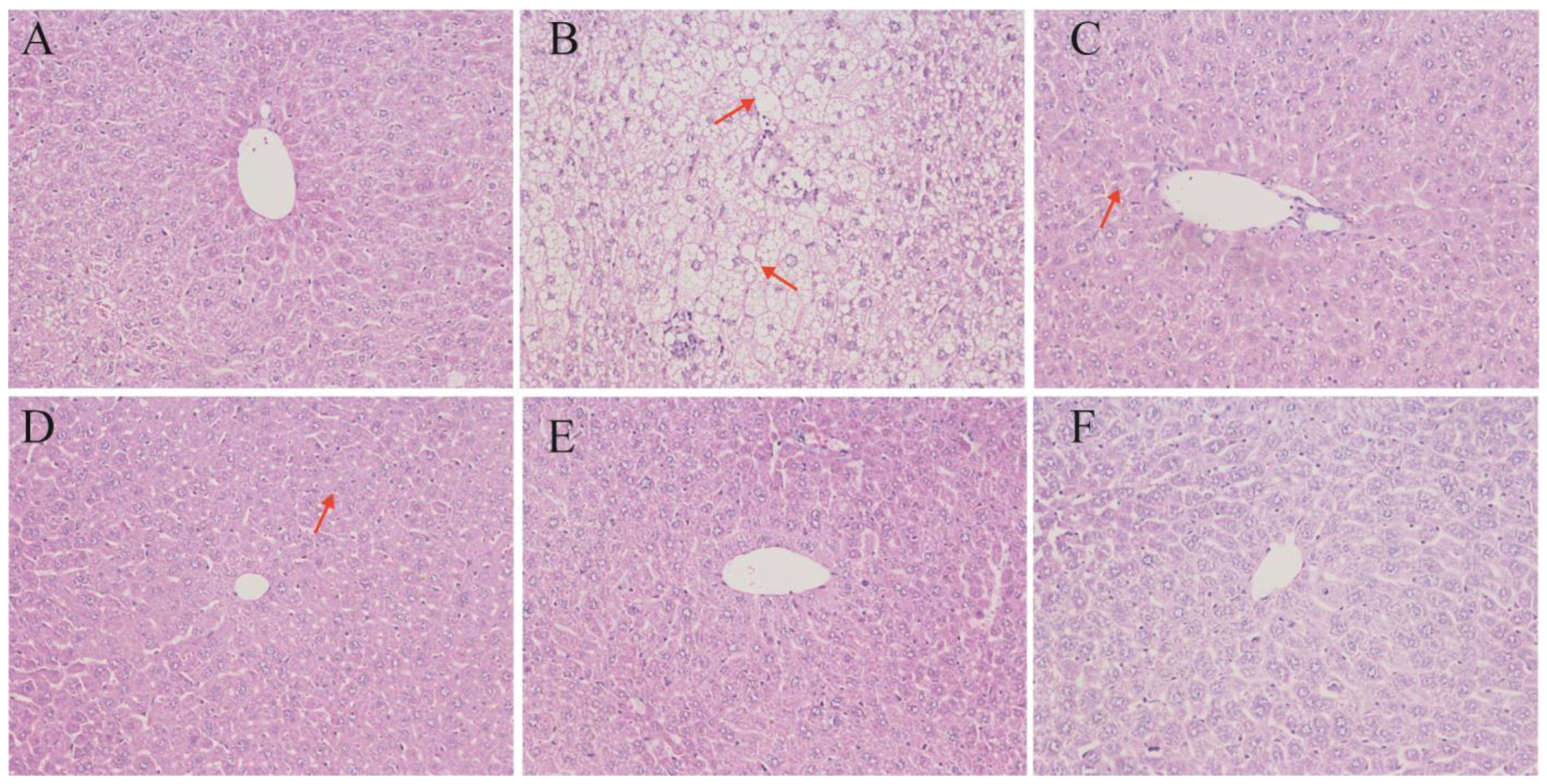

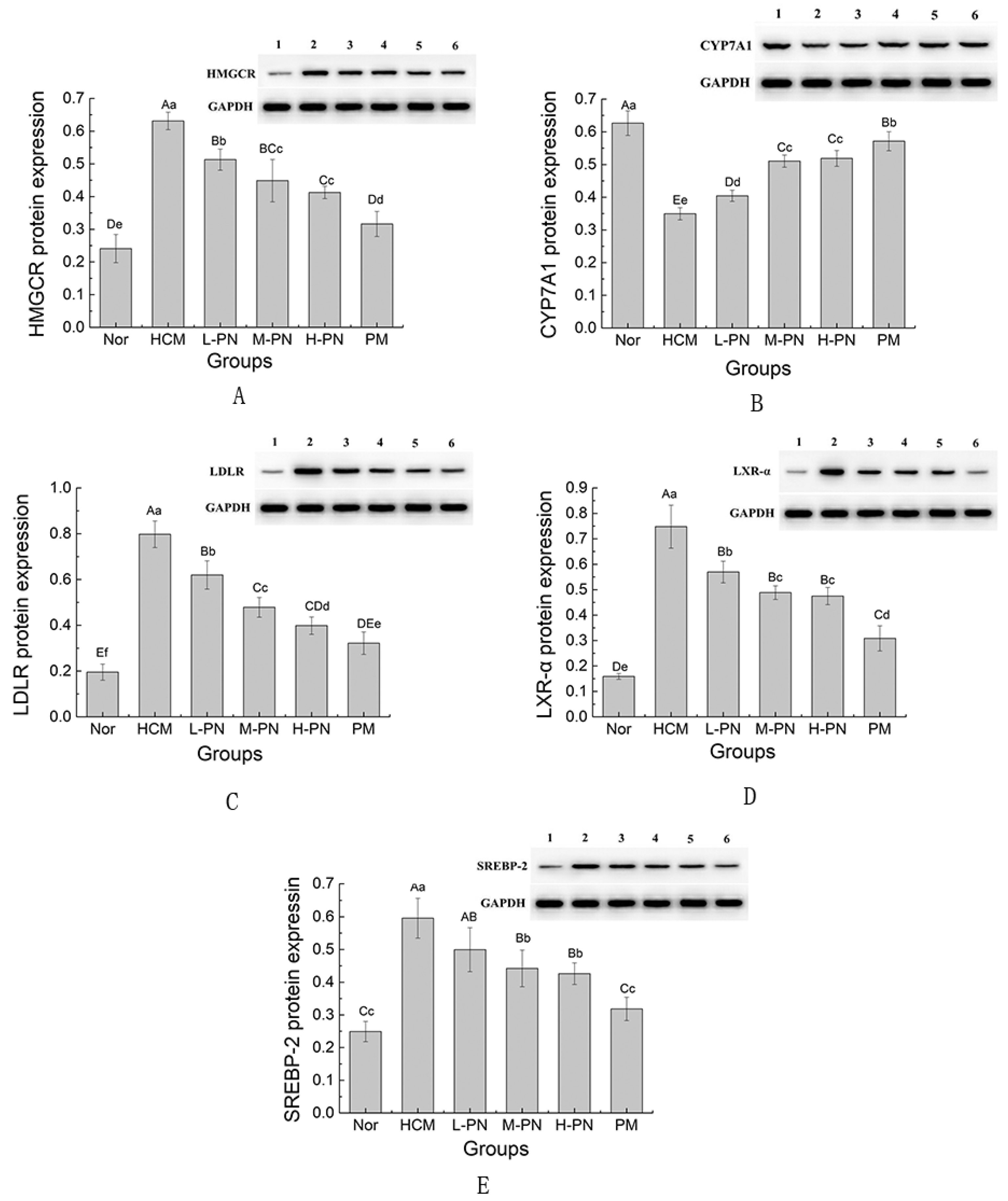
| Groups | Diets |
|---|---|
| Nor | standard diet |
| L-PN | HFD + 4.00 mg/mL of PN (0.5 mL) |
| M-PN | HFD + 8.25 mg/mL of PN (0.5 mL) |
| H-PN | HFD + 12.50 mg/mL of PN (0.5 mL) |
| HCM | HFD + 0.5 mL normal saline |
| PM | HFD + 0.40 mg/mL simvastatin (0.5 mL) |
| Ingredient | Nor | HFD |
|---|---|---|
| Casein | 200 | 200 |
| DL-Methionine | 3 | 3 |
| Corn Starch | 600 | 410 |
| Maltodextrin | 50 | 50 |
| Cellulose | 50 | 50 |
| Sucrose | 1 | 1 |
| Lard | 45 | 225 |
| Cholesterol | 0 | 10 |
| Mineral Mix AIN93 | 23.6 | 23.6 |
| Vitamin Mix AIN93 | 14.4 | 14.4 |
| Choline | 1 | 1 |
| Potassium Citrate | 10 | 10 |
| NaCl | 2 | 2 |
| Fat (%, w/w) | 10 | 45 |
| Energy (kcal/g diet) | 3.87 | 4.69 |
| Primer Name | Sequence (5′-3′) | Amplified Fragment Size (bp) |
|---|---|---|
| SREBP2 | F: CAGACCTAGACCTCGC | 244 |
| R: CTGCTCTTAGCCTCAT | ||
| LDLR | F: GTCTGCTCCCCAACCT | 264 |
| R: TCCTCCTCTTTACGCC | ||
| HMGCR | F: AATAAACCAAACCCCG | 264 |
| R: GACAGCCAAAAGGAAG | ||
| CYP7A1 | F: GAGAAGGAAAGTAGGTGAA | 174 |
| R: AGGGAGTTTGTGATGAAG | ||
| LXRa | F: ATGTTTCTCCTGATTCTGC | 140 |
| R: CTCCAACCCTATCCCTAA | ||
| β Actin | F: CCCATCTACGAGGGCTAT | 145 |
| R: TGTCACGCACGATTTCC |
| Group | Steatosis (0–3) | Inflammation (0–3) | Ballooning (0–2) | Total NAS (0–8) |
|---|---|---|---|---|
| Nor | 0 | 0 | 0 | 0 |
| HCM | 3 | 2 | 2 | 7 |
| PM | 1 | 1 | 1 | 3 |
| L-PN | 2 | 1 | 1 | 4 |
| M-PN | 1 | 1 | 1 | 3 |
| H-PN | 1 | 0–1 | 0–1 | 2–3 |
| Testing Index | Nor | HCM | L-PN | M-PN | H-PN | PM |
|---|---|---|---|---|---|---|
| Cholesterol content (mg/g feces) | 1.26 ± 0.03 A | 11.79 ± 0.24 B | 28.80 ± 0.17 C | 32.73 ± 0.34 D | 36.01 ± 0.34 E | 36.90 ± 0.24 F |
| Sterol content (mg/g feces) | --- | --- | 6.90 ± 0.14 | 10.04 ± 0.05 | 13.07 ± 0.08 | --- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, A.; Pan, W.; Jiao, W.; Peng, K.; Wang, C.; Zhang, C.; Zhang, J. Nano-Encapsulated Phytosterols Ameliorate Hypercholesterolemia in Mice via Dual Modulation of Cholesterol Metabolism Pathways. Nutrients 2025, 17, 2086. https://doi.org/10.3390/nu17132086
Zhu A, Pan W, Jiao W, Peng K, Wang C, Zhang C, Zhang J. Nano-Encapsulated Phytosterols Ameliorate Hypercholesterolemia in Mice via Dual Modulation of Cholesterol Metabolism Pathways. Nutrients. 2025; 17(13):2086. https://doi.org/10.3390/nu17132086
Chicago/Turabian StyleZhu, Aixia, Wenjing Pan, Wenjia Jiao, Kai Peng, Chunwei Wang, Chi Zhang, and Jiaqi Zhang. 2025. "Nano-Encapsulated Phytosterols Ameliorate Hypercholesterolemia in Mice via Dual Modulation of Cholesterol Metabolism Pathways" Nutrients 17, no. 13: 2086. https://doi.org/10.3390/nu17132086
APA StyleZhu, A., Pan, W., Jiao, W., Peng, K., Wang, C., Zhang, C., & Zhang, J. (2025). Nano-Encapsulated Phytosterols Ameliorate Hypercholesterolemia in Mice via Dual Modulation of Cholesterol Metabolism Pathways. Nutrients, 17(13), 2086. https://doi.org/10.3390/nu17132086







