Weizmannia coagulans SA9: A Novel Strategy to Alleviate Type 2 Diabetes
Abstract
1. Introduction
2. Materials and Methods
2.1. Strain Source and Preparation
2.2. Preparation and Functions of Cell-Free Supernatant (CFS), Cell-Free Extract (CFE), and Cell Metabolite Supernatant (CMS)
2.3. Assessment of α-Glucosidase Inhibitory Activity
2.4. Assessment of α-Amylase Inhibition Capacity
2.5. Untargeted Metabolomics and Active Compound Identification
2.6. Animal and Experimental Design
2.7. Observation of Urinary Output in Mice
2.8. Oral Glucose Tolerance Test (OGTT) and Insulin Tolerance Test (ITT)
2.9. Sample Collection
2.10. Analysis of Blood Biochemical Parameters
2.11. Tissue Histopathological Analysis
2.12. Gut Microbiota Analysis
2.13. Statistical Analysis
3. Results
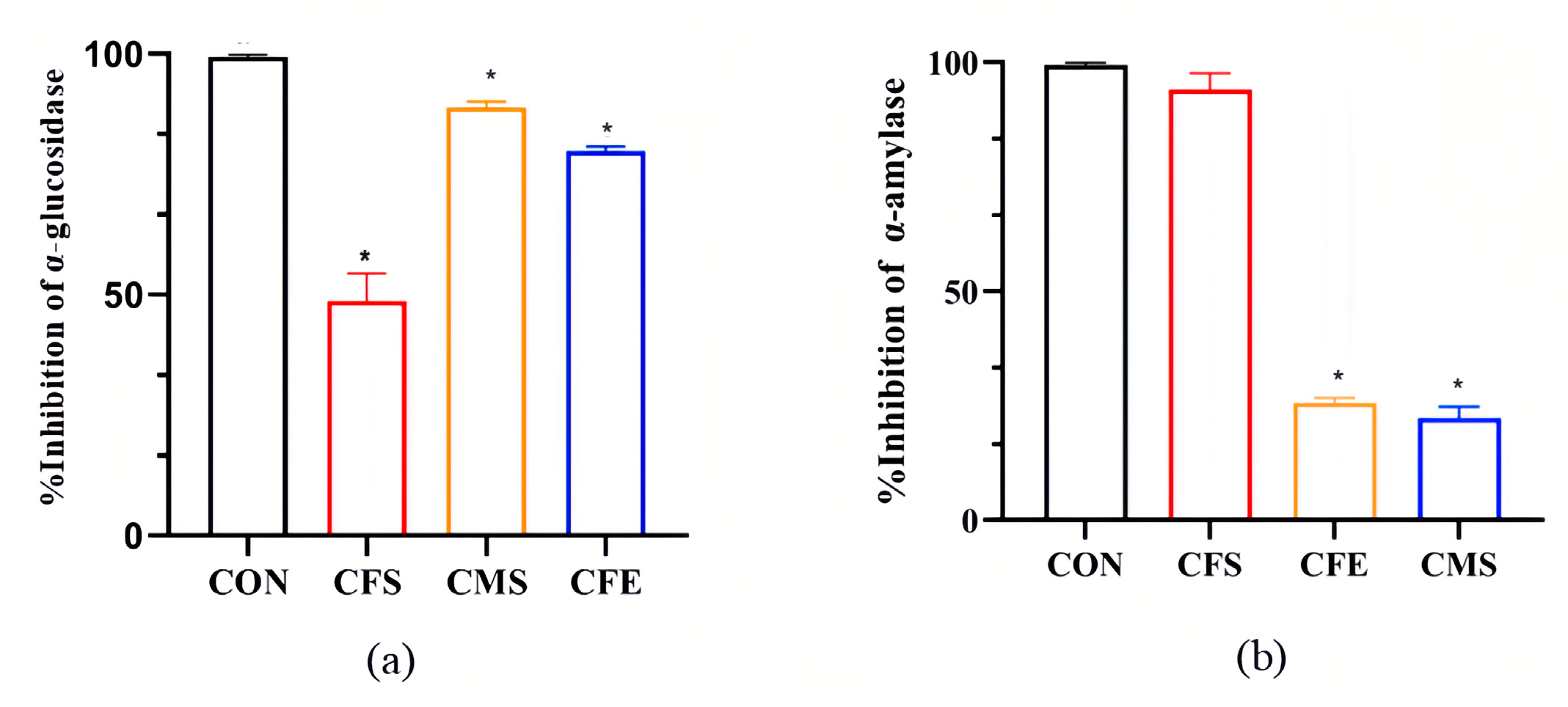
3.1. Significant Inhibition of α-Glucosidase and α-Amylase by W. coagulans SA9
3.2. The Metabolic Products of W. coagulans SA9 Contain a Variety of Potent Anti-Hyperglycemic Substances
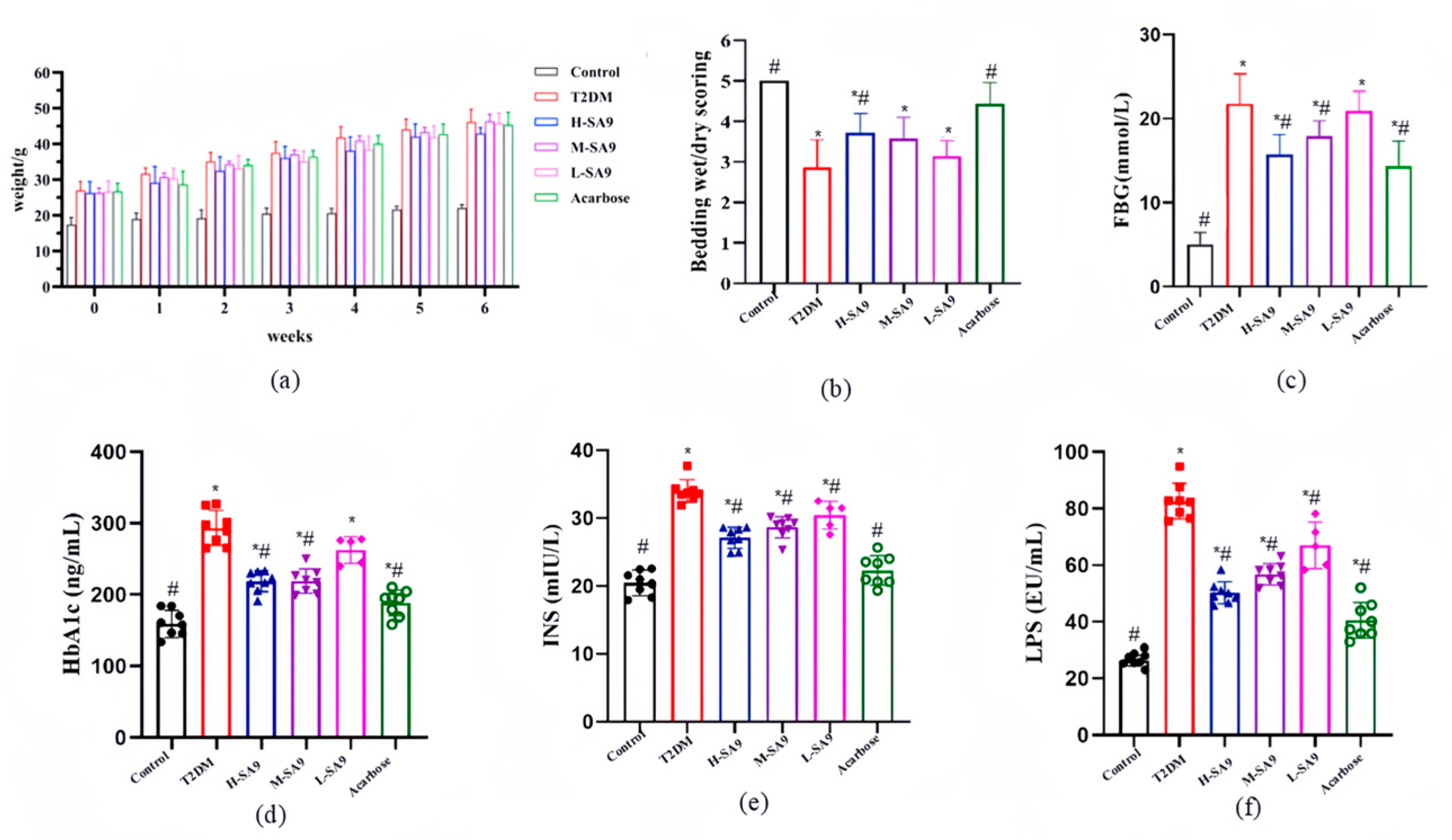
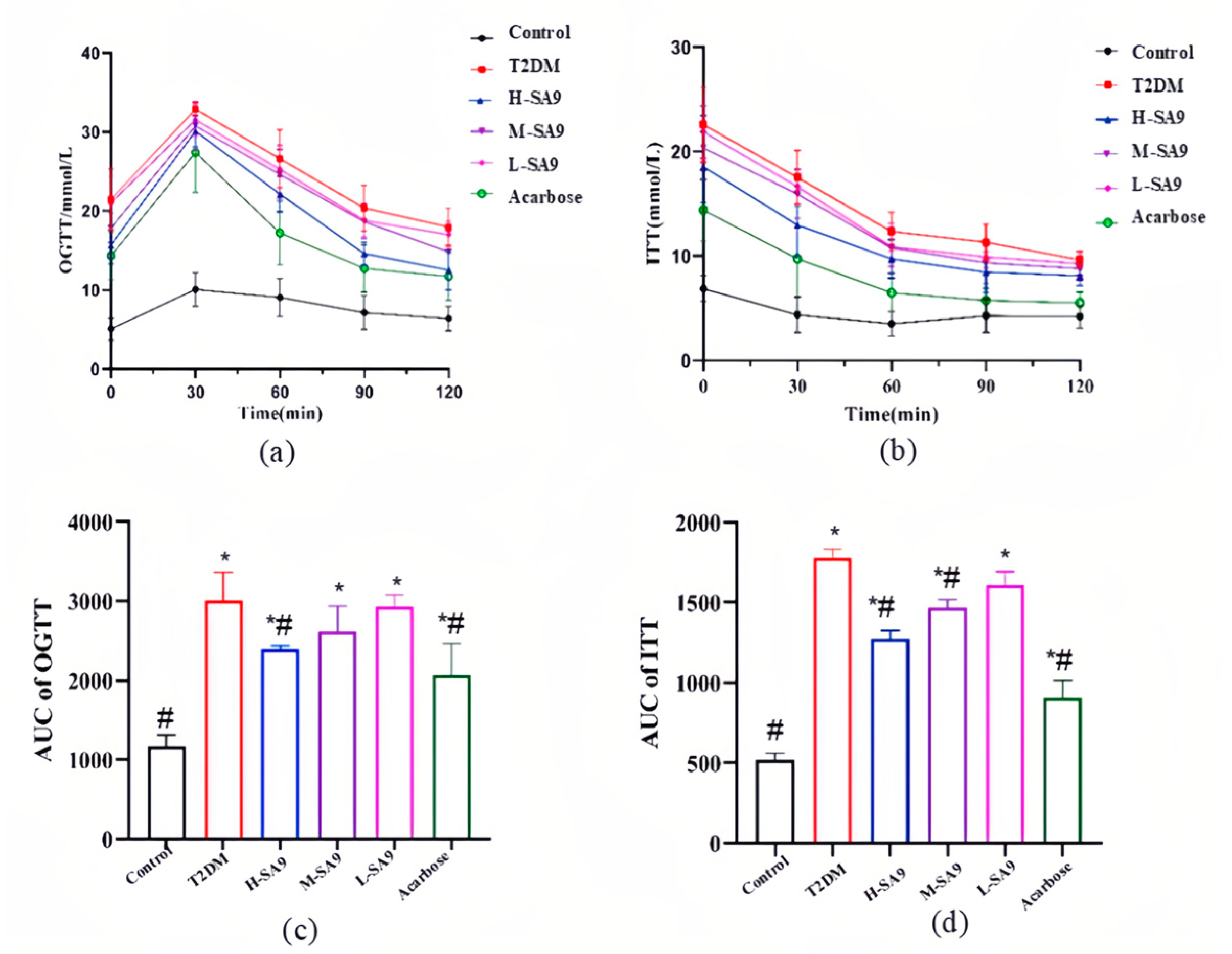
3.3. Positive Effects of W. coagulans SA9 on Diabetic Symptoms in db/db Mice
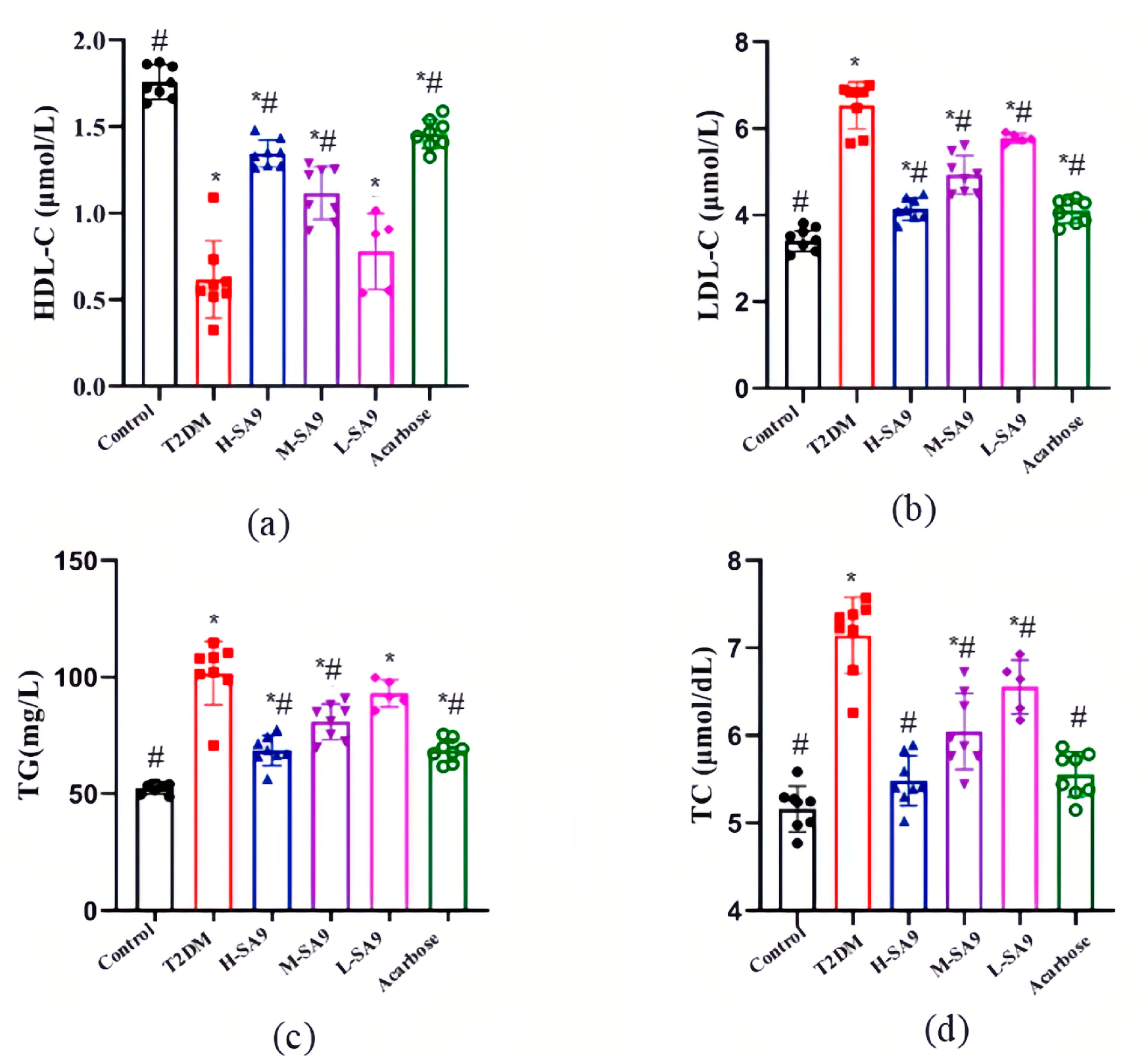
3.4. W. coagulans SA9 Alleviates Dyslipidemia in db/db Mice
3.5. W. coagulans SA9 Alleviates Systemic Inflammation in db/db Mice
3.6. W. coagulans SA9 Protects Islet Structure in db/db Mice
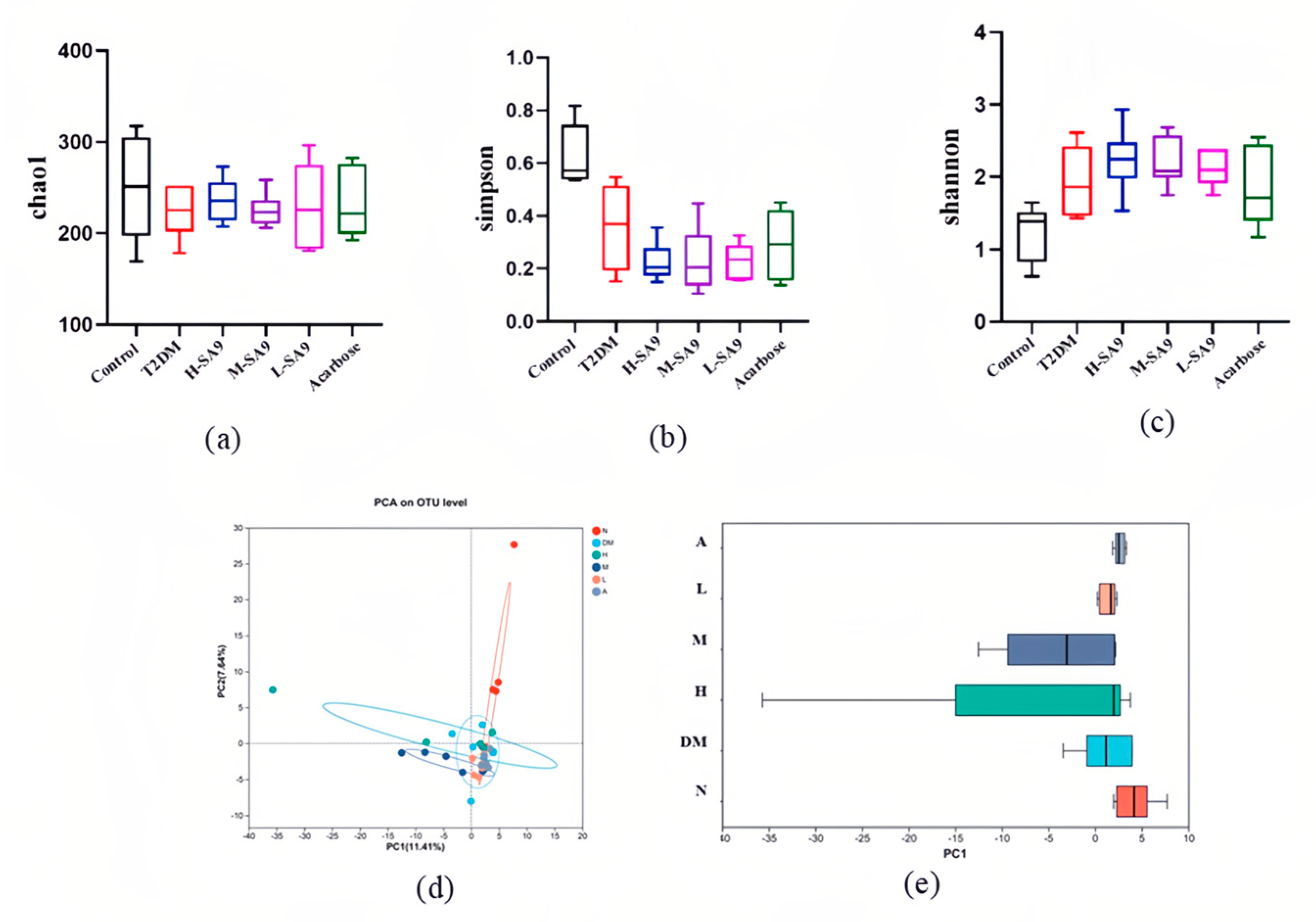
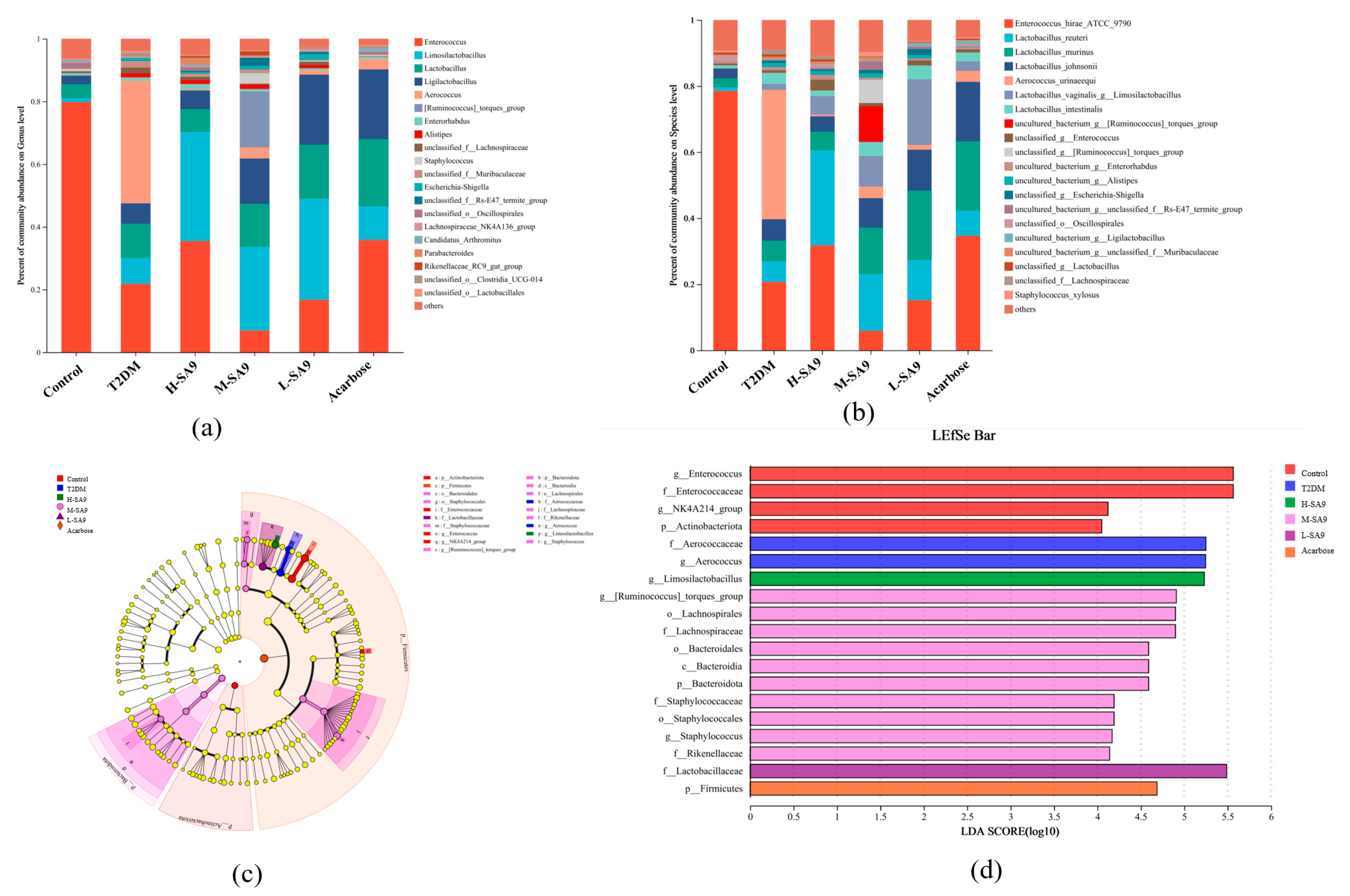
3.7. Regulatory Effects of W. coagulans SA9 on the Gut Microbiota of db/db Mice
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ogurtsova, K.; Guariguata, L.; Barengo, N.C.; Ruiz, P.L.-D.; Sacre, J.W.; Karuranga, S.; Sun, H.; Boyko, E.J.; Magliano, D.J. IDF Diabetes Atlas: Global Estimates of Undiagnosed Diabetes in Adults for 2021. Diabetes Res. Clin. Pract. 2022, 183, 109118. [Google Scholar] [CrossRef]
- Ahmad, E.; Lim, S.; Lamptey, R.; Webb, D.R.; Davies, M.J. Type 2 Diabetes. Lancet 2022, 400, 1803–1820. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhuang, S.; Jiang, S.; Zhang, J.; Chen, Y. Preparation of Nanocomposite Peptide and Its Inhibitory Effect on Myocardial Injury in Type-II Diabetic Rats. J. Nanosci. Nanotechnol. 2021, 21, 1378–1384. [Google Scholar] [CrossRef] [PubMed]
- Bakker, W.; Eringa, E.C.; Sipkema, P.; van Hinsbergh, V.W.M. Endothelial Dysfunction and Diabetes: Roles of Hyperglycemia, Impaired Insulin Signaling and Obesity. Cell Tissue Res. 2009, 335, 165–189. [Google Scholar] [CrossRef]
- Frykenberg, M.R.; Schneider, A.; Bashirelahi, N. What Every Dentist Should Know about Metformin, Diabetes, and Cancer. Gen. Dent. 2015, 63, 70–72. [Google Scholar] [PubMed]
- Slovacek, L.; Pavlik, V.; Slovackova, B. The Effect of Sibutramine Therapy on Occurrence of Depression Symptoms among Obese Patients. Nutr. Metab. Cardiovasc. Dis. 2008, 18, e43–e44. [Google Scholar] [CrossRef]
- Usman, B.; Sharma, N.; Satija, S.; Mehta, M.; Vyas, M.; Khatik, G.L.; Khurana, N.; Hansbro, P.M.; Williams, K.; Dua, K. Recent Developments in Alpha-Glucosidase Inhibitors for Management of Type-2 Diabetes: An Update. Curr. Pharm. Des. 2019, 25, 2510–2525. [Google Scholar] [CrossRef]
- Liang, H.; Hussey, S.E.; Sanchez-Avila, A.; Tantiwong, P.; Musi, N. Effect of Lipopolysaccharide on Inflammation and Insulin Action in Human Muscle. PLoS ONE 2013, 8, e63983. [Google Scholar] [CrossRef]
- Ragul, K.; Kandasamy, S.; Devi, P.B.; Shetty, P.H. Evaluation of Functional Properties of Potential Probiotic Isolates from Fermented Brine Pickle. Food Chem. 2020, 311, 126057. [Google Scholar] [CrossRef]
- Pan, Z.-H.; Ning, D.-S.; Fu, Y.-X.; Li, D.-P.; Zou, Z.-Q.; Xie, Y.-C.; Yu, L.-L.; Li, L.-C. Preparative Isolation of Piceatannol Derivatives from Passion Fruit (Passiflora Edulis) Seeds by High-Speed Countercurrent Chromatography Combined with High-Performance Liquid Chromatography and Screening for α-Glucosidase Inhibitory Activities. J. Agric. Food Chem. 2020, 68, 1555–1562. [Google Scholar] [CrossRef]
- Lee, S.; Kim, M. Leuconostoc mesenteroides MKSR isolated from kimchi possesses α-glucosidase inhibitory activity, antioxidant activity, and cholesterol-lowering effects. LWT 2019, 116, 108570. [Google Scholar] [CrossRef]
- Zhu, Y.-P.; Yamaki, K.; Yoshihashi, T.; Ohnishi Kameyama, M.; Li, X.-T.; Cheng, Y.-Q.; Mori, Y.; Li, L.-T. Purification and Identification of 1-Deoxynojirimycin (DNJ) in Okara Fermented by Bacillus Subtilis B2 from Chinese Traditional Food (Meitaoza). J. Agric. Food Chem. 2010, 58, 4097–4103. [Google Scholar] [CrossRef]
- Qiang, Z.; Truong, M.; Meynen, K.; Murphy, P.A.; Hendrich, S. Efficacy of a Mycotoxin Binder against Dietary Fumonisin, Deoxynivalenol, and Zearalenone in Rats. J. Agric. Food Chem. 2011, 59, 7527–7533. [Google Scholar] [CrossRef]
- Koh, W.Y.; Utra, U.; Ahmad, R.; Rather, I.A.; Park, Y.-H. Evaluation of Probiotic Potential and Anti-Hyperglycemic Properties of a Novel Lactobacillus Strain Isolated from Water Kefir Grains. Food Sci. Biotechnol. 2018, 27, 1369–1376. [Google Scholar] [CrossRef] [PubMed]
- Hummel, K.P.; Dickie, M.M.; Coleman, D.L. Diabetes, a New Mutation in the Mouse. Science 1966, 153, 1127–1128. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Li, Y.; Li, P.; Wang, M.; Wang, J.; Tang, Z.; Wang, T.; Luo, L.; Wang, C.; Wang, T.; et al. Research Progress in the Relationship between Type 2 Diabetes Mellitus and Intestinal Flora. Biomed. Pharmacother. 2019, 117, 109138. [Google Scholar] [CrossRef]
- Murali, S.K.; Mansell, T.J. Next Generation Probiotics: Engineering Live Biotherapeutics. Biotechnol. Adv. 2024, 72, 108336. [Google Scholar] [CrossRef]
- Bang, W.Y.; Ban, O.-H.; Lee, B.S.; Oh, S.; Park, C.; Park, M.-K.; Jung, S.K.; Yang, J.; Jung, Y.H. Genomic-, Phenotypic-, and Toxicity-Based Safety Assessment and Probiotic Potency of Bacillus Coagulans IDCC 1201 Isolated from Green Malt. J. Ind. Microbiol. Biotechnol. 2021, 48, kuab026. [Google Scholar] [CrossRef]
- Hsieh, R.-H.; Chien, Y.-J.; Lan, W.-Y.; Lin, Y.-K.; Lin, Y.-H.; Chiang, C.-F.; Yang, M.-T. Bacillus coagulans TCI711 Supplementation Improved Nonalcoholic Fatty Liver by Modulating Gut Microbiota: A Randomized, Placebo-Controlled, Clinical Trial. Curr. Dev. Nutr. 2024, 8, 102083. [Google Scholar] [CrossRef]
- Wu, Y.; Bai, Z.; Jin, Y.; Zhu, H.; Dong, Y.; Gu, S.; Jin, Y. A Randomized, Double-Blind, Placebo-Controlled Clinical Study to Evaluate the Efficacy and Safety of Weizmannia coagulans BC99 in the Treatment of Chronic Constipation in Adults. Front. Nutr. 2024, 11, 1395083. [Google Scholar] [CrossRef]
- Majeed, M.; Nagabhushanam, K.; Arumugam, S.; Chadalavada, N.; Seepana, J.; Annamalai, T.; Murali, A.; Prakasan, P.; Mundkur, L. Probiotic Weizmannia coagulans MTCC 5856 as Adjunct Therapy in Children’s Acute Diarrhea—A Randomized, Double-Blind, Placebo-Controlled Study. Front. Pediatr. 2024, 11, 1338126. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Kumar, V.; Nayak, S.K.; Wadhwa, P.; Kaur, P.; Sahu, S.K. Alpha-Amylase as Molecular Target for Treatment of Diabetes Mellitus: A Comprehensive Review. Chem. Biol. Drug Des. 2021, 98, 539–560. [Google Scholar] [CrossRef]
- Wang, X.; Han, M.; Zhang, M.; Wang, Y.; Ren, Y.; Yue, T.; Gao, Z. In vitro evaluation of the hypoglycemic properties of lactic acid bacteria and its fermentation adaptability in apple juice. LWT 2021, 136, 110363. [Google Scholar] [CrossRef]
- Won, G.; Choi, S.-I.; Park, N.; Kim, J.-E.; Kang, C.-H.; Kim, G.-H. In Vitro Antidiabetic, Antioxidant Activity, and Probiotic Activities of Lactiplantibacillus plantarum and Lacticaseibacillus paracasei Strains. Curr. Microbiol. 2021, 78, 3181–3191. [Google Scholar] [CrossRef]
- Frediansyah, A.; Nurhayati, R.; Sholihah, J. Lactobacillus pentosus isolated from Muntingia calabura shows inhibition activity toward alpha-glucosidase and alpha-amylase in intra and extracellular level. In IOP Conference Series Earth and Environmental Science; IOP Publishing: Bristol, UK, 2019; Volume 251. [Google Scholar] [CrossRef]
- Kimura, T.; Nakagawa, K.; Kubota, H.; Kojima, Y.; Goto, Y.; Yamagishi, K.; Oita, S.; Oikawa, S.; Miyazawa, T. Food-Grade Mulberry Powder Enriched with 1-Deoxynojirimycin Suppresses the Elevation of Postprandial Blood Glucose in Humans. J. Agric. Food Chem. 2007, 55, 5869–5874. [Google Scholar] [CrossRef] [PubMed]
- Hafizur, R.M.; Hameed, A.; Shukrana, M.; Raza, S.A.; Chishti, S.; Kabir, N.; Siddiqui, R.A. Cinnamic Acid Exerts Anti-Diabetic Activity by Improving Glucose Tolerance in Vivo and by Stimulating Insulin Secretion in Vitro. Phytomedicine 2015, 22, 297–300. [Google Scholar] [CrossRef]
- Yang, M.Y.; Zhang, X.Q.; Ma, Y.; Shen, J.; Song, J.R.; Zhu, H.L. Purification and partial characterization of a glycoprotein alpha-amylase inhibitor from white kidney bean (Phaseolus vulgaris L.). J. Food Biochem. 2008, 32, 72–84. [Google Scholar] [CrossRef]
- Chandrasekhar, C.; Rajpurohit, H.; Javaji, K.; Kuncha, M.; Setti, A.; Ali, A.Z.; Tiwari, A.K.; Misra, S.; Kumar, C.G. Anti-Hyperglycemic and Genotoxic Studies of 1-O-Methyl Chrysophanol, a New Anthraquinone Isolated from Amycolatopsis Thermoflava Strain SFMA-103. Drug Chem. Toxicol. 2021, 44, 148–160. [Google Scholar] [CrossRef]
- Kang, M.-G.; Yi, S.-H.; Lee, J.-S. Production and Characterization of a New α-Glucosidase Inhibitory Peptide from Aspergillus Oryzae N159-1. Mycobiology 2013, 41, 149–154. [Google Scholar] [CrossRef]
- McGee-Lawrence, M.E.; Wenger, K.H.; Misra, S.; Davis, C.L.; Pollock, N.K.; Elsalanty, M.; Ding, K.; Isales, C.M.; Hamrick, M.W.; Wosiski-Kuhn, M.; et al. Whole-Body Vibration Mimics the Metabolic Effects of Exercise in Male Leptin Receptor–Deficient Mice. Endocrinology 2017, 158, 1160–1171. [Google Scholar] [CrossRef]
- Wang, G.Q. Lactobacillus plantarum SHY130 isolated from yak yogurt attenuates hyperglycemia in C57BL/6J mice by regulating the enteroinsular axis. Food Funct. 2022, 13, 675–687. [Google Scholar] [CrossRef]
- Sabico, S.; Al-Mashharawi, A.; Al-Daghri, N.M.; Wani, K.; Amer, O.E.; Hussain, D.S.; Ahmed Ansari, M.G.; Masoud, M.S.; Alokail, M.S.; McTernan, P.G. Effects of a 6-Month Multi-Strain Probiotics Supplementation in Endotoxemic, Inflammatory and Cardiometabolic Status of T2DM Patients: A Randomized, Double-Blind, Placebo-Controlled Trial. Clin. Nutr. 2019, 38, 1561–1569. [Google Scholar] [CrossRef]
- Stewart, M.W.; Laker, M.F.; Dyer, R.G.; Game, F.; Mitcheson, J.; Winocour, P.H.; Alberti, K.G. Lipoprotein Compositional Abnormalities and Insulin Resistance in Type II Diabetic Patients with Mild Hyperlipidemia. Arter. Thromb. 1993, 13, 1046–1052. [Google Scholar] [CrossRef]
- Sas, K.M.; Kayampilly, P.; Byun, J.; Nair, V.; Hinder, L.M.; Hur, J.; Zhang, H.; Lin, C.; Qi, N.R.; Michailidis, G.; et al. Tissue-Specific Metabolic Reprogramming Drives Nutrient Flux in Diabetic Complications. JCI Insight 2016, 1, e86976. [Google Scholar] [CrossRef]
- Sharma, P.; Bhardwaj, P.; Singh, R. Administration of Lactobacillus casei and Bifidobacterium bifidum Ameliorated Hyperglycemia, Dyslipidemia, and Oxidative Stress in Diabetic Rats. Int. J. Prev. Med. 2016, 7, 102. [Google Scholar] [CrossRef]
- Hanes, W.M.; Olofsson, P.S.; Kwan, K.; Hudson, L.K.; Chavan, S.S.; Pavlov, V.A.; Tracey, K.J. Galantamine Attenuates Type 1 Diabetes and Inhibits Anti-Insulin Antibodies in Nonobese Diabetic Mice. Mol. Med. 2015, 21, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Donath, M.Y.; Shoelson, S.E. Type 2 Diabetes as an Inflammatory Disease. Nat. Rev. Immunol. 2011, 11, 98–107. [Google Scholar] [CrossRef]
- Montazeri, S.; Nalliah, S.; Radhakrishnan, A.K. Is There a Genetic Variation Association in the IL-10 and TNF Alpha Promoter Gene with Gestational Diabetes Mellitus? Hereditas 2010, 147, 94–102. [Google Scholar] [CrossRef]
- Feizollahzadeh, S.; Ghiasvand, R.; Rezaei, A.; Khanahmad, H.; Sadeghi, A.; Hariri, M. Effect of Probiotic Soy Milk on Serum Levels of Adiponectin, Inflammatory Mediators, Lipid Profile, and Fasting Blood Glucose Among Patients with Type II Diabetes Mellitus. Probiotics Antimicrob. Proteins 2017, 9, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Guo, X.; Zhang, J.; Yuan, Q.; Chen, S. Lactobacillus paracasei Modulates the Gut Microbiota and Improves Inflammation in Type 2 Diabetic Rats. Food Funct. 2021, 12, 6809–6820. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Obin, M.S.; Zhao, L. The Gut Microbiota, Obesity and Insulin Resistance. Mol. Asp. Med. 2013, 34, 39–58. [Google Scholar] [CrossRef]
- Simon, M.-C.; Strassburger, K.; Nowotny, B.; Kolb, H.; Nowotny, P.; Burkart, V.; Zivehe, F.; Hwang, J.-H.; Stehle, P.; Pacini, G.; et al. Intake of Lactobacillus reuteri Improves Incretin and Insulin Secretion in Glucose-Tolerant Humans: A Proof of Concept. Diabetes Care 2015, 38, 1827–1834. [Google Scholar] [CrossRef]
- Nicolas, G.R.; Chang, P.V. Deciphering the Chemical Lexicon of Host-Gut Microbiota Interactions. Trends Pharmacol. Sci. 2019, 40, 430–445. [Google Scholar] [CrossRef]
- Adami, A.; Cavazzoni, V. Occurrence of Selected Bacterial Groups in the Faeces of Piglets Fed with Bacillus Coagulans as Probiotic. J. Basic. Microbiol. 1999, 39, 3–9. [Google Scholar] [CrossRef]



| Mouse Bedding | Scores |
|---|---|
| Dry | 5 |
| Mostly dry | 4 |
| Partly wet | 3 |
| Mostly wet | 2 |
| Wet | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Wang, J.; Tan, Y.; Cai, C.; Yang, X.; Dong, S.; Hong, J.; Fang, X.; Wei, H.; Liao, Z. Weizmannia coagulans SA9: A Novel Strategy to Alleviate Type 2 Diabetes. Nutrients 2025, 17, 2081. https://doi.org/10.3390/nu17132081
Wang L, Wang J, Tan Y, Cai C, Yang X, Dong S, Hong J, Fang X, Wei H, Liao Z. Weizmannia coagulans SA9: A Novel Strategy to Alleviate Type 2 Diabetes. Nutrients. 2025; 17(13):2081. https://doi.org/10.3390/nu17132081
Chicago/Turabian StyleWang, Linhao, Jie Wang, Yewei Tan, Changyu Cai, Xiaohua Yang, Sashuang Dong, Jiaqi Hong, Xiang Fang, Hong Wei, and Zhenlin Liao. 2025. "Weizmannia coagulans SA9: A Novel Strategy to Alleviate Type 2 Diabetes" Nutrients 17, no. 13: 2081. https://doi.org/10.3390/nu17132081
APA StyleWang, L., Wang, J., Tan, Y., Cai, C., Yang, X., Dong, S., Hong, J., Fang, X., Wei, H., & Liao, Z. (2025). Weizmannia coagulans SA9: A Novel Strategy to Alleviate Type 2 Diabetes. Nutrients, 17(13), 2081. https://doi.org/10.3390/nu17132081






