High Dietary Phosphorus Impairs Bone Microarchitecture and Induces Alterations in the LGR4–R-Spondins Axis in Rats with Normal Renal Function
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
2.2. Phosphate and PTH Treatment of Osteoblast Cultures
2.3. Western Blot
2.4. Animal Model
2.5. Biochemical Markers
2.6. Micro-Computed Tomography (Micro-CT)
2.7. Bone Histology and Histomorphometry
2.8. RNA Isolation, Reverse Transcription and Quantitative PCR
2.9. Immunohistochemistry
2.10. Statistical Analysis
3. Results
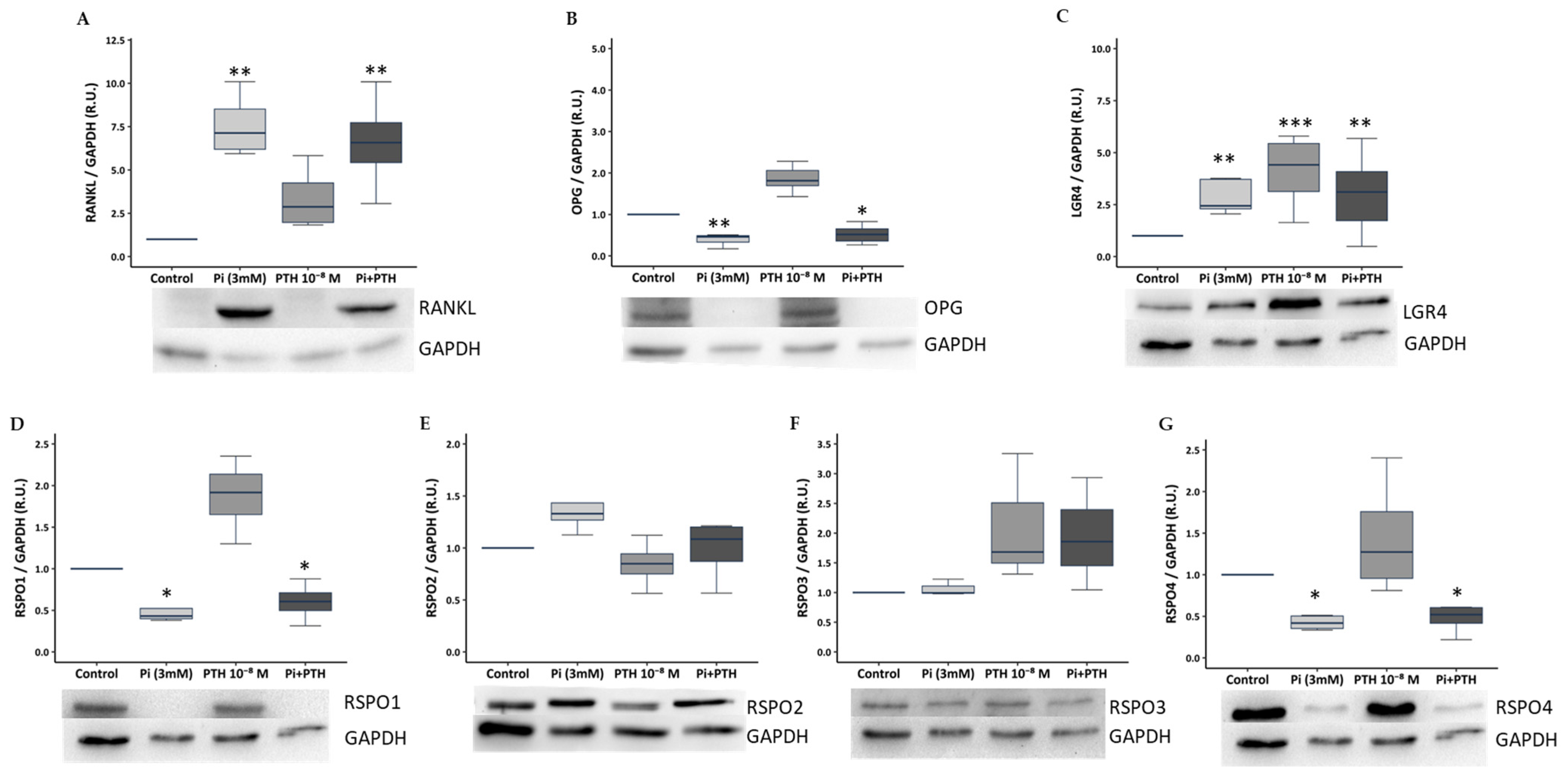
3.1. Phosphate and PTH Modulate Bone Remodelling Markers of the RANKL/OPG/LGR4 Axis and RSPOs Family in UMR-106 Osteoblasts
3.2. High Dietary Phosphorus Alters Mineral Homeostasis in Rats
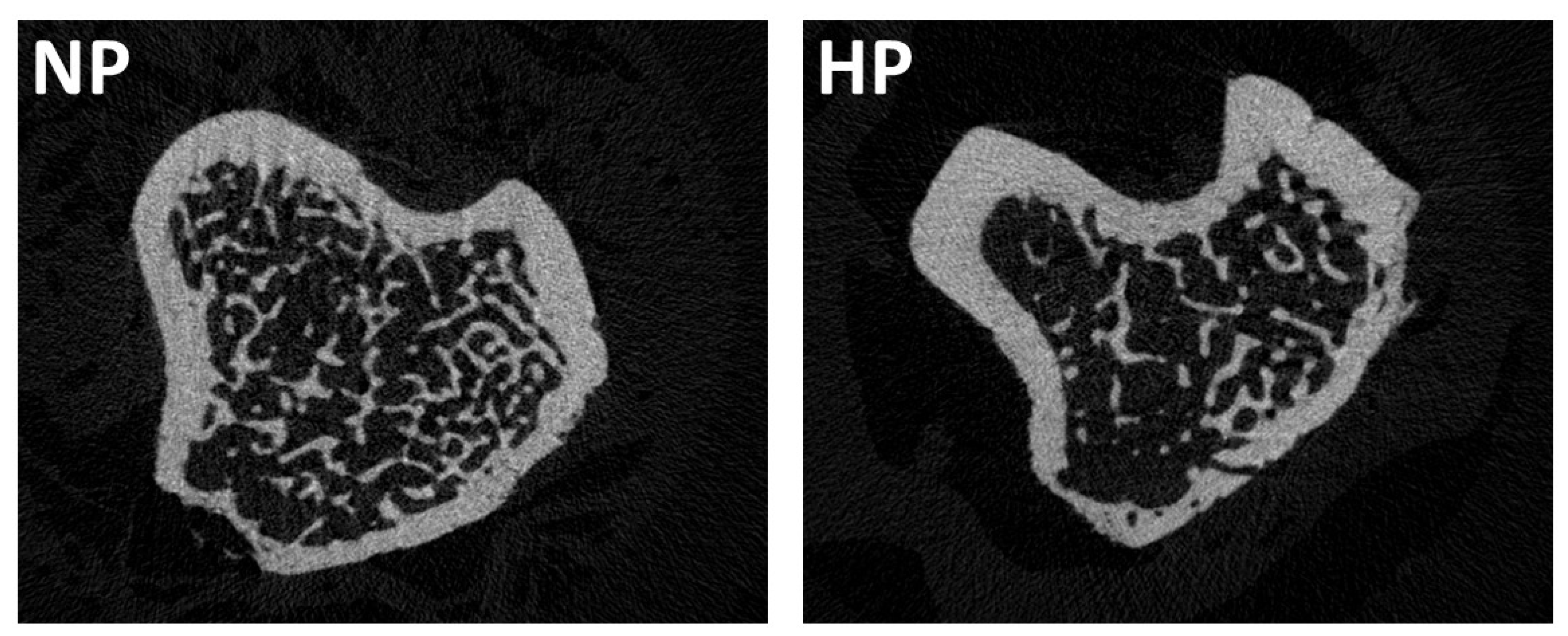
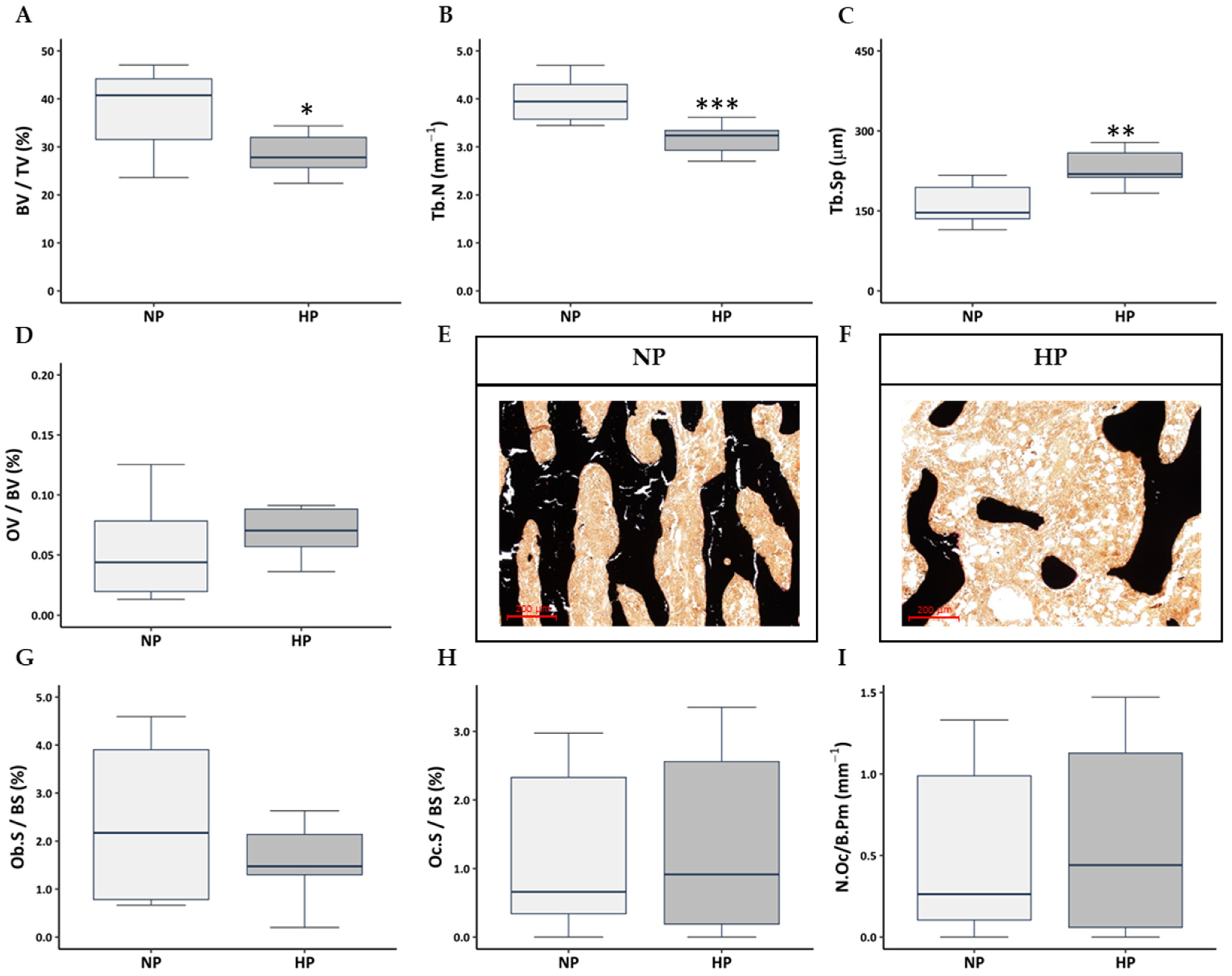
3.3. Influence of High Dietary Phosphorus on Bone Mass and Trabecular and Cortical Bone Architecture
3.4. Influence of High Dietary Phosphorus on Bone Morphology, Osteoblast and Osteoclast Number
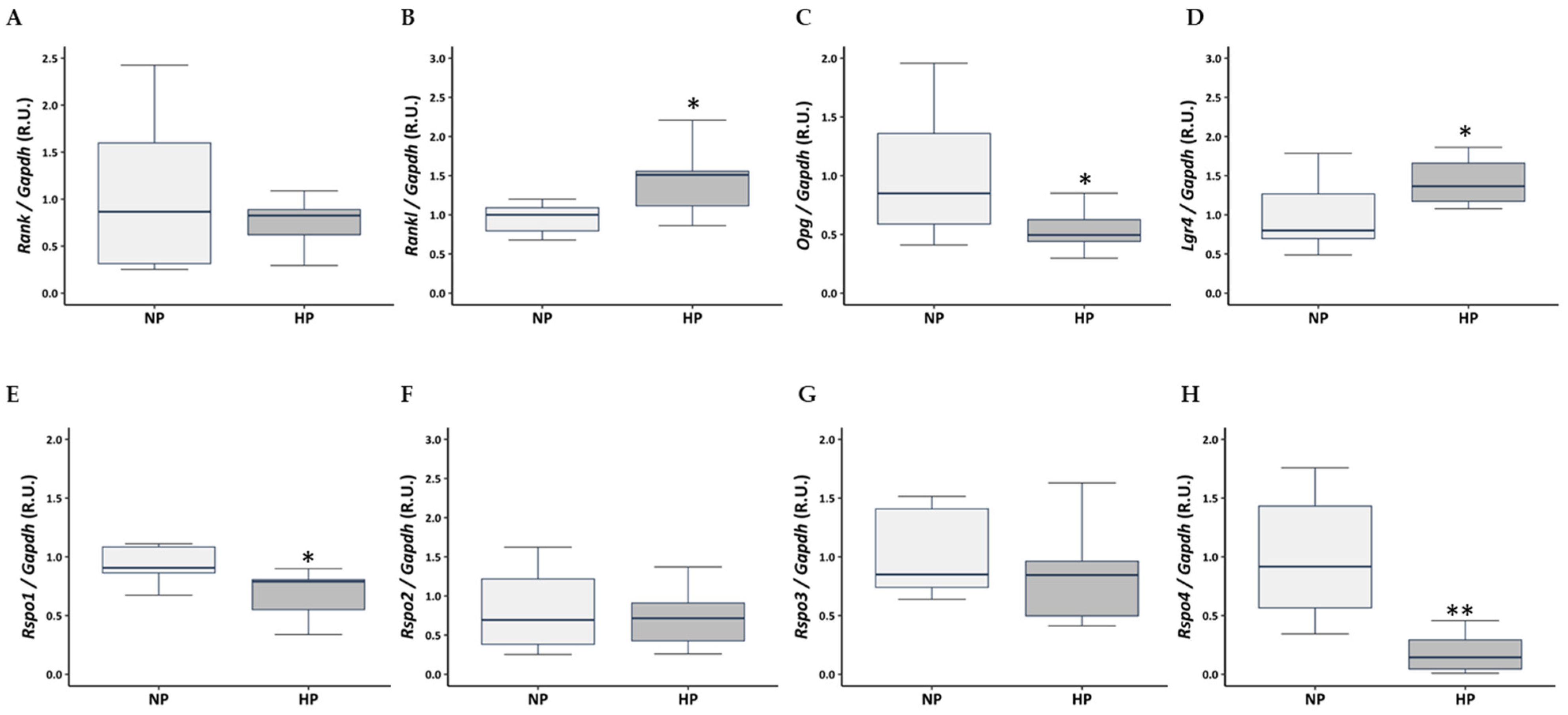
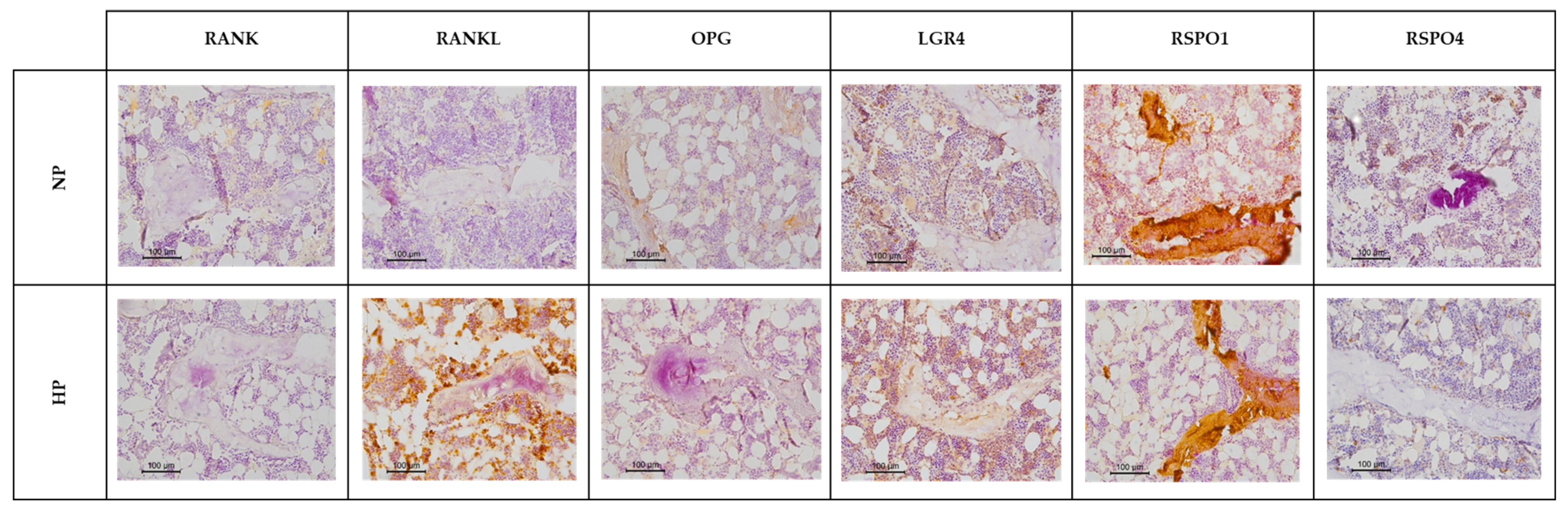
3.5. Influence of High Dietary Phosphorus on the RANKL/OPG/LGR4 Axis and RSPO Expression in Rat Tibia
3.6. Influence of High Dietary Phosphorus on Circulating Levels of RANKL, OPG, LGR4, RSPO1, and RSPO4
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Calvo, M.S.; Uribarri, J. Public health impact of dietary phosphorus excess on bone and cardiovascular health in the general population. Am. J. Clin. Nutr. 2013, 98, 6–15. [Google Scholar] [CrossRef]
- Almaden, Y.; Canalejo, A.; Hernandez, A.; Ballesteros, E.; Garcia-Navarro, S.; Torres, A.; Rodriguez, M. Direct effect of phosphorus on PTH secretion from whole rat parathyroid glands in vitro. J. Bone Min. Res. 1996, 11, 970–976. [Google Scholar] [CrossRef]
- Almaden, Y.; Hernandez, A.; Torregrosa, V.; Canalejo, A.; Sabate, L.; Fernandez Cruz, L.; Campistol, J.M.; Torres, A.; Rodriguez, M. High phosphate level directly stimulates parathyroid hormone secretion and synthesis by human parathyroid tissue in vitro. J. Am. Soc. Nephrol. 1998, 9, 1845–1852. [Google Scholar] [CrossRef]
- Kilav, R.; Silver, J.; Naveh-Many, T. Parathyroid hormone gene expression in hypophosphatemic rats. J. Clin. Investig. 1995, 96, 327–333. [Google Scholar] [CrossRef]
- Slatopolsky, E.; Brown, A.; Dusso, A. Pathogenesis of secondary hyperparathyroidism. Kidney Int. Suppl. 1999, 73, S14–S19. [Google Scholar] [CrossRef]
- Vorland, C.J.; Stremke, E.R.; Moorthi, R.N.; Hill Gallant, K.M. Effects of Excessive Dietary Phosphorus Intake on Bone Health. Curr. Osteoporos. Rep. 2017, 15, 473–482. [Google Scholar] [CrossRef]
- Levin, A.; Bakris, G.L.; Molitch, M.; Smulders, M.; Tian, J.; Williams, L.A.; Andress, D.L. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: Results of the study to evaluate early kidney disease. Kidney Int. 2007, 71, 31–38. [Google Scholar] [CrossRef]
- Choi, Y.; Yoo, J.H.; Lee, Y.; Bae, M.K.; Kim, H.J. Calcium-Phosphate Crystals Promote RANKL Expression via the Downregulation of DUSP1. Mol. Cells 2019, 42, 183–188. [Google Scholar] [CrossRef]
- Kanatani, M.; Sugimoto, T.; Kano, J.; Kanzawa, M.; Chihara, K. Effect of high phosphate concentration on osteoclast differentiation as well as bone-resorbing activity. J. Cell Physiol. 2003, 196, 180–189. [Google Scholar] [CrossRef]
- Boyce, B.F.; Xing, L. Biology of RANK, RANKL, and osteoprotegerin. Arthritis Res. Ther. 2007, 9 (Suppl. 1), S1. [Google Scholar] [CrossRef]
- Luo, J.; Yang, Z.; Ma, Y.; Yue, Z.; Lin, H.; Qu, G.; Huang, J.; Dai, W.; Li, C.; Zheng, C.; et al. LGR4 is a receptor for RANKL and negatively regulates osteoclast differentiation and bone resorption. Nat. Med. 2016, 22, 539–546. [Google Scholar] [CrossRef]
- Lim, W. LGR4 (GPR48): The Emerging Inter-Bridge in Osteoimmunology. Biomedicines 2025, 13, 607. [Google Scholar] [CrossRef]
- de Lau, W.; Barker, N.; Low, T.Y.; Koo, B.K.; Li, V.S.; Teunissen, H.; Kujala, P.; Haegebarth, A.; Peters, P.J.; van de Wetering, M.; et al. Lgr5 homologues associate with Wnt receptors and mediate R-spondin signalling. Nature 2011, 476, 293–297. [Google Scholar] [CrossRef]
- Carmon, K.S.; Gong, X.; Lin, Q.; Thomas, A.; Liu, Q. R-spondins function as ligands of the orphan receptors LGR4 and LGR5 to regulate Wnt/beta-catenin signaling. Proc. Natl. Acad. Sci. USA 2011, 108, 11452–11457. [Google Scholar] [CrossRef]
- Erben, R.G. Embedding of bone samples in methylmethacrylate: An improved method suitable for bone histomorphometry, histochemistry, and immunohistochemistry. J. Histochem. Cytochem. 1997, 45, 307–313. [Google Scholar] [CrossRef]
- Bemenderfer, T.B.; Harris, J.S.; Condon, K.W.; Li, J.; Kacena, M.A. Processing and Sectioning Undecalcified Murine Bone Specimens. In Skeletal Development and Repair: Methods and Protocols; Hilton, M.J., Ed.; Springer: New York, NY, USA, 2021; pp. 231–257. [Google Scholar]
- Dirckx, N.; Tower, R.J.; Mercken, E.M.; Vangoitsenhoven, R.; Moreau-Triby, C.; Breugelmans, T.; Nefyodova, E.; Cardoen, R.; Mathieu, C.; Van der Schueren, B.; et al. Vhl deletion in osteoblasts boosts cellular glycolysis and improves global glucose metabolism. J. Clin. Investig. 2018, 128, 1087–1105. [Google Scholar] [CrossRef]
- Dejaeger, M.; Bohm, A.M.; Dirckx, N.; Devriese, J.; Nefyodova, E.; Cardoen, R.; St-Arnaud, R.; Tournoy, J.; Luyten, F.P.; Maes, C. Integrin-Linked Kinase Regulates Bone Formation by Controlling Cytoskeletal Organization and Modulating BMP and Wnt Signaling in Osteoprogenitors. J. Bone Min. Res. 2017, 32, 2087–2102. [Google Scholar] [CrossRef]
- Dempster, D.W.; Compston, J.E.; Drezner, M.K.; Glorieux, F.H.; Kanis, J.A.; Malluche, H.; Meunier, P.J.; Ott, S.M.; Recker, R.R.; Parfitt, A.M. Standardized nomenclature, symbols, and units for bone histomorphometry: A 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J. Bone Min. Res. 2013, 28, 2–17. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Aloia, J.F.; Vaswani, A.; Yeh, J.K.; Ellis, K.; Cohn, S.H. Total body phosphorus in postmenopausal women. Miner. Electrolyte Metab. 1984, 10, 73–76. [Google Scholar]
- Calvo, M.S.; Moshfegh, A.J.; Tucker, K.L. Assessing the health impact of phosphorus in the food supply: Issues and considerations. Adv. Nutr. 2014, 5, 104–113. [Google Scholar] [CrossRef]
- Institute of Medicine Standing Committee on the Scientific Evaluation of Dietary Reference, I. The National Academies Collection: Reports funded by National Institutes of Health. In Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride; National Academy of Sciences: Washington, DC, USA, 1997. [Google Scholar]
- Tucker, K.L.; Morita, K.; Qiao, N.; Hannan, M.T.; Cupples, L.A.; Kiel, D.P. Colas, but not other carbonated beverages, are associated with low bone mineral density in older women: The Framingham Osteoporosis Study. Am. J. Clin. Nutr. 2006, 84, 936–942. [Google Scholar] [CrossRef]
- Kristensen, M.; Jensen, M.; Kudsk, J.; Henriksen, M.; Mølgaard, C. Short-term effects on bone turnover of replacing milk with cola beverages: A 10-day interventional study in young men. Osteoporos. Int. 2005, 16, 1803–1808. [Google Scholar] [CrossRef]
- Wyshak, G. Teenaged girls, carbonated beverage consumption, and bone fractures. Arch. Pediatr. Adolesc. Med. 2000, 154, 610–613. [Google Scholar] [CrossRef]
- Wyshak, G.; Frisch, R.E. Carbonated beverages, dietary calcium, the dietary calcium/phosphorus ratio, and bone fractures in girls and boys. J. Adolesc. Health 1994, 15, 210–215. [Google Scholar] [CrossRef]
- Amato, D.; Maravilla, A.; Montoya, C.; Gaja, O.; Revilla, C.; Guerra, R.; Paniagua, R. Acute effects of soft drink intake on calcium and phosphate metabolism in immature and adult rats. Rev. Investig. Clin. 1998, 50, 185–189. [Google Scholar]
- García-Contreras, F.; Paniagua, R.; Avila-Díaz, M.; Cabrera-Muñoz, L.; Martínez-Muñiz, I.; Foyo-Niembro, E.; Amato, D. Cola beverage consumption induces bone mineralization reduction in ovariectomized rats. Arch. Med. Res. 2000, 31, 360–365. [Google Scholar] [CrossRef]
- Bergwitz, C.; Jüppner, H. Regulation of phosphate homeostasis by PTH, vitamin D, and FGF23. Annu. Rev. Med. 2010, 61, 91–104. [Google Scholar] [CrossRef]
- Ovejero, D.; Hartley, I.R.; de Castro Diaz, L.F.; Theng, E.; Li, X.; Gafni, R.I.; Collins, M.T. PTH and FGF23 Exert Interdependent Effects on Renal Phosphate Handling: Evidence From Patients With Hypoparathyroidism and Hyperphosphatemic Familial Tumoral Calcinosis Treated With Synthetic Human PTH 1-34. J. Bone Min. Res. 2022, 37, 179–184. [Google Scholar] [CrossRef]
- Georgiadou, E.; Marketou, H.; Trovas, G.; Dontas, I.; Papaioannou, N.; Makris, K.; Galanos, A.; Papavassiliou, A.G. Effect of Calcitriol on FGF23 Level in Healthy Adults and its Dependence on Phosphate Level. In Vivo 2017, 31, 145–150. [Google Scholar] [CrossRef]
- Westerberg, P.A.; Linde, T.; Wikström, B.; Ljunggren, O.; Stridsberg, M.; Larsson, T.E. Regulation of fibroblast growth factor-23 in chronic kidney disease. Nephrol. Dial. Transplant. 2007, 22, 3202–3207. [Google Scholar] [CrossRef]
- Evenepoel, P.; Meijers, B.; Viaene, L.; Bammens, B.; Claes, K.; Kuypers, D.; Vanderschueren, D.; Vanrenterghem, Y. Fibroblast growth factor-23 in early chronic kidney disease: Additional support in favor of a phosphate-centric paradigm for the pathogenesis of secondary hyperparathyroidism. Clin. J. Am. Soc. Nephrol. 2010, 5, 1268–1276. [Google Scholar] [CrossRef]
- Kumar, R.; Thompson, J.R. The regulation of parathyroid hormone secretion and synthesis. J. Am. Soc. Nephrol. 2011, 22, 216–224. [Google Scholar] [CrossRef]
- Barrera-Baena, P.; Rodríguez-García, M.; Rodríguez-Rubio, E.; González-Llorente, L.; Ortiz, A.; Zoccali, C.; Locatelli, F.; Floege, J.; Cohen-Solal, M.; Ferreira, M.A.; et al. Serum phosphate is associated with increased risk of bone fragility fractures in haemodialysis patients. Nephrol. Dial. Transplant. 2024, 39, 618–626. [Google Scholar] [CrossRef]
- Kwon, Y.E.; Choi, H.Y.; Kim, S.; Ryu, D.R.; Oh, H.J. Fracture risk in chronic kidney disease: A Korean population-based cohort study. Kidney Res. Clin. Pract. 2019, 38, 220–228. [Google Scholar] [CrossRef]
- Bajwa, N.M.; Sanchez, C.P.; Lindsey, R.C.; Watt, H.; Mohan, S. Cortical and trabecular bone are equally affected in rats with renal failure and secondary hyperparathyroidism. BMC Nephrol. 2018, 19, 24. [Google Scholar] [CrossRef]
- Lau, W.L.; Linnes, M.; Chu, E.Y.; Foster, B.L.; Bartley, B.A.; Somerman, M.J.; Giachelli, C.M. High phosphate feeding promotes mineral and bone abnormalities in mice with chronic kidney disease. Nephrol. Dial. Transplant. 2013, 28, 62–69. [Google Scholar] [CrossRef]
- Carrillo-Lopez, N.; Panizo, S.; Alonso-Montes, C.; Martinez-Arias, L.; Avello, N.; Sosa, P.; Dusso, A.S.; Cannata-Andia, J.B.; Naves-Diaz, M. High-serum phosphate and parathyroid hormone distinctly regulate bone loss and vascular calcification in experimental chronic kidney disease. Nephrol. Dial. Transplant. 2019, 34, 934–941. [Google Scholar] [CrossRef]
- Batista, D.G.; Neves, K.R.; Graciolli, F.G.; dos Reis, L.M.; Graciolli, R.G.; Dominguez, W.V.; Neves, C.L.; Magalhães, A.O.; Custódio, M.R.; Moysés, R.M.; et al. The bone histology spectrum in experimental renal failure: Adverse effects of phosphate and parathyroid hormone disturbances. Calcif. Tissue Int. 2010, 87, 60–67. [Google Scholar] [CrossRef]
- Yang, Q.; Wei, Z.; Wei, X.; Zhang, J.; Tang, Y.; Zhou, X.; Liu, P.; Dou, C.; Luo, F. The age-related characteristics in bone microarchitecture, osteoclast distribution pattern, functional and transcriptomic alterations of BMSCs in mice. Mech. Ageing Dev. 2023, 216, 111877. [Google Scholar] [CrossRef]
- Schaffler, M.B.; Choi, K.; Milgrom, C. Aging and matrix microdamage accumulation in human compact bone. Bone 1995, 17, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.H.; Chen, L.R.; Chen, K.H. Osteoporosis Due to Hormone Imbalance: An Overview of the Effects of Estrogen Deficiency and Glucocorticoid Overuse on Bone Turnover. Int. J. Mol. Sci. 2022, 23, 1376. [Google Scholar] [CrossRef] [PubMed]
- Kivell, T.L. A review of trabecular bone functional adaptation: What have we learned from trabecular analyses in extant hominoids and what can we apply to fossils? J. Anat. 2016, 228, 569–594. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.C.; Sakata, T.; Pfleger, L.L.; Bencsik, M.; Halloran, B.P.; Bikle, D.D.; Nissenson, R.A. PTH differentially regulates expression of RANKL and OPG. J. Bone Min. Res. 2004, 19, 235–244. [Google Scholar] [CrossRef]
- Fu, Q.; Jilka, R.L.; Manolagas, S.C.; O’Brien, C.A. Parathyroid hormone stimulates receptor activator of NFkappa B ligand and inhibits osteoprotegerin expression via protein kinase A activation of cAMP-response element-binding protein. J. Biol. Chem. 2002, 277, 48868–48875. [Google Scholar] [CrossRef]
- Ben-awadh, A.N.; Delgado-Calle, J.; Tu, X.; Kuhlenschmidt, K.; Allen, M.R.; Plotkin, L.I.; Bellido, T. Parathyroid hormone receptor signaling induces bone resorption in the adult skeleton by directly regulating the RANKL gene in osteocytes. Endocrinology 2014, 155, 2797–2809. [Google Scholar] [CrossRef]
- Lee, S.K.; Lorenzo, J.A. Regulation of receptor activator of nuclear factor-kappa B ligand and osteoprotegerin mRNA expression by parathyroid hormone is predominantly mediated by the protein kinase a pathway in murine bone marrow cultures. Bone 2002, 31, 252–259. [Google Scholar] [CrossRef]
- Mozar, A.; Haren, N.; Chasseraud, M.; Louvet, L.; Mazière, C.; Wattel, A.; Mentaverri, R.; Morlière, P.; Kamel, S.; Brazier, M.; et al. High extracellular inorganic phosphate concentration inhibits RANK-RANKL signaling in osteoclast-like cells. J. Cell Physiol. 2008, 215, 47–54. [Google Scholar] [CrossRef]
- M’Baya-Moutoula, E.; Louvet, L.; Metzinger-Le Meuth, V.; Massy, Z.A.; Metzinger, L. High inorganic phosphate concentration inhibits osteoclastogenesis by modulating miR-223. Biochim. Biophys. Acta 2015, 1852, 2202–2212. [Google Scholar] [CrossRef]
- Styrkarsdottir, U.; Thorleifsson, G.; Sulem, P.; Gudbjartsson, D.F.; Sigurdsson, A.; Jonasdottir, A.; Oddsson, A.; Helgason, A.; Magnusson, O.T.; Walters, G.B.; et al. Nonsense mutation in the LGR4 gene is associated with several human diseases and other traits. Nature 2013, 497, 517–520. [Google Scholar] [CrossRef]
- Schuijers, J.; Clevers, H. Adult mammalian stem cells: The role of Wnt, Lgr5 and R-spondins. EMBO J. 2012, 31, 2685–2696. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Villabrille, S.; Martín-Carro, B.; Martín-Vírgala, J.; Rodríguez-Santamaria, M.D.M.; Baena-Huerta, F.; Muñoz-Castañeda, J.R.; Fernández-Martín, J.L.; Alonso-Montes, C.; Naves-Díaz, M.; Carrillo-López, N.; et al. Novel Biomarkers of Bone Metabolism. Nutrients 2024, 16, 605. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.Y.; Hong, Y.S.; Park, S.H.; Ju, J.H. Increased serum alkaline phosphatase levels correlate with high disease activity and low bone mineral density in patients with axial spondyloarthritis. Semin. Arthritis Rheum. 2015, 45, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Shu, J.; Tan, A.; Li, Y.; Huang, H.; Yang, J. The correlation between serum total alkaline phosphatase and bone mineral density in young adults. BMC Musculoskelet. Disord. 2022, 23, 467. [Google Scholar] [CrossRef]
- Hofbauer, L.C.; Schoppet, M. Clinical implications of the osteoprotegerin/RANKL/RANK system for bone and vascular diseases. JAMA 2004, 292, 490–495. [Google Scholar] [CrossRef]
- Azizieh, F.Y.; Shehab, D.; Jarallah, K.A.; Gupta, R.; Raghupathy, R. Circulatory Levels of RANKL, OPG, and Oxidative Stress Markers in Postmenopausal Women With Normal or Low Bone Mineral Density. Biomark. Insights 2019, 14, 1177271919843825. [Google Scholar] [CrossRef]
- Xu, S.; Wang, Y.; Lu, J.; Xu, J. Osteoprotegerin and RANKL in the pathogenesis of rheumatoid arthritis-induced osteoporosis. Rheumatol. Int. 2012, 32, 3397–3403. [Google Scholar] [CrossRef]
- Li, B.; Yao, Q.; Guo, S.; Ma, S.; Dong, Y.; Xin, H.; Wang, H.; Liu, L.; Chang, W.; Zhang, Y. Type 2 diabetes with hypertensive patients results in changes to features of adipocytokines: Leptin, Irisin, LGR4, and Sfrp5. Clin. Exp. Hypertens. 2019, 41, 645–650. [Google Scholar] [CrossRef]
- Sullivan, C.; Sayre, S.S.; Leon, J.B.; Machekano, R.; Love, T.E.; Porter, D.; Marbury, M.; Sehgal, A.R. Effect of food additives on hyperphosphatemia among patients with end-stage renal disease: A randomized controlled trial. JAMA 2009, 301, 629–635. [Google Scholar] [CrossRef]
- de Fornasari, M.L.; Dos Santos Sens, Y.A. Replacing Phosphorus-Containing Food Additives With Foods Without Additives Reduces Phosphatemia in End-Stage Renal Disease Patients: A Randomized Clinical Trial. J. Ren. Nutr. 2017, 27, 97–105. [Google Scholar] [CrossRef]
| NP | HP | p | |
|---|---|---|---|
| n | 8 | 10 | |
| Creatinine clearance (mL/min) | 2.92 [2.76, 3.21] | 2.72 [2.20, 3.15] | 0.248 |
| Serum phosphate (mg/dL) | 3.78 [3.45, 4.04] | 4.36 [4.08, 4.59] | 0.037 |
| Serum calcium (mg/dL) | 10.19 [10.00, 10.32] | 10.11 [9.98, 10.31] | 0.823 |
| Serum PTH (pg/mL) | 234.43 [179.52, 314.21] | 407.70 [374.72, 579.11] | 0.003 |
| Calcitriol (pg/mL) | 5.00 [5.00, 5.41] | 15.90 [13.48, 18.18] | <0.001 |
| FGF23 (pg/mL) | 55.41 [38.57, 89.70] | 72.09 [41.91, 78.45] | 0.773 |
| FE phosphate % | 0.13 [0.07, 0.54] | 23.02 [20.22, 26.19] | <0.001 |
| FE calcium % | 0.69 [0.53, 0.84] | 0.27 [0.22, 0.30] | 0.008 |
| NP | HP | p | |
|---|---|---|---|
| n | 8 | 10 | |
| BMD (g/cm3) | 0.41 [0.35, 0.42] | 0.31 [0.31, 0.38] | 0.004 |
| TMD (g/cm3) | 1.22 [1.20, 1.23] | 1.20 [1.20, 1.22] | 0.470 |
| Trabecular structure | |||
| Tb.N (mm−1) | 3.04 [2.70, 3.17] | 2.34 [2.19, 2.83] | 0.006 |
| BS/TV (mm−1) | 10.75 [9.69, 11.01] | 8.47 [7.72, 10.03] | 0.006 |
| Conn.Dn (mm−3) | 171.29 [146.10, 182.75] | 100.74 [89.48, 139.88] | 0.014 |
| BV/TV (%) | 22.27 [17.88, 23.00] | 18.03 [16.97, 20.82] | 0.030 |
| Tb.Th (µm) | 71.85 [70.33, 73.44] | 74.48 [72.91, 77.86] | 0.011 |
| Tb.Sp (µm) | 226.04 [214.61, 249.20] | 290.22 [247.72, 329.97] | 0.004 |
| Tb.Po (%) | 77.73 [77.00, 82.12] | 81.97 [79.18, 83.03] | 0.030 |
| SMI | 1.22 [1.18, 1.33] | 1.36 [1.30, 1.51] | 0.037 |
| Tb.Pf | 9.88 [9.69, 11.68] | 11.10 [10.23, 12.26] | 0.425 |
| Cortical structure | |||
| Ct.Th (µm) | 673.05 [622.70, 690.59] | 644.97 [613.89, 655.72] | 0.382 |
| Ct.Po.Dm (µm) | 113.75 [58.52, 157.87] | 94.65 [64.02, 138.48] | 0.909 |
| Ct.Po (%) | 0.82 [0.29, 1.19] | 0.42 [0.33, 0.73] | 0.382 |
| BV/TV (%) | 99.18 [98.81, 99.71] | 99.58 [99.27, 99.67] | 0.382 |
| BS/BV (mm−1) | 5.47 [5.20, 5.68] | 5.42 [5.34, 5.65] | 0.909 |
| NP | HP | p | |
|---|---|---|---|
| n | 8 | 10 | |
| RANK (%) | 0.84 [0.14, 2.29] | 0.41 [0.08, 1.34] | 0.465 |
| RANKL (%) | 4.61 [2.07, 15.94] | 34.07 [12.75, 41.62] | 0.021 |
| OPG (%) | 1.48 [0.84, 2.04] | 0.84 [0.65, 1.08] | 0.150 |
| LGR4 (%) | 7.64 [4.48, 8.26] | 13.70 [13.20, 17.24] | 0.002 |
| RSPO1 (%) | 22.63 [20.43, 32.06] | 19.94 [17.65, 20.92] | 0.153 |
| RSPO1* (%) | 9.54 [8.99, 15.94] | 2.41 [1.72, 2.85] | 0.003 |
| RSPO4 (%) | 5.86 [5.41, 11.11] | 3.55 [3.01, 3.94] | 0.011 |
| NP | HP | p | |
|---|---|---|---|
| n | 8 | 10 | |
| Serum RANKL (pg/mL) | 16.00 [11.38, 18.45] | 26.74 [23.43, 38.28] | 0.039 |
| Serum OPG (pg/mL) | 2301.50 [2015.20, 3899.15] | 3191.60 [2400.80, 4927.60] | 0.315 |
| Serum LGR4 (ng/mL) | 3.08 [2.35, 3.67] | 2.67 [1.91, 2.99] | 0.248 |
| Serum RSPO1 (ng/mL) | 0.40 [0.34, 0.46] | 0.21 [0.16, 0.31] | 0.003 |
| Serum RSPO4 (pg/mL) | 137.90 [120.60, 166.83] | 80.15 [71.58, 111.92] | 0.002 |
| Serum RANKL/OPG (%) | 0.61 [0.36, 0.88] | 0.60 [0.49, 1.04] | 0.479 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Villabrille, S.; Baena-Huerta, F.; Suárez-Fernández, L.; Nefyodova, E.; Calvó, P.; González-García, N.; Gil-Peña, H.; Gómez-Alonso, C.; Alonso-Montes, C.; Naves-Díaz, M.; et al. High Dietary Phosphorus Impairs Bone Microarchitecture and Induces Alterations in the LGR4–R-Spondins Axis in Rats with Normal Renal Function. Nutrients 2025, 17, 2049. https://doi.org/10.3390/nu17122049
Fernández-Villabrille S, Baena-Huerta F, Suárez-Fernández L, Nefyodova E, Calvó P, González-García N, Gil-Peña H, Gómez-Alonso C, Alonso-Montes C, Naves-Díaz M, et al. High Dietary Phosphorus Impairs Bone Microarchitecture and Induces Alterations in the LGR4–R-Spondins Axis in Rats with Normal Renal Function. Nutrients. 2025; 17(12):2049. https://doi.org/10.3390/nu17122049
Chicago/Turabian StyleFernández-Villabrille, Sara, Francisco Baena-Huerta, Laura Suárez-Fernández, Elena Nefyodova, Paula Calvó, Nerea González-García, Helena Gil-Peña, Carlos Gómez-Alonso, Cristina Alonso-Montes, Manuel Naves-Díaz, and et al. 2025. "High Dietary Phosphorus Impairs Bone Microarchitecture and Induces Alterations in the LGR4–R-Spondins Axis in Rats with Normal Renal Function" Nutrients 17, no. 12: 2049. https://doi.org/10.3390/nu17122049
APA StyleFernández-Villabrille, S., Baena-Huerta, F., Suárez-Fernández, L., Nefyodova, E., Calvó, P., González-García, N., Gil-Peña, H., Gómez-Alonso, C., Alonso-Montes, C., Naves-Díaz, M., Maes, C., Carrillo-López, N., & Panizo, S. (2025). High Dietary Phosphorus Impairs Bone Microarchitecture and Induces Alterations in the LGR4–R-Spondins Axis in Rats with Normal Renal Function. Nutrients, 17(12), 2049. https://doi.org/10.3390/nu17122049






