Silkworm Enzyme Hydrolysates Improve Memory in MCI Models via CREB-BDNF Signaling and Enhanced Brain Mitochondrial Function
Abstract
1. Introduction
2. Materials and Methods
2.1. Purchase Golden Silk HongJam and Production of Its Enzymatic Hydrolysate
2.2. Analysis Protocol for the Marker Compound of GS-EHS
2.3. Animals and Experimental Substances
2.4. Behavior Test Protocols
2.4.1. Y-Maze Spontaneous Alteration Assays
2.4.2. Passive Avoidance Tests (PATs)
2.4.3. Novel Objective Recognition Assays (NORs)
time to objects × 100%,
2.5. The Mouse Brain Dissections
2.6. Mitochondria Extraction, Mitochondria Complex I–IV Activity, and ATP Quantification Protocols
2.6.1. MitoCom I&II Activity Assays
ε of DCIP: 19.1 mM−1,
2.6.2. MitoCom III&IV Activity Assays
blank)/min]/[V of sample (mL) × protein concentration],
concentration)],
0.2: reaction volume (mL), 0.002: sample volume (mL),
21.84: ΔзmM between ferrocytochrome C and ferricytochrome C at 550 nm.
2.6.3. The ATP Quantification Protocol
2.7. Western Blot Analysis
2.8. Total Esterase Activity Assay
dilution factors]/[ε × V sample (mL)]/sample protein concentration,
ε for NB = 1.226544 mM−1 cm−1,
2.9. Reactive Oxygen Species (ROS) Quantification Protocol
concentration]),
2.10. Real Time Quantitative PCR (RT-q-PCR) Protocol
2.11. Statistical Analysis
3. Results
3.1. Significantly Enhanced Fear-Aggravated Memory in the MCI Mouse Models Supplemented with GS-EHS
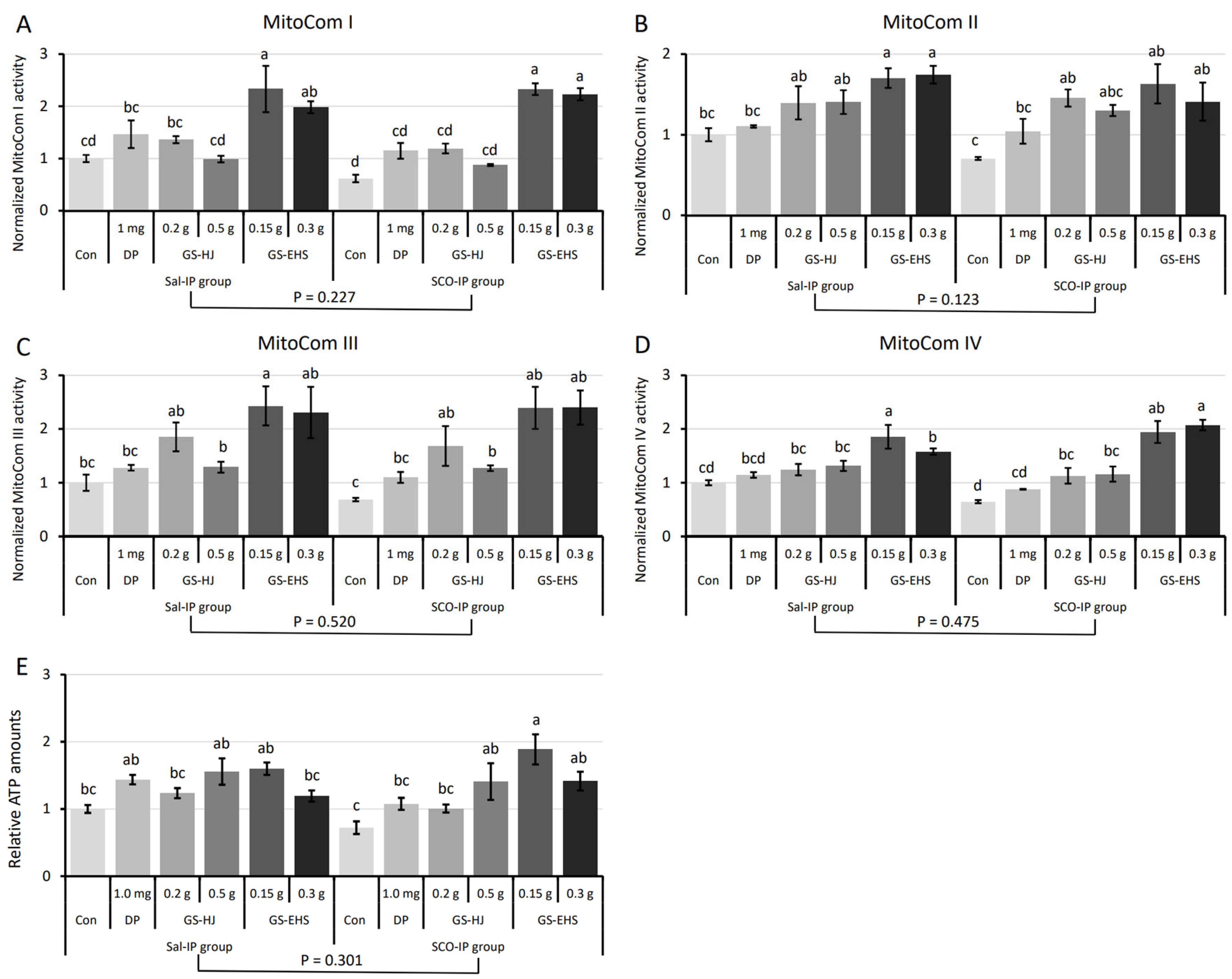
3.2. Significantly Enhanced MitoCom I–IV Activities and ATP Amounts in MCI Mouse Brains Supplemented with GS-EHS
3.2.1. MitoCom I–IV Activity Assay Results
3.2.2. ATP Quantification Assay Results
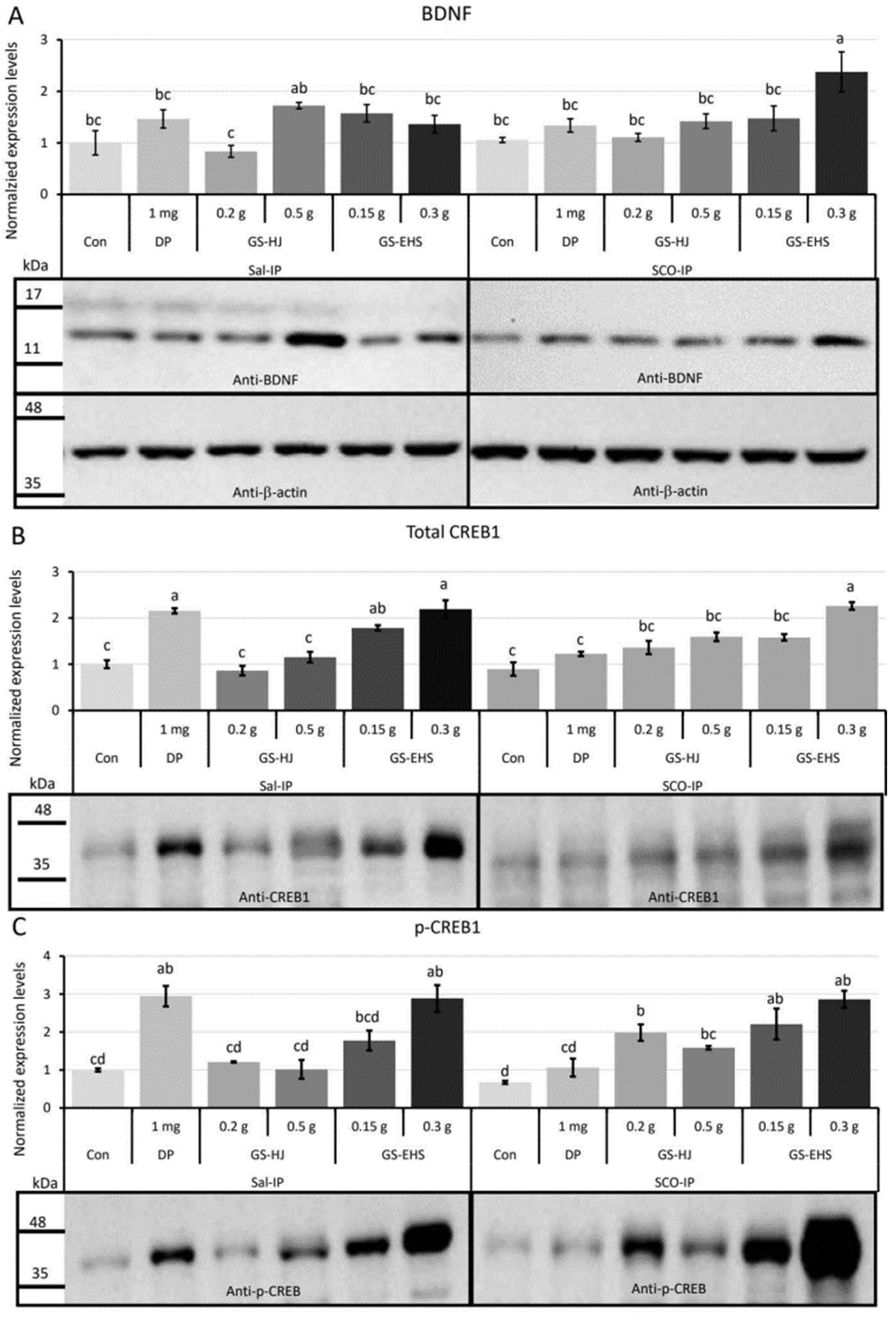
3.3. The Activated BDNF and CREB Signaling in the Mouse Brains Supplemented with GS-EHS
3.3.1. BDNF Quantification Results
3.3.2. CREB1 Quantification Results
3.3.3. p-CREB1 Quantification Results
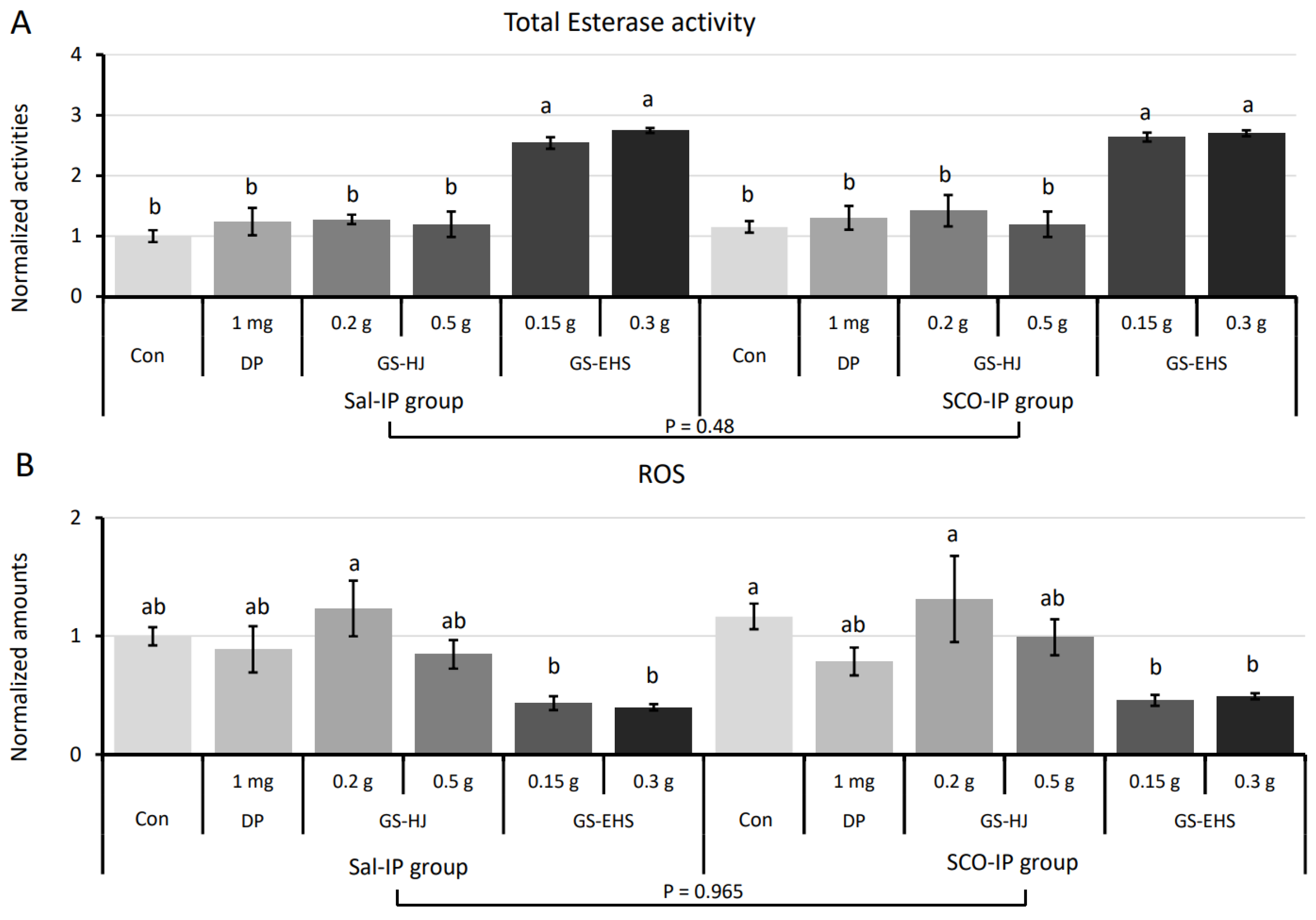
3.4. Increased Total Esterase Activity and Reduced ROS Amounts in GS-EHS-Supplemented Mouse Brains
3.4.1. Total Esterase Activity Assay Results
3.4.2. Reactive Oxygen Species Quantification Results
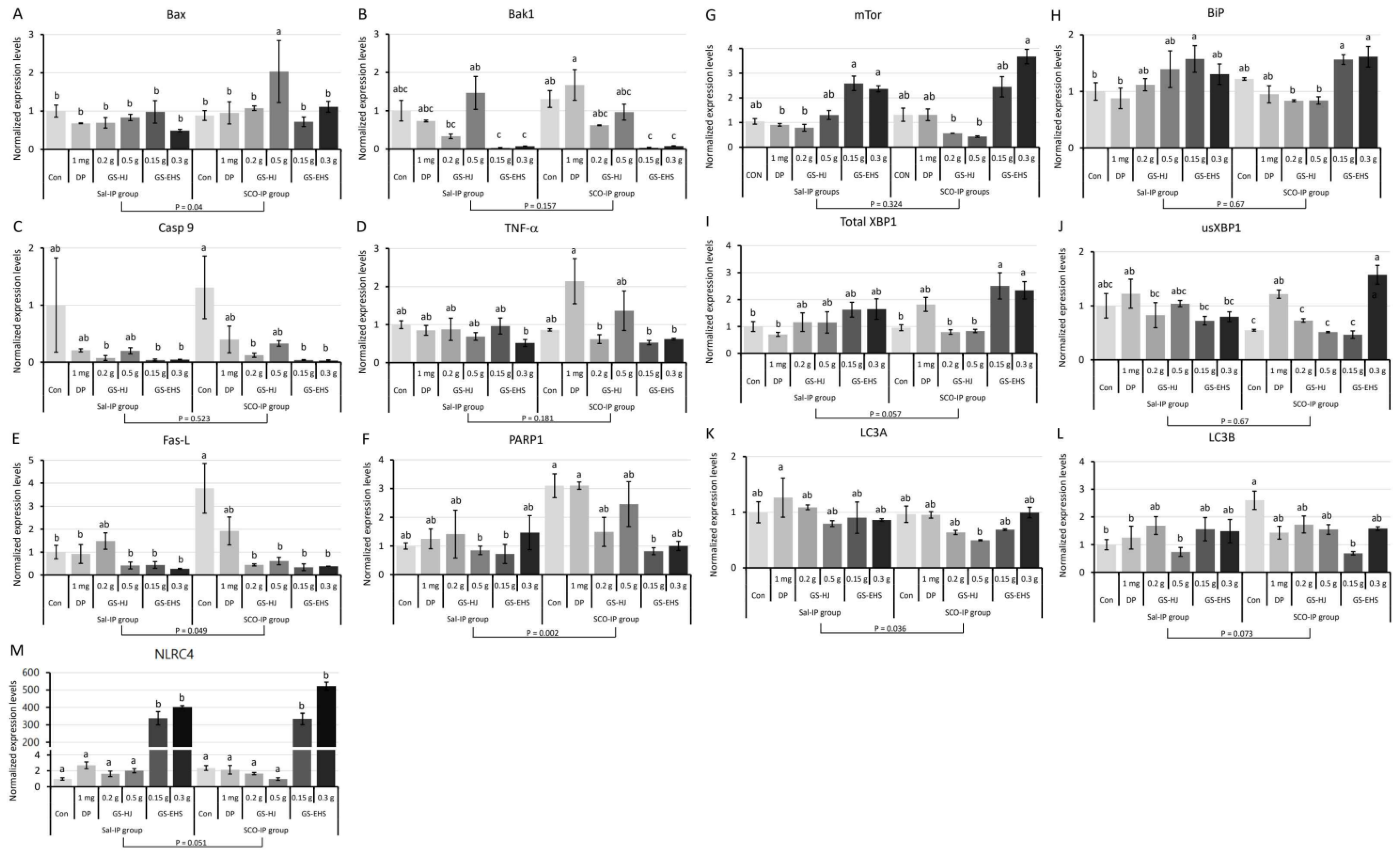
3.5. Supplementation GS-EHS Altered Expression Levels of Genes in Type I, Type II, and Inflammatory Programmed Cell Death Pathways in Mouse Brains
3.5.1. Type I PCD-Related Gene Expression Changes (Bax, Bak1, Casp9, TNF-α, Fas-L, PARP1)
3.5.2. Type II PCD-Associated Gene Expression Changes (mTOR, Bip, XBP1, sXBP1, usXBP1, LC3A, LC3B)
3.5.3. Inflammatory PCD-Associated Gene Expression Changes (IL-10R2, NLRC4, UCP2)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| A600nm | The absorbance at 600 nm |
| AD | Alzheimer’s disease |
| Bak1 | BCL2 antagonist killer 1 |
| Bax | BCL2-associated X protein |
| BDNF | Brain-derived neurotrophic factor |
| BW | Body weight |
| Casp 9 | Caspase 9 |
| CREB1 | Cyclic AMP response element binding protein 1 |
| DCIP | Dichlorophenolindophenol |
| DP | Donepezil |
| DTT | Dithiothreitol |
| Fas-L | Fatty acid synthetase-ligand |
| GS | Golden Silk HongJam |
| GS-EHS | Enzyme hydrolysates of GS |
| GY | Glycine–tyrosine |
| IL-10R2 | Interleukin 10 receptor, beta subunit |
| LC3A | Microtubule-associated protein 1 light chain 3 α |
| LC3B | Microtubule-associated protein 1 light chain 3 β |
| MCI | Mild cognitive impairment |
| MEB | Mitochondria extraction buffer |
| MitoCom | Mitochondria complex |
| mTOR | Mammalian target of rapamycin |
| NLRC4 | NLR family caspase recruitment domain containing protein 4 |
| NOR | Novel objective recognition assay |
| PAT | Passive avoidance test |
| PBT | 0.1 M phosphate buffer with 1.0% Triton X-100 |
| PCD | Programmed cell death |
| ROS | Reactive oxygen species |
| Sal-IP | Intraperitoneal injection of saline |
| SAR | Spontaneous alteration rate |
| SCO-IP | Intraperitoneal injection of scopolamine |
| sXBP1 | Spliced XBP1 |
| TNF-α | Tumor necrosis factor alpha |
| UCP2 | Uncoupling Protein 2 |
| UHPLC-MS/MS | Ultra-high performance liquid chromatography-tandem mass spectrometry |
| UPR | Unfolded protein response |
| usXBP1 | Un-spliced XBP1 |
| XBP1 | X-box-binding protein 1 |
References
- Kontis, V.; Bennett, J.E.; Mathers, C.D.; Li, G.; Foreman, K.; Ezzati, M. Future life expectancy in 35 industrialised countries: Projections with a Bayesian model ensemble. Lancet 2017, 389, 1323–1335. [Google Scholar] [CrossRef]
- Rakesh, G.; Szabo, S.T.; Alexopoulos, G.S.; Zannas, A.S. Strategies for dementia prevention: Latest evidence and implications. Ther. Adv. Chronic Dis. 2017, 8, 121–136. [Google Scholar] [CrossRef]
- Farias, S.T.; Mungas, D.; Reed, B.R.; Harvey, D.; DeCarli, C. Progression of mild cognitive impairment to dementia in clinic- vs community-based cohorts. Arch. Neurol. 2009, 66, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- Reuben, D.B.; Kremen, S.; Maust, D.T. Dementia prevention and treatment: A narrative review. JAMA Intern. Med. 2024, 184, 563–572. [Google Scholar] [CrossRef]
- Park, S.J.; Kim, K.-Y.; Baik, M.-Y.; Koh, Y.H. Sericulture and the edible-insect industry can help humanity survive: Insects are more than just bugs, food, or feed. Food Sci. Biotechnol. 2022, 31, 657–668. [Google Scholar] [CrossRef]
- Ji, S.-D.; Kim, S.-B.; Kim, K.-Y.; Kim, N.-S.; Kim, S.-W.; Jo, Y.-Y.; Kim, J.-G.; Kim, Y.-K.; Seok, Y.-S.; Lim, J.R.; et al. Contents of nutrients in ultra-fine powders of steamed and lyophilized mature silkworms generated by four silkworm varieties. J. Asia-Pac. Entomol. 2019, 22, 969–974. [Google Scholar] [CrossRef]
- Inoue, S.; Tanaka, K.; Arisaka, F.; Kimura, S.; Ohtomo, K.; Mizuno, S. Silk Fibroin of Bombyx mori is secreted, assembling a high molecular mass elementary unit consisting of H-chain, L-chain, and P25, with a 6:6:1 molar ratio. J. Biol. Chem. 2000, 275, 40517–40528. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.K.; Lee, B.Y.; Bucci, L.R.; Stohs, S.J. Effect of a Fibroin enzymatic hydrolysate on memory improvement: A placebo-controlled, double-blind study. Nutrients 2018, 10, 233. [Google Scholar] [CrossRef]
- Kang, Y.K.; Lee, W.J.; Kang, B.H.; Kang, H.N. Memory-enhancing fffects of silk Fibroin-derived peptides in scopolamine-treated mice. J. Microbiol. Biotechn. 2013, 23, 1779–1784. [Google Scholar] [CrossRef]
- Nguyen, P.; Kim, K.-Y.; Kim, A.Y.; Choi, B.-H.; Osabutey, A.F.; Park, Y.H.; Lee, H.-T.; Ji, S.D.; Koh, Y.H. Mature silkworm powders ameliorated scopolamine-induced amnesia by enhancing mitochondrial functions in the brains of mice. J. Funct. Foods 2020, 67, 103886. [Google Scholar] [CrossRef]
- Nguyen, P.; Kim, K.-Y.; Kim, A.Y.; Kang, S.; Osabutey, A.F.; Jin, H.; Guo, Y.; Park, H.; Suh, J.-W.; Koh, Y.H. The additive memory and healthspan enhancement effects by the combined treatment of mature silkworm powders and Korean angelica extracts. J. Ethnopharmacol. 2021, 281, 114520. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.A.H.; Nguyen, T.T.T.; Kim, Y.H.; Koh, Y.H. Preventive, ameliorative, and therapeutic effects of steamed mature silkworms on metabolic disorders caused by loss of apolipoprotein E. npj Sci. Food 2025, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]
- Leger, M.; Quiedeville, A.; Bouet, V.; Haelewyn, B.; Boulouard, M.; Schumann-Bard, P.; Freret, T. Object recognition test in mice. Nat. Protoc. 2013, 8, 2531–2537. [Google Scholar] [CrossRef]
- Nguyen, T.T.T.; Jang, B.; Kim, S.-R.; Kang, S.-K.; Kim, K.-Y.; Kim, Y.H.; Koh, Y.H. Enhanced Immune Functions of in vitro human natural killer cells and splenocytes in immunosuppressed mice supplemented with mature silkworm products. Nutrients 2025, 17, 417. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.M. Drug Metabolism and Pharmacogenetics. In Pharmacology and Physiology for Anesthesia, 2nd ed.; Hemmings, H.C., Egan, T.D., Eds.; Elsevier: Philadelphia, PA, USA, 2019; pp. 70–90. [Google Scholar] [CrossRef]
- Bardaweel, S.K.; Gul, M.; Alzweiri, M.; Ishaqat, A.; HA, A.L.; Bashatwah, R.M. Reactive oxygen species: The dual role in physiological and pathological conditions of the human body. Eurasian J. Med. 2018, 50, 193–201. [Google Scholar] [CrossRef]
- Newton, K.; Strasser, A.; Kayagaki, N.; Dixit, V.M. Cell death. Cell 2024, 187, 235–256. [Google Scholar] [CrossRef]
- Panwar, V.; Singh, A.; Bhatt, M.; Tonk, R.K.; Azizov, S.; Raza, A.S.; Sengupta, S.; Kumar, D.; Garg, M. Multifaceted role of mTOR (mammalian target of rapamycin) signaling pathway in human health and disease. Signal Transduct. Target. Ther. 2023, 8, 375. [Google Scholar] [CrossRef]
- Kopp, M.C.; Larburu, N.; Durairaj, V.; Adams, C.J.; Ali, M.M.U. UPR proteins IRE1 and PERK switch BiP from chaperone to ER stress sensor. Nat. Struct. Mol. Biol. 2019, 26, 1053–1062. [Google Scholar] [CrossRef]
- Wang, F.M.; Chen, Y.J.; Ouyang, H.J. Regulation of unfolded protein response modulator XBP1s by acetylation and deacetylation. Biochem. J. 2011, 433, 245–252. [Google Scholar] [CrossRef]
- Bertheloot, D.; Latz, E.; Franklin, B.S. Necroptosis, pyroptosis and apoptosis: An intricate game of cell death. Cell Mol. Immunol. 2021, 18, 1106–1121. [Google Scholar] [CrossRef] [PubMed]
- Bajo, R.; Pusil, S.; López, M.E.; Canuet, L.; Pereda, E.; Osipova, D.; Maestú, F.; Pekkonen, E. Scopolamine effects on functional brain connectivity: A pharmacological model of Alzheimer’s disease. Sci. Rep. 2015, 5, 9748. [Google Scholar] [CrossRef]
- Miravalles, C.; Cannon, D.M.; Hallahan, B. The effect of scopolamine on memory and attention: A systematic review and meta-analysis. Eur. Psychiatry 2025, 68, e50. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.S. The cellular and molecular processes associated with scopolamine-induced memory deficit: A model of Alzheimer’s biomarkers. Life Sci. 2019, 233, 116695. [Google Scholar] [CrossRef] [PubMed]
- Sakudoh, T.; Sezutsu, H.; Nakashima, T.; Kobayashi, I.; Fujimoto, H.; Uchino, K.; Banno, Y.; Iwano, H.; Maekawa, H.; Tamura, T.; et al. Carotenoid silk coloration is controlled by a carotenoid-binding protein, a product of the Yellow blood gene. Proc. Natl. Acad. Sci. USA 2007, 104, 8941–8946. [Google Scholar] [CrossRef]
- Ji, S.D.; Kim, N.S.; Lee, J.Y.; Kim, M.J.; Kweon, H.; Sung, G.; Kang, P.D.; Kim, K.Y. Development of processing technology for edible mature silkworm. J. Seric. Entomol. Sci. 2015, 53, 38–43. [Google Scholar] [CrossRef][Green Version]
- Qi, Y.; Wang, H.; Wei, K.; Yang, Y.; Zheng, R.Y.; Kim, I.S.; Zhang, K.Q. A review of structure construction of silk fibroin biomaterials from single structures to multi-level structures. Int. J. Mol. Sci. 2017, 18, 237. [Google Scholar] [CrossRef]
- Aksakal, B.; Akdere, Ü.; Günay, S.D.; Çağın, T.; Taşseven, Ç. Influence of repeating sequence on structural and thermal stability of crystalline domain of Bombyx mori silk fibroin. Mater. Res. Express. 2019, 6, 125356. [Google Scholar] [CrossRef]
- Hu, L.; Han, Y.; Ling, S.; Huang, Y.; Yao, J.; Shao, Z.; Chen, X. Direct Observation of native silk fibroin conformation in silk gland of Bombyx mori silkworm. ACS Biomater. Sci. Eng. 2020, 6, 1874–1879. [Google Scholar] [CrossRef]
- Yeo, J.-H.; Lee, K.-G.; Kweon, H.; Woo, S.-O.; Han, S.-M.; Lee, Y.-W.; Kim, J.-I.; Kim, S.-S.; Demura, M. Cognitive ability enhancement effects in rats by B. mori fibroin enzymatic hydrolysate. J. Sericultural Entomol. Sci. 2004, 46, 23–27. [Google Scholar]
- Mai, L.X.; Kang, S.-K.; Jo, Y.-Y.; Nguyen, P.; Kim, A.-Y.; Kim, K.-Y.; Kim, N.-S.; Koh, Y.H. An alkaline protease-digestion of silkworm powder enhances its effects over healthspan, autophagy, and mitochondria function in a rotenone-induced Drosophila Model. Front. Nutr. 2022, 9, 808295. [Google Scholar] [CrossRef]
- Cheng, L.; Shi, C.; Li, X.; Matsui, T. Impact of Peptide Transport and Memory Function in the Brain. Nutrients 2024, 16, 2947. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, S.; Sun, N.; Zhu, B.; Lin, S. Neuroprotective function of a novel hexapeptide QMDDQ from Shrimp via activation of the PKA/CREB/BNDF signaling pathway and its structure-activity relationship. J. Agric. Food Chem. 2020, 68, 6759–6769. [Google Scholar] [CrossRef]
- Amakye, W.K.; Hou, C.; Xie, L.; Lin, X.; Gou, N.; Yuan, E.; Ren, J. Bioactive Anti-aging agents and the identification of new anti-oxidant soybean peptides. Food Biosci. 2021, 42, 101194. [Google Scholar] [CrossRef]
- Chai, H.-J.; Wu, C.-J.; Yang, S.-H.; Li, T.-L.; Sun Pan, B. Peptides from hydrolysate of lantern fish (Benthosema pterotum) proved neuroprotective in vitro and in vivo. J. Funct. Foods 2016, 24, 438–449. [Google Scholar] [CrossRef]
- Zhao, Y.; Dong, Y.; Ge, Q.; Cui, P.; Sun, N.; Lin, S. Neuroprotective effects of NDEELNK from sea cucumber ovum against scopolamine-induced PC12 cell damage through enhancing energy metabolism and upregulation of the PKA/BDNF/NGF signaling pathway. Food Funct. 2021, 12, 7676–7687. [Google Scholar] [CrossRef]
- Abbott, N.J.; Rönnbäck, L.; Hansson, E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 2006, 7, 41–53. [Google Scholar] [CrossRef]
- Chen, W.N.; Yeong, K.Y. Scopolamine, a toxin-induced experimental model, used for research in Alzheimer’s disease. CNS Neurol. Disord. Drug Targets 2020, 19, 85–93. [Google Scholar] [CrossRef]
- Lu, C.; Wang, Y.; Xu, T.; Li, Q.; Wang, D.; Zhang, L.; Fan, B.; Wang, F.; Liu, X. Genistein ameliorates scopolamine-induced amnesia in mice through the regulation of the cholinergic neurotransmission, antioxidant system and the ERK/CREB/BDNF signaling. Front. Pharmacol. 2018, 9, 1153. [Google Scholar] [CrossRef]
- Liao, Y.; Bae, H.J.; Park, J.H.; Zhang, J.; Koo, B.; Lim, M.K.; Han, E.H.; Lee, S.H.; Jung, S.Y.; Lew, J.H.; et al. Aster glehni extract ameliorates scopolamine-induced cognitive impairment in mice. J. Med. Food 2019, 22, 685–695. [Google Scholar] [CrossRef]
- Chen, W.; Zhao, H.; Li, Y. Mitochondrial dynamics in health and disease: Mechanisms and potential targets. Sig. Transduct. Target. Ther. 2023, 8, 333. [Google Scholar] [CrossRef]
- Vringer, E.; Tait, S.W.G. Mitochondria and cell death-associated inflammation. Cell Death Differ. 2023, 30, 304–312. [Google Scholar] [CrossRef]
- Kanev, P.-B.; Atemin, A.; Stoynov, S.; Aleksandrov, R. PARP1 roles in DNA repair and DNA replication: The basi(c)s of PARP inhibitor efficacy and resistance. Semin. Oncol. 2024, 51, 2–18. [Google Scholar] [CrossRef]
- Huang, D.; Kraus, W.L. The expanding universe of PARP1-mediated molecular and therapeutic mechanisms. Mol. Cell 2022, 82, 2315–2334. [Google Scholar] [CrossRef]
- Wen, J.; Xuan, B.; Liu, Y.; Wang, L.; He, L.; Meng, X.; Zhou, T.; Wang, Y. Updating the NLRC4 inflammasome: From bacterial infections to autoimmunity and cancer. Front. Immunol. 2021, 12, 702527. [Google Scholar] [CrossRef]
- Matico, R.E.; Yu, X.; Miller, R.; Somani, S.; Ricketts, M.D.; Kumar, N.; Steele, R.A.; Medley, Q.; Berger, S.; Faustin, B.; et al. Structural basis of the human NAIP/NLRC4 inflammasome assembly and pathogen sensing. Nat. Struct. Mol. Biol. 2024, 31, 82–91. [Google Scholar] [CrossRef]
- Sasaki, Y.; Otsuka, K.; Arimochi, H.; Tsukumo, S.-I.; Yasutomo, K. Distinct roles of IL-1β and IL-18 in NLRC4-Induced autoinflammation. Front. Immunol. 2020, 11, 591713. [Google Scholar] [CrossRef]
- Hoshino, K.; Uchinami, Y.; Uchida, Y.; Saito, H.; Morimoto, Y. Interleukin-1β modulates synaptic transmission and synaptic plasticity during the acute phase of sepsis in the senescence-accelerated mouse hippocampus. Front. Aging Neurosci. 2021, 13, 637703. [Google Scholar] [CrossRef]
- Freeman, L.; Guo, H.; David, C.N.; Brickey, W.J.; Jha, S.; Ting, J.P.-Y. NLR members NLRC4 and NLRP3 mediate sterile inflammasome activation in microglia and astrocytes. J. Exp. Med. 2017, 214, 1351–1370. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.-H.; Phuong, N.; Hoang, N.M.A.; Kim, H.-J.; Baik, M.-Y.; Koh, Y.H. Silkworm Enzyme Hydrolysates Improve Memory in MCI Models via CREB-BDNF Signaling and Enhanced Brain Mitochondrial Function. Nutrients 2025, 17, 2044. https://doi.org/10.3390/nu17122044
Kim Y-H, Phuong N, Hoang NMA, Kim H-J, Baik M-Y, Koh YH. Silkworm Enzyme Hydrolysates Improve Memory in MCI Models via CREB-BDNF Signaling and Enhanced Brain Mitochondrial Function. Nutrients. 2025; 17(12):2044. https://doi.org/10.3390/nu17122044
Chicago/Turabian StyleKim, Yoo-Hee, Nguyen Phuong, Nguyen Minh Anh Hoang, Hye-Jin Kim, Moo-Yeol Baik, and Young Ho Koh. 2025. "Silkworm Enzyme Hydrolysates Improve Memory in MCI Models via CREB-BDNF Signaling and Enhanced Brain Mitochondrial Function" Nutrients 17, no. 12: 2044. https://doi.org/10.3390/nu17122044
APA StyleKim, Y.-H., Phuong, N., Hoang, N. M. A., Kim, H.-J., Baik, M.-Y., & Koh, Y. H. (2025). Silkworm Enzyme Hydrolysates Improve Memory in MCI Models via CREB-BDNF Signaling and Enhanced Brain Mitochondrial Function. Nutrients, 17(12), 2044. https://doi.org/10.3390/nu17122044





