Abstract
Background/Objectives: Poor diet is a leading modifiable cause of chronic disease in the US. In addition to targeting nutrients of concern (saturated fat, added sugars, and sodium), nutrients with both inadequate intakes and associations with major health outcomes require identification. We aimed to identify priority nutrients to address both malnutrition and diet-related disease in the US population. Methods: An established method for identifying priority nutrients across multiple demographic groups was adapted for the US population. This method evaluates and scores nutrients consumed at insufficient or excessive levels, with proposed revised requirements, and shows associations with established health priorities, based on the degree of deviation from recommendations and the number of linked health priorities. Priority nutrients were defined as those scoring in the top 25%. For each priority nutrient, a comparison of intake levels against the Dietary Reference Intake (DRI) was conducted. Results: There were 21 of 24 nutrients with consumption below recommended levels in at least one demographic group. Certain nutrients, such as dietary fiber, vitamin D, and choline, exhibited particularly high inadequacy rates, exceeding 90% throughout different life stages. The highest priority nutrients included vitamin D, vitamin E, calcium, magnesium, and dietary fiber, with vitamin D, omega-3 fatty acids, zinc, folate, and potassium showing priority for specific demographic groups. Comparing current intake levels with those known to benefit health priorities indicated that higher intakes of vitamin D, vitamin E, and calcium could be beneficial. Conclusions: Ten essential nutrients play a role in the prevention of diet-related disease, yet are consumed inadequately across the US population, suggesting that the prioritization of these nutrients can help to address the burden of chronic disease. Priority nutrients should be considered in diet and nutrition policies and guidelines.
1. Introduction
Poor diet is a leading modifiable cause of mortality and morbidity in the United States of America (US) [1]. Poor diet is directly related to both malnutrition (inadequate, excess, or imbalanced nutrient intake) and diet-related chronic diseases, including (but not limited to) type 2 diabetes (T2D), cardiovascular disease (CVD), obesity, and some cancers [2,3]. The issue of nutrient inadequacy is significant [4] with multiple key nutrients, as well as the foods and dietary patterns that support these nutrients being under-consumed [3,5,6]. Such inadequacies are globally prevalent [4], expected to worsen over time [7], and correlate with rising rates of chronic disease [8]. While current efforts to address poor diet and epidemic levels of diet-related disease are largely focused on excessive energy, sodium, sugars, and saturated and trans fats [9,10], there is a recognized need for further action in the US, including evidence-based policy solutions [11]. Current strategies, as recommended by health professionals, nutrition scientists, and policy-makers, include implementing a “food as medicine” approach, where nutrition guidelines and dietary prescriptions are targeted towards the individual, their food choices, and priority health issues [11]. An essential step towards tackling both malnutrition and diet-related disease in the US involves identifying the nutrients that should be prioritized, considering both inadequate intakes and the association with specific health outcomes, for each key demographic group.
The prioritization of nutrients to address both malnutrition and diet-related disease has been published for Australia (AUS) and New Zealand (NZ) [12]. This research identified 22 nutrient inadequacies across demographic groups. Some of these inadequate nutrients show associations with high priority diet-related health outcomes across the lifespan; for example, dietary fiber with cancer [13,14] and cardiometabolic health [15], calcium with bone growth during childhood and bone mass maintenance with aging [16,17], and vitamin D with cognitive function [18], breast cancer risk [19], depression [20], and metabolic health [21]. Within the AUS/NZ data, nine nutrients (vitamin D, calcium, omega-3 fatty acids, magnesium, folate, dietary fiber, iron, selenium, and zinc) showed a high priority in at least one demographic group, based on the specific level of intake and health priorities of that group. Six nutrients (vitamin D, calcium, omega-3 fatty acids, magnesium, folate, dietary fiber) were classified as a priority across the entire population, indicating that a set of nutrients could benefit all individuals, regardless of sex and/or age, informing both national and demographic-specific recommendations and guidelines. The application of this methodology to the US population can uncover nutrients with high potential to affect meaningful benefits in diet-related diseases, both across the lifespan (for each demographic group) and for the overall population.
Meeting Dietary Reference Intakes (DRIs) is used as a measure of nutrient adequacy. However, even if recommended intakes are met, nutrient inadequacy may also be present in instances where there is reduced nutrient absorption, bioactivity, and/or increased excretion. In addition, increased intakes of some nutrients may provide health benefits beyond a frank deficiency. For example, the maintenance requirement for protein has been suggested to be too low, based on the re-evaluation of nitrogen balance data, new data from isotope tracer studies [22,23,24], and the influence of increased protein intake on clinical functional outcomes such as sarcopenia [24]. In AUS/NZ, suggested dietary targets (SDTs) for the prevention of chronic disease have been developed for some nutrients [25], representing increased requirements relative to reference values that may provide additional benefit. Priority nutrient recommendations need to be accompanied by knowledge of the magnitude of intake that can support not only inadequacy, but also the prevention of diet-related disease, including the possibility that increased intake targets may be necessary for some nutrients, and specific to demographic groups and/or health outcomes. The identification of priority nutrients in the American population will highlight the specific nutrients that can be targeted across demographic groups for meaningful impacts on population health, thus helping to inform research and policy.
The objective of this research is to use our previously published methodology [12], adapted to the American context, to determine the priority nutrients to address to contribute to the prevention of malnutrition and chronic disease for the US population overall, and for specific life stages, represented by demographic groups (age/sex). It was hypothesized that nutrient shortfalls would be prevalent, and that each demographic group would be characterized by a specific set of priority nutrients. We also hypothesized that a select set of key nutrients would show relevance to malnutrition and diet-related disease across the entire lifespan.
2. Materials and Methods
2.1. Conceptual Summary
The purpose of this research was to undertake a holistic assessment of nutrients with the highest potential to concurrently address both malnutrition and diet-related disease in the US population, across the lifespan. A five-step methodological approach, based on the four-step method used to identify priority nutrients for the AUS/NZ populations, as previously described by Starck et al. [12] was reproduced (Figure 1). In addition to modification for the US context, a fifth step was added to investigate the intake of each priority nutrient identified and compare this intake to current recommendations, to determine if increased dietary targets specific to disease prevention may be of benefit. Each of the five steps is outlined in Figure 1.
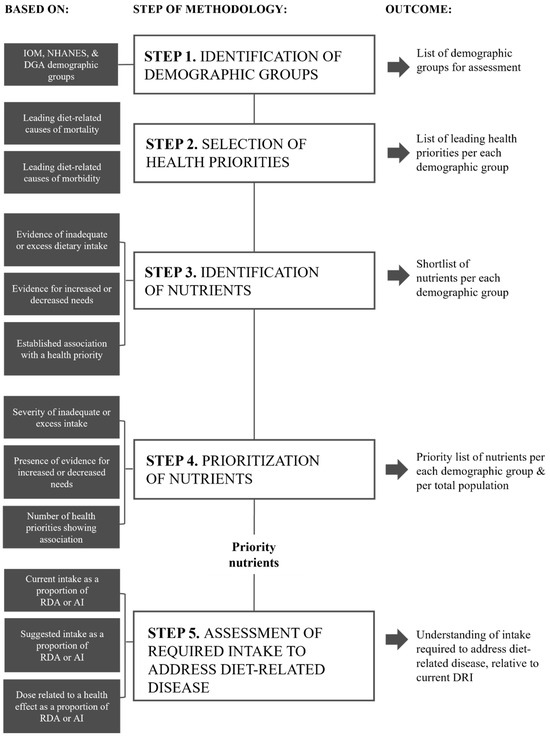
Figure 1.
Overview of the five-step methodological approach used to identify priority nutrients for the US.
2.2. Identification of Demographic Groups (Step 1)
Demographic groups were defined based on the life stages used by the National Academies Institute of Medicine Dietary Reference Intakes (DRIs) [26], National Health and Nutrition Examination Survey (NHANES) nutrient intake data [27,28] and the Dietary Guidelines for Americans [29]. The final selected demographic groups considered both consistency in DRIs across ages and sexes, as well as the biological needs of each life stage and their clinical relevance for nutrient requirements. Age and sex groupings were collapsed if there were no clinically relevant differences in nutrient requirements between sexes of the same age group or consecutive age groups. Age and sex groupings were split if one or more sub-populations of a group were differentiated biologically.
2.3. Selection of Health Priorities (Step 2)
The approach applied to the selection of health priorities for each demographic group was developed to allow for the identification of a broad scope of health outcomes (including both physical and psychological conditions), thus aligning with the holistic intent of the research. Health priorities for each demographic group were informed by national US data collected by the Centers for Disease Control and Prevention (CDC) [30,31], the health priorities laid out by the US Department of Health and Human Services [32], and an examination of the scientific literature [33,34,35]. Health priorities were selected if they were the leading causes of death, non-fatal physical morbidity, and mental or cognitive ill-health, which are known to be modifiable by diet. Health outcomes existing as a subtype of a more significant disease or set of health issues were collapsed within one umbrella health outcome. Health outcomes with the highest prevalence of mortality and/or morbidity and the highest association with diet were prioritized. The maximum number of included health priorities for any one demographic group was five. This number was selected based on balancing multiple goals: to focus on major health outcomes, maintain a manageable and concise scope of research, and allow for the specific health priorities for each demographic group to be identified. To incorporate the large number of diet-related health issues and diseases identified as a national priority for older adults, cognitive function, bone health, and sarcopenia were collapsed within one group defined as ‘independence’.
2.4. Identification of Nutrients (Step 3)
Nutrients included in the assessment (N = 24) were those with an Estimated Average Requirement (EAR) or Adequate Intake (AI) outlined by the Institute of Medicine DRIs [26,36,37] (Table 1).

Table 1.
Nutrients included in the assessment.
The omega-3 fatty acids docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) were added based on the previous AUS/NZ research [12] showing consistent associations with high-priority health outcomes. As per the previous methodology, the following exclusions were observed: energy, due to its dependence on more than one nutrient; sodium, saturated fat, and sugars, due to being established nutrients of concern in the population; water, due to the impact of additional beverages, including those containing nutrients, on hydration status. Pantothenic acid, biotin, chromium, fluoride, manganese, and molybdenum were not included due to an absence of national intake data. For each of the included nutrients per each demographic group, evidence was collected in three areas: 1. Evidence of inadequate or excess dietary intake (as mean intake and percentage of the demographic group with intake less than the EAR or AI, as applicable, or greater than the Tolerable Upper Intake Level (UL), respectively); 2. Scientific evidence for increased or decreased needs, including that addressed within the current DRIs (such as limited utilization by a demographic group) and where a revision of requirements (relative to the NRVs) has been suggested in the literature; and 3. An established association (beneficial or adverse) with one or more of the health priorities identified.
2.4.1. Dietary Intake: Inadequate or Excess
Intake data were obtained from the What We Eat in America (WWEIA) database [28], the dietary component of the NHANES dataset. NHANES dietary intake data were initially collected by trained interviewers using the 5-step USDA Automated Multiple-Pass Method (AMPM) over two days of 24 h dietary recall. The Day 1 interview was conducted in person, and the Day 2 interview was conducted by telephone 3 to 10 days later, on a different day of the week from the Day 1 interview. To identify inadequate or excess nutrients, the most recent intake data available for each nutrient, including information provided within the dataset regarding the proportion of the population with intake below the EAR or AI, or above the UL, were used. For the majority of nutrients, this was the NHANES 2017 to March 2020 pre-pandemic data release [27]. For pregnant and lactating women, the most recent NHANES data are 2003–2006 for select nutrients (dietary fiber, vitamin D, calcium, phosphorus, and magnesium) [28]; this was supplemented by a targeted literature search via the PubMed database for peer-reviewed original research of nationally representative US data. More recent NHANES datasets have excluded pregnant and lactating women due to the sample being too small to allow for reliable estimates. For iodine, intake data from the Global Dietary Database were accessed [38]. These data include median intake and 5th and 95th percentile intakes for five-year age and sex groupings.
For each nutrient and each demographic group, mean intake data and the proportion of the population not meeting the EAR or AI or exceeding the UL (where appropriate) were obtained, as provided within the NHANES reports. For iodine, the percentile of intake corresponding to the EAR was approximated using the mean intake data, 95% confidence interval, and z-score tables, according to the formula: z = (x − µ)/σ, where z = z-score, x = EAR, µ = mean, and σ = standard deviation, calculated as the 95% confidence interval divided by 3.92.
2.4.2. Evidence for Revised Needs: Increased or Decreased
Evidence for revised (increased or decreased) nutrient needs was defined as there being consensus in the scientific literature that the DRI (EAR, RDI, or AI) is too low or high for a demographic group, or that the demographic group may benefit from intakes above or below the specified requirement. Evidence was obtained via a targeted literature search of peer-reviewed studies using the PubMed database. Consensus in the scientific literature was defined as consistency in relevant reviews, randomized controlled trials, balance studies, or a position statement from a national or global authority providing a sound rationale for an increased or decreased requirement of a specific nutrient relative to the DRIs. For some identified nutrients, the rationale and evidence for increased needs were already accounted for by the DRIs and therefore reflected in the level of inadequacy reported by the collected dietary intake data. In these cases, the relevant nutrients were not included as having increased needs to avoid double scoring in the following step (Step 4).
2.4.3. Health Priority Association: Beneficial or Adverse
A targeted literature search of the PubMed (with Medline) database was undertaken for systematic literature reviews (SLRs) of primarily randomized controlled trials (RCTs) or prospective cohort studies showing an association of a nutrient with one of the identified health priorities. The search was focused on capturing the most recent and highest-level evidence for each nutrient and health priority combination up to September 2024, rather than all evidence published to date. Evidence was limited to the demographic group or groups with that specific health priority, according to the age ranges covered by the included research. Search strategies are listed in Supplementary Table S1. Only SLRs of RCTs or prospective cohort studies were eligible for inclusion to ensure that the highest level of available evidence was captured, based on the Oxford Centre for Evidence-Based Medicine (OCEBM) framework [39]. The search and data selection include both beneficial and adverse associations. Neutral and inconclusive associations were not included. Associations for sub-groups (e.g., vegetable vs. cereal fiber and animal vs. plant protein) were not considered. For example, where a systematic review assessed associations between total fiber, as well as fiber from individual food sources (such as fruits, vegetables, nuts and seeds, and cereal foods), association data for total fiber only were used. Where multiple SLRs reported conflicting findings for the same nutrient-health priority association, the evidence was considered to be inconclusive.
2.5. Prioritization of Nutrients (Step 4)
Nutrient prioritization was carried out using a points-based scoring matrix as previously described [12], with adaptations (Table 2). Each nutrient in each demographic group was awarded points in each of the three areas to produce three sub-scores: dietary intake (inadequate or excess), revised needs (increased or decreased), and overall association with health (beneficial minus adverse). The three sub-scores were summed to produce a total priority score. The dietary intake sub-score was calculated to reflect the magnitude of inadequacy or excess of a demographic group relative to the EAR or AI (at least 20% or 50% not meeting the EAR or AI, as applicable, or exceeding the UL, respectively). A demographic group contained more than one age/sex group, and scoring was based on the highest level of inadequacy or excess to ensure that the most at-risk group was accounted for. When the percentage inadequacy or percentile data were not available, a mean intake less than or greater than the DRI for that group was taken to represent inadequacy or excess, respectively.

Table 2.
Quantitative scoring matrix for nutrient prioritization.
Up to one additional point was awarded for evidence of increased or decreased needs, as appropriate, and up to five additional points were awarded for established effects on one or more health priorities. The health association sub-score was the difference between the score for beneficial associations and that for adverse associations. The maximum and minimum total priority scores for any nutrient were 10 and −10, respectively. Positive scoring represented a beneficial nutrient, whereas negative scoring was taken to represent a nutrient of concern. The highest priority nutrients for a demographic group were defined as those scoring in the top 25% for that group, based on the absolute score (positive or negative). The highest priority nutrients across the entire population (all included demographic groups) were determined by summing the total scores per nutrient across all demographic groups. A maximum score of 80 was achievable for a population-level priority nutrient. Where multiple nutrients achieved the same score, preventing clear distinction according to the number of nutrients in the top quartile, the priority nutrient to be included was based on sub-score values: that with the highest level of inadequate or excess intake in the first instance, followed by the highest number of health associations, and then the presence of evidence for increased or decreased needs.
2.6. Assessment of Required Intake to Address Diet-Related Disease (Step 5)
The purpose of Step 5 was to determine, for each priority nutrient, if an increased intake might be beneficial to address diet-related disease. For each priority nutrient, per each demographic group, the mean intake, the level of the suggested revised requirement, and the minimum dose associated with an effect on a health priority were expressed as a proportion of the Recommended Dietary Allowance (RDA) or AI for that nutrient (proportional intake), with the RDA/AI defined as being equal to 1. The RDA was used instead of the EAR to reflect the intake that is considered to be sufficient to meet the requirements of nearly all (97–98%) of the population [26]. For beneficial nutrients where the minimum dose was greater than the UL for a nutrient, the UL was taken to be the minimum dose given the established risks associated with exceeding the UL. These risks and limitations associated with the collected data were noted. For demographic groups comprising more than one age and/or sex group (as defined by national reference intakes or guidelines [26,27,28,29]), the RDA reflecting the greatest need was used to represent the demographic group to capture the highest level of inadequacy. If a nutrient was associated with multiple health priorities for a demographic, with a variation in the minimum dose across health priorities, the mean minimum dose was calculated and used. Similarly, if there were multiple suggestions for an increased need, the mean suggested level of need was used. If there were no data for a specific characteristic (for example, no level of inadequacy identified), the RDA or AI, as appropriate, was assumed to be applicable. The proportional intake for a population-level priority nutrient was calculated as the mean proportional intake ±SD for all demographic groups. Proportional intakes per each of the mean intake, level of suggested revised requirement, and minimum dose associated with an effect on a health priority were compared to assess the suitability of current RDIs to address diet-related diseases.
3. Results
3.1. Identification of Demographic Groups (Step 1)
A total of eight demographic groups were selected for this research: children (males and females) 4 to 8 years; adolescent males 9–18 years; adolescent females 9–18 years; adult males 19–70 years; adult females 19–50 years; pregnancy and lactation 19–50 years; menopause and post-menopause 51–70 years; and older adults (males and females) >70 years. Children under 4 years of age were excluded. Demographic groups were based predominantly on the age/sex groups defined by the Institute of Medicine (IOM) [26,36,37], with the collapse of some of these to produce larger demographic groupings: Male and female children were grouped together due to having similar intake needs for the majority of nutrients and an absence of major hormonal changes; Adolescent age groups of 9–13 years and 14–18 years were collapsed into one group (for each of males and females) due to having similar nutrient needs and hormonal changes appearing before the age of 13 in many individuals [40]. Adult male age groups of 19–30, 31–50, and 51–70 years were collapsed due to having similar nutrient requirements. Female demographic groups were defined based on the major life stages of fertility, pregnancy and lactation, and menopause and post-menopause. Male and female older adults greater than 70 years were collapsed due to an absence of menstrual fluctuations in women by this age.
3.2. Selection of Health Priorities (Step 2)
The final health priorities selected for each demographic group, including the health outcomes covered by each major health priority, are shown in Table 3.

Table 3.
Health priorities and included health outcomes for each demographic group.
Many health priorities featured across multiple demographic groups, such as cardiovascular health, metabolic health, cancer, mood, and mental health. Most demographic groups were characterized by one or more specific health priorities, such as fetal development for pregnancy and lactation, and fertility for adult females aged 19–50 years. For some health priorities, specificity was found within the included health outcomes; for example, while children and male and female adolescents shared a ‘growth and development’ health priority, pubertal development was included for male and female adolescents, while neurodevelopmental disorders were present for children. The health priority of ‘independence’ for older adults was constructed based on there being many aging-related health outcomes that influenced the ability of an individual to remain living independently, including physical mobility (such as muscle mass and osteoarthritis), resilience-related outcomes (such as frailty and risk of falls), and cognitive function.
3.3. Identification of Nutrients (Step 3)
A total of 21 nutrients (out of 24; 87.5%) were identified to have inadequate intake (N = 21), evidence for increased needs (N = 7), or an association with one or more health priorities (N = 14, 58%). Three nutrients showed beneficial and adverse associations (protein, folate, and omega-3 fatty acids), and one nutrient (copper) showed adverse associations only (cardiovascular health). The identified nutrients are summarized for each demographic group in Table 4, with additional detail provided in Supplementary Tables S2–S4.

Table 4.
Nutrients identified as either having inadequate intakes 1, increased needs 2, or association with health priorities 3 by demographic group.
Inadequate intakes were prominent across the demographic groups, with adolescent females showing the greatest number of inadequacies (N = 19), followed by pregnancy and lactation (N = 16). The demographic group with the lowest number of inadequate nutrients was children (N = 8). Some nutrients had very high levels of inadequacy (over 90% not meeting the EAR or AI) across all demographic groups, including dietary fiber, vitamin D, and choline (Supplementary Table S2). No nutrients were identified as being of concern, where intake was above the UL for more than 20% of a demographic group.
Nutrients with evidence for increased needs were identified for all demographic groups (Supplementary Table S3). In particular, there was evidence for increased protein and magnesium requirements across all demographic groups, and for zinc in all demographic groups except children. Some nutrients were suggested to have an increased need specific to one demographic group, such as vitamin D for menopause and post-menopause, and vitamin B12 for older adults. No evidence suggesting decreased needs for any nutrient for any demographic group with respect to the relevant DRIs was identified.
Over half of the included nutrients were associated with at least one health priority, with select nutrients showing associations with multiple health priorities across and within demographic groups (Supplementary Table S4). For example, vitamin D was beneficial for growth and development and respiratory health for both children and adolescents, metabolic health for all demographic groups, mood and mental health for all demographic groups except older adults, cardiovascular health for all demographic groups except children and adolescents, and cancer for all demographic groups except children. Significant associations were also found for dietary fiber, folate, and omega-3 fatty acids. Adverse associations were found for folate (increased risk for gestational diabetes mellitus [48] in pregnancy and lactation, and ADHD children and adolescents have higher blood folate levels [49]), omega-3 fatty acids (increased risk of atrial fibrillation with supplementation [50], and high doses of vitamin D (increased risk of falls and hip fracture in older adults [51,52]). Higher vs. lower protein intake was found to increase the risk of early menarche in adolescent females [53], and elevated blood copper levels were associated with an increased risk of stroke, coronary artery disease mortality, and CVD mortality in adults [54].
Figure 2 depicts the number of demographic groups with inadequate intake (Figure 2A), increased needs (Figure 2A), and association with a health priority, for each nutrient. Seven nutrients had inadequate intake across all demographic groups: dietary fiber, vitamin D, vitamin E, choline, calcium, iodine, and potassium; some nutrients (such as protein and vitamin B12) were consumed inadequately in one demographic group. Increased needs were most prevalent for protein and magnesium, with a suggested increase in the DRI for all demographic groups. Similarly, the health of all demographic groups was found to benefit from vitamin D, vitamin E, calcium, and zinc. While omega-3 fatty acids and folate showed adverse health associations across four demographic groups, beneficial associations were present in more demographic groups (eight and six, respectively). Copper had only adverse associations in half of the demographic groups (all adult groups except pregnancy and lactation, Supplementary Table S4).
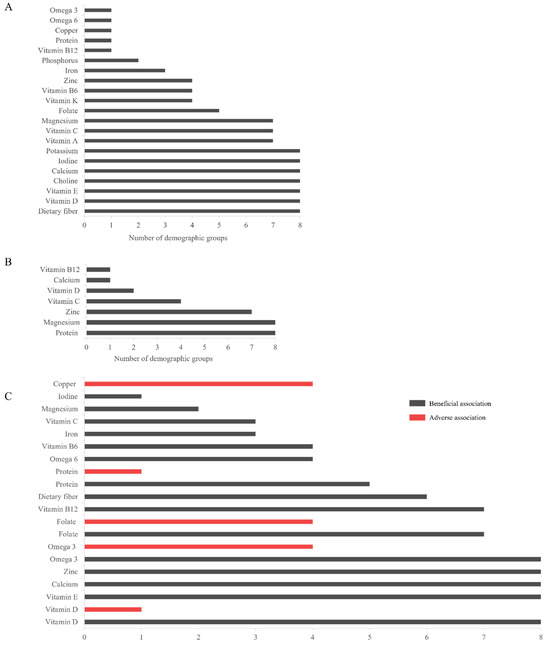
Figure 2.
The number of demographic groups with each of (A) inadequate intake; (B) increased needs; and (C) association with health priorities, for each identified nutrient. Both beneficial (gray bars) and adverse (red bars) health associations are presented for each applicable nutrient. Abbreviations: omega-3, omega-3 fatty acids; omega-6, and omega-6 fatty acids.
3.4. Prioritization of Nutrients (Step 4)
The highest priority nutrients by demographic group, and for the overall population, are shown in Figure 3. Sub-scores for dietary intake (red), revised needs (light gray), and health association (dark gray) are also presented. All priority nutrients were beneficial; no priority nutrients of concern were identified. A heatmap as a visual representation of nutrient scoring and prioritization across the demographic groups is provided in Supplementary Figure S1.
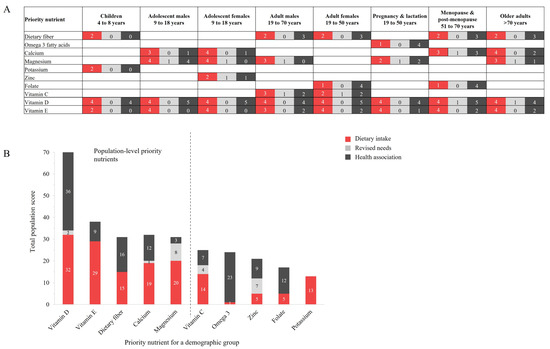
Figure 3.
The priority nutrients for (A) each demographic group; and (B) the total population, including sub-scores for each of dietary intake (red), revised needs (light gray), and association with health priorities (dark gray). The highest priority nutrients for the overall population (left of dashed line, (B) are vitamin D, vitamin E, dietary fiber, calcium, and magnesium. Abbreviations: Omega-3, omega-3 fatty acids.
Ten nutrients emerged as a priority to address malnutrition and diet-related disease in at least one demographic group (Figure 3A); these were dietary fiber, omega-3 fatty acids, calcium, magnesium, potassium, zinc, folate, vitamin C, vitamin D, and vitamin E. Vitamins D and E were priority nutrients for all demographic groups, while potassium (children), zinc (adolescent females), and omega-3 fatty acids (pregnancy and lactation) were priority nutrients for one demographic group only. Sub-scores for the majority of priority nutrients were limited to dietary intakes and health association, with some priority nutrients achieving priority status solely due to inadequate dietary intake, such as dietary fiber, potassium, and vitamin E for children.
The highest priority nutrients across the overall US population were dietary fiber, vitamin D, vitamin E, calcium, and magnesium (Figure 3B), with vitamin D having the highest total priority score at 71 out of 80, almost double the next highest priority nutrient (vitamin E, with 38 points) and more than double that of other priority nutrients. Vitamin D showed high levels of both dietary inadequacy and beneficial health priority associations. This pattern was also reflected by dietary fiber, while vitamin E, calcium, and magnesium had scores that were predominated by dietary inadequacy.
3.5. Assessment of Required Intake to Address Diet-Related Disease (Step 5)
Table 5 summarizes the magnitude of dietary intake, suggested increased need, and dose range associated with beneficial effects on each health priority, for each priority nutrient in each demographic group. The proportional intake (PI) of each, relative to the RDA or AI (as applicable), is also shown. For most priority nutrients, dietary intake had a PI less than one (reflecting inadequacy), while the mean minimum dose associated with beneficial effects on a health priority was greater than one, reflecting that an increased daily intake relative to the DRI may be required to address diet-related disease. The exception to this was dietary fiber, for which a modest increase in daily intake was found to impart significant benefits for multiple health priorities, including metabolic health (at least 3 g/day [15]), cancer (10 g/day [55]), and cardiovascular health (at least 3 g/day [15,55,56]); the PI range was 0.6–0.99 (representing a total fiber intake of 60–99% of the AI) across applicable demographic groups.

Table 5.
Summary of intake 1, suggested increase 2, dose range associated with a beneficial effect on a health priority 3, and estimation of proportional intake 4 for each priority nutrient for each demographic group.
Integration of proportional intake values across the demographic groups for each of the population-level priority nutrients is depicted graphically in Figure 4. Comparison of mean PI values for dietary intake (red bars), level of revised needs (light gray bars), and minimum dose associated with a health priority (dark gray bars) shows that the current DRI (standardized, dashed line) for dietary fiber and magnesium aligns with the minimum dose found to address diet-related disease. For these nutrients, increased dietary intake to meet the DRI is required to address the health priorities of each relevant demographic group. While variability is high (large error bars), an increased intake, relative to the DRI, of vitamin D, vitamin E, and calcium may provide additional benefit in reducing the risk of and/or preventing diet-related disease.
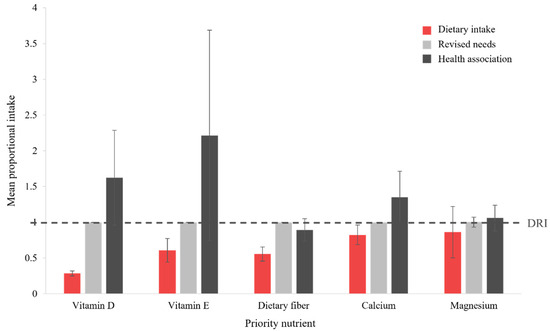
Figure 4.
Comparison to the DRI (RDA or AI, as applicable, dashed line) for each population-level priority nutrient, for dietary intake (red bars), suggested level of revised needs (light gray bars), and minimum dose showing an association with a health priority (dark gray bars). Columns represent the mean proportional intake across all demographic groups, with error bars indicating the associated SD. Abbreviations: AI, Adequate Intake; DRI, Dietary Reference Intake; RDA, Recommended Dietary Allowance.
4. Discussion
This research provides an evidence-based, holistic approach to the prioritization of nutrients that can be targeted to address both malnutrition and diet-related disease in the US. These priority nutrients have been identified across demographic groups and for the overall population, enabling both demographic-specific and nationwide recommendations that can help to close these nutrient gaps. Nutrient inadequacies were highly prevalent across all demographic groups, indicating a key gap in nutrient sufficiency, even within a developed nation. While ten nutrients were found to be the highest priority across the demographic groups, five nutrients were identified to be of utmost importance across the overall American population, showing both a high level of inadequate intake and multiple associations with health priorities. These priority nutrients were vitamin D, vitamin E, dietary fiber, calcium, and magnesium. Vitamin D and E were priority nutrients for every demographic group. A comparison of current and recommended intakes with intakes showing beneficial associations with major health priorities indicated that increased dietary targets for vitamin D, vitamin E, and calcium may be beneficial. Initiatives that increase the intake of priority nutrients to address malnutrition and diet-related disease in the US are needed. Recommendations can further be tailored to each demographic group by health professionals and governing bodies via food-based guidelines.
It is well-established that poor diet is a leading cause of mortality and morbidity related to chronic diseases in the US, such as T2D, CVD, obesity, musculoskeletal disorders, and some cancers [1,11]. Consistent with these data, metabolic health, cardiovascular health, and cancer were found to be major health priorities across almost all demographic groups in the current research, and multiple nutrient-health associations for these health outcomes were identified. However, the novel contribution of the research presented here is that many of the nutrients found to confer protective effects for diet-related disease were also consumed inadequately: the proportion of the population with an intake less than the EAR was over 97% for some nutrients. Thus, micronutrient inadequacies, particularly of nutrients that play a role in the prevention of chronic disease, are likely to be a critical factor in improving diet-related health in the US. Preventative approaches have been highlighted as a key priority for all stakeholders involved in public health, including physicians, nurses, hospital systems, policy-makers, health insurance companies, patients and their families, and advocacy groups [1]. Hence, this research can assist stakeholders in the prioritization of nutrients as part of a preventative solution to both nationwide malnutrition and diet-related disease rates: vitamin D, vitamin E, calcium, magnesium, and dietary fiber. Of these, calcium, dietary fiber, and vitamin D are considered to be nutrients of public health concern for the general US population because low intakes are associated with health concerns [29], adding weight to their priority status.
While the term ‘malnutrition’ has long been associated with protein-energy and micronutrient deficiency in developing countries, particularly for children and pregnant women [177], it is clear that nutrient inadequacies are prominent across the lifespan in developed countries such as AUS, NZ [12], and the US. Similarly, a recent analysis of global micronutrient intakes reported high rates of inadequacy for multiple micronutrients, especially (but not limited to) vitamin A, iodine, and calcium across the lifespan and regardless of a country’s economic status [4]. Although frank deficiencies such as goiter (iodine) and night blindness (vitamin A) are unlikely in developed countries, inadequate nutrient intake is a risk factor for deficiency [4], and is suggestive of dietary patterns that are unable to optimally support health. The World Health Organization defines malnutrition as deficiencies, excesses, or imbalances in a person’s intake of energy and/or nutrients [178]; thus, both micronutrient inadequacy and excess consumption of nutrients of concern (added sugars, sodium, and saturated fat) contribute to malnutrition in the US. It is possible that diet-related diseases are an overt symptom of malnutrition. In this study, 21 of the 24 assessed nutrients (87.5%) were consumed below recommended levels (at least 20% or 50% of the population consuming less than the EAR or AI, respectively) in one or more demographic groups. While seven nutrients showed inadequacy across all demographic groups (vitamin D, vitamin E, calcium, choline, iodine, magnesium, potassium, and dietary fiber), a set of inadequacies characterized each demographic group, indicating that targeted recommendations across the lifespan may also be useful. For example, it is concerning that adolescent females had the highest number of inadequate nutrients (N = 19), a life stage where development remains a key health priority. Similarly, there were 16 inadequate nutrients among the pregnancy and lactation demographic group. This highlights a need for increased monitoring and understanding of the nutritional status of this vulnerable population group, particularly as the data were collected primarily from the scientific literature, given that NHANES does not report on pregnancy and lactation. While children had the lowest number of inadequate nutrients (N = 8), this is a key demographic group for early preventative intervention, where addressing these insufficiencies supports health later in life and can help to establish healthy dietary patterns. The findings support the assertion that nutrient inadequacy is a significant factor underpinning the relationship between diet and chronic disease in the US population.
It is important to recognize that, in addition to nutrient requirements, health priorities and nutrient-health associations also vary by age and sex, resulting in a unique set of priority nutrients for each of the eight demographic groups. Nutritional targets that can address the specific nutrition and diet-related health priorities of each life stage are important. While many priority nutrients were common throughout the demographic groups, some showed specificity to one group only, including potassium (children), zinc (adolescent females), and omega-3 fatty acids (pregnancy and lactation). Similarly, some nutrients showed adverse associations with specific demographic groups, such as folate for pregnancy/lactation and children/adolescents, vitamin D for older adults, and omega-3 fatty acids for all adults. It is important to note that these adverse associations were limited to high intakes and/or blood levels of each nutrient or its supplemental form, with intake levels much higher than the observed average intakes for each relevant demographic group [28]. For folate, adverse associations were primarily for the highest levels of red blood cell or serum folate, with limited information regarding actual folate intake [48,49]. Personalized nutrition and ‘food as medicine’ approaches have been recognized as playing an important role in addressing the diet-related disease status of the US [11]. The prioritization of foods that contain priority nutrients can be utilized by health professionals, including medical practitioners, to provide nutrition recommendations that are more specific to life stages. These priority nutrients are valuable for policy development and government-led health initiatives, to ensure tangible outcomes and optimize return on investment, as well as the prioritization of research funding and product re-formulation and development. These findings may also support international collaboration efforts between countries, given the similarity in priority nutrients across countries. AUS/NZ [12] had four priority nutrients in common with the USA: vitamin D, calcium, magnesium, and dietary fiber. A recent analysis of the global prevalence of micronutrient inadequacy found high rates for both calcium (66%, 5 billion people) and magnesium (31%, 2.4 billion people) [4]; fiber and vitamin D were not included in this research. While focused research on additional countries is needed, the evidence suggests that key nutrients may be targeted and specified within international recommendations to have a global impact on health.
A key component of the nutrient prioritization is the translation of nutrient needs into dietary patterns [11], with a focus on the foods that can provide priority nutrients. For example, prioritizing recommendations for foods such as fatty fish, eggs, and UV-exposed mushrooms would assist with intakes for vitamin D. Dairy, including milk and milk products provide the priority nutrients calcium, magnesium, and vitamin D (via fortification), and vegetables, grains, nuts, and seeds, provide magnesium, dietary fiber, and vitamin E [29,179,180]. Prioritizing foods rich in priority nutrients has increased importance given the rise in consumption of foods high in nutrients of concern (added sugars, saturated fat, and sodium), alongside a decline in grains, lean protein, and dairy in the US [181,182], particularly post the COVID pandemic [183]. For some priority nutrients, this may be exacerbated by recommendations to limit the consumption of animal food sources and an increasing movement towards a plant-based diet, as well as differences in nutrient bioavailability between animal and plant foods [26,184]. Thus, in addition to efforts to reduce the intake of foods high in nutrients of concern, an increased focus on the consumption of foods rich in priority nutrients is necessary. To support priority nutrient intake, supplementation or food fortification may also be required for some demographic groups, for example, older adults who find it difficult to consume an adequate intake of food overall, or during pregnancy and lactation. While the intent of this analysis was not to assess the suitability of current DRIs, intakes greater than the currently recommended for calcium, vitamin D, and vitamin E were found to be of additional health benefit. There was a high degree of variability at the population level, due to specific findings per each age/sex grouping, highlighting the need for the provision of demographic-level recommendations and guidance. For example, high supplemental doses of vitamin D may have an adverse effect on older adults. These findings increase the importance of a focus on foods providing these nutrients for the purpose of diet-related disease prevention.
This research contains both strengths and limitations. The applicability of the methodology to both the US population and each demographic group specifically allows for priority nutrient recommendations to be made at both the national level and for specific life stages. The stepwise methodology lends itself to consistency, for comparison between countries and different age/sex groupings, as well as modification as appropriate to any country or demographic group. Further research employing this methodology can answer key questions about the optimal nutritional approach to support health in a given situation; for example, the approach could focus specifically on the priority nutrients to address digestive issues in athletes, or depression in middle-aged males, with the quality of the findings depending primarily on the breadth of scope and available data. While survey data used for the assessment of dietary nutrient intake were the most recently available [27], these data were pre-COVID and may not accurately reflect the current post-COVID situation. In addition, only the highest priority health outcomes were included in the assessment, preventing the inclusion of secondary diet-related health outcomes that may be important for sub-populations of some demographic groups.
Finally, due to the system’s level nature of research, covering multiple nutrients, health associations, and demographic groups, the search carried out for nutrient-health associations was targeted rather than systematic, and thus limited to the research identified at the time of investigation. Similarly, there was no formal assessment of study quality or certainty of evidence, limiting the ability to make direct recommendations regarding the inclusion and dose of priority nutrients within nutrition guidelines. The methodology can be reproduced with increased detail for one or more demographic groups or sub-groups, allowing for additional health outcomes and/or a systematic approach to be included in the analysis. This would be the recommended approach, including a formal assessment of evidence quality (such as AMSTAR) and certainty (such as GRADE) prior to providing specific population guidance. Further iterations of the methodology can also focus on subtypes of nutrients based on dietary source, which were not addressed here; for example, animal vs. plant protein and vegetable vs. cereal fiber. Regardless of the multiple refinements that could be made, the analysis presented provides an overarching assessment of the priority nutrients for the US population, an important step in addressing modifiable causes of morbidity and mortality.
5. Conclusions
This research identifies ten key nutrients showing both inadequate intakes and a role in the prevention of diet-related disease in the US population, with five nutrients common across all demographic groups. These priority nutrients can be targeted for increased consumption as part of the strategy to improve public health outcomes. This includes priority nutrients in American nutrition policies and dietary guidelines, as well as diet-related initiatives and programs are warranted. A framework that can be adapted to support these initiatives has been presented. Trends that exclude foods rich in priority foods may contribute to and/or potentially exacerbate existing inadequacies.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu17121957/s1, Table S1. Search strategy for each of the nutrients and bioactives with each identified health priority; Table S2. Dietary intake data detailing inadequately consumed nutrients for each demographic group; Table S3. Summary of evidence identified for nutrients where an increased need over the DRI has been identified for each demographic group; Table S4. Summary of evidence identified for nutrients showing an association with one or more health priorities for each demographic group; Figure S1. Heatmap showing the level of inadequate intake (I), suggested increased need (IN), and number of associations with health priorities (HP) for each included nutrient within each demographic group. Nutrient scoring and therefore the degree of need for prioritization increases from green to dark red.
Author Contributions
Conceptualization, C.S.S. and T.C.; methodology, C.S.S. and T.C.; formal analysis, C.S.S.; investigation, C.S.S.; resources, C.S.S.; data curation, C.S.S.; writing—original draft preparation, C.S.S.; writing—review and editing, C.S.S., T.C., E.B., E.D. and F.F.-M.; project administration, C.S.S. and F.F.-M.; funding acquisition, F.F.-M. All authors have read and agreed to the published version of the manuscript.
Funding
The authors declare that financial support was received for the research, authorship, and/or publication of this article. This project has been funded by a research grant from The a2 Milk Company. The funding body had no contribution to the methodology, data analysis, drafting of the manuscript, or interpretation of the findings.
Institutional Review Board Statement
Ethical review and approval were not applicable for this study in accordance with the local legislation and institutional requirements.
Informed Consent Statement
Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Data Availability Statement
The data presented in this study were derived from resources available in the public domain: https://www.ars.usda.gov/northeast-area/beltsville-md-bhnrc/beltsville-human-nutrition-research-center/food-surveys-research-group/docs/wweia-usual-intake-data-tables/. Data were accessed on 12 January 2023.
Conflicts of Interest
The authors declare no conflicts of interest. All authors independently work for FOODiQ Global, which gains funding for projects from government, not-for-profits, professional, community, and industry organizations. All authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| AI | Adequate Intake |
| AUS | Australia |
| CDC | Centers for Disease Control and Prevention |
| CVD | cardiovascular disease |
| DHA | docosahexaenoic acid |
| EPA | eicosapentaenoic acid |
| EAR | Estimated Average Requirement |
| DRI | Dietary Reference Intake |
| IU | International units |
| NHANES | National Health and Nutrition Examination Survey |
| NRV | Nutrient Reference Value |
| NZ | New Zealand |
| RCT | randomized controlled trial |
| RDA | Recommended Dietary Allowance |
| SDT | Suggested Dietary Target |
| SLR | systematic literature review |
| T2D | type 2 diabetes |
| UL | Tolerable Upper Intake Level |
| US | United States of America |
| WWEIA | What We Eat in America |
References
- Mokdad, A.H.; Ballestros, K.; Echko, M.; Glenn, S.; Olsen, H.E.; Mullany, E.; Lee, A.; Khan, A.R.; Ahmadi, A.; Ferrari, A.J.; et al. The State of US Health, 1990–2016: Burden of Diseases, Injuries, and Risk Factors Among US States. JAMA 2018, 319, 1444–1472. [Google Scholar] [CrossRef] [PubMed]
- Chong, B.; Jayabaskaran, J.; Kong, G.; Chan, Y.H.; Chin, Y.H.; Goh, R.; Kannan, S.; Ng, C.H.; Loong, S.; Kueh, M.T.W.; et al. Trends and predictions of malnutrition and obesity in 204 countries and territories: An analysis of the Global Burden of Disease Study 2019. eClinicalMedicine 2023, 57, 101850. [Google Scholar] [CrossRef] [PubMed]
- Afshin, A.; Sur, P.J.; Fay, K.A.; Cornaby, L.; Ferrara, G.; Salama, J.S.; Mullany, E.C.; Abate, K.H.; Abbafati, C.; Abebe, Z.; et al. Health effects of dietary risks in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019, 393, 1958–1972. [Google Scholar] [CrossRef] [PubMed]
- Passarelli, S.; Free, C.M.; Shepon, A.; Beal, T.; Batis, C.; Golden, C.D. Global estimation of dietary micronutrient inadequacies: A modelling analysis. Lancet Glob. Health 2024, 12, e1590–e1599. [Google Scholar] [CrossRef]
- Reider, C.A.; Chung, R.Y.; Devarshi, P.P.; Grant, R.W.; Hazels Mitmesser, S. Inadequacy of Immune Health Nutrients: Intakes in US Adults, the 2005–2016 NHANES. Nutrients 2020, 12, 1735. [Google Scholar] [CrossRef]
- Drake, V. Micronutrient Inadequacies in the US Population: An Overview: Oregon State University. 2018. Available online: https://lpi.oregonstate.edu/mic/micronutrient-inadequacies/overview#micronutrient-deficiencies-inadequacies (accessed on 12 December 2022).
- Freedman, M.R.; Fulgoni, V.L.; Lieberman, H.R. Temporal changes in micronutrient intake among United States Adults, NHANES 2003 through 2018: A cross-sectional study. Am. J. Clin. Nutr. 2024, 119, 1309–1320. [Google Scholar] [CrossRef]
- Benavidez, G.; Zahnd, W.; Hung, P.; Eberth, J. Chronic Disease Prevalence in the US: Sociodemographic and Geographic Variations by Zip Code Tabulation Area. Prev. Chronic Dis. 2024, 21, 230267. [Google Scholar] [CrossRef]
- US Food and Drug Administration. FDA Is Working to Combat the Epidemic of Diet-Related Chronic Disease Through Our Nutrition Efforts. 2023. Available online: https://www.fda.gov/news-events/fda-voices/fda-working-combat-epidemic-diet-related-chronic-disease-through-our-nutrition-efforts (accessed on 6 June 2024).
- Abreu, R.; Oliveira, C.B.; Costa, J.A.; Brito, J.; Teixeira, V.H. Effects of dietary supplements on athletic performance in elite soccer players: A systematic review. J. Int. Soc. Sports Nutr. 2023, 20, 2236060. [Google Scholar] [CrossRef]
- Matthews, E.D.; Kurnat-Thoma, E.L. U.S. food policy to address diet-related chronic disease. Front. Public Health 2024, 12, 1339859. [Google Scholar] [CrossRef]
- Starck, C.S.; Cassettari, T.; Beckett, E.; Marshall, S.; Fayet-Moore, F. Priority nutrients to address malnutrition and diet-related diseases in Australia and New Zealand. Front. Nutr. 2024, 11, 1370550. [Google Scholar] [CrossRef]
- Ma, Y.; Hu, M.; Zhou, L.; Ling, S.; Li, Y.; Kong, B.; Huang, P. Dietary fiber intake and risks of proximal and distal colon cancers: A meta-analysis. Medicine 2018, 97, e11678. [Google Scholar] [CrossRef] [PubMed]
- Farvid, M.S.; Spence, N.D.; Holmes, M.D.; Barnett, J.B. Fiber consumption and breast cancer incidence: A systematic review and meta-analysis of prospective studies. Cancer 2020, 126, 3061–3075. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Zhang, G.; Qian, S.; Zhang, Q.; Tan, M. Associations between dietary fiber intake and cardiovascular risk factors: An umbrella review of meta-analyses of randomized controlled trials. Front. Nutr. 2022, 9, 972399. [Google Scholar] [CrossRef] [PubMed]
- Cormick, G.; Betran, A.P.; Romero, I.B.; Cormick, M.S.; Belizán, J.M.; Bardach, A.; Ciapponi, A. Effect of Calcium Fortified Foods on Health Outcomes: A Systematic Review and Meta-Analysis. Nutrients 2021, 13, 316. [Google Scholar] [CrossRef]
- Wu, J.; Xu, L.; Lv, Y.; Dong, L.; Zheng, Q.; Li, L. Quantitative analysis of efficacy and associated factors of calcium intake on bone mineral density in postmenopausal women. Osteoporos. Int. 2017, 28, 2003–2010. [Google Scholar] [CrossRef]
- Al Khalifah, R.; Alsheikh, R.; Alnasser, Y.; Alsheikh, R.; Alhelali, N.; Naji, A.; Al Backer, N. The impact of vitamin D food fortification and health outcomes in children: A systematic review and meta-regression. Syst. Rev. 2020, 9, 144. [Google Scholar] [CrossRef]
- Song, D.; Deng, Y.; Liu, K.; Zhou, L.; Li, N.; Zheng, Y.; Hao, Q.; Yang, S.; Wu, Y.; Zhai, Z.; et al. Vitamin D intake, blood vitamin D levels, and the risk of breast cancer: A dose-response meta-analysis of observational studies. Aging 2019, 11, 12708–12732. [Google Scholar] [CrossRef]
- Jamilian, H.; Amirani, E.; Milajerdi, A.; Kolahdooz, F.; Mirzaei, H.; Zaroudi, M.; Ghaderi, A.; Asemi, Z. The effects of vitamin D supplementation on mental health, and biomarkers of inflammation and oxidative stress in patients with psychiatric disorders: A systematic review and meta-analysis of randomized controlled trials. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 94, 109651. [Google Scholar] [CrossRef]
- Qi, K.J.; Zhao, Z.T.; Zhang, W.; Yang, F. The impacts of vitamin D supplementation in adults with metabolic syndrome: A systematic review and meta-analysis of randomized controlled trials. Front. Pharmacol. 2022, 13, 1033026. [Google Scholar] [CrossRef]
- Elango, R.; Humayun, M.A.; Ball, R.O.; Pencharz, P.B. Evidence that protein requirements have been significantly underestimated. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 52–57. [Google Scholar] [CrossRef]
- Humayun, M.A.; Elango, R.; Ball, R.O.; Pencharz, P.B. Reevaluation of the protein requirement in young men with the indicator amino acid oxidation technique. Am. J. Clin. Nutr. 2007, 86, 995–1002. [Google Scholar] [CrossRef] [PubMed]
- Weiler, M.; Hertzler, S.R.; Dvoretskiy, S. Is It Time to Reconsider the U.S. Recommendations for Dietary Protein and Amino Acid Intake? Nutrients 2023, 15, 838. [Google Scholar] [CrossRef] [PubMed]
- National Health and Medical Research Council. Nutrient Reference Values for Australia and New Zealand Including Recommended Dietary Intakes; National Health and Medical Research Council: Canberra, Australia, 2005.
- National Academies Institute of Medicine. Dietary Reference Intakes: The Essential Guide to Nutrient Requirements; The National Academies: Washington, DC, USA, 2006. [Google Scholar]
- USDA Agricultual Research Service. Usual Nutrient Intake from Food and Beverages by Gender and Age, What We Eat in America, NHANES 2017-March 2020 Prepandemic; United States Department of Agriculture: Washington, DC, USA, 2023.
- USDA Agricultual Research Service. WWEIA Data Tables; USDA Agricultural Research Service: Beltsville, MD, USA, 2022.
- US Department of Agriculture and US Department of Health and Human Services. Dietary Guidelines for Americans 2020–2025, 9th ed.; USDA: Washington, DC, USA, 2020.
- Centers for Disease Control and Prevention. Vital Signs, Pregnancy-Related Deaths. 2019. Available online: https://www.cdc.gov/maternal-mortality/php/data-research/ (accessed on 12 January 2023).
- Centers for Disease Control and Prevention. National Center for Health Statistics, FastStats. 2020. Available online: https://www.cdc.gov/nchs/fastats/default.htm (accessed on 12 January 2023).
- US Department of Health and Human Services and Office of Disease Prevention and Health Promotion. Healthy People 2030, Populations: US Government. 2022. Available online: https://health.gov/healthypeople/objectives-and-data/browse-objectives#populations (accessed on 12 January 2023).
- Miller, G.F.; Coffield, E.; Leroy, Z.; Wallin, R. Prevalence and Costs of Five Chronic Conditions in Children. J. Sch. Nurs. 2016, 32, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Newacheck, P.W.; McManus, M.A.; Fox, H.B. Prevalence and Impact of Chronic Illness Among Adolescents. Am. J. Dis. Child. 1991, 145, 1367–1373. [Google Scholar] [CrossRef]
- US Preventive Services Task Force. Hormone Therapy for the Prevention of Chronic Conditions in Postmenopausal Women: Recommendation Statement. Am. Fam. Physician 2005, 72, 311–316. [Google Scholar]
- Institute of Medicine. Dietary Reference Intakes for Calcium and Vitamin D; Institute of Medicine: Washington, DC, USA, 2011. [Google Scholar]
- National Academies of Science Engineering and Medicine. Dietary Reference Intakes for Sodium and Potassium; National Academies of Science Engineering and Medicine: Washington, DC, USA, 2019. [Google Scholar]
- Gerald, J.; Dorothy, R.; Global Dietary Database. Friedman School of Nutrition Science and Policy at Tufts University. 2024. Available online: https://globaldietarydatabase.org/ (accessed on 6 June 2024).
- OCEBM Levels of Evidence Working Group. The Oxford Levels of Evidence 2. Available online: https://www.cebm.ox.ac.uk/resources/levels-of-evidence/ocebm-levels-of-evidence (accessed on 28 May 2025).
- Breehl, L.; Caban, O. Physiology, Puberty; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- USDA Agricultual Research Service. Dietary Fiber (g): Usual Nutrient Intakes from Food and Water, 2003–2006, Compared to Adequate Intakes. 2006. Available online: https://www.ars.usda.gov/ARSUserFiles/80400530/pdf/usual/usual_nutrient_intake_dietary_fiber_2003-06.pdf (accessed on 12 January 2023).
- USDA Agricultual Research Service. What We Eat in America, NHANES 2005–2006. Usual Nutrient Intakes from Food and Water Compared to 1997 Dietary Reference Intakes for Vitamin D, Calcium, Phosphorus, and Magnesium; US Department of Agriculture: Washington, DC, USA, 2009. Available online: https://www.ars.usda.gov/ARSUserFiles/80400530/pdf/usual/usual_nutrient_intake_vitD_ca_phos_mg_2005-06.pdf (accessed on 12 January 2023).
- Bailey, R.L.; Pac, S.G.; Fulgoni, V.L., III; Reidy, K.C.; Catalano, P.M. Estimation of Total Usual Dietary Intakes of Pregnant Women in the United States. JAMA Netw. Open 2019, 2, e195967. [Google Scholar] [CrossRef]
- Higgins, K.A.; Bi, X.; Davis, B.J.; Barraj, L.M.; Scrafford, C.G.; Murphy, M.M. Adequacy of total usual micronutrient intakes among pregnant women in the United States by level of dairy consumption, NHANES 2003–2016. Nutr. Health 2022, 28, 621–631. [Google Scholar] [CrossRef]
- Pratt, N.; Durham, H.; Sherry, C. Nutrient Intakes from Food of Lactating Women Do Not Meet Many Dietary Recommendations Important for Infant Development and Maternal Health. Food Nutr. Sci. 2014, 5, 1644–1651. [Google Scholar] [CrossRef][Green Version]
- Sun, H.; Weaver, C.M. Iodine Intake Trends in United States Girls and Women between 2011 and 2020. J. Nutr. 2024, 154, 928–939. [Google Scholar] [CrossRef]
- Zhang, Z.; Fulgoni, V.L.; Kris-Etherton, P.M.; Mitmesser, S.H. Dietary Intakes of EPA and DHA Omega-3 Fatty Acids among US Childbearing-Age and Pregnant Women: An Analysis of NHANES 2001–2014. Nutrients 2018, 10, 416. [Google Scholar] [CrossRef]
- Li, N.; Jiang, J.; Guo, L. Effects of maternal folate and vitamin B12 on gestational diabetes mellitus: A dose-response meta-analysis of observational studies. Eur. J. Clin. Nutr. 2022, 76, 1502–1512. [Google Scholar] [CrossRef] [PubMed]
- Prades, N.; Varela, E.; Flamarique, I.; Deulofeu, R.; Baeza, I. Water-soluble vitamin insufficiency, deficiency and supplementation in children and adolescents with a psychiatric disorder: A systematic review and meta-analysis. Nutr. Neurosci. 2023, 26, 85–107. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Liu, M.; Yang, D.; Zhang, Y.; An, F. Efficacy and Safety of Omega-3 Fatty Acids in the Prevention of Cardiovascular Disease: A Systematic Review and Meta-analysis. Cardiovasc. Drugs Ther. 2024, 38, 799–817. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; He, R.; Zheng, X. Effect of vitamin D, calcium, or combined supplementation on fall prevention: A systematic review and updated network meta-analysis. BMC Geriatr. 2024, 24, 390. [Google Scholar] [CrossRef]
- de Souza, M.M.; Moraes Dantas, R.L.; Leão Durães, V.; Defante, M.L.R.; Mendes, T.B. Vitamin D Supplementation and the Incidence of Fractures in the Elderly Healthy Population: A Meta-analysis of Randomized Controlled Trials. J. Gen. Intern. Med. 2024, 39, 2829–2836. [Google Scholar] [CrossRef]
- Nguyen, N.T.K.; Fan, H.Y.; Tsai, M.C.; Tung, T.H.; Huynh, Q.T.V.; Huang, S.Y.; Chen, Y.C. Nutrient Intake through Childhood and Early Menarche Onset in Girls: Systematic Review and Meta-Analysis. Nutrients 2020, 12, 2544. [Google Scholar] [CrossRef]
- Zhao, H.; Mei, K.; Hu, Q.; Wu, Y.; Xu, Y.; Yu, P.; Deng, Y.; Zhu, W.; Yan, Z.; Liu, X.; et al. Circulating copper levels and the risk of cardio-cerebrovascular diseases and cardiovascular and all-cause mortality: A systematic review and meta-analysis of longitudinal studies. Env. Pollut. 2024, 340, 122711. [Google Scholar] [CrossRef]
- Mirrafiei, A.; Jayedi, A.; Shab-Bidar, S. Total and different dietary fiber subtypes and the risk of all-cause, cardiovascular, and cancer mortality: A dose-response meta-analysis of prospective cohort studies. Food Funct. 2023, 14, 10667–10680. [Google Scholar] [CrossRef]
- Kim, Y.; Je, Y. Dietary fibre intake and mortality from cardiovascular disease and all cancers: A meta-analysis of prospective cohort studies. Arch. Cardiovasc. Dis. 2016, 109, 39–54. [Google Scholar] [CrossRef]
- Winzenberg, T.; Powell, S.; Shaw, K.A.; Jones, G. Effects of vitamin D supplementation on bone density in healthy children: Systematic review and meta-analysis. BMJ 2011, 342, c7254. [Google Scholar] [CrossRef]
- Winzenberg, T.M.; Powell, S.; Shaw, K.A.; Jones, G. Vitamin D supplementation for improving bone mineral density in children. Cochrane Database Syst. Rev. 2010, 10, Cd006944. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Fuleihan, G.E.; Cai, G.; Lamberg-Allardt, C.; Viljakainen, H.T.; Rahme, M.; Grønborg, I.M.; Andersen, R.; Khadilkar, A.; Zulf, M.M.; et al. Vitamin D supplementation for improving bone density in vitamin D-deficient children and adolescents: Systematic review and individual participant data meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2023, 118, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Xu, Y.; Zhang, X.; Zhang, L.; Wu, Y.; Wang, X.; Zhu, C. The effect of vitamin D supplementation in treatment of children with autism spectrum disorder: A systematic review and meta-analysis of randomized controlled trials. Nutr. Neurosci. 2022, 25, 835–845. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhou, Q.; Zhang, G.; Tian, X.; Li, Y.; Wang, Z.; Zhao, Y.; Chen, Y.; Luo, Z. Vitamin D Supplementation and Allergic Diseases during Childhood: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 3947. [Google Scholar] [CrossRef]
- Shah, V.P.; Nayfeh, T.; Alsawaf, Y.; Saadi, S.; Farah, M.; Zhu, Y.; Firwana, M.; Seisa, M.; Wang, Z.; Scragg, R.; et al. A Systematic Review Supporting the Endocrine Society Clinical Practice Guidelines on Vitamin D. J. Clin. Endocrinol. Metab. 2024, 109, 1961–1974. [Google Scholar] [CrossRef]
- Gou, H.; Wang, Y.; Liu, Y.; Peng, C.; He, W.; Sun, X. Efficacy of vitamin D supplementation on child and adolescent overweight/obesity: A systematic review and meta-analysis of randomized controlled trials. Eur. J. Pediatr. 2023, 182, 255–264. [Google Scholar] [CrossRef]
- Soltani, S.; Beigrezaei, S.; Abdollahi, S.; Clark, C.C.T.; Ashoori, M. Oral vitamin D supplementation and body weight in children and adolescents: A systematic review and meta-analysis of randomized controlled trials. Eur. J. Pediatr. 2023, 182, 1977–1989. [Google Scholar] [CrossRef]
- Głąbska, D.; Kołota, A.; Lachowicz, K.; Skolmowska, D.; Stachoń, M.; Guzek, D. Vitamin D Supplementation and Mental Health in Inflammatory Bowel Diseases and Irritable Bowel Syndrome Patients: A Systematic Review. Nutrients 2021, 13, 3662. [Google Scholar] [CrossRef]
- Chen, Z.; Peng, C.; Mei, J.; Zhu, L.; Kong, H. Vitamin D can safely reduce asthma exacerbations among corticosteroid-using children and adults with asthma: A systematic review and meta-analysis of randomized controlled trials. Nutr. Res. 2021, 92, 49–61. [Google Scholar] [CrossRef]
- Liu, M.; Wang, J.; Sun, X. A Meta-Analysis on Vitamin D Supplementation and Asthma Treatment. Front. Nutr. 2022, 9, 860628. [Google Scholar] [CrossRef]
- Guo, X.F.; Zhao, T.; Han, J.M.; Li, S.; Li, D. Vitamin D and liver cancer risk: A meta-analysis of prospective studies. Asia Pac. J. Clin. Nutr. 2020, 29, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Lu, H.; Cheng, Y. To identify the association between dietary vitamin D intake and serum levels and risk or prognostic factors for melanoma-systematic review and meta-analysis. BMJ Open 2022, 12, e052442. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yang, N.; Yuan, M. Dietary and circulating vitamin D and risk of renal cell carcinoma: A meta-analysis of observational studies. Int. Braz. J. Urol. 2021, 47, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Winzenberg, T.; Shaw, K.; Fryer, J.; Jones, G. Effects of calcium supplementation on bone density in healthy children: Meta-analysis of randomised controlled trials. BMJ 2006, 333, 775. [Google Scholar] [CrossRef]
- Nielsen, F.H. The Problematic Use of Dietary Reference Intakes to Assess Magnesium Status and Clinical Importance. Biol. Trace Elem. Res. 2019, 188, 52–59. [Google Scholar] [CrossRef]
- Armah, S.M. Fractional zinc absorption for men, women, and adolescents is overestimated in the current dietary reference intakes. J. Nutr. 2016, 146, 1276–1280. [Google Scholar] [CrossRef]
- Meli, A.M.; Ali, A.; Mhd Jalil, A.M.; Mohd Yusof, H.; Tan, M.M.C. Effects of Physical Activity and Micronutrients on Cognitive Performance in Children Aged 6 to 11 Years: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Medicina 2021, 58, 57. [Google Scholar] [CrossRef]
- Tsang, B.L.; Holsted, E.; McDonald, C.M.; Brown, K.H.; Black, R.; Mbuya, M.N.N.; Grant, F.; Rowe, L.A.; Manger, M.S. Effects of Foods Fortified with Zinc, Alone or Cofortified with Multiple Micronutrients, on Health and Functional Outcomes: A Systematic Review and Meta-Analysis. Adv. Nutr. 2021, 12, 1821–1837. [Google Scholar] [CrossRef]
- McRae, M.P. Dietary Fiber Intake and Type 2 Diabetes Mellitus: An Umbrella Review of Meta-analyses. J. Chiropr. Med. 2018, 17, 44–53. [Google Scholar] [CrossRef]
- Reynolds, A.N.; Akerman, A.P.; Mann, J. Dietary fibre and whole grains in diabetes management: Systematic review and meta-analyses. PLoS Med. 2020, 17, e1003053. [Google Scholar] [CrossRef]
- Ramezani, F.; Pourghazi, F.; Eslami, M.; Gholami, M.; Mohammadian Khonsari, N.; Ejtahed, H.S.; Larijani, B.; Qorbani, M. Dietary fiber intake and all-cause and cause-specific mortality: An updated systematic review and meta-analysis of prospective cohort studies. Clin. Nutr. 2024, 43, 65–83. [Google Scholar] [CrossRef] [PubMed]
- Veronese, N.; Solmi, M.; Caruso, M.G.; Giannelli, G.; Osella, A.R.; Evangelou, E.; Maggi, S.; Fontana, L.; Stubbs, B.; Tzoulaki, I. Dietary fiber and health outcomes: An umbrella review of systematic reviews and meta-analyses. Am. J. Clin. Nutr. 2018, 107, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Watling, C.Z.; Wojt, A.; Florio, A.A.; Butera, G.; Albanes, D.; Weinstein, S.J.; Huang, W.Y.; Parisi, D.; Zhang, X.; Graubard, B.I.; et al. Fiber and whole grain intakes in relation to liver cancer risk: An analysis in 2 prospective cohorts and systematic review and meta-analysis of prospective studies. Hepatology 2024, 80, 552–565. [Google Scholar] [CrossRef] [PubMed]
- Li, D.B.; Hao, Q.Q.; Hu, H.L. The relationship between dietary fibre and stroke: A meta-analysis. J. Stroke Cerebrovasc. Dis. 2023, 32, 107144. [Google Scholar] [CrossRef]
- Hujoel, P.P.; Hujoel, M.L.A. Vitamin C and scar strength: Analysis of a historical trial and implications for collagen-related pathologies. Am. J. Clin. Nutr. 2022, 115, 8–17. [Google Scholar] [CrossRef]
- Levine, M.; Conry-Cantilena, C.; Wang, Y.; Welch, R.W.; Washko, P.W.; Dhariwal, K.R.; Park, J.B.; Lazarev, A.; Graumlich, J.F.; King, J.; et al. Vitamin C pharmacokinetics in healthy volunteers: Evidence for a recommended dietary allowance. Proc. Natl. Acad. Sci. USA 1996, 93, 3704–3709. [Google Scholar] [CrossRef]
- Yosaee, S.; Keshtkaran, Z.; Abdollahi, S.; Shidfar, F.; Sarris, J.; Soltani, S. The effect of vitamin C supplementation on mood status in adults: A systematic review and meta-analysis of randomized controlled clinical trials. Gen. Hosp. Psychiatry 2021, 71, 36–42. [Google Scholar] [CrossRef]
- Ashor, A.W.; Siervo, M.; Lara, J.; Oggioni, C.; Afshar, S.; Mathers, J.C. Effect of vitamin C and vitamin E supplementation on endothelial function: A systematic review and meta-analysis of randomised controlled trials. Br. J. Nutr. 2015, 113, 1182–1194. [Google Scholar] [CrossRef]
- Jayedi, A.; Rashidy-Pour, A.; Parohan, M.; Zargar, M.S.; Shab-Bidar, S. Dietary and circulating vitamin C, vitamin E, β-carotene and risk of total cardiovascular mortality: A systematic review and dose-response meta-analysis of prospective observational studies. Public Health Nutr. 2019, 22, 1872–1887. [Google Scholar] [CrossRef]
- Guzek, D.; Kołota, A.; Lachowicz, K.; Skolmowska, D.; Stachoń, M.; Głąbska, D. Influence of Vitamin D Supplementation on Mental Health in Diabetic Patients: A Systematic Review. Nutrients 2021, 13, 3678. [Google Scholar] [CrossRef]
- Guzek, D.; Kołota, A.; Lachowicz, K.; Skolmowska, D.; Stachoń, M.; Głąbska, D. Effect of Vitamin D Supplementation on Depression in Adults: A Systematic Review of Randomized Controlled Trials (RCTs). Nutrients 2023, 15, 951. [Google Scholar] [CrossRef] [PubMed]
- Mikola, T.; Marx, W.; Lane, M.M.; Hockey, M.; Loughman, A.; Rajapolvi, S.; Rocks, T.; O’Neil, A.; Mischoulon, D.; Valkonen-Korhonen, M.; et al. The effect of vitamin D supplementation on depressive symptoms in adults: A systematic review and meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2023, 63, 11784–11801. [Google Scholar] [CrossRef] [PubMed]
- Srifuengfung, M.; Srifuengfung, S.; Pummangura, C.; Pattanaseri, K.; Oon-Arom, A.; Srisurapanont, M. Efficacy and acceptability of vitamin D supplements for depressed patients: A systematic review and meta-analysis of randomized controlled trials. Nutrition 2023, 108, 111968. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Xu, F.; Xia, X.; Xiong, A.; Dai, D.; Ling, Y.; Sun, R.; Qiu, L.; Ding, Y.; Xie, Z. The effect of vitamin D supplementation on primary depression: A meta-analysis. J. Affect. Disord. 2024, 344, 653–661. [Google Scholar] [CrossRef]
- Dominguez, L.J.; Veronese, N.; Marrone, E.; Di Palermo, C.; Iommi, C.; Ruggirello, R.; Caffarelli, C.; Gonnelli, S.; Barbagallo, M. Vitamin D and Risk of Incident Type 2 Diabetes in Older Adults: An Updated Systematic Review and Meta-Analysis. Nutrients 2024, 16, 1561. [Google Scholar] [CrossRef]
- Musazadeh, V.; Zarezadeh, M.; Ghalichi, F.; Kalajahi, F.H.; Ghoreishi, Z. Vitamin D supplementation positively affects anthropometric indices: Evidence obtained from an umbrella meta-analysis. Front. Nutr. 2022, 9, 980749. [Google Scholar] [CrossRef]
- Arayici, M.E.; Basbinar, Y.; Ellidokuz, H. Vitamin D Intake, Serum 25-Hydroxyvitamin-D (25(OH)D) Levels, and Cancer Risk: A Comprehensive Meta-Meta-Analysis Including Meta-Analyses of Randomized Controlled Trials and Observational Epidemiological Studies. Nutrients 2023, 15, 2722. [Google Scholar] [CrossRef]
- Ismail, N.H.; Mussa, A.; Al-Khreisat, M.J.; Mohamed Yusoff, S.; Husin, A.; Johan, M.F.; Islam, M.A. The Global Prevalence of Vitamin D Deficiency and Insufficiency in Patients with Multiple Myeloma: A Systematic Review and Meta-Analysis. Nutrients 2023, 15, 3227. [Google Scholar] [CrossRef]
- Jung, S.; Jin, S.; Je, Y. Vitamin D Intake, Blood 25-Hydroxyvitamin D, and Risk of Ovarian Cancer: A Meta-Analysis of Observational Studies. J. Womens Health 2023, 32, 561–573. [Google Scholar] [CrossRef]
- Chen, Q.Y.; Kim, S.; Lee, B.; Jeong, G.; Lee, D.H.; Keum, N.; Manson, J.E.; Giovannucci, E.L. Post-Diagnosis Vitamin D Supplement Use and Survival among Cancer Patients: A Meta-Analysis. Nutrients 2022, 14, 3418. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, L.; Wang, D.; Yan, N.; Li, C.; Wu, M.; Wang, F.; Mi, B.; Chen, F.; Jia, W.; et al. Omega-3 polyunsaturated fatty acid biomarkers and risk of type 2 diabetes, cardiovascular disease, cancer, and mortality. Clin. Nutr. 2022, 41, 1798–1807. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, A.S.; Brown, T.J.; Brainard, J.S.; Biswas, P.; Thorpe, G.C.; Moore, H.J.; Deane, K.H.; Summerbell, C.D.; Worthington, H.V.; Song, F.; et al. Omega-3 fatty acids for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2020, 3, Cd003177. [Google Scholar] [CrossRef] [PubMed]
- Bernasconi, A.A.; Wiest, M.M.; Lavie, C.J.; Milani, R.V.; Laukkanen, J.A. Effect of Omega-3 Dosage on Cardiovascular Outcomes: An Updated Meta-Analysis and Meta-Regression of Interventional Trials. Mayo Clin. Proc. 2021, 96, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.U.; Khan, M.U.; Riaz, H.; Valavoor, S.; Zhao, D.; Vaughan, L.; Okunrintemi, V.; Riaz, I.B.; Khan, M.S.; Kaluski, E.; et al. Effects of Nutritional Supplements and Dietary Interventions on Cardiovascular Outcomes: An Umbrella Review and Evidence Map. Ann. Intern. Med. 2019, 171, 190–198. [Google Scholar] [CrossRef]
- Marston, N.A.; Giugliano, R.P.; Im, K.; Silverman, M.G.; O’Donoghue, M.L.; Wiviott, S.D.; Ference, B.A.; Sabatine, M.S. Association Between Triglyceride Lowering and Reduction of Cardiovascular Risk Across Multiple Lipid-Lowering Therapeutic Classes: A Systematic Review and Meta-Regression Analysis of Randomized Controlled Trials. Circulation 2019, 140, 1308–1317. [Google Scholar] [CrossRef]
- Sohouli, M.H.; Roshan, M.M.; Olusola, O.F.; Fatahi, S.; Omidi, H.R.; Sharifi, P.; Hekmatdoost, A.; Kutbi, E.; Abu-Zaid, A. Impact of Omega-3 supplementation on homocysteine levels in humans: A systematic review and meta-regression analysis of randomized controlled trials. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 2013–2025. [Google Scholar] [CrossRef]
- Xu, Q.; Du, L.; Gu, H.; Ji, M.; Zhan, L. The effect of omega-3 polyunsaturated fatty acids on stroke treatment and prevention: A systematic review and meta-analysis. Nutr. Hosp. 2022, 39, 924–935. [Google Scholar] [CrossRef]
- Yokoyama, Y.; Kuno, T.; Morita, S.X.; Slipczuk, L.; Takagi, H.; Briasoulis, A.; Latib, A.; Bangalore, S.; Heffron, S.P. Eicosapentaenoic Acid for Cardiovascular Events Reduction- Systematic Review and Network Meta-Analysis of Randomized Controlled Trials. J. Cardiol. 2022, 80, 416–422. [Google Scholar] [CrossRef]
- Yu, F.; Qi, S.; Ji, Y.; Wang, X.; Fang, S.; Cao, R. Effects of omega-3 fatty acid on major cardiovascular outcomes: A systematic review and meta-analysis. Medicine 2022, 101, e29556. [Google Scholar] [CrossRef]
- Luo, S.; Hou, H.; Wang, Y.; Li, Y.; Zhang, L.; Zhang, H.; Jin, Q.; Wu, G.; Wang, X. Effects of omega-3, omega-6, and total dietary polyunsaturated fatty acid supplementation in patients with atherosclerotic cardiovascular disease: A systematic review and meta-analysis. Food Funct. 2024, 15, 1208–1222. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, X.; Zhou, N.; Shen, Y.; Li, B.; Chen, B.E.; Li, X. Association Between Omega-3 Fatty Acid Intake and Dyslipidemia: A Continuous Dose-Response Meta-Analysis of Randomized Controlled Trials. J. Am. Heart Assoc. 2023, 12, e029512. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Xu, H.; Xia, J.; Lu, Y.; Xu, D.; Sun, J.; Wang, Y.; Liao, W.; Sun, G. Effect of Alpha-Linolenic Acid Supplementation on Cardiovascular Disease Risk Profile in Individuals with Obesity or Overweight: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Adv. Nutr. 2023, 14, 1644–1655. [Google Scholar] [CrossRef]
- Arabi, S.M.; Bahari, H.; Chambari, M.; Bahrami, L.S.; Mohaildeen Gubari, M.I.; Watts, G.F.; Sahebkar, A. Omega-3 fatty acids and endothelial function: A GRADE-assessed systematic review and meta-analysis. Eur. J. Clin. Investig. 2024, 54, e14109. [Google Scholar] [CrossRef]
- Lee, Y.S.; Park, J.W.; Joo, M.; Moon, S.; Kim, K.; Kim, M.G. Effects of Omega-3 Fatty Acids on Flow-mediated Dilatation and Carotid Intima Media Thickness: A Meta-analysis. Curr. Atheroscler. Rep. 2023, 25, 629–641. [Google Scholar] [CrossRef] [PubMed]
- Emami, M.R.; Safabakhsh, M.; Alizadeh, S.; Asbaghi, O.; Khosroshahi, M.Z. Effect of vitamin E supplementation on blood pressure: A systematic review and meta-analysis. J. Hum. Hypertens. 2019, 33, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.; Liu, L.; Jian, Z.; Ma, Y.; Li, H.; Jin, X.; Liao, B.; Wang, K. Vitamin E and Multiple Health Outcomes: An Umbrella Review of Meta-Analyses. Nutrients 2023, 15, 3301. [Google Scholar] [CrossRef]
- Fu, J.; Sun, J.; Zhang, C. Vitamin D supplementation and risk of stroke: A meta-analysis of randomized controlled trials. Front. Neurol. 2022, 13, 970111. [Google Scholar] [CrossRef]
- Pei, Y.Y.; Zhang, Y.; Peng, X.C.; Liu, Z.R.; Xu, P.; Fang, F. Association of Vitamin D Supplementation with Cardiovascular Events: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 3158. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, P.; Jie, Y.; Sun, Y.; Wang, X.; Fan, Y. Predictive value of 25-hydroxyvitamin D level in patients with coronary artery disease: A meta-analysis. Front. Nutr. 2022, 9, 984487. [Google Scholar] [CrossRef]
- Mattumpuram, J.; Maniya, M.T.; Faruqui, S.K.; Ahmed, A.; Jaiswal, V.; Harshakumar, S.P. Cardiovascular and Cerebrovascular Outcomes with Vitamin D Supplementation: A Systematic Review and Meta-Analysis. Curr. Probl. Cardiol. 2024, 49 Pt C, 102119. [Google Scholar] [CrossRef]
- Qi, S.; Luo, X.; Liu, S.; Ling, B.; Si, M.; Jin, H. Effect of vitamin B(2), vitamin C, vitamin D, vitamin E and folic acid in adults with essential hypertension: A systematic review and network meta-analysis. BMJ Open 2024, 14, e074511. [Google Scholar] [CrossRef] [PubMed]
- Rasouli, M.A.; Darvishzadehdaledari, S.; Alizadeh, Z.; Moradi, G.; Gholami, F.; Mahmoudian, A. Vitamin D Supplementation and Cardiovascular Disease Risks in More Than 134000 Individuals in 29 Randomized Clinical Trials and 157000 Individuals in 30 Prospective Cohort Studies: An Updated Systematic Review and Meta-analysis. J. Res. Health Sci. 2023, 23, e00594. [Google Scholar] [CrossRef] [PubMed]
- Serra, M.O.; de Macedo, L.R.; Silva, M.; Lautner, R.Q. Effect of Vitamin D supplementation on blood pressure in hypertensive individuals with hypovitaminosis D: A systematic review and meta-analysis. J. Hypertens. 2024, 42, 594–604. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Zhao, C.; Li, J.; Li, Y. A systematic review and meta-analysis of the linkage between low vitamin D and the risk as well as the prognosis of stroke. Brain Behav. 2024, 14, e3577. [Google Scholar] [CrossRef]
- Iliuta, F.; Pijoan, J.I.; Lainz, L.; Exposito, A.; Matorras, R. Women’s vitamin D levels and IVF results: A systematic review of the literature and meta-analysis, considering three categories of vitamin status (replete, insufficient and deficient). Hum. Fertil. 2022, 25, 228–246. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, J.; Wan, Q.; Huang, J.; Han, T.; Qu, T.; Yu, L.L. Influence of Vitamin D supplementation on reproductive outcomes of infertile patients: A systematic review and meta-analysis. Reprod. Biol. Endocrinol. 2023, 21, 17. [Google Scholar] [CrossRef]
- Moridi, I.; Chen, A.; Tal, O.; Tal, R. The Association between Vitamin D and Anti-Müllerian Hormone: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 1567. [Google Scholar] [CrossRef]
- Altaf, R.; Gonzalez, I.; Rubino, K.; Nemec, E.C., II. Folate as adjunct therapy to SSRI/SNRI for major depressive disorder: Systematic review & meta-analysis. Complement. Ther. Med. 2021, 61, 102770. [Google Scholar] [CrossRef]
- Lam, N.S.K.; Long, X.X.; Li, X.; Saad, M.; Lim, F.; Doery, J.C.; Griffin, R.C.; Galletly, C. The potential use of folate and its derivatives in treating psychiatric disorders: A systematic review. Biomed. Pharmacother. 2022, 146, 112541. [Google Scholar] [CrossRef]
- Aydoğdu, G.S.; Akyakar, B.; Kalaycı, Z.; Uçar, A.; Gezmen-Karadağ, M. Folic Acid as a Potential Vitamin in Glycemic Control: A Systematic Review. Curr. Nutr. Rep. 2024, 13, 729–750. [Google Scholar] [CrossRef]
- Lei, J.; Ren, F.; Li, W.; Guo, X.; Liu, Q.; Gao, H.; Pang, Y.; He, Y.; Guo, J.; Zeng, J. Use of folic acid supplementation to halt and even reverse the progression of gastric precancerous conditions: A meta-analysis. BMC Gastroenterol. 2022, 22, 370. [Google Scholar] [CrossRef] [PubMed]
- Moazzen, S.; Dolatkhah, R.; Tabrizi, J.S.; Shaarbafi, J.; Alizadeh, B.Z.; de Bock, G.H.; Dastgiri, S. Folic acid intake and folate status and colorectal cancer risk: A systematic review and meta-analysis. Clin. Nutr. 2018, 37, 1926–1934. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Xu, P.; Zhang, D.; Liu, K.; Song, D.; Zheng, Y.; Yang, S.; Li, N.; Hao, Q.; Wu, Y.; et al. Association of folate intake and plasma folate level with the risk of breast cancer: A dose-response meta-analysis of observational studies. Aging 2020, 12, 21355–21375. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Guo, Y.; Chen, Y.; Lin, Y.; Lu, Y.; Guo, Q. The effect of B-vitamins on the prevention and treatment of cardiovascular diseases: A systematic review and meta-analysis. Nutr. Rev. 2024, 82, 1386–1401. [Google Scholar] [CrossRef]
- Ribamar, A.; Almeida, B.; Soares, A.; Peniche, B.; Jesus, P.; Cruz, S.P.D.; Ramalho, A. Relationship between vitamin D deficiency and both gestational and postpartum depression. Nutr. Hosp. 2020, 37, 1238–1245. [Google Scholar] [CrossRef]
- Tan, Q.; Liu, S.; Chen, D. Poor vitamin D status and the risk of maternal depression: A dose-response meta-analysis of observational studies. Public Health Nutr. 2021, 24, 2161–2170. [Google Scholar] [CrossRef]
- Chan, K.Y.; Wong, M.M.H.; Pang, S.S.H.; Lo, K.K.H. Dietary supplementation for gestational diabetes prevention and management: A meta-analysis of randomized controlled trials. Arch. Gynecol. Obs. 2021, 303, 1381–1391. [Google Scholar] [CrossRef]
- Chien, M.C.; Huang, C.Y.; Wang, J.H.; Shih, C.L.; Wu, P. Effects of vitamin D in pregnancy on maternal and offspring health-related outcomes: An umbrella review of systematic review and meta-analyses. Nutr. Diabetes 2024, 14, 35. [Google Scholar] [CrossRef]
- Gallo, S.; McDermid, J.M.; Al-Nimr, R.I.; Hakeem, R.; Moreschi, J.M.; Pari-Keener, M.; Stahnke, B.; Papoutsakis, C.; Handu, D.; Cheng, F.W. Vitamin D Supplementation during Pregnancy: An Evidence Analysis Center Systematic Review and Meta-Analysis. J. Acad. Nutr. Diet. 2020, 120, 898–924.e894. [Google Scholar] [CrossRef]
- Irwinda, R.; Hiksas, R.; Lokeswara, A.W.; Wibowo, N. Vitamin D supplementation higher than 2000 IU/day compared to lower dose on maternal-fetal outcome: Systematic review and meta-analysis. Womens Health 2022, 18, 17455057221111066. [Google Scholar] [CrossRef]
- Palacios, C.; Kostiuk, L.L.; Cuthbert, A.; Weeks, J. Vitamin D supplementation for women during pregnancy. Cochrane Database Syst. Rev. 2024, 7, Cd008873. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Chen, Z.; Hu, Y.; Wang, Y.; Wu, Y.; Lian, F.; Li, H.; Yang, J.; Xu, X. The effects of vitamin D supplementation on glycemic control and maternal-neonatal outcomes in women with established gestational diabetes mellitus: A systematic review and meta-analysis. Clin. Nutr. 2021, 40, 3148–3157. [Google Scholar] [CrossRef] [PubMed]
- Fogacci, S.; Fogacci, F.; Banach, M.; Michos, E.D.; Hernandez, A.V.; Lip, G.Y.H.; Blaha, M.J.; Toth, P.P.; Borghi, C.; Cicero, A.F.G. Vitamin D supplementation and incident preeclampsia: A systematic review and meta-analysis of randomized clinical trials. Clin. Nutr. 2020, 39, 1742–1752. [Google Scholar] [CrossRef] [PubMed]
- Gunabalasingam, S.; De Almeida Lima Slizys, D.; Quotah, O.; Magee, L.; White, S.L.; Rigutto-Farebrother, J.; Poston, L.; Dalrymple, K.V.; Flynn, A.C. Micronutrient supplementation interventions in preconception and pregnant women at increased risk of developing pre-eclampsia: A systematic review and meta-analysis. Eur. J. Clin. Nutr. 2023, 77, 710–730. [Google Scholar] [CrossRef]
- Wu, C.; Song, Y.; Wang, X. Vitamin D Supplementation for the Outcomes of Patients with Gestational Diabetes Mellitus and Neonates: A Meta-Analysis and Systematic Review. Int. J. Clin. Pract. 2023, 2023, 1907222. [Google Scholar] [CrossRef]
- Bahardoust, M.; Salari, S.; Ghotbi, N.; Rahimpour, E.; Haghmoradi, M.; Alipour, H.; Soleimani, M. Association between prenatal vitamin D deficiency with dental caries in infants and children: A systematic review and meta-analysis. BMC Pregnancy Childbirth 2024, 24, 256. [Google Scholar] [CrossRef]
- Luo, C.; Sun, Y.; Zeng, Z.; Liu, Y.; Peng, S. Vitamin D supplementation in pregnant women or infants for preventing allergic diseases: A systematic review and meta-analysis of randomized controlled trials. Chin. Med. J. 2022, 135, 276–284. [Google Scholar] [CrossRef]
- Shi, D.; Wang, D.; Meng, Y.; Chen, J.; Mu, G.; Chen, W. Maternal vitamin D intake during pregnancy and risk of asthma and wheeze in children: A systematic review and meta-analysis of observational studies. J. Matern. Fetal Neonatal Med. 2021, 34, 653–659. [Google Scholar] [CrossRef]
- Sobczak, M.; Pawliczak, R. Relationship between vitamin D and asthma from gestational to adulthood period: A meta-analysis of randomized clinical trials. BMC Pulm. Med. 2023, 23, 212. [Google Scholar] [CrossRef]
- Bi, W.G.; Nuyt, A.M.; Weiler, H.; Leduc, L.; Santamaria, C.; Wei, S.Q. Association Between Vitamin D Supplementation During Pregnancy and Offspring Growth, Morbidity, and Mortality: A Systematic Review and Meta-analysis. JAMA Pediatr. 2018, 172, 635–645. [Google Scholar] [CrossRef]
- Liu, Y.; Ding, C.; Xu, R.; Wang, K.; Zhang, D.; Pang, W.; Tu, W.; Chen, Y. Effects of vitamin D supplementation during pregnancy on offspring health at birth: A meta-analysis of randomized controlled trails. Clin. Nutr. 2022, 41, 1532–1540. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Lin, Y.; Lu, J.; Lian, X.; Guo, Y.; Han, L.; Guo, Y. Effects of vitamin D supplementation during pregnancy on bone health and offspring growth: A systematic review and meta-analysis of randomized controlled trials. PLoS ONE 2022, 17, e0276016. [Google Scholar] [CrossRef] [PubMed]
- Moon, R.J.; Green, H.D.; D’Angelo, S.; Godfrey, K.M.; Davies, J.H.; Curtis, E.M.; Cooper, C.; Harvey, N.C. The effect of pregnancy vitamin D supplementation on offspring bone mineral density in childhood: A systematic review and meta-analysis. Osteoporos. Int. 2023, 34, 1269–1279. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Huang, Y. Magnesium supplementation for glycemic status in women with gestational diabetes: A systematic review and meta-analysis. Gynecol. Endocrinol. 2022, 38, 202–206. [Google Scholar] [CrossRef]
- Mocking, R.J.T.; Steijn, K.; Roos, C.; Assies, J.; Bergink, V.; Ruhé, H.G.; Schene, A.H. Omega-3 Fatty Acid Supplementation for Perinatal Depression: A Meta-Analysis. J. Clin. Psychiatry 2020, 81, 19r13106. [Google Scholar] [CrossRef]
- Sun, J.; Wang, J.; Ma, W.; Miao, M.; Sun, G. Effects of Additional Dietary Fiber Supplements on Pregnant Women with Gestational Diabetes: A Systematic Review and Meta-Analysis of Randomized Controlled Studies. Nutrients 2022, 14, 4626. [Google Scholar] [CrossRef]
- Bakouei, F.; Delavar, M.A.; Mashayekh-Amiri, S.; Esmailzadeh, S.; Taheri, Z. Efficacy of n-3 fatty acids supplementation on the prevention of pregnancy induced-hypertension or preeclampsia: A systematic review and meta-analysis. Taiwan. J. Obs. Gynecol. 2020, 59, 8–15. [Google Scholar] [CrossRef]
- Liu, W.; Gao, M.; Yang, S.; Sun, C.; Bi, Y.; Li, Y.; Wang, J.; Yuan, X. Effects of omega-3 supplementation on glucose and lipid metabolism in patients with gestational diabetes: A meta-analysis of randomized controlled trials. J. Diabetes Complicat. 2023, 37, 108451. [Google Scholar] [CrossRef]
- Abdelrahman, M.A.; Osama, H.; Saeed, H.; Madney, Y.M.; Harb, H.S.; Abdelrahim, M.E.A. Impact of n-3 polyunsaturated fatty acid intake in pregnancy on maternal health and birth outcomes: Systematic review and meta-analysis from randomized controlled trails. Arch. Gynecol. Obs. 2023, 307, 249–262. [Google Scholar] [CrossRef]
- Bilgundi, K.; Viswanatha, G.L.; Purushottam, K.M.; John, J.; Kamath, A.P.; Kishore, A.; Nayak, P.G.; Nandakumar, K. Docosahexaenoic Acid and Pregnancy: A Systematic Review and Meta-Analysis of the Association with Improved Maternal and Fetal Health. Nutr. Res. 2024, 128, 82–93. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, C.; Wang, Y.; Li, Y. Does vitamin E prevent asthma or wheeze in children: A systematic review and meta-analysis. Paediatr. Respir. Rev. 2018, 27, 60–68. [Google Scholar] [CrossRef]
- Montgomery, S.C.; Streit, S.M.; Beebe, M.L.; Maxwell IV, P.J. Micronutrient needs of the elderly. Nutr. Clin. Pract. 2014, 29, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Jayedi, A.; Daneshvar, M.; Jibril, A.T.; Sluyter, J.D.; Waterhouse, M.; Romero, B.D.; Neale, R.E.; Manson, J.E.; Shab-Bidar, S. Serum 25(OH)D Concentration, Vitamin D Supplementation, and Risk of Cardiovascular Disease and Mortality in Patients with Type 2 Diabetes or Prediabetes: A Systematic Review and Dose-Response Meta-Analysis. Am. J. Clin. Nutr. 2023, 118, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Gaugris, S.; Heaney, R.P.; Boonen, S.; Kurth, H.; Bentkover, J.D.; Sen, S.S. Vitamin D inadequacy among post-menopausal women: A systematic review. QJM 2005, 98, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Habibi Ghahfarrokhi, S.; Mohammadian-Hafshejani, A.; Sherwin, C.M.T.; Heidari-Soureshjani, S. Relationship between serum vitamin D and hip fracture in the elderly: A systematic review and meta-analysis. J. Bone Min. Metab. 2022, 40, 541–553. [Google Scholar] [CrossRef]
- Liu, C.; Kuang, X.; Li, K.; Guo, X.; Deng, Q.; Li, D. Effects of combined calcium and vitamin D supplementation on osteoporosis in postmenopausal women: A systematic review and meta-analysis of randomized controlled trials. Food Funct. 2020, 11, 10817–10827. [Google Scholar] [CrossRef]
- Reis, A.R.; Santos, R.K.F.; Dos Santos, C.B.; Santos, B.D.C.; de Carvalho, G.B.; Brandão-Lima, P.N.; de Oliveira, E.S.A.M.; Pires, L.V. Supplementation of vitamin D isolated or calcium-associated with bone remodeling and fracture risk in postmenopausal women without osteoporosis: A systematic review of randomized clinical trials. Nutrition 2023, 116, 112151. [Google Scholar] [CrossRef]
- Cormick, G.; Ciapponi, A.; Harbron, J.; Perez, S.M.; Vazquez, P.; Rivo, J.; Metzendorf, M.I.; Althabe, F.; Belizán, J.M. Calcium supplementation for people with overweight or obesity. Cochrane Database Syst. Rev. 2024, 5, Cd012268. [Google Scholar] [CrossRef]
- Ghoreishy, S.M.; Bagheri, A.; Nejad, M.M.; Larijani, B.; Esmaillzadeh, A. Association between calcium intake and risk of breast cancer: An updated systematic review and dose-response meta-analysis of cohort studies. Clin. Nutr. ESPEN 2023, 55, 251–259. [Google Scholar] [CrossRef]
- Bristow, S.M.; Bolland, M.J.; Gamble, G.D.; Leung, W.; Reid, I.R. Dietary calcium intake and change in bone mineral density in older adults: A systematic review of longitudinal cohort studies. Eur. J. Clin. Nutr. 2022, 76, 196–205. [Google Scholar] [CrossRef]
- ter Borg, S.; Verlaan, S.; Hemsworth, J.; Mijnarends, D.M.; Schols, J.M.; Luiking, Y.C.; de Groot, L.C. Micronutrient intakes and potential inadequacies of community-dwelling older adults: A systematic review. Br. J. Nutr. 2015, 113, 1195–1206. [Google Scholar] [CrossRef] [PubMed]
- D’Ecclesiis, O.; Gavioli, C.; Martinoli, C.; Raimondi, S.; Chiocca, S.; Miccolo, C.; Bossi, P.; Cortinovis, D.; Chiaradonna, F.; Palorini, R.; et al. Vitamin D and SARS-CoV2 infection, severity and mortality: A systematic review and meta-analysis. PLoS ONE 2022, 17, e0268396. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.Y.; Cheng, Y.C.; Chiu, C.C.; Liu, H.C.; Huang, M.C.; Tu, Y.K.; Kuo, P.H. Effects of Vitamin D Supplementation on Cognitive Outcomes: A Systematic Review and Meta-Analysis. Neuropsychol. Rev. 2024, 34, 568–580. [Google Scholar] [CrossRef] [PubMed]
- Bergink, A.P.; Zillikens, M.C.; Van Leeuwen, J.P.; Hofman, A.; Uitterlinden, A.G.; van Meurs, J.B. 25-Hydroxyvitamin D and osteoarthritis: A meta-analysis including new data. Semin. Arthritis Rheum. 2016, 45, 539–546. [Google Scholar] [CrossRef]
- Cao, Y.; Winzenberg, T.; Nguo, K.; Lin, J.; Jones, G.; Ding, C. Association between serum levels of 25-hydroxyvitamin D and osteoarthritis: A systematic review. Rheumatology 2013, 52, 1323–1334. [Google Scholar] [CrossRef]
- Hung, K.C.; Wang, L.K.; Lin, Y.T.; Yu, C.H.; Chang, C.Y.; Sun, C.K.; Chen, J.Y. Association of preoperative vitamin D deficiency with the risk of postoperative delirium and cognitive dysfunction: A meta-analysis. J. Clin. Anesth. 2022, 79, 110681. [Google Scholar] [CrossRef]
- Wang, K.; Xia, C.; Zhou, L.; Zheng, Y.; Wang, X.; Cheng, L. The Association between Vitamin D Deficiency and the Risk of Mortality after Hip Fractures: A Systematic Review and Meta-Analysis. J. Nutr. Sci. Vitaminol. 2024, 70, 89–97. [Google Scholar] [CrossRef]
- Xiong, A.; Li, H.; Lin, M.; Xu, F.; Xia, X.; Dai, D.; Sun, R.; Ling, Y.; Qiu, L.; Wang, R.; et al. Effects of active vitamin D analogues on muscle strength and falls in elderly people: An updated meta-analysis. Front. Endocrinol. 2024, 15, 1327623. [Google Scholar] [CrossRef]
- Chen, F.; Wang, J.; Cheng, Y.; Li, R.; Wang, Y.; Chen, Y.; Scott, T.; Tucker, K.L. Magnesium and Cognitive Health in Adults: A Systematic Review and Meta-Analysis. Adv. Nutr. 2024, 15, 100272. [Google Scholar] [CrossRef]
- Müller, O.; Krawinkel, M. Malnutrition and health in developing countries. Can. Med. Assoc. J. 2005, 173, 279–286. [Google Scholar] [CrossRef]
- World Health Organization. Malnutrition. Available online: https://www.who.int/health-topics/malnutrition#tab=tab_1 (accessed on 6 June 2024).
- Dietary Guidelines for Americans. Food Sources of Select Nutrients. Available online: https://www.dietaryguidelines.gov/resources/2020-2025-dietary-guidelines-online-materials/food-sources-select-nutrients (accessed on 6 June 2024).
- Smith, N.W.; Fletcher, A.J.; Hill, J.P.; McNabb, W.C. Modeling the Contribution of Milk to Global Nutrition. Front. Nutr. 2022, 8, 716100. [Google Scholar] [CrossRef] [PubMed]
- Juul, F.; Parekh, N.; Martinez-Steele, E.; Monteiro, C.A.; Chang, V.W. Ultra-processed food consumption among US adults from 2001 to 2018. Am. J. Clin. Nutr. 2022, 115, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Stewart, H.; Kuchler, F.; Dong, D.; Cessna, J. Examining the Decline in U.S. Per Capita Consumption of Fluid Cow’s Milk, 2003–2018; US Department of Agriculture: Washington, DC, USA, 2021. Available online: https://www.ers.usda.gov/publications/pub-details?pubid=102446 (accessed on 6 June 2024).
- Monroe-Lord, L.; Harrison, E.; Ardakani, A.; Duan, X.; Spechler, L.; Jeffery, T.D.; Jackson, P. Changes in Food Consumption Trends among American Adults since the COVID-19 Pandemic. Nutrients 2023, 15, 1769. [Google Scholar] [CrossRef] [PubMed]
- Chungchunlam, S.M.S.; Moughan, P.J. Comparative bioavailability of vitamins in human foods sourced from animals and plants. Crit. Rev. Food Sci. Nutr. 2024, 64, 11590–11625. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).