Lactase-Treated A2 Milk as a Feasible Conventional Milk Alternative: Results of a Randomized Controlled Crossover Trial to Assess Tolerance, Gastrointestinal Distress, and Preference for Milks Varying in Casein Types and Lactose Content
Abstract
1. Introduction
2. Materials and Methods
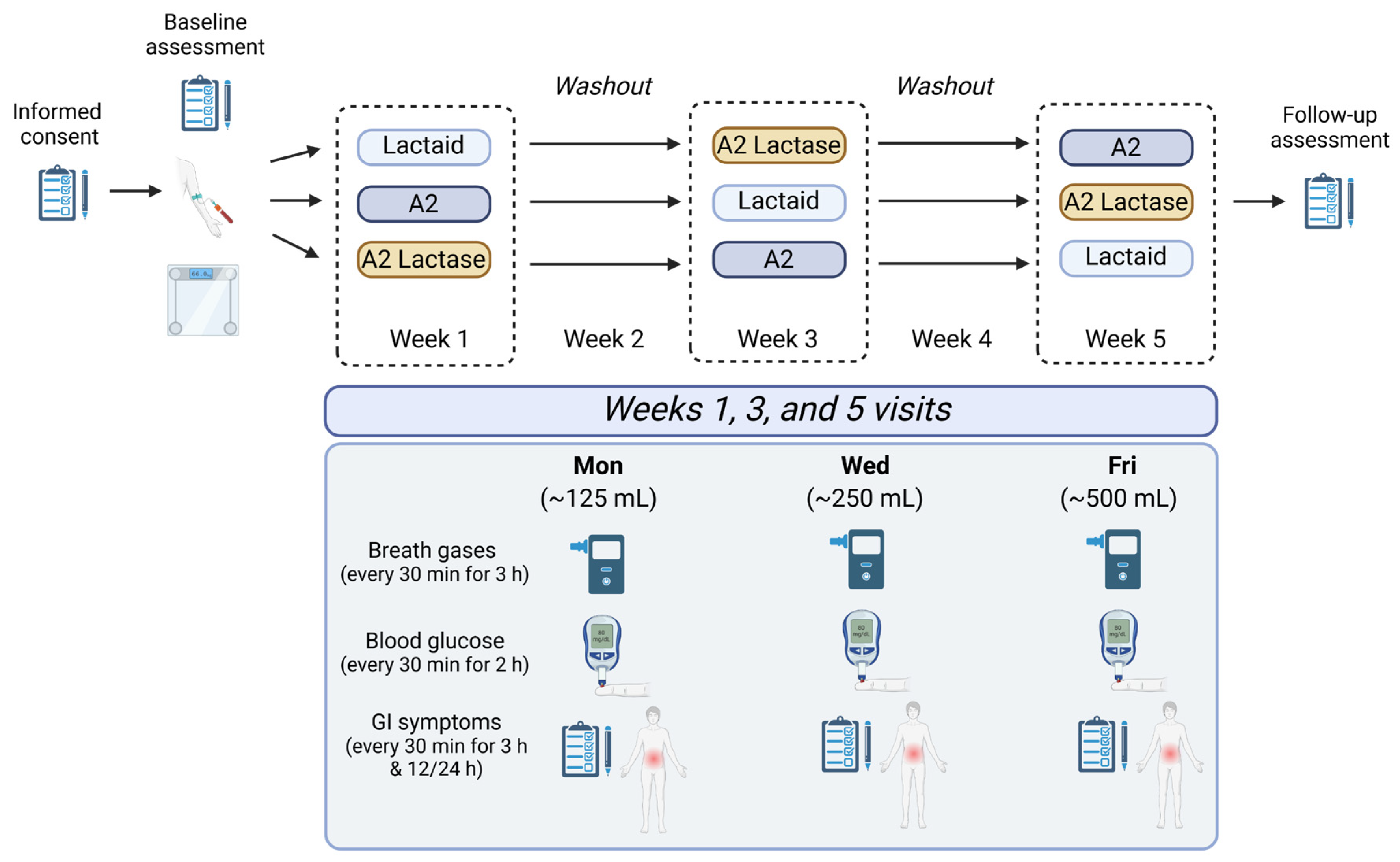
2.1. Study Design and Interventions
2.1.1. Participants
2.1.2. Sample Preparation
2.2. Study Measures
2.2.1. Gastrointestinal Symptom Scores
2.2.2. Breath Gases
2.2.3. Blood Glucose Levels
2.2.4. Milk Sensory Scale
2.2.5. Lactose Tolerance
2.2.6. Milk Sample Composition
2.3. Adverse Events
2.4. Statistical Analyses
3. Results
3.1. Participant Eligibility, Enrollment, and Randomization
3.2. Consumer Preference Across Samples
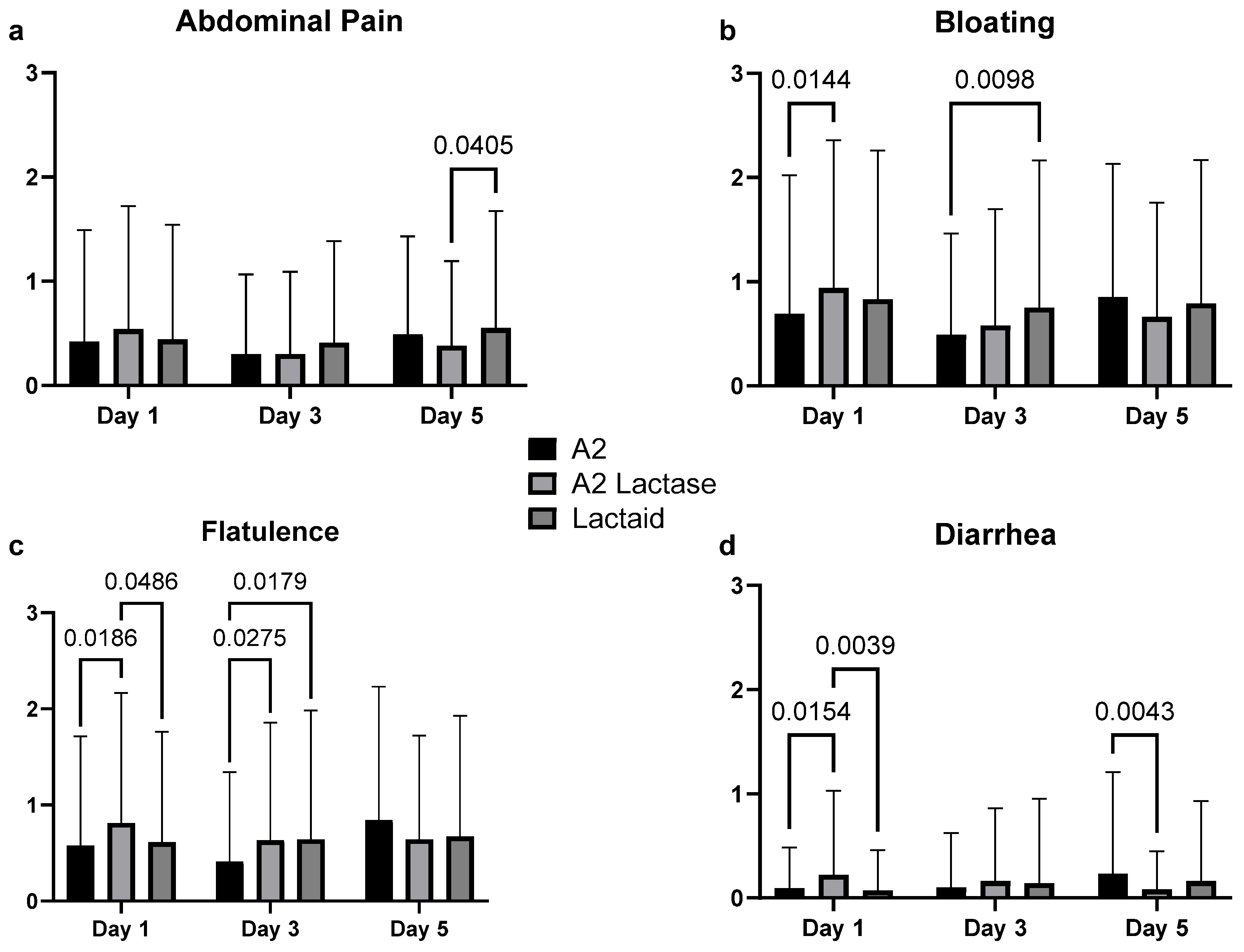
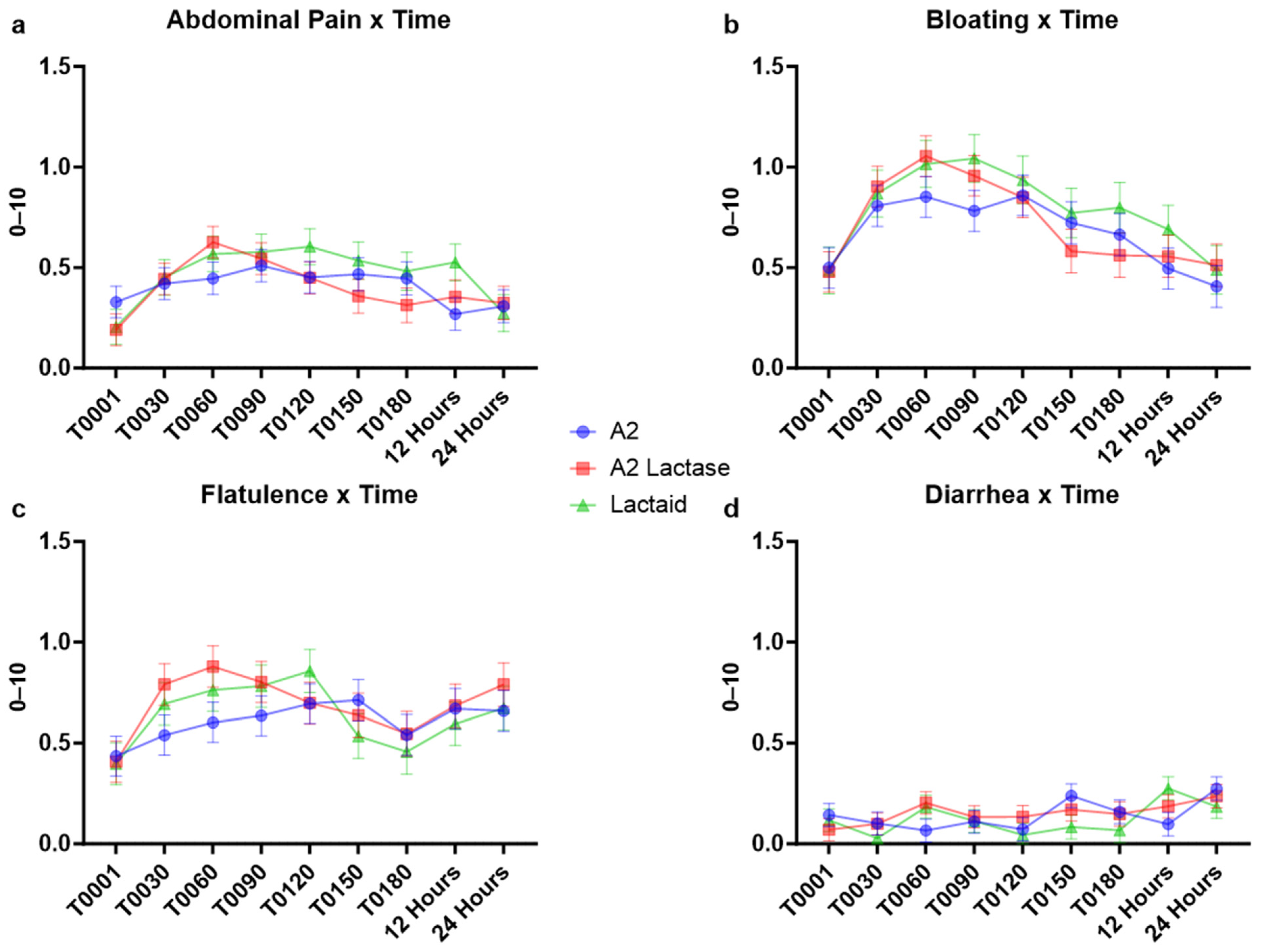
3.3. Gastrointestinal Symptoms in Response to Milk Types
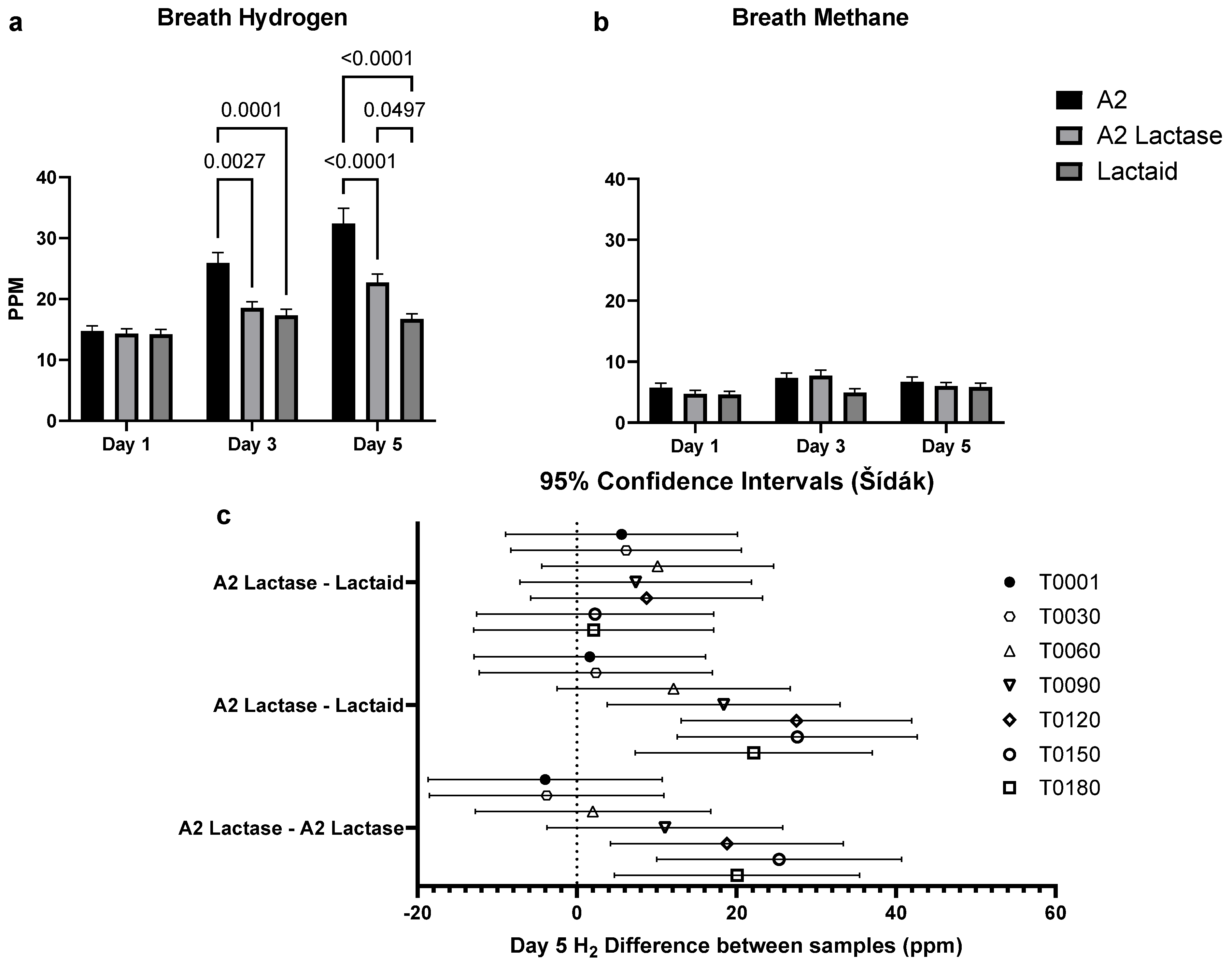
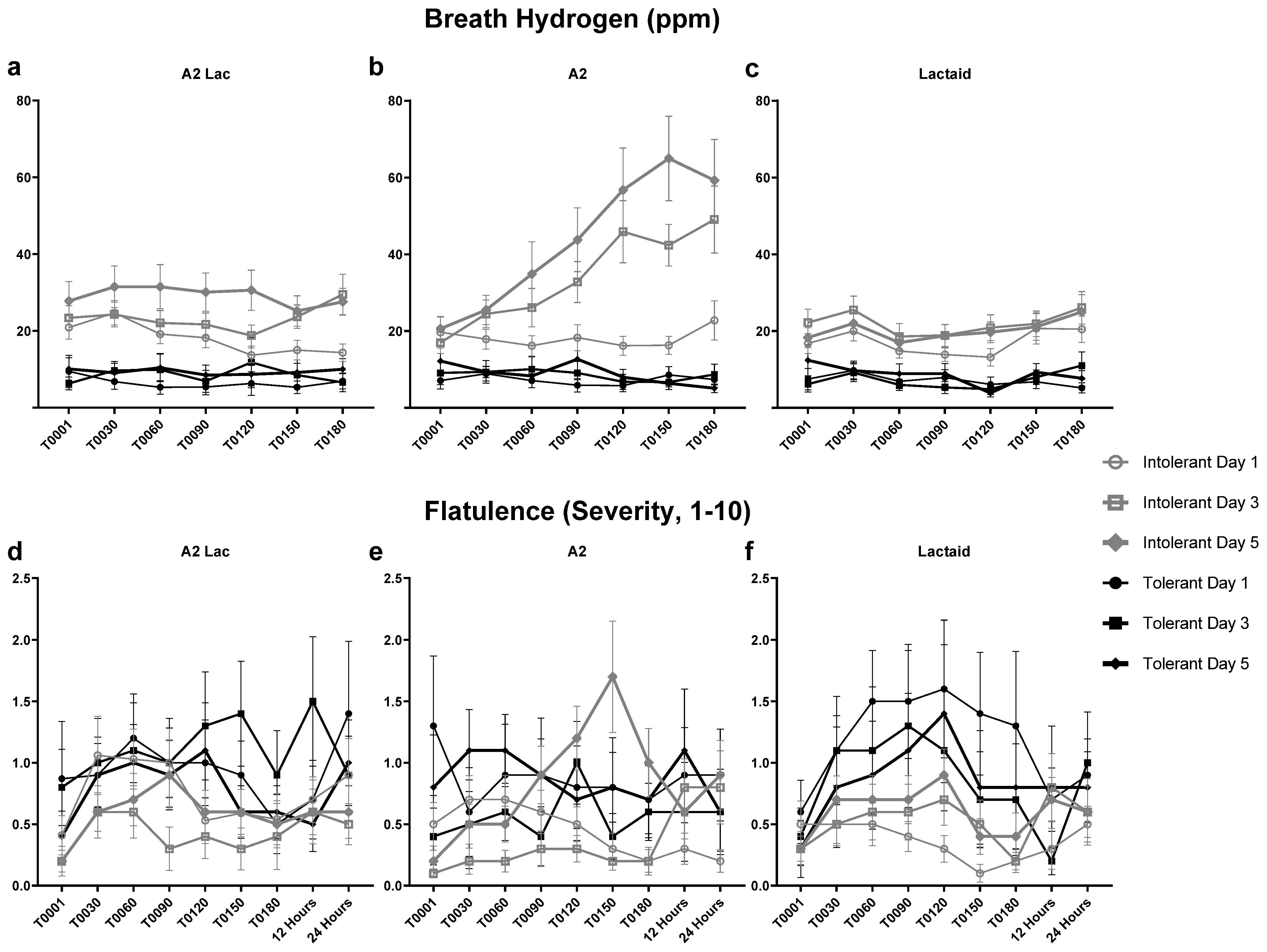
3.4. Breath Hydrogen in Response to Milk Type over Time
3.5. Blood Glucose Response to Milk Samples
3.6. Breath Hydrogen and Flatulence Response to Milk Type Between Lactose Intolerant Individuals
4. Discussion
5. Limitations and Future Research
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABC | Ammonium bicarbonate |
| AGC | Automatic gain control |
| A2 Lac | Lactose-free A2 milk |
| ANOVA | Analysis of variance |
| BCM-7 | β-casomorphin-7 |
| BMI | Body mass index |
| DTT | Dithiothreitol |
| ESI-MS/MS | Electrospray ionization tandem mass spectrometry |
| FA | Formic acid |
| GI | Gastrointestinal |
| H2 | Hydrogen |
| HCD | Higher-energy collisional dissociation |
| IRB | Institutional Review Board |
| JAR | Just-About-Right |
| Lac | Lactaid (lactose-free conventional milk) |
| m/z | Mass-to-charge ratio |
| MDPI | Multidisciplinary Digital Publishing Institute |
| MS | Mass spectrometry |
| PPM | Parts per million |
| RF | Radio frequency |
| SPSS | Statistical Package for the Social Sciences |
| SD | Standard deviation |
| TFA | Trifluoroacetic acid |
| UHPLC | Ultra-high-performance liquid chromatography |
| UHT | Ultra-high temperature |
| VAS | Visual analog scale |
References
- Simoes, C.D.; Maukonen, J.; Scott, K.P.; Virtanen, K.A.; Pietilainen, K.H.; Saarela, M. Impact of a very low-energy diet on the fecal microbiota of obese individuals. Eur. J. Nutr. 2014, 53, 1421–1429. [Google Scholar] [CrossRef] [PubMed]
- Misselwitz, B.; Butter, M.; Verbeke, K.; Fox, M.R. Update on lactose malabsorption and intolerance: Pathogenesis, diagnosis and clinical management. Gut 2019, 68, 2080–2091. [Google Scholar] [CrossRef]
- Swagerty, D.L., Jr.; Walling, A.D.; Klein, R.M. Lactose intolerance. Am. Fam. Physician 2002, 65, 1845–1851. [Google Scholar] [PubMed]
- Dekker, P.J.; Koenders, D.; Bruins, M.J. Lactose-free dairy products: Market developments, production, nutrition and health benefits. Nutrients 2019, 11, 551. [Google Scholar] [CrossRef]
- Sharp, E.; D’Cunha, N.M.; Ranadheera, C.S.; Vasiljevic, T.; Panagiotakos, D.B.; Naumovski, N. Effects of lactose-free and low-lactose dairy on symptoms of gastrointestinal health: A systematic review. Int. Dairy J. 2021, 114, 104936. [Google Scholar] [CrossRef]
- Pal, S.; Woodford, K.; Kukuljan, S.; Ho, S. Milk intolerance, beta-casein and lactose. Nutrients 2015, 7, 7285–7297. [Google Scholar] [CrossRef]
- Giribaldi, M.; Lamberti, C.; Cirrincione, S.; Giuffrida, M.G.; Cavallarin, L. A2 milk and BCM-7 peptide as emerging parameters of milk quality. Front. Nutr. 2022, 9, 842375. [Google Scholar] [CrossRef]
- Kaskous, S. A1-and A2-Milk and their effect on human health. J. Food Eng. Technol. 2020, 9, 15–21. [Google Scholar] [CrossRef]
- Fernández-Rico, S.; Mondragón, A.d.C.; López-Santamarina, A.; Cardelle-Cobas, A.; Regal, P.; Lamas, A.; Ibarra, I.S.; Cepeda, A.; Miranda, J.M. A2 Milk: New Perspectives for Food Technology and Human Health. Foods 2022, 11, 2387. [Google Scholar] [CrossRef]
- Sodhi, M.; Mukesh, M.; Kataria, R.S.; Mishra, B.P.; Joshii, B.K. Milk proteins and human health: A1/A2 milk hypothesis. Indian J. Endocrinol. Metab. 2012, 16, 856. [Google Scholar] [CrossRef]
- Milan, A.M.; Shrestha, A.; Karlström, H.J.; Martinsson, J.A.; Nilsson, N.J.; Perry, J.K.; Day, L.; Barnett, M.P.; Cameron-Smith, D. Comparison of the impact of bovine milk β-casein variants on digestive comfort in females self-reporting dairy intolerance: A randomized controlled trial. Am. J. Clin. Nutr. 2020, 111, 149–160. [Google Scholar] [CrossRef]
- Sheng, X.; Li, Z.; Ni, J.; Yelland, G. Effects of conventional milk versus milk containing only A2 β-casein on digestion in Chinese children: A randomized study. J. Pediatr. Gastroenterol. Nutr. 2019, 69, 375. [Google Scholar] [CrossRef]
- Ramakrishnan, M.; Eaton, T.K.; Sermet, O.M.; Savaiano, D.A. Milk containing A2 β-casein only, as a single meal, causes fewer symptoms of lactose intolerance than milk containing A1 and A2 β-caseins in subjects with lactose maldigestion and intolerance: A randomized, double-blind, crossover trial. Nutrients 2020, 12, 3855. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Xu, L.; Lu, X.; Yelland, G.W.; Ni, J.; Clarke, A.J. Effects of milk containing only A2 beta casein versus milk containing both A1 and A2 beta casein proteins on gastrointestinal physiology, symptoms of discomfort, and cognitive behavior of people with self-reported intolerance to traditional cows’ milk. Nutr. J. 2015, 15, 35. [Google Scholar] [CrossRef] [PubMed]
- Popescu, L.; Bulgaru, V.; Siminiuc, R.J.F.; Sciences, N. Effect of temperature, pH and amount of enzyme used in the lactose hydrolysis of milk. Food Nutr. Sci. 2021, 12, 1243–1254. [Google Scholar] [CrossRef]
- Shrestha, A.; Samuelsson, L.M.; Sharma, P.; Day, L.; Cameron-Smith, D.; Milan, A.M. Comparing Response of Sheep and Cow Milk on Acute Digestive Comfort and Lactose Malabsorption: A Randomized Controlled Trial in Female Dairy Avoiders. Front. Nutr. 2021, 8, 603816. [Google Scholar] [CrossRef]
- Lim, S.H.; Chin, N.L.; Sulaiman, A.; Tay, C.H.; Wong, T.H. Sensory Analysis for Cow Milk Product Development Using High Pressure Processing (HPP) in the Dairy Industry. Foods 2022, 11, 1233. [Google Scholar] [CrossRef]
- Lawless, H.T.; Heymann, H. Sensory Evaluation of Food Principles and Practices; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Chávez-Servín, J.L.; Castellote, A.I.; López-Sabater, M.C. Analysis of mono- and disaccharides in milk-based formulae by high-performance liquid chromatography with refractive index detection. J. Chromatogr. A 2004, 1043, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Rainville, P.; Liu, K.; Pointer, B. Determination of lactose in low-lactose and lactose-free dairy products using LC-MS. J. Food Compos. Anal. 2021, 100, 103824. [Google Scholar] [CrossRef]
- Czank, C.; Simmer, K.; Hartmann, P.E. A method for standardizing the fat content of human milk for use in the neonatal intensive care unit. Int. Breastfeed. J. 2009, 4, 3. [Google Scholar] [CrossRef]
- Liu, Z.; Pan, S.; Wu, P.; Li, M.; Liang, D. Determination of A1 and A2 β-Casein in Milk Using Characteristic Thermolytic Peptides via Liquid Chromatography-Mass Spectrometry. Molecules 2023, 28, 5200. [Google Scholar] [CrossRef] [PubMed]
- Rappsilber, J.; Mann, M.; Ishihama, Y. Protocol for micro-purification, enrichment, pre-fractionation and storage of peptides for proteomics using StageTips. Nat. Protoc. 2007, 2, 1896–1906. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.; Woodford, K.; Kukuljan, S.; Pal, S. Comparative effects of A1 versus A2 beta-casein on gastrointestinal measures: A blinded randomised cross-over pilot study. Eur. J. Clin. Nutr. 2014, 68, 994–1000. [Google Scholar] [CrossRef]
- Choi, Y.; Kim, N.; Song, C.H.; Kim, S.; Lee, D.H. The Effect of A2 Milk on Gastrointestinal Symptoms in Comparison to A1/A2 Milk: A Single-center, Randomized, Double-blind, Cross-over Study. J. Cancer Prev. 2024, 29, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Pereira, P.C. Milk nutritional composition and its role in human health. Nutrition 2014, 30, 619–627. [Google Scholar] [CrossRef]
- Willett, W.C.; Ludwig, D.S. Milk and health. N. Engl. J. Med. 2020, 382, 644–654. [Google Scholar] [CrossRef]


| Nutrients | A2 | A2 Lactase | Lactaid |
|---|---|---|---|
| Lactose, g/240 mL | 12.9 | 0.0 | 0.3 |
| Glucose and galactose, g/240 mL | 0.0 | 15.0 | 13.4 |
| A1 β-casein as % total β-casein | 0.0 | 0.0 | 23.9 |
| Sequence | A | B | C | D | E | F | All | p |
|---|---|---|---|---|---|---|---|---|
| N = 9 | N = 8 | N = 9 | N = 8 | N = 7 | N = 7 | N = 48 | ||
| Sex | 0.089 | |||||||
| Male | 4 (44.4%) | 1 (12.5%) | 3 (33.3%) | 1 (12.5%) | 5 (71.4%) | 1 (14.3%) | 15 (31.3%) | |
| Female | 5 (55.6%) | 7 (87.5%) | 6 (66.7%) | 7 (87.5%) | 2 (28.6%) | 6(85.7%) | 33 (68.8%) | |
| Ethnicity | 0.195 | |||||||
| White | 4 (44.4%) | 4 (50.0%) | 3 (33.3%) | 4 (50.0%) | 3 (42.9%) | 4 (57.1%) | 22 (45.8%) | |
| Black | 0 (0.0%) | 4 (50.0%) | 1 (11.1%) | 1 (12.5%) | 1 (14.3%) | 0 (0.0%) | 7 (14.6%) | |
| Asian | 2 (22.2%) | 0 (0.0%) | 3 (33.3%) | 1 (12.5%) | 3 (42.9%) | 0 (0.0%) | 9 (18.8%) | |
| Hispanic | 2 (22.2%) | 0 (0.0%) | 1 (11.1%) | 2 (25.0%) | 0 (0.0%) | 3 (42.9%) | 8 (16.7%) | |
| Latino | 0 (0.0%) | 0 (0.0%) | 1 (11.1%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (2.1%) | |
| White/Latino | 1 (11.1%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (2.1%) | |
| Age | 31 (7.14) | 33 (9.55) | 29 (5.36) | 31 (8.21) | 25 (3.10) | 33 (16.40) | 31 (8.97) | 0.814 |
| BMI | 29.0 (7.45) | 25.0 (6.73) | 26.2 (3.34) | 28.1 (6.46) | 27.1 (3.42) | 28.1 (5.84) | 27.2 (5.70) | 0.405 |
| Lactose-Intolerant | 7 (77.8%) | 6 (75%) | 6 (66.7%) | 3 (37.5%) | 5 (71.4%) | 6 (85.7%) | 33 (68.8%) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robinson, L.A.; Cavanah, A.M.; Lennon, S.; Mattingly, M.L.; Anglin, D.A.; Boersma, M.D.; Roberts, M.D.; Frugé, A.D. Lactase-Treated A2 Milk as a Feasible Conventional Milk Alternative: Results of a Randomized Controlled Crossover Trial to Assess Tolerance, Gastrointestinal Distress, and Preference for Milks Varying in Casein Types and Lactose Content. Nutrients 2025, 17, 1946. https://doi.org/10.3390/nu17121946
Robinson LA, Cavanah AM, Lennon S, Mattingly ML, Anglin DA, Boersma MD, Roberts MD, Frugé AD. Lactase-Treated A2 Milk as a Feasible Conventional Milk Alternative: Results of a Randomized Controlled Crossover Trial to Assess Tolerance, Gastrointestinal Distress, and Preference for Milks Varying in Casein Types and Lactose Content. Nutrients. 2025; 17(12):1946. https://doi.org/10.3390/nu17121946
Chicago/Turabian StyleRobinson, Laura A., Aidan M. Cavanah, Sarah Lennon, Madison L. Mattingly, Derick A. Anglin, Melissa D. Boersma, Michael D. Roberts, and Andrew Dandridge Frugé. 2025. "Lactase-Treated A2 Milk as a Feasible Conventional Milk Alternative: Results of a Randomized Controlled Crossover Trial to Assess Tolerance, Gastrointestinal Distress, and Preference for Milks Varying in Casein Types and Lactose Content" Nutrients 17, no. 12: 1946. https://doi.org/10.3390/nu17121946
APA StyleRobinson, L. A., Cavanah, A. M., Lennon, S., Mattingly, M. L., Anglin, D. A., Boersma, M. D., Roberts, M. D., & Frugé, A. D. (2025). Lactase-Treated A2 Milk as a Feasible Conventional Milk Alternative: Results of a Randomized Controlled Crossover Trial to Assess Tolerance, Gastrointestinal Distress, and Preference for Milks Varying in Casein Types and Lactose Content. Nutrients, 17(12), 1946. https://doi.org/10.3390/nu17121946










