Function of Yogurt Fermented with the Lactococcus lactis 11/19-B1 Strain in Improving the Lipid Profile and Intestinal Microbiome in Hemodialysis Patients
Abstract
1. Introduction
2. Materials and Methods
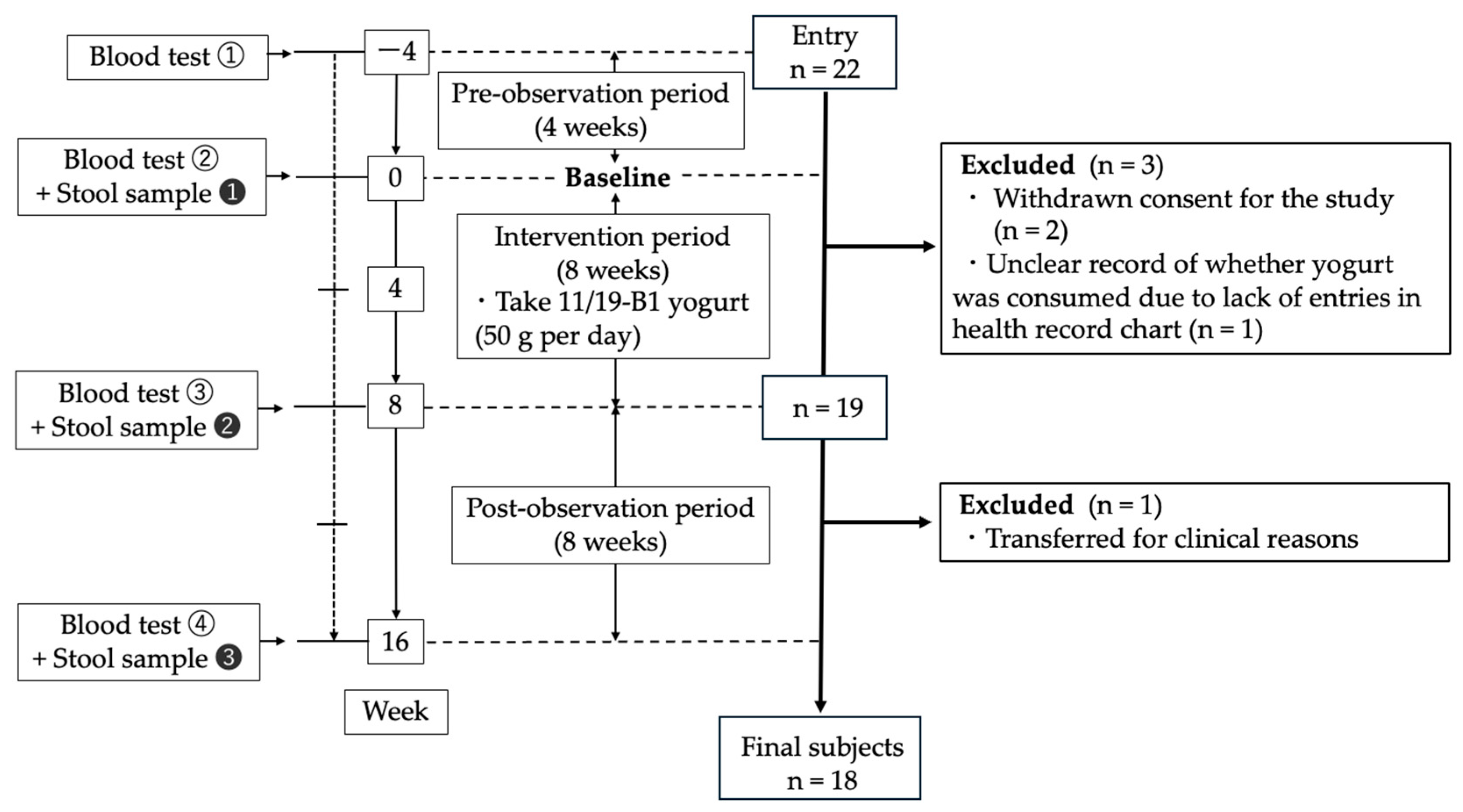
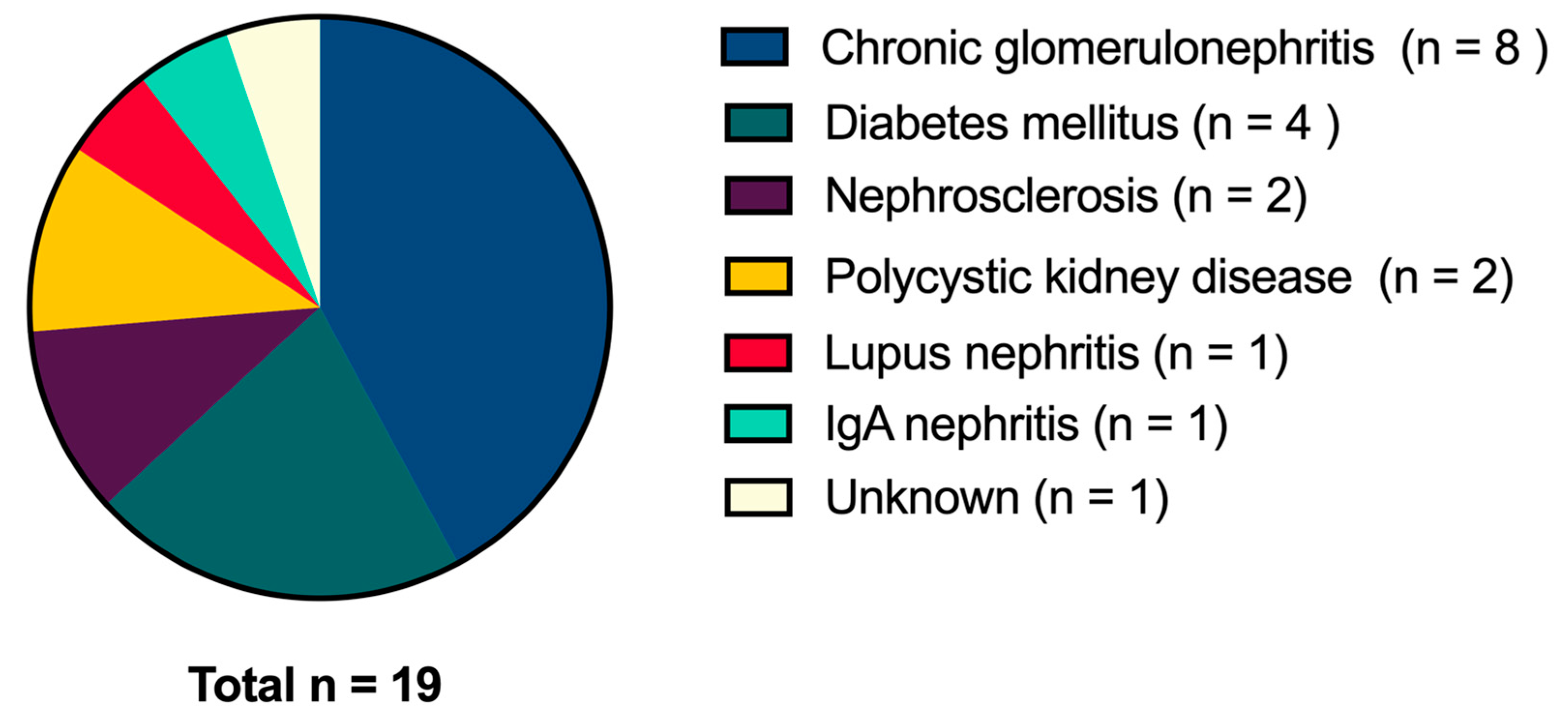
2.1. Subjects and Study Design
2.2. Yogurt
2.3. Blood and Stool Samples
2.4. Analyses of Serum Uremic Toxin Concentrations (Indoxyl Sulfate and TMAO)
2.5. Statistical Analysis
2.6. Procedure for 16S rRNA Sequencing
2.7. Gut Microbiome Statistical Analysis
3. Results
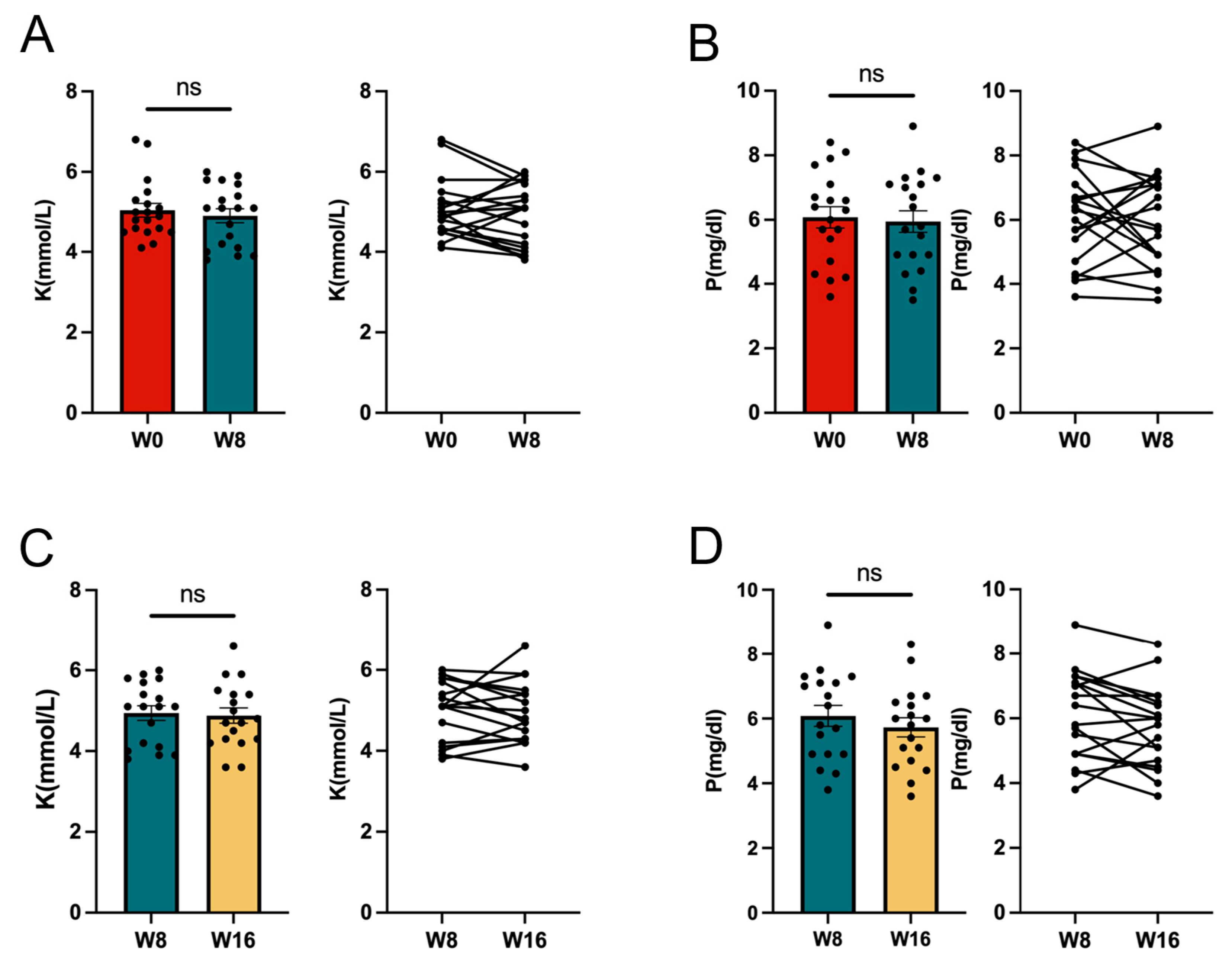
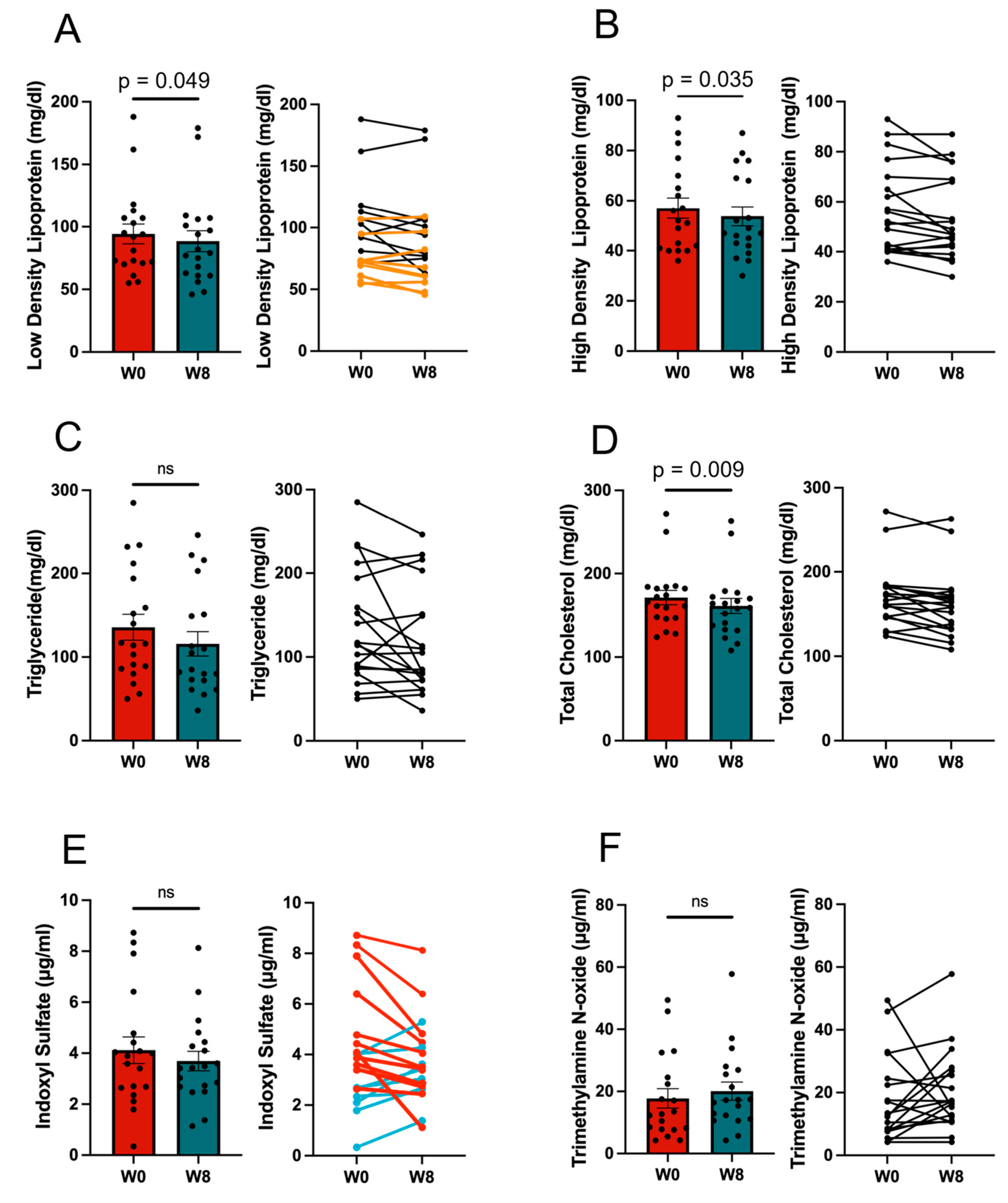
3.1. Effect of the 11/19-B1 Yogurt on Serum Findings
3.2. Effect of 11/19-B1 Yogurt Intake on Stool Characteristics
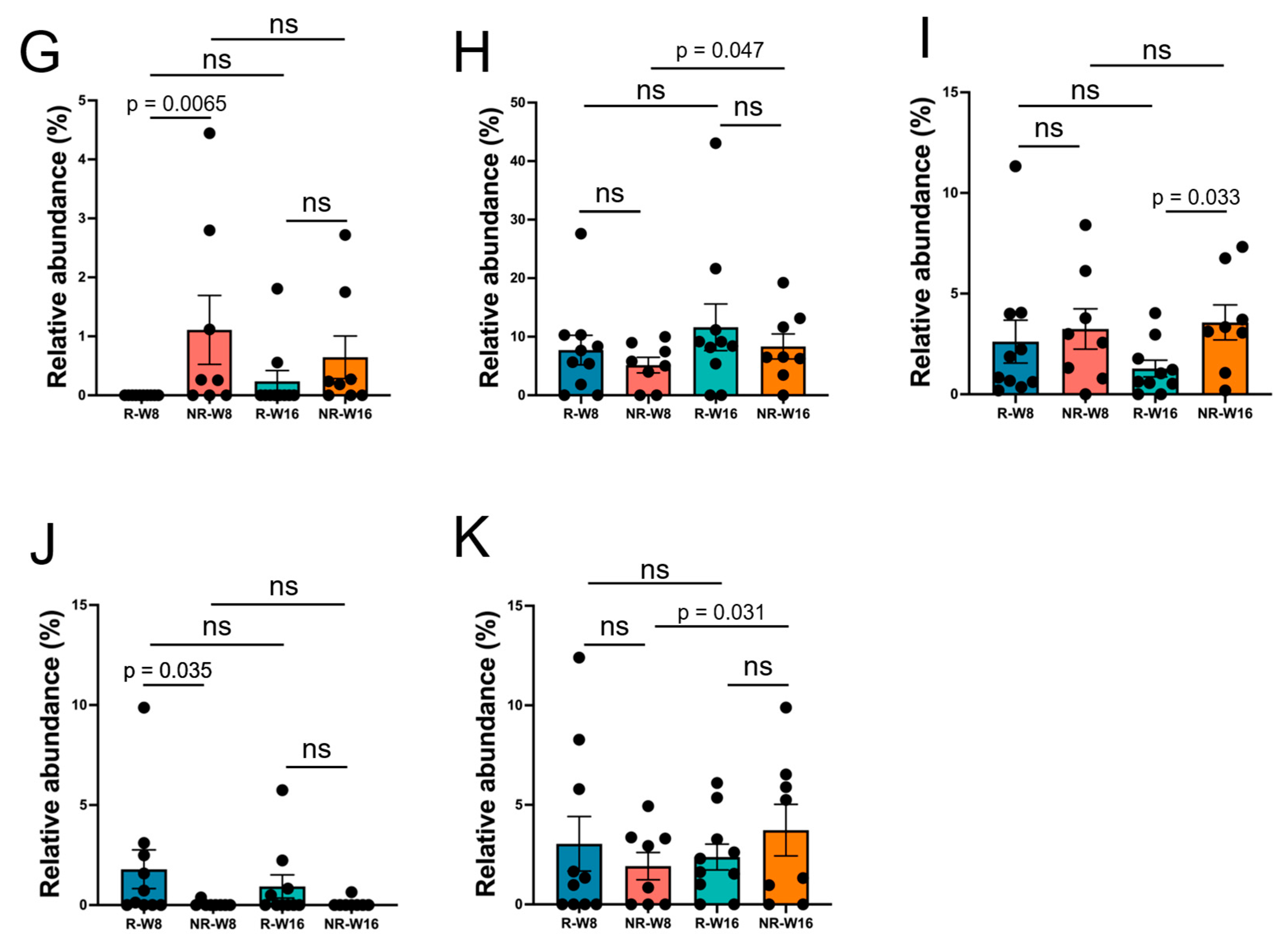
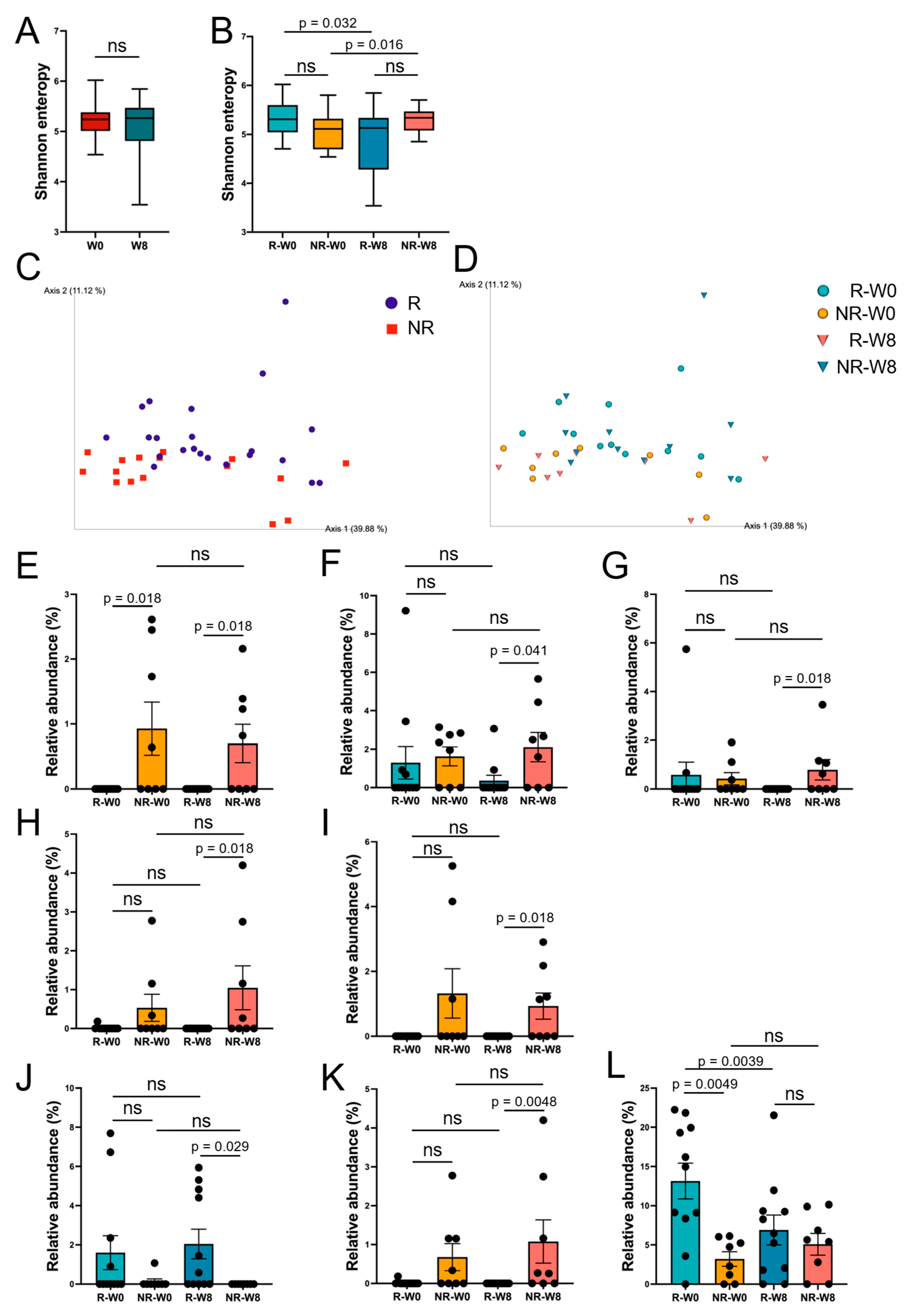
3.3. Gut Microbiome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A




References
- Francis, A.; Harhay, M.N.; Ong, A.C.M.; Tummalapalli, S.L.; Ortiz, A.; Fogo, A.B.; Fliser, D.; Roy-Chaudhury, P.; Fontana, M.; Nangaku, M.; et al. Chronic Kidney Disease and the Global Public Health Agenda: An International Consensus. Nat. Rev. Nephrol. 2024, 20, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Masaki, T.; Hansahusa, N.; Abe, M.; Jyoki, N.; Hoshino, J.; Taniguchi, M.; Kikuchi, K.; Hasegawa, T.; Goto, S.; Komada, H.; et al. 2023 Annual Dialysis Data Report, JSDT Renal Data Registry. Nihon Toseki Igakkai Zasshi 2024, 57, 543–620. [Google Scholar]
- Nagai, K.; Asahi, K.; Iseki, K.; Yamagata, K. Estimating the Prevalence of Definitive Chronic Kidney Disease in the Japanese General Population. Clin. Exp. Nephrol. 2021, 25, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Wu, L.; Tang, M.-Y.; Liu, Y.-F.; Liu, L.; Liu, X.-Y.; Zhang, C.; Huang, L. Indoxyl Sulfate in Atherosclerosis. Toxicol. Lett. 2023, 383, 204–212. [Google Scholar] [CrossRef]
- Canyelles, M.; Borràs, C.; Rotllan, N.; Tondo, M.; Escolà-Gil, J.C.; Blanco-Vaca, F. Gut Microbiota-Derived TMAO: A Causal Factor Promoting Atherosclerotic Cardiovascular Disease? Int. J. Mol. Sci. 2023, 24, 1940. [Google Scholar] [CrossRef]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal Microbiota Metabolism of L-Carnitine, a Nutrient in Red Meat, Promotes Atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef]
- Al-Waiz, M.; Mikov, M.; Mitchell, S.C.; Smith, R.L. The Exogenous Origin of Trimethylamine in the Mouse. Metabolism 1992, 41, 135–136. [Google Scholar] [CrossRef]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; Dugar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.-M.; et al. Gut Flora Metabolism of Phosphatidylcholine Promotes Cardiovascular Disease. Nature 2011, 472, 57–63. [Google Scholar] [CrossRef]
- Meyer, T.W.; Hostetter, T.H. Uremic Solutes from Colon Microbes. Kidney Int. 2012, 81, 949–954. [Google Scholar] [CrossRef]
- Mafra, D.; Lobo, J.C.; Barros, A.F.; Koppe, L.; Vaziri, N.D.; Fouque, D. Role of Altered Intestinal Microbiota in Systemic Inflammation and Cardiovascular Disease in Chronic Kidney Disease. Future Microbiol. 2014, 9, 399–410. [Google Scholar] [CrossRef]
- Kim, K.; Kim, J.-E.; Kim, J.H.; Ahn, S.H.; Jung, C.Y.; Hwang, S.D.; Lee, S.W.; Song, J.H. Real-World Evidence of Constipation and Laxative Use in the Korean Population with Chronic Kidney Disease from a Common Data Model. Sci. Rep. 2024, 14, 6610. [Google Scholar] [CrossRef] [PubMed]
- Noce, A.; Marrone, G.; Di Daniele, F.; Ottaviani, E.; Wilson Jones, G.; Bernini, R.; Romani, A.; Rovella, V. Impact of Gut Microbiota Composition on Onset and Progression of Chronic Non-Communicable Diseases. Nutrients 2019, 11, 1073. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, F.P.B.; de Luna Freire, M.O.; de Oliveira Coutinho, D.; de Santana Cirilo, M.A.; de Brito Alves, J.L. Gut Dysbiosis and Probiotic Therapy in Chronic Kidney Disease: A Comprehensive Review. Probiotics Antimicrob. Proteins 2024. [Google Scholar]
- Mortensen, M.B.; Nordestgaard, B.G. Elevated LDL Cholesterol and Increased Risk of Myocardial Infarction and Atherosclerotic Cardiovascular Disease in Individuals Aged 70–100 Years: A Contemporary Primary Prevention Cohort. Lancet 2020, 396, 1644–1652. [Google Scholar] [CrossRef]
- Nishiyama, K.; Kobayashi, T.; Sato, Y.; Watanabe, Y.; Kikuchi, R.; Kanno, R.; Koshizuka, T.; Miyazaki, N.; Ishioka, K.; Suzutani, T. A Double-Blind Controlled Study to Evaluate the Effects of Yogurt Enriched with Lactococcus Lactis 11/19-B1 and Bifidobacterium Lactis on Serum Low-Density Lipoprotein Level and Antigen-Specific Interferon-γ Releasing Ability. Nutrients 2018, 10, 1778. [Google Scholar] [CrossRef]
- Suzuki, T.; Nishiyama, K.; Kawata, K.; Sugimoto, K.; Isome, M.; Suzuki, S.; Nozawa, R.; Ichikawa, Y.; Watanabe, Y.; Suzutani, T. Effect of the Lactococcus Lactis 11/19-B1 Strain on Atopic Dermatitis in a Clinical Test and Mouse Model. Nutrients 2020, 12, 763. [Google Scholar] [CrossRef]
- Ishioka, K.; Miyazaki, N.; Nishiyama, K.; Suzutani, T. Characterization of Lactococcus lactis 11/19-B1 isolated from Kiwifruits as a Potential Probiotic and Paraprobiotic. Microorganisms 2023, 11, 2949. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Uemura, M.; Hayashi, F.; Ishioka, K.; Ihara, K.; Yasuda, K.; Okazaki, K.; Omata, J.; Suzutani, T.; Hirakawa, Y.; Chiang, C.; et al. Obesity and Mental Health Improvement Following Nutritional Education Focusing on Gut Microbiota Composition in Japanese Women: A Randomized Controlled Trial. Eur. J. Nutr. 2019, 58, 3291–3302. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt Removes Adapter Sequences from High-Throughput Sequencing Reads. EMBnet J. 2011, 17, 10. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Lozupone, C.; Lladser, M.E.; Knights, D.; Stombaugh, J.; Knight, R. UniFrac: An Effective Distance Metric for Microbial Community Comparison. ISME J. 2011, 5, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Bokulich, N.A.; Kaehler, B.D.; Rideout, J.R.; Dillon, M.; Bolyen, E.; Knight, R.; Huttley, G.A.; Gregory Caporaso, J. Optimizing Taxonomic Classification of Marker-Gene Amplicon Sequences with QIIME 2’s q2-Feature-Classifier Plugin. Microbiome 2018, 6, 90. [Google Scholar] [CrossRef] [PubMed]
- Robeson, M.S., 2nd; O’Rourke, D.R.; Kaehler, B.D.; Ziemski, M.; Dillon, M.R.; Foster, J.T.; Bokulich, N.A. RESCRIPt: Reproducible Sequence Taxonomy Reference Database Management. PLoS Comput. Biol. 2021, 17, e1009581. [Google Scholar] [CrossRef]
- Pruesse, E.; Quast, C.; Knittel, K.; Fuchs, B.M.; Ludwig, W.; Peplies, J.; Glöckner, F.O. SILVA: A Comprehensive Online Resource for Quality Checked and Aligned Ribosomal RNA Sequence Data Compatible with ARB. Nucleic Acids Res. 2007, 35, 7188–7196. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA Ribosomal RNA Gene Database Project: Improved Data Processing and Web-Based Tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Inoue, K.; Abe, S.; Fukunaga, T. Current State of Cause of Death Determinations in Japan and the Need to List the Precise Underlying Cause of Death. Med. Sci. Law 2023, 63, 114–119. [Google Scholar] [CrossRef]
- Hung, S.-C.; Kuo, K.-L.; Wu, C.-C.; Tarng, D.-C. Indoxyl Sulfate: A Novel Cardiovascular Risk Factor in Chronic Kidney Disease. J. Am. Heart Assoc. 2017, 6, e005022. [Google Scholar] [CrossRef]
- Sun, X.; Jiao, X.; Ma, Y.; Liu, Y.; Zhang, L.; He, Y.; Chen, Y. Trimethylamine N-Oxide Induces Inflammation and Endothelial Dysfunction in Human Umbilical Vein Endothelial Cells via Activating ROS-TXNIP-NLRP3 Inflammasome. Biochem. Biophys. Res. Commun. 2016, 481, 63–70. [Google Scholar] [CrossRef]
- Dou, L.; Bertrand, E.; Cerini, C.; Faure, V.; Sampol, J.; Vanholder, R.; Berland, Y.; Brunet, P. The Uremic Solutes P-Cresol and Indoxyl Sulfate Inhibit Endothelial Proliferation and Wound Repair. Kidney Int. 2004, 65, 442–451. [Google Scholar] [CrossRef]
- Brial, F.; Alzaid, F.; Sonomura, K.; Kamatani, Y.; Meneyrol, K.; Le Lay, A.; Péan, N.; Hedjazi, L.; Sato, T.-A.; Venteclef, N.; et al. The Natural Metabolite 4-Cresol Improves Glucose Homeostasis and Enhances β-Cell Function. Cell Rep. 2020, 30, 2306–2320.e5. [Google Scholar] [CrossRef] [PubMed]
- Roager, H.M.; Licht, T.R. Microbial Tryptophan Catabolites in Health and Disease. Nat. Commun. 2018, 9, 3294. [Google Scholar] [CrossRef] [PubMed]
- Shivani, S.; Kao, C.-Y.; Chattopadhyay, A.; Chen, J.-W.; Lai, L.-C.; Lin, W.-H.; Lu, T.-P.; Huang, I.-H.; Tsai, M.-H.; Teng, C.-H.; et al. Uremic Toxin-Producing Bacteroides Species Prevail in the Gut Microbiota of Taiwanese CKD Patients: An Analysis Using the New Taiwan Microbiome Baseline. Front. Cell. Infect. Microbiol. 2022, 12, 726256. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.K.; Vasquez, R.; Kim, S.H.; Hwang, I.-C.; Song, J.H.; Park, J.H.; Kim, I.H.; Kang, D.-K. Multispecies Probiotics Alter Fecal Short-Chain Fatty Acids and Lactate Levels in Weaned Pigs by Modulating Gut Microbiota. J. Anim. Sci. Technol. 2021, 63, 1142–1158. [Google Scholar] [CrossRef]
- Castillo-Rodriguez, E.; Fernandez-Prado, R.; Esteras, R.; Perez-Gomez, M.V.; Gracia-Iguacel, C.; Fernandez-Fernandez, B.; Kanbay, M.; Tejedor, A.; Lazaro, A.; Ruiz-Ortega, M.; et al. Impact of Altered Intestinal Microbiota on Chronic Kidney Disease Progression. Toxins 2018, 10, 300. [Google Scholar] [CrossRef]
- Einhorn, L.M.; Zhan, M.; Hsu, V.D.; Walker, L.D.; Moen, M.F.; Seliger, S.L.; Weir, M.R.; Fink, J.C. The Frequency of Hyperkalemia and Its Significance in Chronic Kidney Disease. Arch. Intern. Med. 2009, 169, 1156–1162. [Google Scholar] [CrossRef]
- Hayes, J.; Kalantar-Zadeh, K.; Lu, J.L.; Turban, S.; Anderson, J.E.; Kovesdy, C.P. Association of Hypo- and Hyperkalemia with Disease Progression and Mortality in Males with Chronic Kidney Disease: The Role of Race. Nephron Clin. Pract. 2012, 120, c8–c16. [Google Scholar] [CrossRef]
- Sinha, A.D.; Agarwal, R. Chronic Renal Disease Progression: Treatment Strategies and Potassium Intake. Semin. Nephrol. 2013, 33, 290–299. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, B.; Zhou, X.; Wang, Y.; Wang, H.; Jia, S.; Zhang, Z.; Chu, C.; Mu, J. Combined Lowering Effects of Rosuvastatin and L. Acidophilus on Cholesterol Levels in Rat. J. Microbiol. Biotechnol. 2019, 29, 473–481. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, Q.; Ren, Y.; Ruan, Z. Effect of Probiotic Lactobacillus on Lipid Profile: A Systematic Review and Meta-Analysis of Randomized, Controlled Trials. PLoS ONE 2017, 12, e0178868. [Google Scholar] [CrossRef]
- Zhao, L.; Shen, Y.; Wang, Y.; Wang, L.; Zhang, L.; Zhao, Z.; Li, S. Lactobacillus Plantarum S9 Alleviates Lipid Profile, Insulin Resistance, and Inflammation in High-Fat Diet-Induced Metabolic Syndrome Rats. Sci. Rep. 2022, 12, 15490. [Google Scholar] [CrossRef] [PubMed]
- Xie, N.; Cui, Y.; Yin, Y.-N.; Zhao, X.; Yang, J.-W.; Wang, Z.-G.; Fu, N.; Tang, Y.; Wang, X.-H.; Liu, X.-W.; et al. Effects of Two Lactobacillus Strains on Lipid Metabolism and Intestinal Microflora in Rats Fed a High-Cholesterol Diet. BMC Complement. Altern. Med. 2011, 11, 53. [Google Scholar]
- Sugimura, T.; Jounai, K.; Ohshio, K.; Tanaka, T.; Suwa, M.; Fujiwara, D. Immunomodulatory Effect of Lactococcus Lactis JCM5805 on Human Plasmacytoid Dendritic Cells. Clin. Immunol. 2013, 149, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Sugimura, T.; Takahashi, H.; Jounai, K.; Ohshio, K.; Kanayama, M.; Tazumi, K.; Tanihata, Y.; Miura, Y.; Fujiwara, D.; Yamamoto, N. Effects of Oral Intake of Plasmacytoid Dendritic Cells-Stimulative Lactic Acid Bacterial Strain on Pathogenesis of Influenza-like Illness and Immunological Response to Influenza Virus. Br. J. Nutr. 2015, 114, 727–733. [Google Scholar] [CrossRef]
- Rysz, J.; Franczyk, B.; Ławiński, J.; Olszewski, R.; Ciałkowska-Rysz, A.; Gluba-Brzózka, A. The Impact of CKD on Uremic Toxins and Gut Microbiota. Toxins 2021, 13, 252. [Google Scholar] [CrossRef]

| Dialysis Patients Group (N = 19) | ||
|---|---|---|
| Age | year | 65.2 ± 17.6 |
| BMI | kg/m2 | 22.1 ± 4.3 |
| Sex | % of male | 58.9 |
| Dialysis period | year | 7.5 ± 7.4 |
| Height | cm | 162.6 ± 9.1 |
| Body weight | kg | 58.4 ± 11.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suzuki, Y.; Ishioka, K.; Nakamura, T.; Miyazaki, N.; Marubashi, S.; Suzutani, T. Function of Yogurt Fermented with the Lactococcus lactis 11/19-B1 Strain in Improving the Lipid Profile and Intestinal Microbiome in Hemodialysis Patients. Nutrients 2025, 17, 1931. https://doi.org/10.3390/nu17111931
Suzuki Y, Ishioka K, Nakamura T, Miyazaki N, Marubashi S, Suzutani T. Function of Yogurt Fermented with the Lactococcus lactis 11/19-B1 Strain in Improving the Lipid Profile and Intestinal Microbiome in Hemodialysis Patients. Nutrients. 2025; 17(11):1931. https://doi.org/10.3390/nu17111931
Chicago/Turabian StyleSuzuki, Yoshiki, Ken Ishioka, Taichi Nakamura, Nozomu Miyazaki, Shigeru Marubashi, and Tatsuo Suzutani. 2025. "Function of Yogurt Fermented with the Lactococcus lactis 11/19-B1 Strain in Improving the Lipid Profile and Intestinal Microbiome in Hemodialysis Patients" Nutrients 17, no. 11: 1931. https://doi.org/10.3390/nu17111931
APA StyleSuzuki, Y., Ishioka, K., Nakamura, T., Miyazaki, N., Marubashi, S., & Suzutani, T. (2025). Function of Yogurt Fermented with the Lactococcus lactis 11/19-B1 Strain in Improving the Lipid Profile and Intestinal Microbiome in Hemodialysis Patients. Nutrients, 17(11), 1931. https://doi.org/10.3390/nu17111931







