Persistent Vitamin D Deficiency in Pediatric Patients with Cystic Fibrosis
Abstract
1. Introduction
2. Materials and Methods
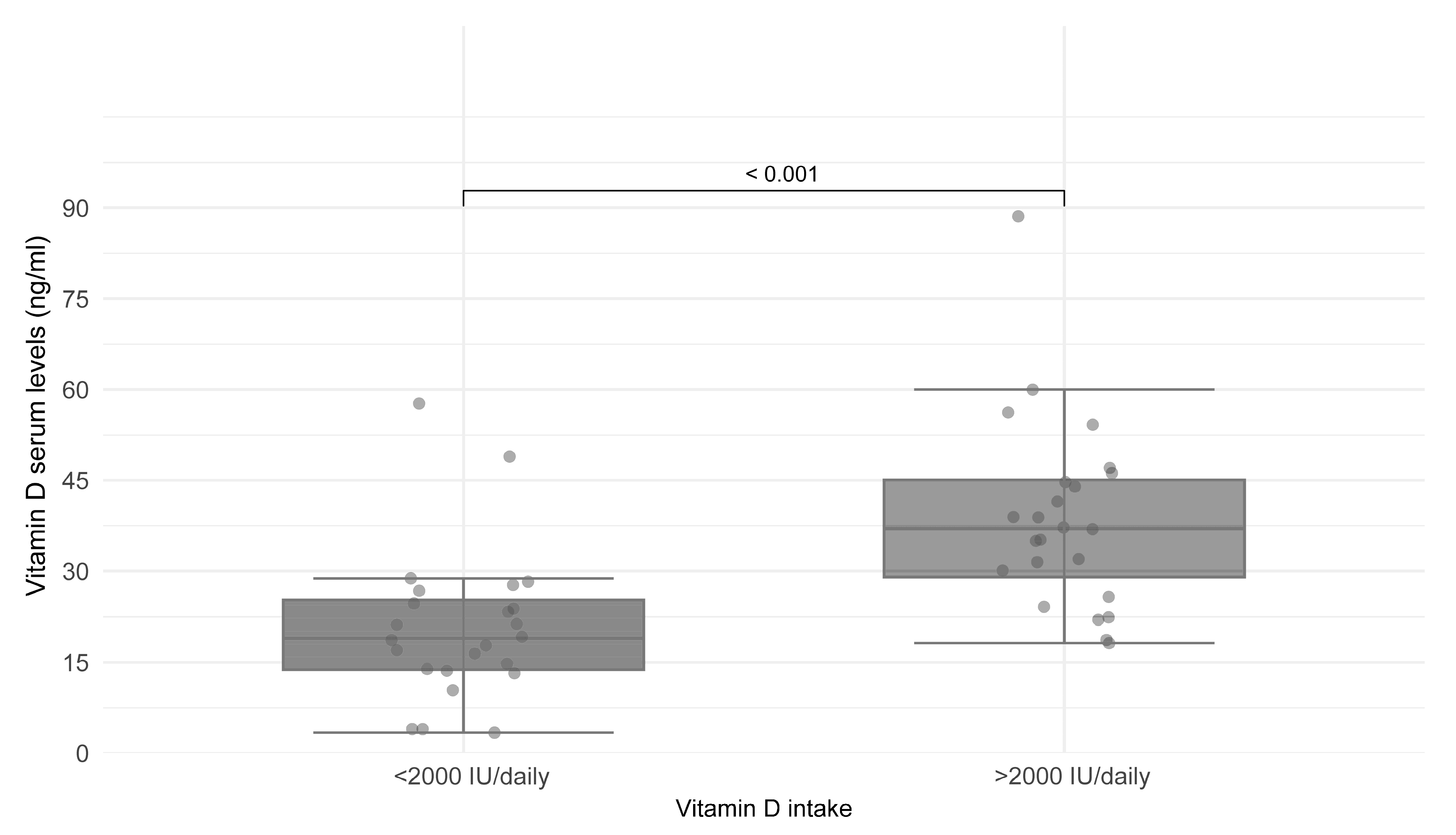
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CF | Cystic fibrosis |
| CFTR | Cystic fibrosis transmembrane conductance gene |
| VitD | Vitamin D |
| PwCF | Patients with cystic fibrosis |
| BMI | Body mass index |
| IU | International Units |
| kg | Kilograms |
| ng | Nanograms |
| mL | Milliliters |
| PERT | Pancreatic enzyme replacement therapy |
References
- Farrell, P.M.; White, T.B.; Derichs, N.; Castellani, C.; Rosenstein, B.J. Cystic Fibrosis Diagnostic Challenges over 4 Decades: Historical Perspectives and Lessons Learned. J. Pediatr. 2017, 181, S16–S26. [Google Scholar] [CrossRef] [PubMed]
- Mirtajani, S.B.; Farnia, P.; Hassanzad, M.; Ghanavi, J.; Farnia, P.; Velayati, A.A. Geographical distribution of cystic fibrosis; The past 70 years of data analyzis. Biomed. Biotechnol. Res. J. (BBRJ) 2017, 1, 105. [Google Scholar]
- Le, T.N. Updates in vitamin D therapy in cystic fibrosis. Curr. Opin. Endocrinol. Diabetes 2018, 25, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Bravo, M.P.; Balboa, P.; Torrejon, C.; Bozzo, R.; Boza, M.L.; Contreras, I.; Jorquera, P.; Astorga, L.; Weisstaub, G. Bone mineral density, lung function, vitamin D and body composition in children and adolescents with cystic fibrosis: A multicenter study. Nutr. Hosp. 2018, 35, 789–795. [Google Scholar] [CrossRef] [PubMed]
- Flores, M.E.; Rivera-Pasquel, M.; Valdez-Sánchez, A.; De la Cruz-Góngora, V.; Contreras-Manzano, A.; Shamah-Levy, T.; Villalpando, S. Vitamin D status in Mexican children 1 to 11 years of age: An update from the Ensanut 2018-19. Salud Publica Mex. 2021, 63, 382–393. [Google Scholar] [CrossRef]
- Turck, D.; Braegger, C.P.; Colombo, C.; Declercq, D.; Morton, A.; Pancheva, R.; Robberecht, E.; Stern, M.; Strandvik, B.; Wolfe, S.; et al. ESPEN-ESPGHAN-ECFS guidelines on nutrition care for infants, children, and adults with cystic fibrosis. Clin. Nutr. 2016, 35, 557–577. [Google Scholar] [CrossRef] [PubMed]
- Juhász, M.F.; Varannai, O.; Németh, D.; Szakács, Z.; Kiss, S.; Izsák, V.D.; Martonosi, Á.R.; Hegyi, P.; Párniczky, A. Vitamin D supplementation in patients with cystic fibrosis: A systematic review and meta-analysis. J. Cyst. Fibros. 2021, 20, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Iniesta, R.R.; Cook, S.; Oversby, G.; Koufaki, P.; Van der Linden, M.L.; Vlachopoulos, D.; Williams, C.A.; Urquhart, D.S. Systematic review and meta-analysis: Associations of vitamin D with pulmonary function in children and young people with cystic fibrosis. Clin. Nutr. ESPEN 2023, 54, 349–373. [Google Scholar] [CrossRef] [PubMed]
- Farahbakhsh, N.; Fatahi, S.; Shirvani, A.; Motaharifard, M.S.; Mohkam, M.; Tabatabaii, S.A.; Khanbabaee, G.; Yaghoobpoor, S.; Davoodi, S.Z.; Hosseini, A.H. Vitamin D deficiency in patients with cystic fibrosis: A systematic review and meta-analysis. J. Heal. Popul. Nutr. 2024, 43, 11. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Bhimavarapu, A.; Alvarez, J.A.; Hunt, W.R.; Tangpricha, V. Changes in bone turnover after high-dose vitamin D supplementation during acute pulmonary exacerbation in cystic fibrosis. Bone 2023, 174, 116835. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, D.G.; García, C.B.; Díaz Martin, J.J.; Treviño, S.J. Suplementacion con vitaminas liposolubles en pacientes con fibrosis quistica: ¿Es suficiente con Aquadek’s ®? Nutr. Hosp. 2015, 31, 1625–1632. [Google Scholar]
- Tangpricha, V.; Kelly, A.; Stephenson, A.; Maguiness, K.; Enders, J.; Robinson, K.A.; Marshall, B.C.; Borowitz, D. An Update on the Screening, Diagnosis, Management, and Treatment of Vitamin D Deficiency in Individuals with Cystic Fibrosis: Evidence-Based Recommendations from the Cystic Fibrosis Foundation. J. Clin. Endocrinol. Metab. 2012, 97, 1082–1093. [Google Scholar] [CrossRef] [PubMed]
- Wilschanski, M.; Munck, A.; Carrion, E.; Cipolli, M.; Collins, S.; Colombo, C.; Declercq, D.; Hatziagorou, E.; Hulst, J.; Kalnins, D.; et al. ESPEN-ESPGHAN-ECFS guideline on nutrition care for cystic fibrosis. Clin. Nutr. 2023, 43, 413–445. [Google Scholar] [CrossRef] [PubMed]
- Sermet-Gaudelus, I.; Bianchi, M.L.; Garabédian, M.; Aris, R.M.; Morton, A.; Hardin, D.S.; Elkin, S.L.; Compston, J.E.; Conway, S.P.; Castanet, M.; et al. European cystic fibrosis bone mineralisation guidelines. J. Cyst. Fibros. 2011, 10, S16–S23. [Google Scholar] [CrossRef] [PubMed]
- Ongaratto, R.; da Rosa, K.M.; Eloi, J.C.; Epifanio, M.; Marostica, P.; Pinto, L.A. Association between hypovitaminosis D and frequency of pulmonary exacerbations in children and adolescents with cystic fibrosis. Einstein 2018, 16, eAO4143. [Google Scholar] [CrossRef] [PubMed]
- Wani, W.A.; Nazir, M.; Bhat, J.I.; Malik, E.-U.; Ahmad, Q.I.; Charoo, B.A.; Ali, S.W. Vitamin D status correlates with the markers of cystic fibrosis-related pulmonary disease. Pediatr. Neonatol. 2019, 60, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Usategui-Martín, R.; De Luis-Román, D.-A.; Fernández-Gómez, J.M.; Ruiz-Mambrilla, M.; Pérez-Castrillón, J.-L. Vitamin D Receptor (VDR) Gene Polymorphisms Modify the Response to Vitamin D Supplementation: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 360. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.J.; Song, J.; Lu, Q.; Murali, S.G.; Gajapathy, M.; Wilk, B.M.; Brown, D.M.; Worthey, E.A.; Farrell, P.M. Genetic factors help explain the variable responses of young children with cystic fibrosis to vitamin D supplements. Clin. Nutr. ESPEN 2022, 51, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Braun, A.T.; Lai, H.J.; Laxova, A.; Biller, J.A.; Hubertz, E.K.; Zhao, Z.; Lu, Q.; Murali, S.; Brown, D.M.; Worthey, E.A.; et al. Vitamin D status and variable responses to supplements depend in part on genetic factors in adults with cystic fibrosis. J. Cyst. Fibros. 2024, 23, 754–757. [Google Scholar] [CrossRef] [PubMed]
| Pediatric Patients with Cystic Fibrosis | N = 48 Median [IQR] |
|---|---|
| Women, n (%) | 27 (56.3) |
| Age, months | 101.5 [68.5–140] |
| Exocrine Pancreatic Insufficiency, n (%) | 43 (89.6) |
| PERT dose, IU/kg/day | 5882.3 [4811–7272] |
| BMI Nutritional Status | |
| Normal, n (%) | 41 (85.4) |
| Undernutrition, n (%) | 5 (10.4) |
| Severe undernutrition n (%) | 2 (4.2) |
| Nutritional BMI goal achieved, n (%) | 14 (29.2) |
| Short stature, n (%) | 15 (31.3) |
| OH-25-Hidroxi-Vitamin D, ng/mL | 26.3 [18.3–38.9] |
| Vitamin D Status | |
| Sufficiency, n (%) | 20 (41.7) |
| Insufficiency, n (%) | 15 (31.3) |
| Deficiency, n (%) | 13 (27) |
| Calcium/creatinine ratio | 0.08 [0.027–0.158] |
| Use of vitamin D supplements, n (%) | 40 (83.3) |
| Duration of supplement use, months | 4 [3–6.75] |
| Vitamin D daily intake, IU | 2050 [250–4350] |
| Sufficiency N = 20 | Insufficiency N = 15 | Deficiency N = 13 | p Value | |
|---|---|---|---|---|
| PERT doses, n (%) | ||||
| <5000 IU/kg/day | 4 (20) | 7 (46.6) | 5 (38.5) | 0.208 * |
| >5000 IU/kg/day | 16 (80) | 8 (53.4) | 8 (61.5) | |
| Vitamin D intake, n (%) | ||||
| <2000 IU/day | 2 (10) | 13 (86.6) | 9 (69.2) | 0.001 * |
| >2000 IU/day | 18 (90) | 2 (13.4) | 4 (30.8) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reyes-Apodaca, M.; Lezana-Fernández, J.L.; Vázquez Frias, R.; Rendón-Macías, M.E.; González-Molina, A.; Rodríguez Espino, B.A.; Núñez-Barrera, I.; Medeiros, M. Persistent Vitamin D Deficiency in Pediatric Patients with Cystic Fibrosis. Nutrients 2025, 17, 1890. https://doi.org/10.3390/nu17111890
Reyes-Apodaca M, Lezana-Fernández JL, Vázquez Frias R, Rendón-Macías ME, González-Molina A, Rodríguez Espino BA, Núñez-Barrera I, Medeiros M. Persistent Vitamin D Deficiency in Pediatric Patients with Cystic Fibrosis. Nutrients. 2025; 17(11):1890. https://doi.org/10.3390/nu17111890
Chicago/Turabian StyleReyes-Apodaca, Magali, José L. Lezana-Fernández, Rodrigo Vázquez Frias, Mario E. Rendón-Macías, Aline González-Molina, Benjamín A. Rodríguez Espino, Isela Núñez-Barrera, and Mara Medeiros. 2025. "Persistent Vitamin D Deficiency in Pediatric Patients with Cystic Fibrosis" Nutrients 17, no. 11: 1890. https://doi.org/10.3390/nu17111890
APA StyleReyes-Apodaca, M., Lezana-Fernández, J. L., Vázquez Frias, R., Rendón-Macías, M. E., González-Molina, A., Rodríguez Espino, B. A., Núñez-Barrera, I., & Medeiros, M. (2025). Persistent Vitamin D Deficiency in Pediatric Patients with Cystic Fibrosis. Nutrients, 17(11), 1890. https://doi.org/10.3390/nu17111890






