Refining Nutritional Assessment Methods for Older Adults: A Pilot Study on Sicilian Long-Living Individuals
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
Variables
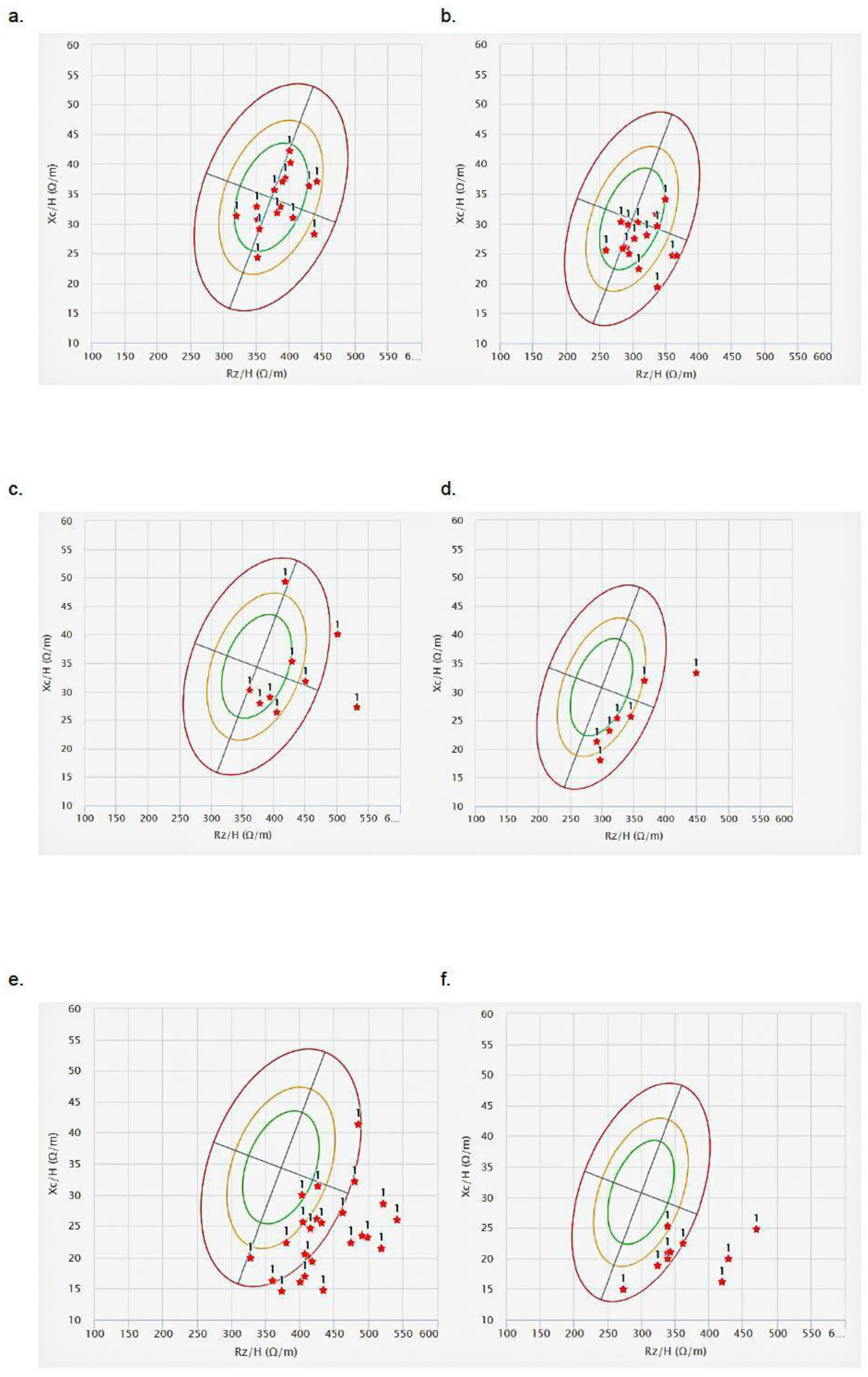
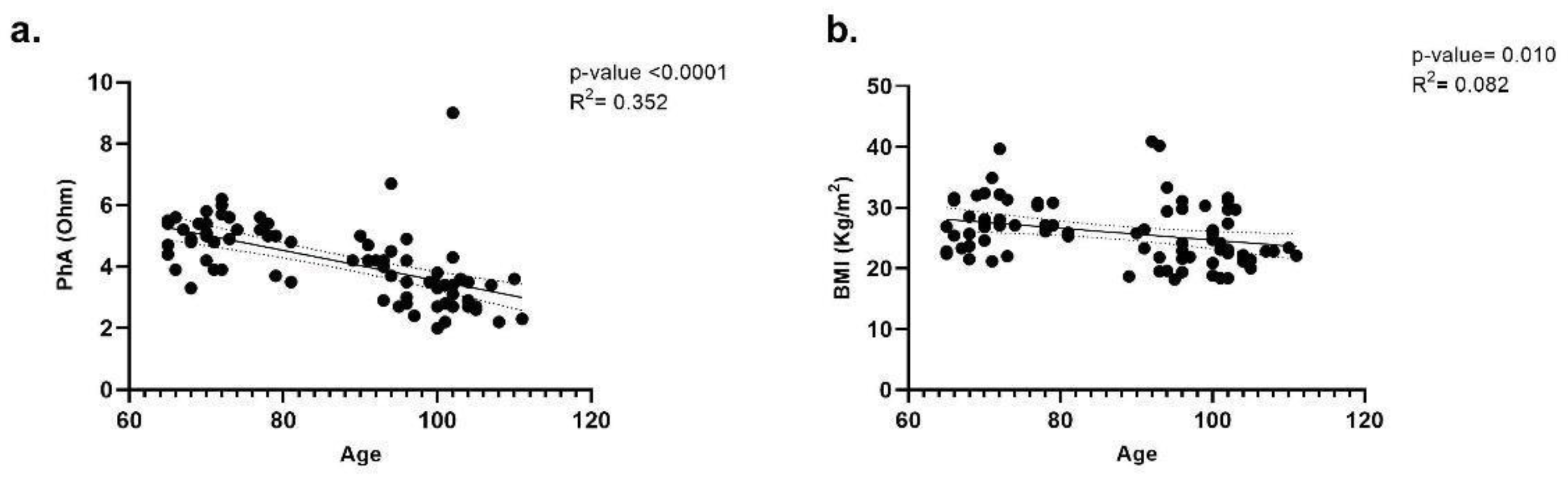
2.3. Anthropometric, BIA, and BIVA Evaluation
2.4. MNA Questionnaire
2.5. CONUT and GNRI Scores
2.6. Bias
2.7. Study Size
2.8. Statistical Analysis
3. Results
3.1. Nutritional Status Evaluation Based on Anthropometric Measures and BIA
3.2. Nutritional Status Evaluation Based on MNA Questionnaire and CONUT and GNRI Scores
4. Discussion
Study Limitations
5. Conclusions
6. Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BIA | Bioimpedance analysis. |
| MNA | Mini Nutritional Assessment. |
| CONUT | Controlling Nutritional Risk Index. |
| GNRI | Geriatric Nutritional Risk Index. |
| ARDs | Age-Related Diseases. |
| DRM | Disease-related malnutrition. |
| LLIs | Long-living individuals. |
| y.o. | Years old. |
| BMI | Body Mass Index. |
| STROBE | Strengthening the Reporting of Observational Studies in Epidemiology. |
| SD | Standard deviation. |
| MMSE | Mini Mental State Examination. |
| BIVA | Bioelectrical Impedance Vector Analysis. |
| PhA | Phase Angle. |
| Rz | Reactance. |
| Xc | Resistance. |
| H | Height. |
| WLo | Ideal Weight. |
| N | Number of individuals for each age. |
| F | Females. |
| M | Males. |
| ESPEN | European Society for Clinical Nutrition and Metabolism. |
| MUST | Malnutrition Universal Screening Tool. |
| GLIM | Global Leadership Initiative on malnutrition criteria. |
References
- Govindaraju, T.; Sahle, B.W.; McCaffrey, T.A.; McNeil, J.J.; Owen, A.J. Dietary Patterns and Quality of Life in Older Adults: A Systematic Review. Nutrients 2018, 10, 971. [Google Scholar] [CrossRef] [PubMed]
- Bruins, M.J.; Van Dael, P.; Eggersdorfer, M. The Role of Nutrients in Reducing the Risk for Noncommunicable Diseases during Aging. Nutrients 2019, 11, 85. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.B.; Silver, R.E.; Das, S.K.; Fielding, R.A.; Gilhooly, C.H.; Jacques, P.F.; Kelly, J.M.; Mason, J.B.; McKeown, N.M.; Reardon, M.A.; et al. Healthy Aging-Nutrition Matters: Start Early and Screen Often. Adv. Nutr. 2021, 12, 1438–1448. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of aging: An expanding universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef]
- Accardi, G.; Aiello, A.; Vasto, S.; Caruso, C. Bioimpedance: A New Approach for Studying Longevity. In Updates in Pathobiology: Causality and Chance in Ageing, Age-Related Diseases and Longevity; Accardi, G., Caruso, C., Eds.; Palermo University Press: Palermo, Italy, 2017; pp. 67–78. [Google Scholar]
- Poulsen, W.; Christensen, K.; Dalgård, C. Dietary patterns and survival to 100+ years: An empty systematic review of cohort and case–control studies. Arch. Public Health 2022, 80, 161. [Google Scholar] [CrossRef]
- Amarya, S.; Singh, K.; Sabharwal, M. Changes during aging and their association with malnutrition. J. Nutr. Gerontol. Geriatr. 2015, 6, 78–84. [Google Scholar] [CrossRef]
- Eurostat. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Population_structure_and_ageing (accessed on 7 May 2025).
- Nuijten, M. Malnutrition in Healthcare: Treatment Pays Off—A Health-Economic Analysis of Malnutrition in the Netherlands; A2M Consultancy: Amsterdam, The Netherlands, 2024. [Google Scholar]
- Pradelli, L.; Zaniolo, O.; Sanfilippo, A.; Lezo, A.; Riso, S.; Zanetti, M. Prevalence and economic cost of malnutrition in Italy: A systematic review and metanalysis from the Italian Society of Artificial Nutrition and Metabolism (SINPE). Nutrition 2023, 108, 111943. [Google Scholar] [CrossRef]
- World Health Organization. Regional Office for Europe. Disease-Related Malnutrition: A Time for Action; World Health Organization Regional Office for Europe: Copenhagen, Denmark, 2023. Available online: https://iris.who.int/handle/10665/375033 (accessed on 7 May 2025).
- Montesanto, A.; De Rango, F.; Pirazzini, C.; Guidarelli, G.; Domma, F.; Franceschi, C.; Passarino, G. Demographic, genetic and phenotypic characteristics of centenarians in Italy: Focus on gender differences. Mech. Aging Dev. 2017, 165, 68–74. [Google Scholar] [CrossRef]
- Aiello, A.; Accardi, G.; Aprile, S.; Caldarella, R.; Carru, C.; Ciaccio, M.; De Vivo, I.; Gambino, C.M.; Ligotti, M.E.; Vasto, S.; et al. Age and Gender-related Variations of Molecular and Phenotypic Parameters in A Cohort of Sicilian Population: From Young to Centenarians. Aging Dis. 2021, 12, 1773–1793. [Google Scholar] [CrossRef]
- Vellas, B.; Guigoz, Y.; Garry, P.J.; Nourhashemi, F.; Bennahum, D.; Lauque, S.; Albarede, J.L. The Mini Nutritional Assessment (MNA) and its use in grading the nutritional state of elderly patients. Nutrition 1999, 15, 116–122. [Google Scholar] [CrossRef]
- Lukaski, H.C.; Bolonchuk, W.W.; Hall, C.B.; Siders, W.A. Validation of tetrapolar bioelectrical impedance method to assess human body composition. J. Appl. Physiol. 1986, 60, 1327–1332. [Google Scholar] [CrossRef] [PubMed]
- Ignacio de Ulíbarri, J.; González-Madroño, A.; de Villar, N.G.; González, P.; González, B.; Mancha, A.; Rodríguez, F.; Fernández, G. CONUT: A tool for controlling nutritional status. First validation in a hospital population. Nutr. Hosp. 2005, 20, 38–45. [Google Scholar] [PubMed]
- Bouillanne, O.; Morineau, G.; Dupont, C.; Coulombel, I.; Vincent, J.P.; Nicolis, I.; Benazeth, S.; Cynober, L.; Aussel, C. Geriatric Nutritional Risk Index: A new index for evaluating at-risk elderly medical patients. Am. J. Clin. Nutr. 2005, 82, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Saragat, B.; Buffa, R.; Mereu, E.; De Rui, M.; Coin, A.; Sergi, G.; Marini, E. Specific bioelectrical impedance vector reference values for assessing body composition in the Italian elderly. Exp. Gerontol. 2014, 50, 52–56. [Google Scholar] [CrossRef]
- Barbosa-Silva, M.C.; Barros, A.J.; Wang, J.; Heymsfield, S.B.; Pierson, R.N., Jr. Bioelectrical impedance analysis: Population reference values for phase angle by age and sex. Am. J. Clin. Nutr. 2005, 82, 49–52. [Google Scholar] [CrossRef]
- Foster, K.R.; Lukaski, H.C. Whole-body impedance—what does it measure? Am. J. Clin. Nutr. 1996, 64, 388S–396S. [Google Scholar] [CrossRef]
- Bera, T.K. Bioelectrical Impedance Methods for Noninvasive Health Monitoring: A Review. J. Med. Eng. 2014, 2014, 381251. [Google Scholar] [CrossRef]
- Piccoli, A.; Pittoni, G.; Facco, E.; Favaro, E.; Pillon, L. Relationship between central venous pressure and bioimpedance vector analysis in critically ill patients. Crit. Care Med. 2000, 28, 132–137. [Google Scholar] [CrossRef]
- Miller, J.; Wells, L.; Nwulu, U.; Currow, D.; Johnson, M.J.; Skipworth, R.J.E. Validated screening tools for the assessment of cachexia, sarcopenia, and malnutrition: A systematic review. Am. J. Clin. Nutr. 2018, 108, 1196–1208. [Google Scholar] [CrossRef]
- Lin, Y.M.; Wang, M.; Sun, N.X.; Liu, Y.Y.; Yin, T.F.; Chen, C. Screening and application of nutritional support in elderly hospitalized patients of a tertiary care hospital in China. PLoS ONE 2019, 14, e0213076. [Google Scholar] [CrossRef]
- Toyokawa, T.; Kubo, N.; Tamura, T.; Sakurai, K.; Amano, R.; Tanaka, H.; Muguruma, K.; Yashiro, M.; Hirakawa, K.; Ohira, M. The pretreatment Controlling Nutritional Status (CONUT) score is an independent prognostic factor in patients with resectable thoracic esophageal squamous cell carcinoma: Results from a retrospective study. BMC Cancer 2016, 16, 722. [Google Scholar] [CrossRef] [PubMed]
- BIVA SOFTWARE 2002. Available online: http://www.renalgate.it/formule_calcolatori/BIVAguide.pdf (accessed on 2 April 2025).
- Kondrup, J.; Allison, S.P.; Elia, M.; Vellas, B.; Plauth, M.; Educational and Clinical Practice Committee, European Society of Parenteral and Enteral Nutrition (ESPEN). ESPEN guidelines for nutrition screening 2002. Clin. Nutr. 2003, 22, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Mattavelli, E.; Verduci, E.; Mascheroni, A.; Corradi, E.; Da Prat, V.; Ammoni, E.; Cereda, D.; Scardoni, A.; Amorosi, A.; Caccialanza, R.; et al. Toward a Pragmatic Multidisciplinary Management of Nutritional Risk in Hospitalized Patients: Initiatives and Proposals of the Clinical Nutrition Network of Lombardy Region. Nutrients 2025, 17, 1472. [Google Scholar] [CrossRef]
- Starace, E.; De Pasquale, G.; Morenghi, E.; Crippa, C.; Matteucci, S.; Pieri, G.; Soekeland, F.; Gibbi, S.M.; Lo Cricchio, G.; Reggiani, F.; et al. Hospital Malnutrition in the Medicine and Neurology Departments: A Complex Challenge. Nutrients 2023, 15, 5061. [Google Scholar] [CrossRef] [PubMed]
- Santomauro, F.; Olimpi, N.; Baggiani, L.; Comodo, N.; Mantero, S.; Bonaccorsi, G. Bioelectrical Impedance Vector Analysis and Mini Nutritional Assessment in elderly nursing home residents. J. Nutr. Health Aging 2011, 15, 163–167. [Google Scholar] [CrossRef]
- Nwosu, A.C.; Mayland, C.R.; Mason, S.; Cox, T.F.; Varro, A.; Stanley, S.; Ellershaw, J. Bioelectrical impedance vector analysis (BIVA) as a method to compare body composition differences according to cancer stage and type. Clin. Nutr. ESPEN 2019, 30, 59–66. [Google Scholar] [CrossRef]
- Bosello, O.; Vanzo, A. Obesity paradox and aging. Eat Weight Disord. 2021, 26, 27–35. [Google Scholar] [CrossRef]
- Dramé, M.; Godaert, L. The Obesity Paradox and Mortality in Older Adults: A Systematic Review. Nutrients 2023, 15, 1780. [Google Scholar] [CrossRef]
- Accardi, G.; Ligotti, M.E.; Candore, G. Phenotypic Aspects of Longevity. In Centenarians: An Example of Positive Biology; Caruso, C., Ed.; Springer Nature: Berlin, Germany, 2019; pp. 23–34. [Google Scholar]
- Pereira da Silva, A.; Matos, A.; Valente, A.; Gil, Â.; Alonso, I.; Ribeiro, R.; Bicho, M.; Gorjão-Clara, J. Body Composition Assessment and Nutritional Status Evaluation in Men and Women Portuguese Centenarians. J. Nutr. Health Aging 2016, 20, 256–266. [Google Scholar] [CrossRef]
- Yalin, S.F.; Gulcicek, S.; Avci, S.; Erkalma Senates, B.; Altiparmak, M.R.; Trabulus, S.; Alagoz, S.; Yavuzer, H.; Doventas, A.; Seyahi, N. Single-frequency and multi-frequency bioimpedance analysis: What is the difference? Nephrology 2018, 23, 438–445. [Google Scholar] [CrossRef]
- Marini, E.; Buffa, R.; Gobbo, L.A.; Salinas-Escudero, G.; Stagi, S.; García-Peña, C.; Sánchez-García, S.; Carrillo-Vega, M.F. Interpopulation Similarity of Sex and Age-Related Body Composition Variations Among Older Adults. Int. J. Environ. Res. Public Health 2020, 17, 6047. [Google Scholar] [CrossRef]
- Takayama, A.; Ishizaka, M.; Kubo, A.; Sato, M.; Yakabi, A.; Tsukahara, S.; Numaguchi, S.; Ishii, H. Association between phase angle and level of independence in daily living among institutionalized super older females requiring nursing care. J. Phys. Ther. Sci. 2024, 36, 526–529. [Google Scholar] [CrossRef]
- Eyre, S.; Stenberg, J.; Wallengren, O.; Keane, D.; Avesani, C.M.; Bosaeus, I.; Clyne, N.; Heimbürger, O.; Indurain, A.; Johansson, A.C.; et al. Bioimpedance analysis in patients with chronic kidney disease. J. Ren. Care 2023, 49, 147–157. [Google Scholar] [CrossRef]
- Guigoz, Y.; Lauque, S.; Vellas, B.J. Identifying the elderly at risk for malnutrition. The Mini Nutritional Assessment. Clin. Geriatr. Med. 2002, 18, 737–757. [Google Scholar] [CrossRef]
- Kozáková, R.; Zeleníková, R. Assessing the nutritional status of the elderly living at home. Eur. Geriatr. Med. 2014, 5, 377–381. [Google Scholar] [CrossRef]
- Nishi, I.; Seo, Y.; Hamada-Harimura, Y.; Yamamoto, M.; Ishizu, T.; Sugano, A.; Sato, K.; Sai, S.; Obara, K.; Suzuki, S.; et al. Geriatric nutritional risk index predicts all-cause deaths in heart failure with preserved ejection fraction. ESC Heart Fail. 2019, 6, 396–405. [Google Scholar] [CrossRef]
- Wang, Q.Q.; Zheng, G.Q.; Yang, D.L.; Liang, Y.F.; Yin, W.J.; Su, S.S. Pretreatment Controlling Nutritional Status Score and Lactate Dehydrogenase as Predictive Markers of Survival in Patients with Malignant Peritoneal Mesothelioma. Nutr. Cancer 2018, 70, 1264–1274. [Google Scholar] [CrossRef]
- Don, B.R.; Kaysen, G. Serum albumin: Relationship to inflammation and nutrition. Semin. Dial. 2004, 17, 432–437. [Google Scholar] [CrossRef]
- Sergi, G.; Coin, A.; Enzi, G.; Volpato, S.; Inelmen, E.M.; Buttarello, M.; Peloso, M.; Mulone, S.; Marin, S.; Bonometto, P. Role of visceral proteins in detecting malnutrition in the elderly. Eur. J. Clin. Nutr. 2006, 60, 203–209. [Google Scholar] [CrossRef]
- Keller, U. Nutritional Laboratory Markers in Malnutrition. J. Clin. Med. 2019, 8, 775. [Google Scholar] [CrossRef]
- Lidoriki, I.; Schizas, D.; Frountzas, M.; Machairas, N.; Prodromidou, A.; Kapelouzou, A.; Karavokyros, I.; Pikoulis, E.; Kales, S.N.; Liakakos, T. GNRI as a Prognostic Factor for Outcomes in Cancer Patients: A Systematic Review of the Literature. Nutr. Cancer 2021, 73, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Su, W.T.; Tsai, C.H.; Huang, C.Y.; Chou, S.E.; Li, C.; Hsu, S.Y.; Hsieh, C.H. Geriatric Nutritional Risk Index as a Prognostic Factor for Mortality in Elderly Patients with Moderate to Severe Traumatic Brain Injuries. Risk Manag. Heal. Policy 2021, 14, 2465–2474. [Google Scholar] [CrossRef] [PubMed]
- Miano, N.; Di Marco, M.; Alaimo, S.; Coppolino, G.; L’Episcopo, G.; Leggio, S.; Scicali, R.; Piro, S.; Purrello, F.; Di Pino, A. Controlling Nutritional Status (CONUT) Score as a Potential Prognostic Indicator of In-Hospital Mortality, Sepsis and Length of Stay in an Internal Medicine Department. Nutrients 2023, 15, 1554. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wang, S.; Liu, W.; Han, K.; Jia, W.; Liu, M.; He, Y. Malnutrition Is an Independent Risk Factor for Low Health-Related Quality of Life Among Centenarians. Front. Med. 2021, 8, 729928. [Google Scholar] [CrossRef]
- Lozada-Martinez, I.D.; Vindas-Meza, L.; Castelblanco-Toro, S.; Salazar-Uribe, J.C.; Anaya, J. The impact of nutritional status on centenarians’ physical, mental, and functional health. Clin. Nutr. Open Sci. 2025, 60, 10–20. [Google Scholar] [CrossRef]
- Formiga, F.; Ferrer, A.; De Ulíbarri Pérez, J.; Badia, T.; Montero, A.; Soldevila, L.; Moreno, R.; Corbella, X. Detecting malnutrition and predicting mortality in the Spanish oldest old: Utility of the Controlling Nutritional Status (CONUT) score compared with the Mini Nutritional Assessment (MNA) score. Eur. Geriatr. Med. 2016, 7, 566–570. [Google Scholar] [CrossRef]
- Lo Buglio, A.; Bellanti, F.; Carmignano, D.F.; Serviddio, G.; Vendemiale, G. Association between Controlling Nutritional Status (CONUT) Score and Body Composition, Inflammation and Frailty in Hospitalized Elderly Patients. Nutrients 2023, 16, 576. [Google Scholar] [CrossRef]
- Uemura, Y.; Shibata, R.; Miyagaki, Y.; Takemoto, K.; Ishikawa, S.; Murohara, T.; Watarai, M. A Comparative Study of Three Nutritional Risk/Screening Indices for Predicting Cardiac Events and Physical Functioning Among Patients with Acute Heart Failure. Int. Heart J. 2022, 63, 541–549. [Google Scholar] [CrossRef]

| Age Groups | p-Value | |||
|---|---|---|---|---|
| 65–80 N = 33 F/M = 16/17 | 81–94 N = 13 F/M = 8/5 | ≥95 N = 34 F/M = 24/10 | ||
| BMI (kg/m2) ± SD | 27.65 ± 4.2 | 26.93 ± 3.9 | 23.91 ± 4.02 | 0.006 (65–80 vs. ≥95) |
| Underweight (%) | 0 | 0 | 6 | |
| Normal weight (%) | 27 | 38 | 68 | |
| Overweight (%) | 39 | 38 | 15 | |
| Obesity (%) | 33 | 8 | 12 | |
| Extreme obesity (%) | 0 | 15 | 0 | |
| Age Groups | |||
|---|---|---|---|
| 65–80 N = 33 F/M = 16/17 | 81–94 N = 13 F/M = 8/5 | ≥95 N = 34 F/M = 24/10 | |
| MNA | |||
| Well fed (%) | 88 | 23 | 26 |
| At risk of malnutrition (%) | 12 | 69 | 65 |
| Malnourished (%) | 0 | 8 | 9 |
| GNRI | |||
| No risk (%) | 100 | 62 | 53 |
| Moderate risk (%) | 0 | 0 | 3 |
| Low risk (%) | 0 | 38 | 44 |
| CONUT | |||
| Normal (%) | 82 | 61.5 | 53 |
| Moderate (%) | 0 | 7.5 | 3 |
| Light (%) | 18 | 31 | 44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aiello, A.; Calabrò, A.; Zarcone, R.; Caruso, C.; Candore, G.; Accardi, G. Refining Nutritional Assessment Methods for Older Adults: A Pilot Study on Sicilian Long-Living Individuals. Nutrients 2025, 17, 1873. https://doi.org/10.3390/nu17111873
Aiello A, Calabrò A, Zarcone R, Caruso C, Candore G, Accardi G. Refining Nutritional Assessment Methods for Older Adults: A Pilot Study on Sicilian Long-Living Individuals. Nutrients. 2025; 17(11):1873. https://doi.org/10.3390/nu17111873
Chicago/Turabian StyleAiello, Anna, Anna Calabrò, Rosa Zarcone, Calogero Caruso, Giuseppina Candore, and Giulia Accardi. 2025. "Refining Nutritional Assessment Methods for Older Adults: A Pilot Study on Sicilian Long-Living Individuals" Nutrients 17, no. 11: 1873. https://doi.org/10.3390/nu17111873
APA StyleAiello, A., Calabrò, A., Zarcone, R., Caruso, C., Candore, G., & Accardi, G. (2025). Refining Nutritional Assessment Methods for Older Adults: A Pilot Study on Sicilian Long-Living Individuals. Nutrients, 17(11), 1873. https://doi.org/10.3390/nu17111873







