Abstract
Background: The association between high-sensitivity C-reactive protein (hsCRP) and activities of daily living (ADL) disability remains unclear. Our study aimed to comprehensively explore the relationship between hsCRP concentrations and the risk of ADL disability, while also identifying potential modifiers of this association in middle-aged and older adults. Methods: We conducted a prospective study involving 16,342 participants aged 50 years and older (mean age: 64 ± 10 years) from the Health and Retirement Study. To investigate the longitudinal association between hsCRP and the risk of ADL disability, we employed Cox proportional hazard regression models, adjusting for a wide range of potential confounders. Subgroups analyses were further conducted to examine interactions across factors such as gender, age, body mass index, smoking status, and drinking status. Results: This study involved a follow-up of 125,858 person-years (median of 8 years; interquartile range: 4–12 years), revealing a total of 4579 incidents of ADL disability. The highest hsCRP concentration was significantly associated with ADL disability after adjustment for covariates (hazard ratio [HR] = 1.25; confidence interval [CI] = 1.14–1.36). The associations between hsCRP and the risk of ADL disability seemed to be somewhat stronger among individuals aged < 65 years and with a BMI ≥ 30 kg/m2 (both p for interaction < 0.05). Conclusions: Our findings indicated that elevated hsCRP concentrations are associated with an increased risk of ADL disability in middle-aged and older adults. HsCRP appears to serve as a biomarker for ADL disability, particularly among individuals with obesity and middle-aged adults.
1. Introduction
High-sensitivity C-reactive protein (hsCRP) is an acute-phase protein synthesized by the liver during inflammatory responses [1]. It has emerged as a reliable biomarker for systemic inflammation due to its relatively stable concentrations, cost-effective detection, and high sensitivity [2]. Previous research has indicated that hsCRP is associated with various adverse health outcomes, including an increased risk of mortality, cardiovascular disease, and higher rates of disability [3,4,5,6].
Physical functional impairment is one of the most common health problems among the elderly in the United States, imposing a considerable burden on both individuals and society [7]. Specifically, activities of daily living (ADL) disability, as the core manifestation of physical functional impairment, has become a crucial factor affecting the quality of life of the elderly [8]. ADL disability is defined as the inability of an individual to independently perform basic tasks in daily life, including essential activities such as dressing, eating, doing laundry, and walking [9]. Current studies mainly focus on the clinical state of ADL disability, which is irreversible and leaves little opportunity for interventions to delay the process; it is valuable to explore the predictable factors to delay the onset and reduce the severity of ADL disability. Recent studies have demonstrated that elevated hsCRP concentrations are associated with the pathogenesis of sarcopenia, frailty, and functional limitations, which are critical determinants of ADL disability [6,10,11]. Despite several investigations into the association between hsCRP and the risk of ADL disability, the findings remain inconsistent [12,13,14,15,16]. For instance, a cross-sectional study conducted among elderly individuals in Chinese communities revealed an inverse association between serum hsCRP and ADL performance (n < 3500) [12]. In addition, a cohort study conducted among a community-residing population aged 70 and older explored the relationship between hsCRP and ADL decline, showing that elevated hsCRP levels were associated with an increased risk of ADL disability (n = 624) [13]. Another study involving 2610 men and women aged 65 and older identified a link between hsCRP levels and ADL disability; however, this association was not significant in women [17]. Furthermore, prior studies have demonstrated a significant positive link between obesity and inflammatory markers like hsCRP [18,19]. Research also indicates that the body’s inflammatory response capability declines with age, leading to notable differences in hsCRP levels across age groups [20]. However, there are certain limitations in the current related research. Most studies adopt a cross-sectional research design, and the sample sizes are generally small. Several confounding factors, such as chronic diseases and lifestyles choices, have not been adequately controlled, which are highly likely to interfere with the determination of the relationship between hsCRP and ADL performance. Additionally, there is a lack of understanding regarding whether the associations between hsCRP and ADL disability differ based on the body mass index (BMI), gender, or age subgroups within population studies.
Therefore, we conducted a prospective cohort study using data from the 2006–2016 Health and Retirement Study (HRS) community cohort. By controlling for confounding factors from multiple aspects, we aimed to explore the association between hsCRP and the risk of ADL disability in individuals aged 50 and above. Additionally, we performed a series of subgroup analyses to explore the modifying effects of various factors, such as age, BMI, smoking status, and drinking status, among others.
2. Methods and Materials
2.1. Study Design and Population
This study used data from the HRS, a nationally representative, community-based, prospective cohort study sample of the United States population aged 50 and older. Information on the study participants and its design has been reported before [19]. In brief, interviews with the participants were conducted in 1992 and then repeated at two-year intervals. From 1994 to 2016, six new groups of participants were incorporated in stages. Commencing in 2006, as part of the HRS, an upgraded face-to-face interview, which involved a biomarker assessment, was carried out. In all the HRS research activities, the Declaration of Helsinki guidelines were adhered to, and procedures were approved by the University of Michigan Health Sciences/Behavioral Sciences Institutional Review Board (IRB) (Protocol: HUM00061128). Before enrolling any participants, written informed consent was collected.
In the present study, we used data from the 2006 to 2016 waves of the HRS. The participants who had missing data on hsCRP and those with hsCRP concentrations of greater than 10 were excluded due to association with acute illness and major trauma; furthermore, individuals with limitations in any of the six ADLs at the baseline were not included in the study. A total of 16,342 individuals, comprising 7178 men and 9164 women, were deemed eligible for inclusion in the study. Figure 1 presents a flowchart of participant enrollment.
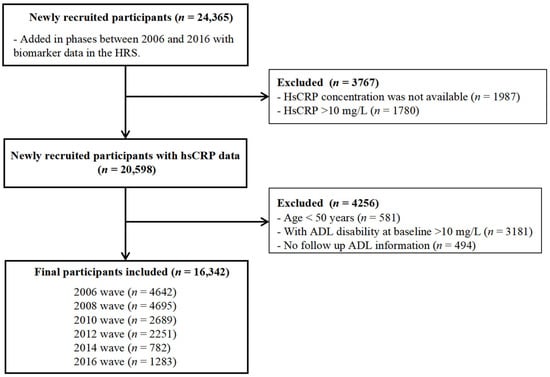
Figure 1.
Flowchart of the sample selection process.
2.2. Measurement of hsCRP
The plasma hsCRP concentration was measured in serum using a latex-particle-enhanced immunoturbidimetric assay kit. The minimum detectable value for the hsCRP concentration stood at 0.035 mg/L. The within-assay variability was 8.1%, and the between-assay variability was 11.0%. All the participants were categorized into four groups according to quartiles. These quartiles were defined as follows: quartile 1 (Q1), with values of less than 0.61 mg/L; quartile 2 (Q2), ranging from 0.61 to 1.29 mg/L; quartile 3 (Q3), from 1.30 to 2.73 mg/L; and quartile 4 (Q4), with values of greater than 2.73 mg/L.
2.3. Assessment of ADL Disability
The ability to perform ADLs was ascertained by a questionnaire that asked the participants of the HRS whether they had any difficulty with the following six tasks: (1) dressing, including putting on shoes and socks; (2) eating, such as cutting your food; (3) using the toilet, such as getting up; (4) bathing or showering; (5) getting into or out of bed; and (6) walking across a room. The respondents were asked to exclude any difficulties expected to last less than 3 months. Following previous studies [21,22], we dichotomized ADL disability into “no limitation = 0” or “at least one limitation = 1”. All the respondents were free of ADL disability at baseline. The participants were defined as having “ADL disability” if one or more limitations emerged during the follow-up period.
2.4. Covariates
We selected a wide range of potential confounders for inclusion in this study by drawing on evidence from prior epidemiological investigations [23,24]. Covariates encompassed sociodemographic details such as age, sex, and race/ethnicity. Lifestyle-related covariates, such as the body mass index (BMI), smoking status, and drinking status, were included. Clinical measures included the concentrations of total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and hemoglobin A1c (HbA1c). Additionally, the score from the 8-item Center for Epidemiologic Studies Depression Scale (CES-D 8) was included. Moreover, chronic conditions, like hypertension, diabetes, cancer, and arthritis, were among the covariates. The BMI was calculated from body weight and height measurements calculated by trained personnel. A BMI < 18.5 kg/m2 was defined as low, a BMI between 18.5 and 24.9 kg/m2 was considered normal, a BMI between 25.0 and 29.9 kg/m2 was classified as overweight, and a BMI ≥ 30.0 kg/m2 was regarded as obese, according to the World Health Organization [25]. The entirety of the data associated with covariates was procured from the structured questionnaire and the biochemistry examinations that were executed at the baseline. The original contributions presented in the study are publicly available. These data can be found here (http://hrsonline.isr.umich.edu, accessed on 25 September 2024).
2.5. Statistical Analysis
Descriptive data were used to summarize the participant characteristics, with continuous variables presented as the means (standard deviation, SD) and categorical variables presented as counts (percentage) stratified by hsCRP quartiles. Cox proportional hazard models were applied to estimate hazard ratios (HRs), and 95% confidence intervals (95% CIs) were applied to estimate the risk of ADL disability according to the hsCRP quartiles, using the lowest quartile as the reference. We also evaluated the HRs for the risk of ADL disability for each 1 mg/L increase in hsCRP. To evaluate potential nonlinear associations, restricted cubic splines (RCSs) with 3 knots were incorporated into the Cox models, and the linearity assumption was tested via likelihood ratio tests comparing the models with linear and spline terms. We used two Cox hazard regression models, which were adjusted for different sets of variables and evaluated using Schoenfeld residual plots. Model 1 tested the association between hsCRP and the risk of ADL disability controlled for age and sex; Model 2 further adjusted for ethnicity, BMI, smoking status, drinking status, regular exercise, HDL-C, TC, HbA1c, CES-D 8 scores, hypertension, diabetes, cancer, and arthritis. To increase the statistical power, we utilized the multiple imputation through chained equations to fill in the missing covariate data [26]. The effect modifications of the associations between each 1 mg/L increase in hsCRP and the risk of ADL disability by sex (men or women), age (<65 or ≥65 years), BMI (obese or non-obese), current smoking status, and current drinking status were assessed by calculating and comparing likelihood ratios for their statistical fit with interaction terms in the multivariable adjusted models. To address any potential Type I error from multiple testing, we adjusted p-values for interaction effects in the subgroup analyses using two methods: (1) Bonferroni correction (dividing α = 0.05 by 2, the number of tests); (2) False Discovery Rate (FDR) control via Benjamini-Hochberg method.
To ensure the robustness of our findings, we performed a series of sensitivity analyses, including excluding all the participants who died within the 2 years prior to follow-up; stratifying individuals by tertiles, quintiles, and clinically relevant categories of hsCRP [27]. All the analyses were conducted using R 4.3.2, and a significant difference was defined as p < 0.05.
3. Results
3.1. Baseline Characteristics
Table 1 provides the details of the participants’ characteristics, with the participants stratified by the quartiles of hsCRP measured at baseline. We followed a cohort of 16,342 participants, with a median age of 64 years old (standard deviation: 10 years), of whom 56% were women. The median value of hsCRP was 1.27 mg/L. The participants with higher hsCRP levels were more likely to be women, of white ethnicity, and current drinkers and to have a higher BMI (all p < 0.05). Compared to the participants without limitation, those who with ADL disability were older, predominantly female, engaged in less regular exercise, and had higher incidences of hypertension, diabetes, cancer, and arthritis (all p < 0.05, Table S1).

Table 1.
Characteristics of study participants.
3.2. HsCRP and the Risk of ADL Disability
The total number of person-years comprising the follow-up was 125,858, and the median follow-up time was 8 years (interquartile range [IQR] = 4–12 years); we recorded 4579 incidents of ADL disability events. The multivariable-adjusted HRs with 95% CIs for ADL disability from the lowest to the highest quartiles of the hsCRP level were 1 (reference), 1.08 (95% CI, 0.99–1.17), 1.24 (95% CI, 1.14–1.35), and 1.52 (95% CI, 1.40–1.65) (p for trend < 0.001) (Table 2). Further analysis demonstrated that each 1 mg/L increment of hsCRP was significantly associated with a 3% increased risk of ADL disability, with an HR (95% CI) of 1.03 (1.01–1.04) (Figure 2). Additionally, a nonlinear and positive association between the hsCRP concentration and the risk of incident ADL disability events was identified using a restricted cubic spline regression (p for nonlinearity < 0.001) (Figure 3).

Table 2.
HRs (95% CI) for ADL disability stratified by baseline hsCRP concentration quartiles.
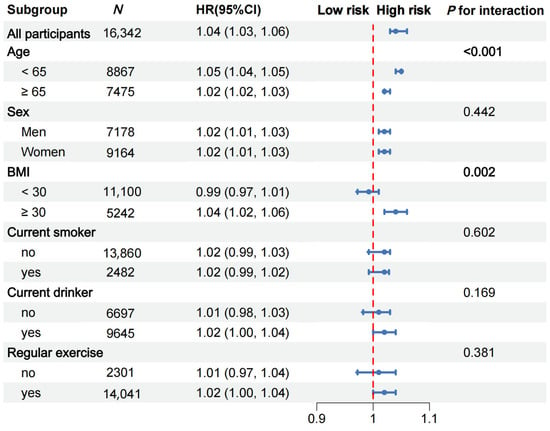
Figure 2.
Subgroup analyses for the hazard ratios (HRs) of ADL disability. Adjusted for age, sex, race, current smoking status, current drinking status, regular exercise, BMI, TC, HDL-C, HbA1c, CES-D 8 score, hypertension, diabetes, cancer, and arthritis.
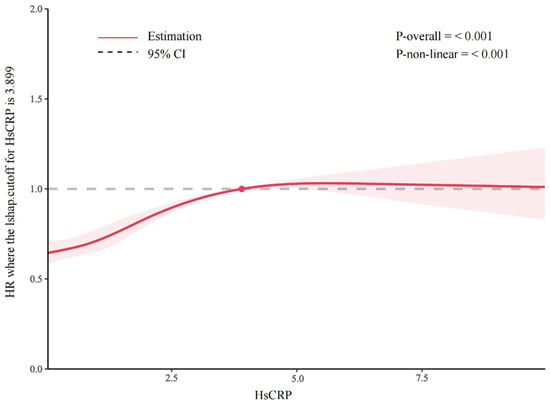
Figure 3.
Hazard ratios (HRs) of hsCRP concentration on ADL disability. The results are from the restricted cubic spline Cox proportional hazard regression model.
3.3. Subgroup Analyses
Subgroup and interaction analyses were performed to identify potential modifying factors. The positive associations between hsCRP and the risk of ADL disability were stronger among those aged < 65 years (p for interaction < 0.001, Figure 2) and those with a BMI ≥ 30 kg/m2 (p for interaction = 0.002; Figure 2). After considering multiple comparisons, the interactions between hsCRP and age, as well as between the CRP and BMI groups, remain significant (p < 0.05) under both the Bonferroni correction and the FDR control (Table S6). However, no significant interaction effects were found for the other four predefined subgroups (all p for interactions > 0.05).
3.4. Sensitivity Analyses
The associations between hsCRP and ADL disability remained consistent when participants who died within 2 years of follow-up were excluded (Table S2). Moreover, when categorizing hsCRP levels as tertiles or quintiles, the results were similar (Tables S3 and S4), and when using clinically relevant categories (Table S5) based on hsCRP, the results remained materially unchanged.
4. Discussion
In this prospective cohort study, we observed that elevated levels of hsCRP were associated with a higher incidence of ADL disability among middle-aged and elderly individuals. Even after thorough adjustment for sociodemographic factors, metabolic biomarkers, lifestyle choices, and chronic disease history, these associations persisted as robust, suggesting that hsCRP levels may play an independent role in the decline of ADL performance. Moreover, our findings highlight that the risk of hsCRP contributing to ADL disability is significantly heightened among obese individuals and those aged under 65 years.
Several studies have assessed the relationship between hsCRP and ADL disability events, and most indicate that individuals who have higher hsCRP levels are likely to have deficits in ADL performance [12,13,14,15,16,28]. Moreover, a prospective study conducted in a Japanese population indicates that hsCRP is favorably associated with physical performance, even within a very low range (<1.0 mg/L) [15]. In addition, these associations have also been found in special populations. In chronic kidney disease patients, higher hsCRP levels increase the risk of ADL disability [29]. Among stroke survivors, baseline hsCRP levels predict ADL recovery [6]. In elderly patients with cognitive impairment, elevated hsCRP is linked to faster ADL decline. By overcoming the sample size and design limitations of previous studies, our study also found a significant association between elevated hsCRP levels and the risk of ADL disability using a large, community-based cohort of middle-aged and older adults. These findings affirm the significant utility of hsCRP, a highly sensitive and readily measurable biomarker, in the early prediction and detection of ADL disability [30]. By identifying individuals with elevated hsCRP levels at an early stage, healthcare professionals can implement evidence-based, proactive interventions. These interventions may encompass personalized exercise regimens designed to enhance physical function, targeted nutritional counseling aimed at modulating inflammatory pathways, and weight management programs aimed at halting the progression of functional impairment and potentially deferring or even preventing the onset of ADL disability.
Moreover, our study found that the association between hsCRP and ADL disability was more pronounced in individuals with obesity. Prior studies have indicated that obesity is known to relate to the length of a life with a disability before death or an increase in the severity of the disability occurs [31,32]. Similarly, a study noted that obese individuals with elevated inflammatory levels experience a faster decline in muscle strength and physical function compared to non-obese individuals, thereby increasing the risk of impaired physical performance [33]. A potential explanation for this is that excessive energy intake may lead the body into a pro-inflammatory state, amplifying the adverse effects of inflammation on physical function and significantly raising the risk of ADL disability [34]. While some studies suggest a higher risk of ADL disability among women, possibly due to relatively lower physical fitness compared to men [35], this pattern was not evident in our study. This discrepancy may be attributed to our focus on individuals aged 50 and above, who are typically in the perimenopausal stage, where female sex hormones may have less influence on ADL disability outcomes [36].
The mechanisms by which hsCRP is associated with the development of functional limitations are not fully understood. They may be mediated through several biological pathways [37,38,39]. First, chronic inflammation, as indicated by elevated hsCRP, can lead to muscle degradation through increased protein catabolism and oxidative stress [39,40]. This process may result in sarcopenia, a condition characterized by the loss of muscle mass and strength, which is a critical determinant of ADL disability among older adults. Second, hsCRP is also linked to the development of various chronic diseases, such as diabetes, cardiovascular disease, and cognitive decline [41]. The inflammation-induced hepatic acute-phase response prioritizes the synthesis of inflammatory proteins such as hsCRP, while inhibiting the production of nutrition-related proteins like albumin and prealbumin, thereby exacerbating malnutrition [42,43]. Inadequate protein intake leads to insufficient nutrient intake in older adults, which in turn activates inflammatory pathways. This process impairs muscle repair capacity, thereby accelerating functional decline [44]. Despite these proposed mechanisms, further research is needed to fully elucidate the complex interplay between hsCRP and functional decline. Future studies should prioritize exploring interventions like dietary changes and lifestyle modifications, which can safeguard muscle function and uphold seniors’ independence. Such efforts are crucial for improving their quality of life and reducing the healthcare burden tied to ADL disability.
5. Strengths and Limitations
This investigation offers several methodological strengths, including a population-based design with adequate power to detect moderate effect sizes. The incorporation of longitudinal biomarker data enhances clinical translatability while controlling for key confounders. Nevertheless, our findings should be considered in the context of several limitations. First, our reliance on single-timepoint hsCRP measurements may not fully capture chronic inflammatory exposure. Second, our study did not consider other potential confounding factors that could influence both hsCRP concentrations and the risk of ADL disability. Third, there may have been a selection bias relating to our participants, as healthier individuals were more likely to be followed-up for a longer period, which could have introduced a healthy survivor bias. Finally, although our study involved a relatively large sample size, it was conducted in a specific geographic region, which may have introduced a regional bias and limits the generalizability of the results to other populations.
6. Conclusions
Our study demonstrates a significant association between elevated hsCRP and the risk of ADL disability among middle-aged and older individuals. The findings highlight the importance of considering inflammation as a potential risk factor for functional decline and suggest that targeted interventions to reduce inflammation may help preserve ADL function in vulnerable populations.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/nu17101732/s1. Table S1. Demographic and clinical characteristics of study participants with varying status of ADL disability; Table S2. HRs (95% CI) for ADL disability stratified by hsCRP quartiles excluding participants who died within 2 years of follow-up; Table S3. HRs (95% CI) of ADL disability by tertiles of high-sensitivity C-reactive protein; Table S4. HRs (95% CI) of ADL disability by quintiles of high-sensitivity C-reactive protein; Table S5. HRs (95% CI) of ADL disability according to clinical categories of high-sensitivity C-reactive protein; Table S6. Subgroup analyses for the HRs (95% CI) of ADL disability for each 1 mg/L increase in high-sensitivity C-reactive protein.
Author Contributions
Conceptualization, S.-M L., L.K., X.-L.T., C.-S.Q., D.-Q.L., H.-M.L. and Z.-H.L.; formal analysis, H.-X.H. and L.-Y.D.; methodology, S.-M L., L.K., X.-L.T., C.-S.Q., D.-Q.L., H.-M.L. and Z.-H.L.; project administration, S.-M L. and Z.-H.L.; writing—original draft, D.-Q.L. and H.-M.L.; writing—review and editing, S.-M.L., L.K., X.-L.T., C.-S.Q., H.-X.H., L.-Y.D., Z.-Y.X., B.-Y.Z., H.-J.C. and Z.-H.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (82204115).
Institutional Review Board Statement
This study used a public use dataset and therefore does not require additional Institutional Review Board approval. The primary data collection for the Health and Retirement Study was approved by the Institutional Review Board at the University of Michigan (Protocol: HUM00061128; Approved through 18 October 2018).
Informed Consent Statement
Patient consent was waived due to informed consent having been obtained from all the participants involved in the study prior to their participation in the original data collection, as documented by the HRS.
Data Availability Statement
The original contributions presented in the study are publicly available. These data can be found here (http://hrsonline.isr.umich.edu, accessed on 25 September 2024).
Acknowledgments
We would like to thank all the HRS participants and all the people involved in building the HRS.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Abbreviations
| HsCRP | high-sensitivity C-reactive protein |
| ADL | activities of daily living |
| BMI | body mass index |
| HR | hazard ratio |
| CI | confidence interval |
| HRS | Health and Retirement Study |
| DBS | Dried blood spot |
| TC | total cholesterol |
| HDL-C | high-density lipoprotein cholesterol |
| HbA1c | hemoglobin A1c |
| CES-D 8 | 8-item Center for Epidemiologic Studies Depression Scale |
| SDs | standard deviations |
| RCSs | restricted cubic splines |
References
- Min, L.; Shao, S.; Wu, X.; Cong, L.; Liu, P.; Zhao, H.; Luo, Y. Anti-inflammatory and anti-thrombogenic effects of atorvastatin in acute ischemic stroke. Neural Regen. Res. 2013, 8, 2144–2154. [Google Scholar]
- Yeung, E.H.; Guan, W.; Zeng, X.; Salas, L.A.; Mumford, S.L.; de Prado Bert, P.; Van Meel, E.R.; Malmberg, A.; Sunyer, J.; Duijts, L.; et al. Cord blood DNA methylation reflects cord blood C-reactive protein levels but not maternal levels: A longitudinal study and meta-analysis. Clin. Epigenet. 2020, 12, 60. [Google Scholar] [CrossRef] [PubMed]
- Lawler, P.R.; Akinkuolie, A.O.; Chandler, P.D.; Moorthy, M.V.; Vandenburgh, M.J.; Schaumberg, D.A.; Lee, I.M.; Glynn, R.J.; Ridker, P.M.; Buring, J.E.; et al. Circulating N-Linked Glycoprotein Acetyls and Longitudinal Mortality Risk. Circ. Res. 2016, 118, 1106–1115. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Speiser, J.L.; Ye, F.; Tsai, M.Y.; Cainzos-Achirica, M.; Nasir, K.; Herrington, D.M.; Shapiro, M.D. High-Sensitivity C-Reactive Protein Modifies the Cardiovascular Risk of Lipoprotein(a): Multi-Ethnic Study of Atherosclerosis. J. Am. Coll. Cardiol. 2021, 78, 1083–1094. [Google Scholar] [CrossRef] [PubMed]
- Peikert, A.; Kaier, K.; Merz, J.; Manhart, L.; Schäfer, I.; Hilgendorf, I.; Hehn, P.; Wolf, D.; Willecke, F.; Sheng, X.; et al. Residual inflammatory risk in coronary heart disease: Incidence of elevated high-sensitive CRP in a real-world cohort. Clin. Res. Cardiol. 2020, 109, 315–323. [Google Scholar] [CrossRef]
- Gu, H.Q.; Yang, K.X.; Lin, J.X.; Jing, J.; Zhao, X.Q.; Wang, Y.L.; Liu, L.P.; Meng, X.; Jiang, Y.; Li, H.; et al. Association between high-sensitivity C-reactive protein, functional disability, and stroke recurrence in patients with acute ischaemic stroke: A mediation analysis. EBioMedicine 2022, 80, 104054. [Google Scholar] [CrossRef]
- Mu, R.; Li, C.; Li, X.; Ke, Y.; Zhao, L.; Chen, L.; Wu, R.; Wu, Z.; Zuo, X.; Xie, Y.; et al. Effectiveness and safety of iguratimod treatment in patients with active rheumatoid arthritis in Chinese: A nationwide, prospective real-world study. Lancet Reg. Health West. Pac. 2021, 10, 100128. [Google Scholar] [CrossRef]
- Hu, Y.; Zhou, F.; Kaminga, A.C.; Yan, S.; Hu, Z. Associations of depressive symptoms and chronic diseases with activities of daily living among middle-aged and older population in China: A population-based cohort study. Front. Psychiatry 2022, 13, 848255. [Google Scholar] [CrossRef]
- Mukamal, K.J.; Siscovick, D.S.; de Boer, I.H.; Ix, J.H.; Kizer, J.R.; Djoussé, L.; Fitzpatrick, A.L.; Tracy, R.P.; Boyko, E.J.; Kahn, S.E.; et al. Metabolic Clusters and Outcomes in Older Adults: The Cardiovascular Health Study. J. Am. Geriatr. Soc. 2018, 66, 289–296. [Google Scholar] [CrossRef]
- Huang, Y.; Zeng, M.; Zhang, L.; Shi, J.; Yang, Y.; Liu, F.; Sun, L.; Xiao, L. Dietary Inflammatory Potential Is Associated with Sarcopenia Among Chronic Kidney Disease Population. Front. Nutr. 2022, 9, 856726. [Google Scholar] [CrossRef]
- Jang, W.; Shin, Y.; Kim, Y. Dietary Pattern Accompanied with a High Food Variety Score Is Negatively Associated with Frailty in Older Adults. Nutrients 2021, 13, 3164. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.S.; Yin, Z.X.; Lyu, Y.B.; Wang, J.L.; Shi, X.M. Association between the hypersensitive C-reactive protein and activities of daily living among elderly adults in longevity areas of China. Zhonghua Yu Fang Yi Xue Za Zhi 2016, 50, 605–610. [Google Scholar] [PubMed]
- Verghese, J.; Holtzer, R.; Lipton, R.B.; Wang, C. High-sensitivity C-reactive protein and mobility disability in older adults. Age Ageing 2012, 41, 541–545. [Google Scholar] [CrossRef]
- Cesari, M.; Penninx, B.W.; Pahor, M.; Lauretani, F.; Corsi, A.M.; Williams, G.R.; Guralnik, J.M.; Ferrucci, L. Inflammatory markers and physical performance in older persons: The InCHIANTI study. J. Gerontol. A Biol. Sci. Med. Sci. 2004, 59, 242–248. [Google Scholar] [CrossRef]
- Niu, K.; Hozawa, A.; Guo, H.; Kuriyama, S.; Ebihara, S.; Yang, G.; Ohmori-Matsuda, K.; Nakaya, N.; Takahashi, H.; Fujita, K.; et al. Serum C-reactive protein even at very low (<1.0 mg/l) concentration is associated with physical performance in a community-based elderly population aged 70 years and over. Gerontology 2008, 54, 260–267. [Google Scholar]
- Sujarwoto, S.; Tampubolon, G. Inflammatory markers and physical performance in middle-aged and older people in Indonesia. Age Ageing 2015, 44, 610–615. [Google Scholar] [CrossRef]
- Takashima, N.; Nakamura, Y.; Miyagawa, N.; Kadota, A.; Saito, Y.; Matsui, K.; Miura, K.; Ueshima, H.; Kita, Y. Association between C-Reactive Protein Levels and Functional Disability in the General Older-Population: The Takashima Study. J. Atheroscler. Thromb. 2023, 30, 56–65. [Google Scholar] [CrossRef]
- Andaku, D.K.; D’Almeida, V.; Carneiro, G.; Hix, S.; Tufik, S.; Togeiro, S.M. Sleepiness, inflammation and oxidative stress markers in middle-aged males with obstructive sleep apnea without metabolic syndrome: A cross-sectional study. Respir. Res. 2015, 16, 3. [Google Scholar] [CrossRef]
- Gidding, S.S.; Keith, S.W.; Falkner, B. Adolescent and adult African Americans have similar metabolic dyslipidemia. J. Clin. Lipidol. 2015, 9, 368–376. [Google Scholar] [CrossRef][Green Version]
- Kasprzak, Ł.; Twardawa, M.; Formanowicz, P.; Formanowicz, D. The Mutual Contribution of 3-NT, IL-18, Albumin, and Phosphate Foreshadows Death of Hemodialyzed Patients in a 2-Year Follow-Up. Antioxidants 2022, 11, 355. [Google Scholar] [CrossRef]
- Peralta, C.A.; Katz, R.; Newman, A.B.; Psaty, B.M.; Odden, M.C. Systolic and diastolic blood pressure, incident cardiovascular events, and death in elderly persons: The role of functional limitation in the Cardiovascular Health Study. Hypertension 2014, 64, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Allaire, S.H.; LaValley, M.P.; Evans, S.R.; O’Connor, G.T.; Kelly-Hayes, M.; Meenan, R.F.; Levy, D.; Felson, D.T. Evidence for decline in disability and improved health among persons aged 55 to 70 years: The Framingham Heart Study. Am. J. Public Health 1999, 89, 1678–1683. [Google Scholar] [CrossRef]
- Li, Z.H.; Zhong, W.F.; Lv, Y.B.; Kraus, V.B.; Gao, X.; Chen, P.L.; Huang, Q.M.; Ni, J.D.; Shi, X.M.; Mao, C.; et al. Associations of plasma high-sensitivity C-reactive protein concentrations with all-cause and cause-specific mortality among middle-aged and elderly individuals. Immun. Ageing 2019, 16, 28. [Google Scholar] [CrossRef]
- Li, Y.; Zhong, X.; Cheng, G.; Zhao, C.; Zhang, L.; Hong, Y.; Wan, Q.; He, R.; Wang, Z. Hs-CRP and all-cause, cardiovascular, and cancer mortality risk: A meta-analysis. Atherosclerosis 2017, 259, 75–82. [Google Scholar] [CrossRef]
- Hautekiet, P.; Saenen, N.D.; Martens, D.S.; Debay, M.; Van der Heyden, J.; Nawrot, T.S.; De Clercq, E.M. A healthy lifestyle is positively associated with mental health and well-being and core markers in ageing. BMC Med. 2022, 20, 328. [Google Scholar] [CrossRef]
- Little, V.G.R.J.; Rubin, D.B. Statistical Analysis with Missing Data. Psychometrika 2022, 87, 1575–1578. [Google Scholar]
- Pearson, T.A.; Mensah, G.A.; Alexander, R.W.; Anderson, J.L.; Cannon, R.O., 3rd; Criqui, M.; Fadl, Y.Y.; Fortmann, S.P.; Hong, Y.; Myers, G.L.; et al. Markers of inflammation and cardiovascular disease: Application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 2003, 107, 499–511. [Google Scholar] [CrossRef]
- Sousa, A.C.; Zunzunegui, M.V.; Li, A.; Phillips, S.P.; Guralnik, J.M.; Guerra, R.O. Association between C-reactive protein and physical performance in older populations: Results from the International Mobility in Aging Study (IMIAS). Age Ageing 2016, 45, 274–280. [Google Scholar] [CrossRef]
- Deme, S.; Janakiraman, B.; Alamer, A.; Wayessa, D.I.; Yitbarek, T.; Sidiq, M. Predictors of functional status and disability among patients living with chronic kidney diseases at St Paul’s hospital millennium medical college, Ethiopia: Findings from a cross-sectional study. BMC Nephrol. 2024, 25, 343. [Google Scholar] [CrossRef]
- Feng, Y.-G.; He, J.-W.; Jiang, L.-Y.; Chen, D.-N.; Wang, A.-J.; Feng, J.-J. Novel sandwich-typed electrochemical immunosensing of C-reactive protein using multiply twinned AuPtRh nanobead chains and nitrogen-rich porous carbon nanospheres decorated with Au nanoparticles. Sens. Actuators B Chem. 2022, 358, 131518. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Saito, Y.; Crimmins, E.M. Changing Impact of Obesity on Active Life Expectancy of Older Americans. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, 1944–1951. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.F.; Giles, J.T.; Weber, D.; Leonard, M.B.; Zemel, B.S.; Long, J.; Ibrahim, S.; Katz, P.P. Assessment of muscle mass relative to fat mass and associations with physical functioning in rheumatoid arthritis. Rheumatology 2017, 56, 981–988. [Google Scholar] [CrossRef] [PubMed]
- You, T.; Wang, X.; Murphy, K.M.; Lyles, M.F.; Demons, J.L.; Yang, R.; Gong, D.W.; Nicklas, B.J. Regional adipose tissue hormone/cytokine production before and after weight loss in abdominally obese women. Obesity 2014, 22, 1679–1684. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H. Variation in the TAS2R38 Bitterness Receptor Gene Was Associated with Food Consumption and Obesity Risk in Koreans. Nutrients 2019, 11, 1973. [Google Scholar] [CrossRef]
- Yin, Z.; Shi, X.; Kraus, V.B.; Brasher, M.S.; Chen, H.; Liu, Y.; Lv, Y.; Zeng, Y. Gender-dependent association of body mass index and waist circumference with disability in the Chinese oldest old. Obesity 2014, 22, 1918–1925. [Google Scholar] [CrossRef]
- Gaskins, A.J.; Wilchesky, M.; Mumford, S.L.; Whitcomb, B.W.; Browne, R.W.; Wactawski-Wende, J.; Perkins, N.J.; Schisterman, E.F. Endogenous reproductive hormones and C-reactive protein across the menstrual cycle: The BioCycle Study. Am. J. Epidemiol. 2012, 175, 423–431. [Google Scholar] [CrossRef]
- Bruunsgaard, H. Physical activity and modulation of systemic low-level inflammation. J. Leukoc. Biol. 2005, 78, 819–835. [Google Scholar] [CrossRef]
- Gonzalez-Freire, M.; de Cabo, R.; Studenski, S.A.; Ferrucci, L. The Neuromuscular Junction: Aging at the Crossroad between Nerves and Muscle. Front. Aging Neurosci. 2014, 6, 208. [Google Scholar] [CrossRef]
- Stam, S.P.; Eisenga, M.F.; Gomes-Neto, A.W.; van Londen, M.; de Meijer, V.E.; van Beek, A.P.; Gansevoort, R.T.; Bakker, S.J. Muscle mass determined from urinary creatinine excretion rate, and muscle performance in renal transplant recipients. J. Cachexia Sarcopenia Muscle 2019, 10, 621–629. [Google Scholar] [CrossRef]
- Musolino, V.; Palus, S.; Tschirner, A.; Drescher, C.; Gliozzi, M.; Carresi, C.; Vitale, C.; Muscoli, C.; Doehner, W.; von Haehling, S.; et al. Megestrol acetate improves cardiac function in a model of cancer cachexia-induced cardiomyopathy by autophagic modulation. J. Cachexia Sarcopenia Muscle 2016, 7, 555–566. [Google Scholar] [CrossRef]
- Chen, J.; Liu, Y.; Luo, H.; Chen, G.; Zheng, Z.; Wang, T.; Hu, X.; Zhao, Y.; Tang, J.; Su, C.; et al. Inflammation Induced by Lipopolysaccharide and Palmitic Acid Increases Cholesterol Accumulation via Enhancing Myeloid Differentiation Factor 88 Expression in HepG2 Cells. Pharmaceuticals 2022, 15, 813. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liang, S.; Guo, X.; Wang, Y.; Chen, X.; Cai, G. Association of the malnutrition-inflammation score with physical function and functional disability in elderly patients with chronic kidney disease. Asia Pac. J. Clin. Nutr. 2023, 32, 57–62. [Google Scholar] [PubMed]
- Vicente, B.M.; Lucio Dos Santos Quaresma, M.V.; Maria de Melo, C.; Lima Ribeiro, S.M. The dietary inflammatory index (DII®) and its association with cognition, frailty, and risk of disabilities in older adults: A systematic review. Clin. Nutr. ESPEN 2020, 40, 7–16. [Google Scholar] [CrossRef]
- Wunderle, C.; Stumpf, F.; Schuetz, P. Inflammation and response to nutrition interventions. JPEN J. Parenter. Enteral. Nutr. 2024, 48, 27–36. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).