Impaired Mitophagy Contributes to Pyroptosis in Sarcopenic Obesity Zebrafish Skeletal Muscle
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Culture Conditions and Experimental Design
2.2. Micro-CT
2.3. Biochemical Analyses
2.4. Swimming Capacity and Oxygen Consumption Measurement
2.5. Open Field Test
2.6. Histological Analysis
2.7. Transmission Electron Microscopy
2.8. Western Blot
2.9. Mitochondrial Respiratory Function
2.10. Statistical Analysis
3. Results
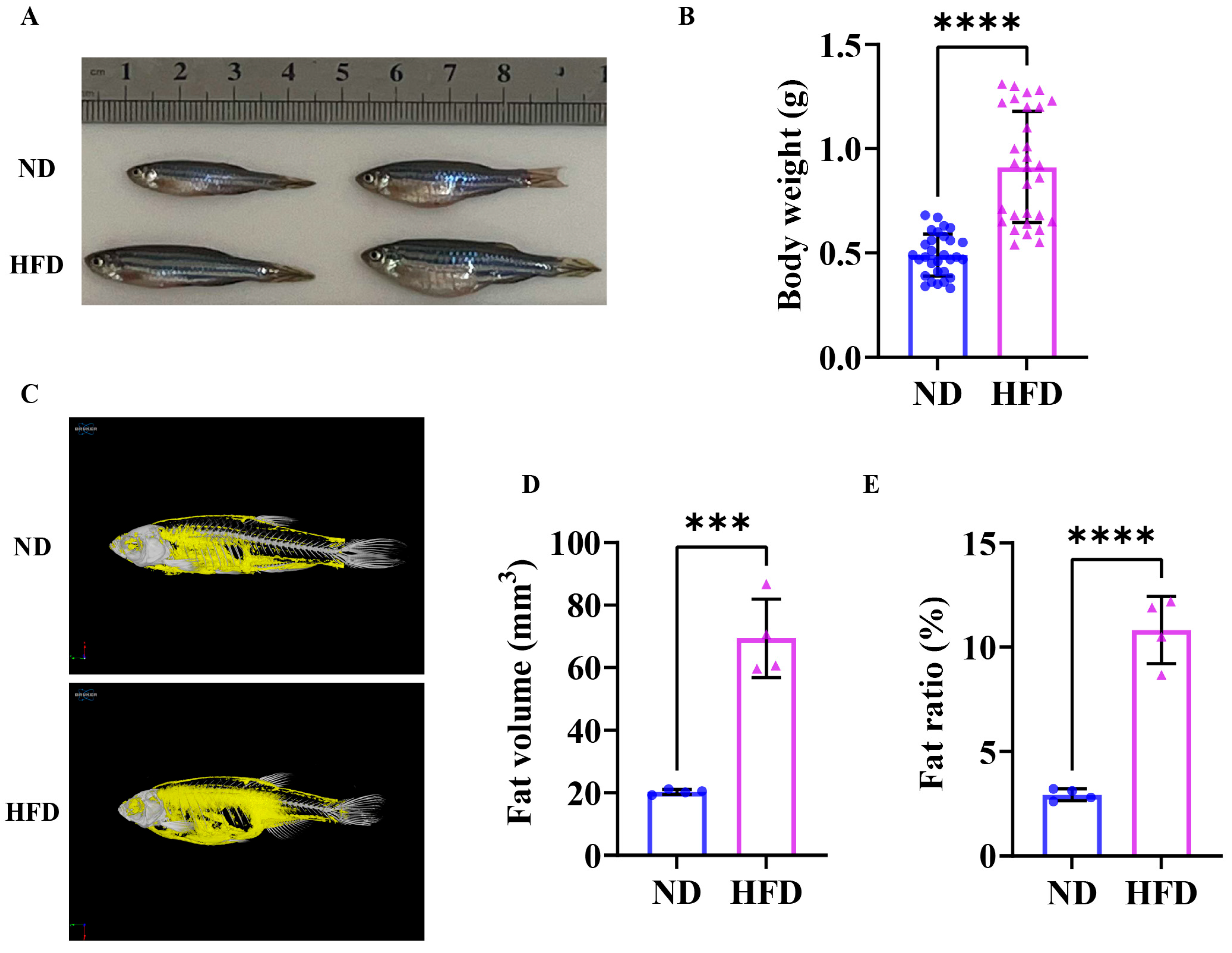
3.1. Sixteen Weeks of a High-Fat Diet Induced Obesity in Zebrafish
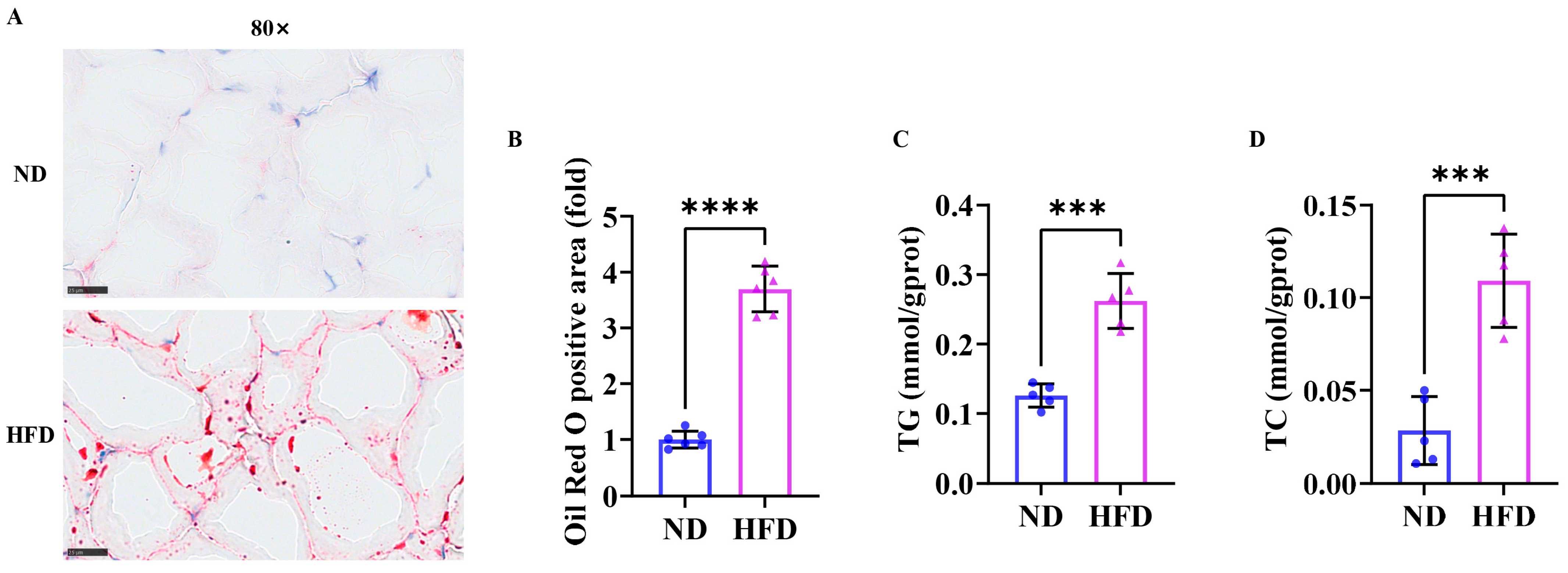
3.2. Sixteen Weeks of a High-Fat Diet Increased Lipid Content in Zebrafish Skeletal Muscle
3.3. Exercise Capacity Is Impaired in Obese Zebrafish
3.4. Sixteen Weeks of a High-Fat Diet Induced Skeletal Muscle Atrophy in Zebrafish
3.5. Sixteen Weeks of a High-Fat Diet Induced Impaired Skeletal Muscle Mitochondrial Function in Obese Zebrafish
3.6. Sixteen Weeks of a High-Fat Diet Induced Impaired Skeletal Muscle Mitophagy in Obese Zebrafish
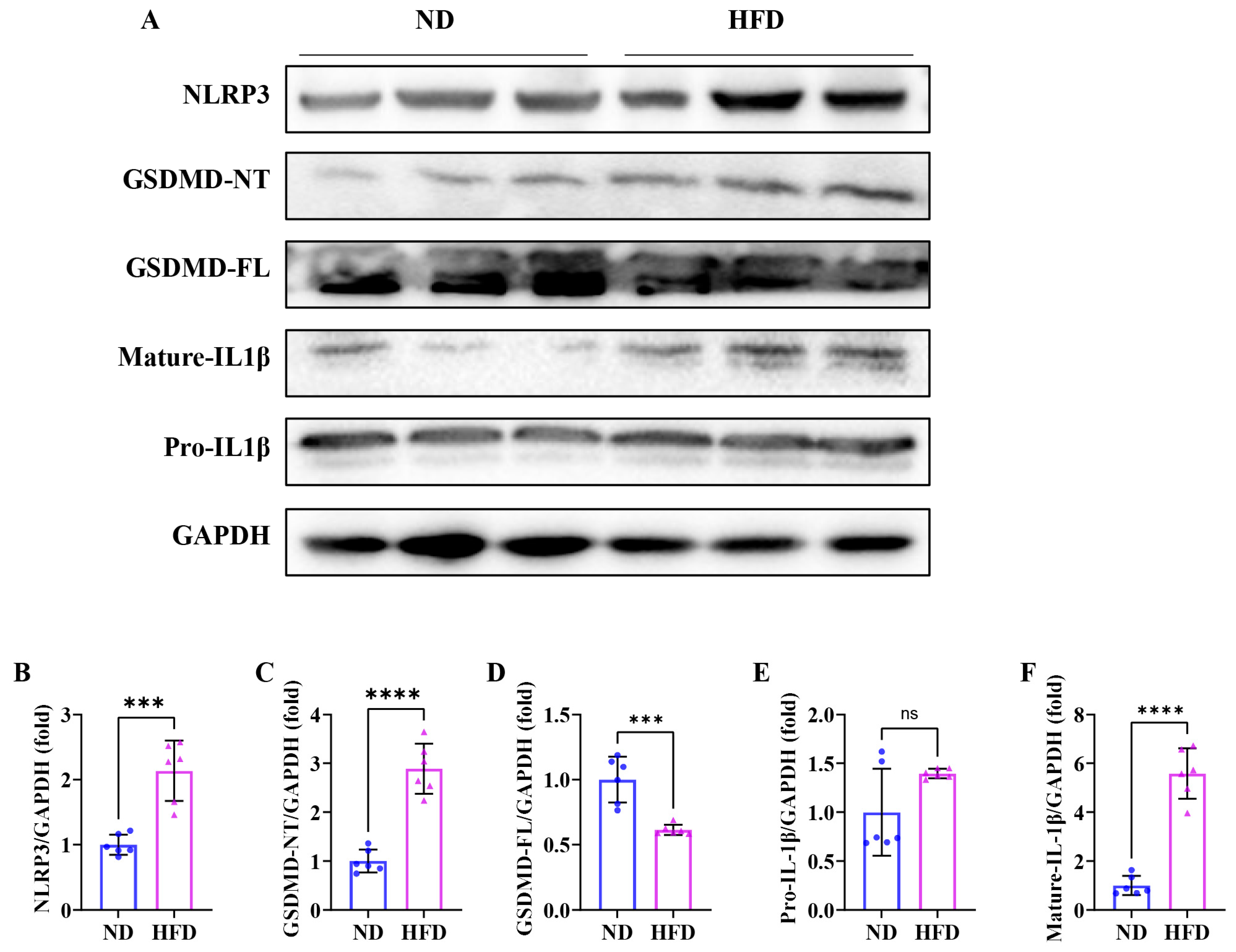
3.7. Sixteen Weeks of a High-Fat Diet Induces Skeletal Muscle Pyroptosis in Obese Zebrafish
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tham, K.W.; Abdul Ghani, R.; Cua, S.C.; Deerochanawong, C.; Fojas, M.; Hocking, S.; Lee, J.; Nam, T.Q.; Pathan, F.; Saboo, B.; et al. Obesity in South and Southeast Asia-A new consensus on care and management. Obes. Rev. 2023, 24, e13520. [Google Scholar] [CrossRef]
- Perdomo, C.M.; Cohen, R.V.; Sumithran, P.; Clement, K.; Fruhbeck, G. Contemporary medical, device, and surgical therapies for obesity in adults. Lancet 2023, 401, 1116–1130. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Ballantyne, C.M. Skeletal muscle inflammation and insulin resistance in obesity. J. Clin. Investig. 2017, 127, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Tournadre, A.; Vial, G.; Capel, F.; Soubrier, M.; Boirie, Y. Sarcopenia. Jt. Bone Spine 2019, 86, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Yoo, A.; Ahn, J.; Kim, M.J.; Seo, H.D.; Hahm, J.H.; Jung, C.H.; Ha, T.Y. Fruit of Schisandra chinensis and its bioactive component schizandrin B ameliorate obesity-induced skeletal muscle atrophy. Food Res. Int. 2022, 157, 111439. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, Y.; Du, C.; Peng, X.; Fan, W.; Chang, B.; Shan, C. Berberine attenuates obesity-induced skeletal muscle atrophy via regulation of FUNDC1 in skeletal muscle of mice. Sci. Rep. 2025, 15, 4918. [Google Scholar] [CrossRef]
- Kalinkovich, A.; Livshits, G. Sarcopenic obesity or obese sarcopenia: A cross talk between age-associated adipose tissue and skeletal muscle inflammation as a main mechanism of the pathogenesis. Ageing Res. Rev. 2017, 35, 200–221. [Google Scholar] [CrossRef]
- Axelrod, C.L.; Dantas, W.S.; Kirwan, J.P. Sarcopenic obesity: Emerging mechanisms and therapeutic potential. Metabolism 2023, 146, 155639. [Google Scholar] [CrossRef]
- Prado, C.M.; Batsis, J.A.; Donini, L.M.; Gonzalez, M.C.; Siervo, M. Sarcopenic obesity in older adults: A clinical overview. Nat. Rev. Endocrinol. 2024, 20, 261–277. [Google Scholar] [CrossRef]
- Batsis, J.A.; Villareal, D.T. Sarcopenic obesity in older adults: Aetiology, epidemiology and treatment strategies. Nat. Rev. Endocrinol. 2018, 14, 513–537. [Google Scholar] [CrossRef]
- Ji, T.; Li, Y.; Ma, L. Sarcopenic Obesity: An Emerging Public Health Problem. Aging Dis. 2022, 13, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Zhang, X.; Liu, N.; Tang, L.; Peng, C.; Chen, X. Pyroptosis: Mechanisms and diseases. Signal Transduct. Target. Ther. 2021, 6, 128. [Google Scholar] [CrossRef] [PubMed]
- Bertheloot, D.; Latz, E.; Franklin, B.S. Necroptosis, pyroptosis and apoptosis: An intricate game of cell death. Cell Mol. Immunol. 2021, 18, 1106–1121. [Google Scholar] [CrossRef]
- Shao, F.; Fitzgerald, K.A. Molecular mechanisms and functions of pyroptosis. J. Mol. Biol. 2022, 434, 167461. [Google Scholar] [CrossRef]
- Xiao, C.; Cao, S.; Li, Y.; Luo, Y.; Liu, J.; Chen, Y.; Bai, Q.; Chen, L. Pyroptosis in microbial infectious diseases. Mol. Biol. Rep. 2023, 51, 42. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, B.A.; Dixit, V.M.; Power, C. Fiery Cell Death: Pyroptosis in the Central Nervous System. Trends Neurosci. 2020, 43, 55–73. [Google Scholar] [CrossRef]
- Wei, Y.; Lan, B.; Zheng, T.; Yang, L.; Zhang, X.; Cheng, L.; Tuerhongjiang, G.; Yuan, Z.; Wu, Y. GSDME-mediated pyroptosis promotes the progression and associated inflammation of atherosclerosis. Nat. Commun. 2023, 14, 929. [Google Scholar] [CrossRef]
- Wei, X.; Xie, F.; Zhou, X.; Wu, Y.; Yan, H.; Liu, T.; Huang, J.; Wang, F.; Zhou, F.; Zhang, L. Role of pyroptosis in inflammation and cancer. Cell Mol. Immunol. 2022, 19, 971–992. [Google Scholar] [CrossRef]
- Jin, H.; Xie, W.; He, M.; Li, H.; Xiao, W.; Li, Y. Pyroptosis and Sarcopenia: Frontier Perspective of Disease Mechanism. Cells 2022, 11, 1078. [Google Scholar] [CrossRef]
- Wu, J.; Lin, S.; Chen, W.; Lian, G.; Wu, W.; Chen, A.; Sagor, M.I.H.; Luo, L.; Wang, H.; Xie, L. TNF-alpha contributes to sarcopenia through caspase-8/caspase-3/GSDME-mediated pyroptosis. Cell Death Discov. 2023, 9, 76. [Google Scholar] [CrossRef]
- Yang, J.; Wang, M.; Shi, L.; Fang, X.; Gao, C.; Ma, L.; Wang, Y.; Ying, S.; Yang, Y. The Stimulator of Interferon Genes Deficiency Attenuates Diabetic Myopathy Through Inhibiting NLRP3-Mediated Pyroptosis. J. Cachexia Sarcopenia Muscle 2025, 16, e13649. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Shi, N.; Cai, C.; Zhang, M.; He, J.; Tan, Y.; Fu, W. The Role of Mitochondria in Pyroptosis. Front. Cell Dev. Biol. 2020, 8, 630771. [Google Scholar] [CrossRef] [PubMed]
- Bock, F.J.; Tait, S.W.G. Mitochondria as multifaceted regulators of cell death. Nat. Rev. Mol. Cell Biol. 2020, 21, 85–100. [Google Scholar] [CrossRef] [PubMed]
- Ashrafi, G.; Schwarz, T.L. The pathways of mitophagy for quality control and clearance of mitochondria. Cell Death Differ. 2013, 20, 31–42. [Google Scholar] [CrossRef]
- Han, X.; Xu, T.; Fang, Q.; Zhang, H.; Yue, L.; Hu, G.; Sun, L. Quercetin hinders microglial activation to alleviate neurotoxicity via the interplay between NLRP3 inflammasome and mitophagy. Redox Biol. 2021, 44, 102010. [Google Scholar] [CrossRef]
- Harris, J.; Deen, N.; Zamani, S.; Hasnat, M.A. Mitophagy and the release of inflammatory cytokines. Mitochondrion 2018, 41, 2–8. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, M.; Wang, X.; Bu, Q.; Wang, Q.; Su, W.; Li, L.; Zhou, H.; Lu, L. XBP1 deficiency promotes hepatocyte pyroptosis by impairing mitophagy to activate mtDNA-cGAS-STING signaling in macrophages during acute liver injury. Redox Biol. 2022, 52, 102305. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Wei, S.; Nguyen, T.H.; Jo, Y.; Zhang, Y.; Park, W.; Gariani, K.; Oh, C.M.; Kim, H.H.; Ha, K.T.; et al. Mitochondria-associated programmed cell death as a therapeutic target for age-related disease. Exp. Mol. Med. 2023, 55, 1595–1619. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Q.; Yang, H.; Zhang, D.; Zhang, Y.; Wang, J.; Liu, J. 5-Heptadecylresorcinol Ameliorates Obesity-Associated Skeletal Muscle Mitochondrial Dysfunction through SIRT3-Mediated Mitophagy. J. Agric. Food Chem. 2023, 71, 16032–16042. [Google Scholar] [CrossRef]
- Zou, Y.Y.; Chen, Z.L.; Sun, C.C.; Yang, D.; Zhou, Z.Q.; Xiao, Q.; Peng, X.Y.; Tang, C.F. A High-Fat Diet Induces Muscle Mitochondrial Dysfunction and Impairs Swimming Capacity in Zebrafish: A New Model of Sarcopenic Obesity. Nutrients 2022, 14, 1975. [Google Scholar] [CrossRef]
- Jin, H.; Oh, H.J.; Lee, B.Y. GABA Prevents Age-Related Sarcopenic Obesity in Mice with High-Fat-Diet-Induced Obesity. Cells 2023, 12, 2146. [Google Scholar] [CrossRef]
- Ren, Q.; Chen, S.; Chen, X.; Niu, S.; Yue, L.; Pan, X.; Li, Z.; Chen, X. An Effective Glucagon-Like Peptide-1 Receptor Agonists, Semaglutide, Improves Sarcopenic Obesity in Obese Mice by Modulating Skeletal Muscle Metabolism. Drug Des. Devel Ther. 2022, 16, 3723–3735. [Google Scholar] [CrossRef] [PubMed]
- Dantas, W.S.; Zunica, E.R.M.; Heintz, E.C.; Vandanmagsar, B.; Floyd, Z.E.; Yu, Y.; Fujioka, H.; Hoppel, C.L.; Belmont, K.P.; Axelrod, C.L.; et al. Mitochondrial uncoupling attenuates sarcopenic obesity by enhancing skeletal muscle mitophagy and quality control. J. Cachexia Sarcopenia Muscle 2022, 13, 1821–1836. [Google Scholar] [CrossRef]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Steffen, L.S.; Guyon, J.R.; Vogel, E.D.; Beltre, R.; Pusack, T.J.; Zhou, Y.; Zon, L.I.; Kunkel, L.M. Zebrafish orthologs of human muscular dystrophy genes. BMC Genomics 2007, 8, 79. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Shang, G.; Wang, W.; Chen, X.; Lou, Q.; Zhai, G.; Li, D.; Du, Z.; Ye, Y.; Jin, X.; et al. Fatty Acid Oxidation in Zebrafish Adipose Tissue Is Promoted by 1alpha,25(OH)(2)D(3). Cell Rep. 2017, 19, 1444–1455. [Google Scholar] [CrossRef]
- Chen, Z.; Zhou, Z.; Peng, X.; Sun, C.; Yang, D.; Li, C.; Zhu, R.; Zhang, P.; Zheng, L.; Tang, C. Cardioprotective responses to aerobic exercise-induced physiological hypertrophy in zebrafish heart. J. Physiol. Sci. 2021, 71, 33. [Google Scholar] [CrossRef]
- Polyzos, S.A.; Margioris, A.N. Sarcopenic obesity. Hormones 2018, 17, 321–331. [Google Scholar] [CrossRef]
- Seth, A.; Stemple, D.L.; Barroso, I. The emerging use of zebrafish to model metabolic disease. Dis. Model. Mech. 2013, 6, 1080–1088. [Google Scholar] [CrossRef]
- Cui, B.; Hui, Y.; Sun, C. Relationship between lipid profiles and reduced handgrip strength (dynapenia) in hospitalized patients with cirrhosis. Eur. J. Gastroenterol. Hepatol. 2023, 35, 575–582. [Google Scholar] [CrossRef]
- Quadrilatero, J. Mitochondria: Key modulators of skeletal muscle remodeling. Semin. Cell Dev. Biol. 2023, 143, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Hood, D.A.; Memme, J.M.; Oliveira, A.N.; Triolo, M. Maintenance of Skeletal Muscle Mitochondria in Health, Exercise, and Aging. Annu. Rev. Physiol. 2019, 81, 19–41. [Google Scholar] [CrossRef] [PubMed]
- Granic, A.; Suetterlin, K.; Shavlakadze, T.; Grounds, M.D.; Sayer, A.A. Hallmarks of ageing in human skeletal muscle and implications for understanding the pathophysiology of sarcopenia in women and men. Clin. Sci. 2023, 137, 1721–1751. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Tang, D.; Qi, B.; Guo, D.; Wang, Y.; Geng, J.; Zhang, X.; Song, L.; Chang, P.; Chen, W.; et al. Mfn2/Hsc70 Complex Mediates the Formation of Mitochondria-Lipid Droplets Membrane Contact and Regulates Myocardial Lipid Metabolism. Adv. Sci. 2024, 11, e2307749. [Google Scholar] [CrossRef]
- Wang, S.H.; Zhu, X.L.; Wang, F.; Chen, S.X.; Chen, Z.T.; Qiu, Q.; Liu, W.H.; Wu, M.X.; Deng, B.Q.; Xie, Y.; et al. LncRNA H19 governs mitophagy and restores mitochondrial respiration in the heart through Pink1/Parkin signaling during obesity. Cell Death Dis. 2021, 12, 557. [Google Scholar] [CrossRef]
- Swalsingh, G.; Pani, P.; Bal, N.C. Structural functionality of skeletal muscle mitochondria and its correlation with metabolic diseases. Clin. Sci. 2022, 136, 1851–1871. [Google Scholar] [CrossRef]
- Pileggi, C.A.; Hooks, B.G.; McPherson, R.; Dent, R.R.M.; Harper, M.E. Targeting skeletal muscle mitochondrial health in obesity. Clin. Sci. 2022, 136, 1081–1110. [Google Scholar] [CrossRef]
- Chatzinikita, E.; Maridaki, M.; Palikaras, K.; Koutsilieris, M.; Philippou, A. The Role of Mitophagy in Skeletal Muscle Damage and Regeneration. Cells 2023, 12, 716. [Google Scholar] [CrossRef]
- Song, H.; Tian, X.; Liu, D.; Liu, M.; Liu, Y.; Liu, J.; Mei, Z.; Yan, C.; Han, Y. CREG1 improves the capacity of the skeletal muscle response to exercise endurance via modulation of mitophagy. Autophagy 2021, 17, 4102–4118. [Google Scholar] [CrossRef]
- Wu, H.; Wang, Y.; Li, W.; Chen, H.; Du, L.; Liu, D.; Wang, X.; Xu, T.; Liu, L.; Chen, Q. Deficiency of mitophagy receptor FUNDC1 impairs mitochondrial quality and aggravates dietary-induced obesity and metabolic syndrome. Autophagy 2019, 15, 1882–1898. [Google Scholar] [CrossRef]
- Zhou, T.; Chang, L.; Luo, Y.; Zhou, Y.; Zhang, J. Mst1 inhibition attenuates non-alcoholic fatty liver disease via reversing Parkin-related mitophagy. Redox Biol. 2019, 21, 101120. [Google Scholar] [CrossRef]
- Gambarotto, L.; Metti, S.; Chrisam, M.; Cerqua, C.; Sabatelli, P.; Armani, A.; Zanon, C.; Spizzotin, M.; Castagnaro, S.; Strappazzon, F.; et al. Ambra1 deficiency impairs mitophagy in skeletal muscle. J. Cachexia Sarcopenia Muscle 2022, 13, 2211–2224. [Google Scholar] [CrossRef] [PubMed]
- Triolo, M.; Hood, D.A. Manifestations of Age on Autophagy, Mitophagy and Lysosomes in Skeletal Muscle. Cells 2021, 10, 1054. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.N.; Padman, B.S.; Lazarou, M. Deciphering the Molecular Signals of PINK1/Parkin Mitophagy. Trends Cell Biol. 2016, 26, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Lazarou, M.; Sliter, D.A.; Kane, L.A.; Sarraf, S.A.; Wang, C.; Burman, J.L.; Sideris, D.P.; Fogel, A.I.; Youle, R.J. The ubiquitin kinase PINK1 recruits autophagy receptors to induce mitophagy. Nature 2015, 524, 309–314. [Google Scholar] [CrossRef]
- Han, R.; Liu, Y.; Li, S.; Li, X.J.; Yang, W. PINK1-PRKN mediated mitophagy: Differences between in vitro and in vivo models. Autophagy 2023, 19, 1396–1405. [Google Scholar] [CrossRef]
- Li, J.; Yang, D.; Li, Z.; Zhao, M.; Wang, D.; Sun, Z.; Wen, P.; Dai, Y.; Gou, F.; Ji, Y.; et al. PINK1/Parkin-mediated mitophagy in neurodegenerative diseases. Ageing Res. Rev. 2023, 84, 101817. [Google Scholar] [CrossRef]
- Li, J.; Lai, M.; Zhang, X.; Li, Z.; Yang, D.; Zhao, M.; Wang, D.; Sun, Z.; Ehsan, S.; Li, W.; et al. PINK1-parkin-mediated neuronal mitophagy deficiency in prion disease. Cell Death Dis. 2022, 13, 162. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, L.; Luo, Y.; Wang, K.; Liu, X.; Xiao, Z.; Zhao, G.; Yao, Y.; Lu, Z. Pink1/Parkin-Mediated Mitophagy Regulated the Apoptosis of Dendritic Cells in Sepsis. Inflammation 2022, 45, 1374–1387. [Google Scholar] [CrossRef]
- Kovacs, S.B.; Miao, E.A. Gasdermins: Effectors of Pyroptosis. Trends Cell Biol. 2017, 27, 673–684. [Google Scholar] [CrossRef]
- Coll, R.C.; Schroder, K.; Pelegrin, P. NLRP3 and pyroptosis blockers for treating inflammatory diseases. Trends Pharmacol. Sci. 2022, 43, 653–668. [Google Scholar] [CrossRef] [PubMed]
- Aluganti Narasimhulu, C.; Singla, D.K. Amelioration of diabetes-induced inflammation mediated pyroptosis, sarcopenia, and adverse muscle remodelling by bone morphogenetic protein-7. J. Cachexia Sarcopenia Muscle 2021, 12, 403–420. [Google Scholar] [CrossRef]
- Jimenez-Gutierrez, G.E.; Martinez-Gomez, L.E.; Martinez-Armenta, C.; Pineda, C.; Martinez-Nava, G.A.; Lopez-Reyes, A. Molecular Mechanisms of Inflammation in Sarcopenia: Diagnosis and Therapeutic Update. Cells 2022, 11, 2359. [Google Scholar] [CrossRef] [PubMed]
- Cox, A.J.; West, N.P.; Cripps, A.W. Obesity, inflammation, and the gut microbiota. Lancet Diabetes Endocrinol. 2015, 3, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Saltiel, A.R.; Olefsky, J.M. Inflammatory mechanisms linking obesity and metabolic disease. J. Clin. Investig. 2017, 127, 1–4. [Google Scholar] [CrossRef]
- Miao, P.; Ruiqing, T.; Yanrong, L.; Zhuwen, S.; Huan, Y.; Qiong, W.; Yongnian, L.; Chao, S. Pyroptosis: A possible link between obesity-related inflammation and inflammatory diseases. J. Cell Physiol. 2022, 237, 1245–1265. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, D.; Li, T.; Yang, F.; Li, Z.; Bai, X.; Wang, Y. The role of NLRP3 inflammasome in inflammation-related skeletal muscle atrophy. Front. Immunol. 2022, 13, 1035709. [Google Scholar] [CrossRef]
- Bernard, N.J. Mitochondria control pyroptosis. Nat. Immunol. 2021, 22, 1071. [Google Scholar] [CrossRef]
- Zhou, B.; Zhang, J.Y.; Liu, X.S.; Chen, H.Z.; Ai, Y.L.; Cheng, K.; Sun, R.Y.; Zhou, D.; Han, J.; Wu, Q. Tom20 senses iron-activated ROS signaling to promote melanoma cell pyroptosis. Cell Res. 2018, 28, 1171–1185. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, P.; Chen, Q.; Huang, Z.; Zou, D.; Zhang, J.; Gao, X.; Lin, Z. Mitochondrial ROS promote macrophage pyroptosis by inducing GSDMD oxidation. J. Mol. Cell Biol. 2019, 11, 1069–1082. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, X.; Zou, Y.; Yang, S.; Chen, Z.; Zhou, Z.; Peng, X.; Tang, C. Impaired Mitophagy Contributes to Pyroptosis in Sarcopenic Obesity Zebrafish Skeletal Muscle. Nutrients 2025, 17, 1711. https://doi.org/10.3390/nu17101711
Tang X, Zou Y, Yang S, Chen Z, Zhou Z, Peng X, Tang C. Impaired Mitophagy Contributes to Pyroptosis in Sarcopenic Obesity Zebrafish Skeletal Muscle. Nutrients. 2025; 17(10):1711. https://doi.org/10.3390/nu17101711
Chicago/Turabian StyleTang, Xiangbin, Yunyi Zou, Siyuan Yang, Zhanglin Chen, Zuoqiong Zhou, Xiyang Peng, and Changfa Tang. 2025. "Impaired Mitophagy Contributes to Pyroptosis in Sarcopenic Obesity Zebrafish Skeletal Muscle" Nutrients 17, no. 10: 1711. https://doi.org/10.3390/nu17101711
APA StyleTang, X., Zou, Y., Yang, S., Chen, Z., Zhou, Z., Peng, X., & Tang, C. (2025). Impaired Mitophagy Contributes to Pyroptosis in Sarcopenic Obesity Zebrafish Skeletal Muscle. Nutrients, 17(10), 1711. https://doi.org/10.3390/nu17101711





