Regulation of Metabolic Aging Through Adenosine Mono Phosphate-Activated Protein Kinase and Mammalian Target of Rapamycin: A Comparative Study of Intermittent Fasting Variations in Obese Young Women
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participant Characteristics
2.3. Intermittent Fasting Protocol
2.4. Outcomes Measurement
2.4.1. Body Composition Assessment
2.4.2. Blood Sampling and Biochemical Analysis
2.5. Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. Effects of Intermittent Fasting on AMPK Levels, mTOR Levels, and Metabolic Age
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jia, W.; Liu, F. Obesity: Causes, Consequences, Treatments, and Challenges. J. Mol. Cell Biol. 2021, 13, 463–465. [Google Scholar] [CrossRef] [PubMed]
- Sari, A.R.; Risdaryanto, R.D.; Pradipta, M.H.; Al Qorni, U.; Rejeki, P.S.; Argarini, R.; Halim, S.; Pranoto, A. Impact of Time-Resricted Feeding and Aerobic Exercise Combination on Promotes Myokine Levels and Improve Body Composition in Obese Women. Retos 2024, 53, 1–10. [Google Scholar] [CrossRef]
- Rejeki, P.S.; Pranoto, A.; Widiatmaja, D.M.; Utami, D.M.; Izzatunnisa, N.; Sugiharto; Lesmana, R.; Halim, S. Combined Aerobic Exercise with Intermittent Fasting Is Effective for Reducing MTOR and Bcl-2 Levels in Obese Females. Sports 2024, 12, 116. [Google Scholar] [CrossRef] [PubMed]
- Dwaib, H.S.; Alzaim, I.; Eid, A.H.; Obeid, O. Modulatory Effect of Intermittent Fasting on Adipose Tissue In Fl Ammation: Amelioration of Cardiovascular Dysfunction in Early Metabolic Impairment. Front. Pharmacol. 2021, 12, 626313. [Google Scholar] [CrossRef]
- Santos, A.L.; Sinha, S. Obesity and Aging: Molecular Mechanisms and Therapeutic Approaches. Ageing Res. Rev. 2021, 67, 101268. [Google Scholar] [CrossRef]
- WHO Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 24 July 2024).
- Badan Penelitian dan Pengembangan Kesehatan Republik Indonesia. Laporan Nasional RISKESDAS 2018; Lembaga Penerbit Balitbangkes: Jakarta, Indonesia, 2018; Available online: https://repository.badankebijakan.kemkes.go.id/id/eprint/3514/1/Laporan%20Riskesdas%202018%20Nasional.pdf (accessed on 30 July 2024).
- Laporan Tematik Survei Kesehatan Indonesia (SKI) Tahun 2023: Potret Indonesia Sehat; Kementerian Kesehatan RI: Jakarta, Indonesia, 2024; Available online: https://repository.badankebijakan.kemkes.go.id/id/eprint/5537/1/SKI%20TEMATIK%202023%20-%20SEPTEMBER.pdf (accessed on 29 April 2025)ISBN 978-623-301-455-7.
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of Aging: An Expanding Universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef]
- Nelson, M.C.; Story, M.; Larson, N.I.; Neumark-Sztainer, D.; Lytle, L.A. Emerging Adulthood and College-aged Youth: An Overlooked Age for Weight-related Behavior Change. Obes. Soc. 2008, 16, 2205–2211. [Google Scholar] [CrossRef]
- Yao, K.; Su, H.; Cui, K.; Gao, Y.; Xu, D.; Wang, Q.; Ha, Z.; Zhang, T.; Chen, S.; Liu, T. Effectiveness of an Intermittent Fasting Diet versus Regular Diet on Fat Loss in Overweight and Obese Middle-Aged and Olderly People without Metabolic Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Nutr. Health Aging 2024, 28, 100165. [Google Scholar] [CrossRef]
- Lobstein, T.; Powis, J.; Jackson-Leach, R. World Obesity Atlas 2024; World Obesity Federation: London, UK, 2024; Available online: https://data.worldobesity.org/publications/?cat=22 (accessed on 24 July 2024).
- Zein, M.; Zein, O.; Diab, R.; Dimachkie, L.; Sahebkar, A.; Al-asmakh, M.; Kobeissy, F.; Eid, A.H. Intermittent Fasting Favorably Modulates Adipokines and Potentially Attenuates Atherosclerosis. Biochem. Pharmacol. 2023, 218, 115876. [Google Scholar] [CrossRef]
- Yuliyanasari, N.; Zamri, E.N.; Rejeki, P.S. The Impact of Ten Days of Periodic Fasting on the Modulation of the Longevity Gene in Overweight and Obese Individuals: A Quasi-Experimental Study. Nutrients 2024, 16, 3112. [Google Scholar] [CrossRef]
- Strilbytska, O.; Klishch, S.; Storey, K.B.; Koliada, A.; Lushchak, O. Intermittent Fasting and Longevity: From Animal Models to Implication for Humans. Ageing Res. Rev. 2024, 96, 102274. [Google Scholar] [CrossRef] [PubMed]
- Anton, S.; Ezzati, A.; Witt, D.; McLaren, C.; Vial, P. The Effects of Intermittent Fasting Regimens in Middle-Age and Older Adults: Current State of Evidence. Exp. Gerontol. 2021, 156, 111617. [Google Scholar] [CrossRef] [PubMed]
- Gabel, K.; Hoddy, K.K.; Haggerty, N.; Song, J.; Kroeger, C.M.; Trepanowski, J.F.; Panda, S.; Varady, K.A. Effects of 8-Hour Time Restricted Feeding on Body Weight and Metabolic Disease Risk Factors in Obese Adults: A Pilot Study. Nutr. Healthy Aging 2018, 4, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Boeckholt, T. Intermittent Fasting (IF) Promotes Longevity Through Alterations of the Mammalian Target of Rapamycin (MTOR) and the Epigenome. 2020. Available online: https://openprairie.sdstate.edu/biomicro_plan-b/25/?fbclid=IwAR1E7gEcK7Oodn5KHI1Ox34OvG2j-sZ4E1Ne14X4EWrPkRoqIsRaLRaIUbs (accessed on 24 September 2024).
- Peng, Y.; Qi, Z.; Xu, Y.; Yang, X.; Cui, Y.; Sun, Q. AMPK and Metabolic Disorders: The Opposite Roles of Dietary Bioactive Components and Food Contaminants. Food Chem. 2024, 437, 137784. [Google Scholar] [CrossRef]
- Ge, Y.; Zhou, M.; Chen, C.; Wu, X.; Wang, X. Role of AMPK Mediated Pathways in Autophagy and Aging. Biochimie 2021, 195, 100–113. [Google Scholar] [CrossRef]
- Wijngaarden, M.A.; van der Zon, G.C.; van Dijk, K.W.; Pijl, H.; Guigas, B. Effects of Prolonged Fasting on AMPK Signaling, Gene Expression, and Mitochondrial Respiratory Chain Content in Skeletal Muscle from Lean and Obese Individuals. Am. J. Physiol.-Endocrinol. Metab. 2013, 304, 1012–1021. [Google Scholar] [CrossRef]
- Dethlefsen, M.M.; Bertholdt, L.; Gudiksen, A.; Stankiewicz, T.; Bangsbo, J.; Van Hall, G.; Plomgaard, P.; Pilegaard, H. Training State and Skeletal Muscle Autophagy in Response to 36 h of Fasting. J. Appl. Physiol. 2018, 125, 1609–1619. [Google Scholar] [CrossRef]
- Storoschuk, K.L.; Lesiuk, D.; Nuttall, J.; LeBouedec, M.; Khansari, A.; Islam, H.; Gurd, B.J. Impact of Fasting on the AMPK and PGC-1α Axis in Rodent and Human Skeletal Muscle: A Systematic Review. Metabolism 2024, 152, 155768. [Google Scholar] [CrossRef]
- Dorling, J.L.; Martin, C.K.; Redman, L.M. Calorie Restriction for Enhanced Longevity: The Role of Novel Dietary Strategies in the Present Obesogenic Environment. Ageing Res. Rev. 2020, 64, 101038. [Google Scholar] [CrossRef]
- Li, K.; Wang, C.; Wang, Y.; Fu, L.; Zhang, N. Future Foods, Dietary Factors and Healthspan. J. Futur. Foods 2022, 3, 75–98. [Google Scholar] [CrossRef]
- Bilibio, B.L.E.; dos Reis, W.R.; Compagnon, L.; de Batista, D.G.; Sulzbacher, L.M.; Pinheiro, J.F.; Ludwig, M.S.; Frizzo, M.N.; Cruzat, V.; Heck, T.G. Effects of Alternate-Day Fasting and Time-Restricted Feeding in Obese Middle-Aged Female Rats. Nutrition 2023, 116, 112198. [Google Scholar] [CrossRef] [PubMed]
- Varady, K.A.; Bhutani, S.; Church, E.C.; Klempel, M.C. Short-Term Modified Alternate-Day Fasting: A Novel Dietary Strategy for Weight Loss and Cardioprotection in Obese Adults. Am. J. Clin. Nutr. 2009, 90, 1138–1143. [Google Scholar] [CrossRef] [PubMed]
- Zeb, F.; Wu, X.; Fatima, S.; Zaman, M.H.; Khan, S.A.; Safdar, M.; Alam, I.; Feng, Q. Time-Restricted Feeding Regulates Molecular Mechanisms with Involvement of Circadian Rhythm to Prevent Metabolic Diseases. Nutrition 2021, 89, 111244. [Google Scholar] [CrossRef] [PubMed]
- Sutton, E.F.; Beyl, R.; Early, K.S.; Cefalu, W.T.; Ravussin, E.; Peterson, C.M. Early Time-Restricted Feeding Improves Insulin Sensitivity, Blood Pressure, and Oxidative Stress Even without Weight Loss in Men with Prediabetes. Cell Metab. 2018, 27, 1212–1221.e3. [Google Scholar] [CrossRef]
- Cienfuegos, S.; Gabel, K.; Kalam, F.; Ezpeleta, M.; Wiseman, E.; Pavlou, V.; Lin, S.; Oliveira, M.L.; Varady, K.A. Effects of 4- and 6-h Time-Restricted Feeding on Weight and Cardiometabolic Health: A Randomized Controlled Trial in Adults with Obesity. Cell Metab. 2020, 32, 366–378.e3. [Google Scholar] [CrossRef]
- Attinà, A.; Leggeri, C.; Paroni, R.; Pivari, F.; Cas, M.D.; Mingione, A.; Dri, M.; Marchetti, M.; Di Renzo, L. Fasting: How to Guide. Nutrients 2021, 13, 1570. [Google Scholar] [CrossRef]
- Koppold, D.A.; Breinlinger, C.; Hanslian, E.; Kessler, C.; Cramer, H.; Khokhar, A.R.; Peterson, C.M.; Tinsley, G.; Vernieri, C.; Bloomer, R.J.; et al. International Consensus on Fasting Terminology. Cell Metab. 2024, 36, 1779–1794.e4. [Google Scholar] [CrossRef]
- Pavlidou, E.; Papadopoulou, S.K.; Seroglou, K.; Giaginis, C. Revised Harris–Benedict Equation: New Human Resting Metabolic Rate Equation. Metabolites 2023, 13, 189. [Google Scholar] [CrossRef]
- Sandeep, K.S.; Singaraju, G.S.; Reddy, V.K.; Mandava, P.; Bhavikati, V.N.; Reddy, R. Evaluation of Body Weight, Body Mass Index, and Body Fat Percentage Changes in Early Stages of Fixed Orthodontic Therapy. J. Int. Soc. Prev. Community Dent. 2016, 6, 349–358. [Google Scholar] [CrossRef]
- Sharifi-Rigi, A.; Zal, F.; Aarabi, M.H.; Rad, N.R.; Naghibalhossaini, F.; Shafiee, S.M.; Aminorroaya, A. The Effects of Astaxanthin on AMPK/Autophagy Axis and Inflammation in Type 2 Diabetes Patients: A Randomized, Double-Blind, Placebo-Controlled Trial. Gene Rep. 2023, 33, 101844. [Google Scholar] [CrossRef]
- Tapan, O.O.; Tapan, U.; Edgunlu, T.; Dogan, E. Evaluation of MTOR Activity in COPD Patients with Emphysema. J. Coll. Physicians Surg. Pakistan 2022, 32, 1448–1453. [Google Scholar] [CrossRef]
- Alandağ, C.; Öztürk, A.; Yulak, F.; Şahin İnan, Z.D.; Özkaraca, M.; Lacın, B.B.; Altun, A. HER-2 SMASH. Cancer Chemother. Pharmacol. 2024, 95, 10. [Google Scholar] [CrossRef] [PubMed]
- Townsend, L.K.; Steinberg, G.R. AMPK and the Endocrine Control of Metabolism. Endocr. Rev. 2023, 44, 910–933. [Google Scholar] [CrossRef] [PubMed]
- Jamshed, H.; Beyl, R.A.; della Manna, D.L.; Yang, E.S.; Ravussin, E.; Peterson, C.M. Early Time-Restricted Feeding Improves 24-Hour. Nutrients 2019, 11, 1234. [Google Scholar] [CrossRef]
- Ma, Y.N.; Jiang, X.; Tang, W.; Song, P. Influence of Intermittent Fasting on Autophagy in the Liver. Biosci. Trends 2023, 17, 335–355. [Google Scholar] [CrossRef]
- Davidson, M.D.; Khetani, S.R. Intermittent Starvation Extends the Functional Lifetime of Primary Human Hepatocyte Cultures. Toxicol. Sci. 2020, 174, 266–277. [Google Scholar] [CrossRef]
- Surugiu, R.; Iancu, M.A.; Vintilescu Ștefănița, B.; Stepan, M.D.; Burdusel, D.; Genunche-Dumitrescu, A.V.; Dogaru, C.A.; Dumitra, G.G. Molecular Mechanisms of Healthy Aging: The Role of Caloric Restriction, Intermittent Fasting, Mediterranean Diet, and Ketogenic Diet—A Scoping Review. Nutrients 2024, 16, 2878. [Google Scholar] [CrossRef] [PubMed]
- Anton, S.D.; Moehl, K.; Donahoo, W.T.; Marosi, K.; Lee, S.A.; Mainous, A.G.; Leeuwenburgh, C.; Mattson, M.P. Flipping the Metabolic Switch: Understanding and Applying the Health Benefits of Fasting. Obesity 2018, 26, 254–268. [Google Scholar] [CrossRef]
- Patterson, R.E.; Sears, D.D. Metabolic Effects of Intermittent Fasting. Annu. Rev. Nutr. 2017, 37, 371–393. [Google Scholar] [CrossRef]
- Longo, V.D.; Panda, S. Fasting, Circadian Rhythms, and Time-Restricted Feeding in Healthy Lifespan. Cell Metab. 2016, 23, 1048–1059. [Google Scholar] [CrossRef]
- Rothschild, J.; Hoddy, K.K.; Jambazian, P.; Varady, K.A. Time-Restricted Feeding and Risk of Metabolic Disease: A Review of Human and Animal Studies. Nutr. Rev. 2014, 72, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Saxton, R.A.; Sabatini, D.M. MTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 169, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhou, X. Research Progress of MTOR Inhibitors. Eur. J. Med. Chem. 2020, 208, 112820. [Google Scholar] [CrossRef] [PubMed]
- Stekovic, S.; Hofer, S.J.; Tripolt, N.; Aon, M.A.; Royer, P.; Pein, L.; Stadler, J.T.; Pendl, T.; Prietl, B.; Url, J.; et al. Alternate Day Fasting Improves Physiological and Molecular Markers of Aging in Healthy, Non-Obese Humans. Cell Metab. 2019, 30, 462–476.e5. [Google Scholar] [CrossRef]
- de Cabo, R.; Mattson, M.P. Effects of Intermittent Fasting on Health, Aging and Disease. N. Engl. J. Med. 2019, 381, 2531–2551. [Google Scholar] [CrossRef]
- Mattson, M.P.; Longo, V.D.; Harvie, M.; States, U.; States, U.; Angeles, L.; States, U.; Cancer, B.; Centre, P.; Kingdom, U. Impact of Intermittent Fasting. Ageing Res. Rev. 2017, 39, 46–58. [Google Scholar] [CrossRef]
- Kotarsky, C.J.; Johnson, N.R.; Mahoney, S.J.; Mitchell, S.L.; Schimek, R.L.; Stastny, S.N.; Hackney, K.J. Time-Restricted Eating and Concurrent Exercise Training Reduces Fat Mass and Increases Lean Mass in Overweight and Obese Adults. Physiol. Rep. 2021, 9, e14868. [Google Scholar] [CrossRef]
- Moro, T.; Tinsley, G.; Pacelli, F.Q.; Marcolin, G.; Bianco, A.; Paoli, A. Twelve Months of Time-Restricted Eating and Resistance Training Improves Inflammatory Markers and Cardiometabolic Risk Factors. Med. Sci. Sports Exerc. 2021, 53, 2577–2585. [Google Scholar] [CrossRef]
- Longo, V.D.; Mattson, M.P. Fasting: Molecular Mechanisms and Clinical Applications. Cell Metab. 2014, 19, 181–192. [Google Scholar] [CrossRef]
- Tivya, S.; Mustafa, N.; Manaf, Z.; Amiliyaton, M. Effect of Intermittent Fasting in Overweight Females on Weight Loss and Metabolic Biomarkers. COVID Endocr. Dis. 2021, 2021, 17. [Google Scholar] [CrossRef]


| Control (n = 7) | ADMF (n = 5) | TRF (n = 8) | p Value | |
|---|---|---|---|---|
| Age, years a | 20.60 ± 1.82 | 21.20 ± 2.28 | 20.4 ± 1.14 | 0.877 |
| Blood pressure, mmHg | ||||
| Sistolic a | 114.40 ± 10.26 | 110.60 ± 8.65 | 122.20 ± 15.88 | 0.185 |
| Diastolic a | 80.20 ± 8.32 | 77.60 ± 2.70 | 84.40 ± 11.41 | 0.471 |
| Fasting Blood Glucose, mg/dL b | 101 (98–139.5) | 102 (92–120) | 95 (88.5–118.5) | 0.878 |
| Haemoglobin, mg/dL a | 12.46 ± 1.54 | 13.2 ± 2.41 | 12.80 ± 1.39 | 0.920 |
| Weight, kg a | 76.49 ± 12.03 | 73.46 ± 11.06 | 79.19 ± 5.73 | 0.591 |
| Height, cm a | 157.80 ± 6.51 | 155.7 ± 6.91 | 161.30 ± 2.71 | 0.397 |
| BMI, kg/m2 a | 30.58 ± 5.25 | 30.08 ± 3.44 | 30.48 ± 2.28 | 0.824 |
| Fat mass, % a | 37.16 ± 3.86 | 36.86 ± 4.69 | 37.58 ± 2.57 | 0.880 |
| Visceral Fat, % b | 9 (7.25–16) | 9.5 (7.75–13) | 10 (8.25–12) | 0.319 |
| AMPK, ng/mL b | 35.29 (29.22–43.30) | 45.64 (36.75–66.83) | 44.29 (29.97–50.92) | 0.546 |
| mTOR, ng/mL b | 1029 (7.25–10.61) | 8.22 (4.89–9.21) | 8.14 (6.72–9.07) | 0.751 |
| Metabolic age, years a | 44.20 ± 6.18 | 44.20 ± 4.44 | 45.40 ± 4.16 | 0.484 |
| Parameter | Group | Pre-Test | Post-Test | p-Value |
|---|---|---|---|---|
| Weight, kg | Control (n = 7) a | 76.49 ± 12.03 | 76.25 ± 11.87 | 0.656 |
| ADMF (n = 5) a | 73.46 ± 11.06 | 72.38 ± 10.82 | 0.086 | |
| TRF (n = 8) a | 79.19 ± 5.73 | 79.57 ± 5.24 | 0.877 | |
| BMI, kg/m2 | Control (n = 7) b | 27.10 (25.10–29.78) | 27.55 (25.1–29.1) | 0.786 |
| ADMF (n = 5) a | 30.38 ± 3.52 | 29.94 ± 3.61 | 0.052 | |
| TRF (n = 8) a | 30.14 ± 2.54 | 30.21 ± 2.58 | 0.351 | |
| Fat Mass, % | Control (n = 7) a | 37.16 ± 3.86 | 37.34 ± 3.82 | 0.907 |
| ADMF (n = 5) a | 36.86 ± 4.69 | 37.26 ± 3.87 | 0.698 | |
| TRF (n = 8) a | 37.58 ± 2.57 | 37.58 ± 2.42 | 0.464 | |
| Visceral Fat, % | Control (n = 7) b | 9 (7.25–16) | 9 (7.25–15.5) | 0.655 |
| ADMF (n = 5) a | 10.20 ± 2.73 | 9.10 ± 3.05 | 0.605 | |
| TRF (n = 8) a | 10.13 ± 2.25 | 10.19 ± 3.58 | 0.961 |
| Variable | Group | Pre-Test | Post-Test | p-Value |
|---|---|---|---|---|
| AMPK levels, ng/mL | Control (n = 7) a | 36.07 ± 7.75 | 41.63 ± 8.24 | 0.364 |
| ADMF (n = 5) b | 45.64 (36.75–66.83) | 37.81 (31.95–56.71) | 0.043 * | |
| TRF (n = 8) a | 41.22 ± 12.05 | 39.45 ± 11.42 | 0.744 | |
| mTOR levels, ng/mL | Control (n = 7) b | 10.29 (7.25–10.61) | 6.12 (2.94–7.64) | 0.128 |
| ADMF (n = 5) a | 7.28 ± 2.3 | 6.42 ± 2.1 | 0.621 | |
| TRF (n = 8) a | 7.94 ± 1.18 | 4.32 ± 1.26 | 0.499 | |
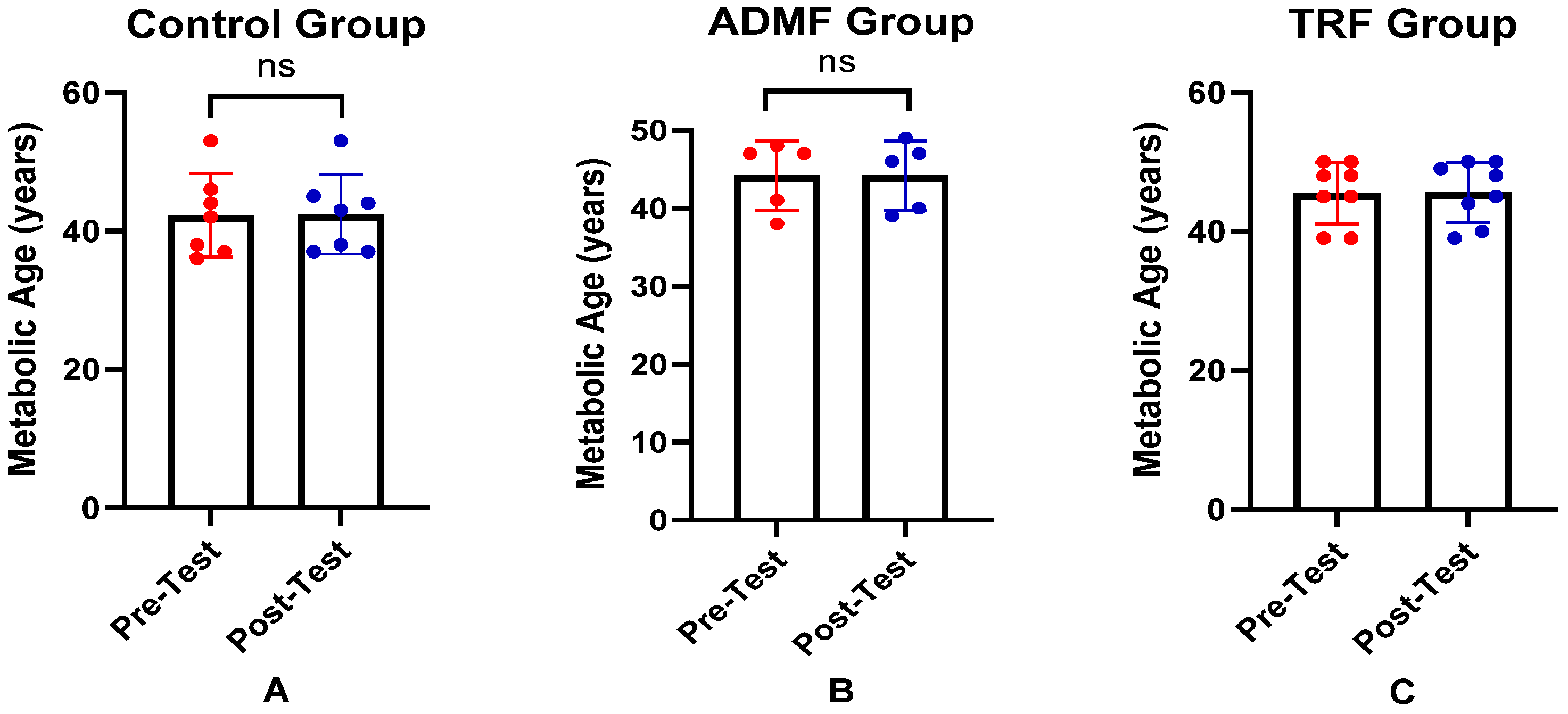
| Metabolic age, ng/mL | Control (n = 7) a | 44.20 ± 6.18 | 44.40 ± 5.73 | 0.604 |
| ADMF (n = 5) a | 44.20 ± 4.44 | 44.20 ± 4.44 | 1.000 | |
| TRF (n = 8) a | 45.40 ± 4.16 | 45.40 ± 3.85 | 0.598 |
| Group | Δ AMPK, ng/mL b | p Value | Δ mTOR, ng/mL a | p Value | Δ Metabolic Age, Years a | p Value |
|---|---|---|---|---|---|---|
| Control (n = 7) | 5.56 ± 10.79 | 0.174 | −3.75 ± 3.68 | 0.618 | 0.20 ± 0.84 | 0.943 |
| ADMF (n = 5) | −7.54 ± 4.49 | −0.86 ± 3.61 | 0.00 ± 1.00 | |||
| TRF (n = 8) | −1.76 ± 13.83 | −3.63 ± 0.43 | 0.00 ± 0.70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Purnomo, S.P.; Rejeki, P.S.; Argarini, R.; Halim, S.; Rachmayanti, D.A.; Permataputri, C.D.A.; Singgih, I.K. Regulation of Metabolic Aging Through Adenosine Mono Phosphate-Activated Protein Kinase and Mammalian Target of Rapamycin: A Comparative Study of Intermittent Fasting Variations in Obese Young Women. Nutrients 2025, 17, 1695. https://doi.org/10.3390/nu17101695
Purnomo SP, Rejeki PS, Argarini R, Halim S, Rachmayanti DA, Permataputri CDA, Singgih IK. Regulation of Metabolic Aging Through Adenosine Mono Phosphate-Activated Protein Kinase and Mammalian Target of Rapamycin: A Comparative Study of Intermittent Fasting Variations in Obese Young Women. Nutrients. 2025; 17(10):1695. https://doi.org/10.3390/nu17101695
Chicago/Turabian StylePurnomo, Sheeny Priska, Purwo Sri Rejeki, Raden Argarini, Shariff Halim, Dian Aristia Rachmayanti, Chy’as Diuranil Astrid Permataputri, and Ivan Kristianto Singgih. 2025. "Regulation of Metabolic Aging Through Adenosine Mono Phosphate-Activated Protein Kinase and Mammalian Target of Rapamycin: A Comparative Study of Intermittent Fasting Variations in Obese Young Women" Nutrients 17, no. 10: 1695. https://doi.org/10.3390/nu17101695
APA StylePurnomo, S. P., Rejeki, P. S., Argarini, R., Halim, S., Rachmayanti, D. A., Permataputri, C. D. A., & Singgih, I. K. (2025). Regulation of Metabolic Aging Through Adenosine Mono Phosphate-Activated Protein Kinase and Mammalian Target of Rapamycin: A Comparative Study of Intermittent Fasting Variations in Obese Young Women. Nutrients, 17(10), 1695. https://doi.org/10.3390/nu17101695






