The Importance of Vitamin D and Magnesium in Athletes
Abstract
1. Introduction
2. Vitamin D in Athletes
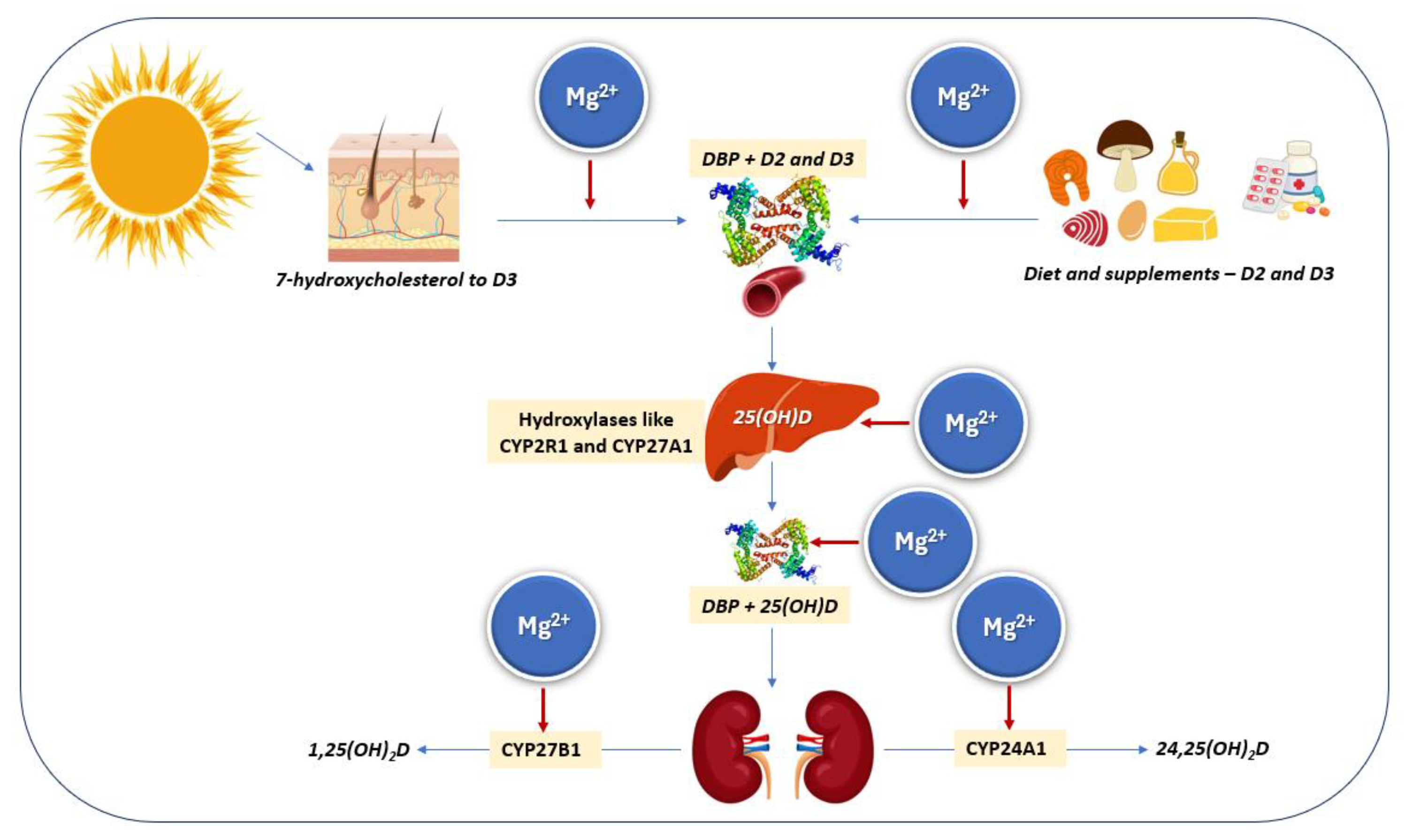
2.1. Synthesis, Sources, Metabolism, and Assessment
2.2. Vitamin D Deficiency in Athletes
2.3. Effects of Vitamin D of Particular Interest for Athletes
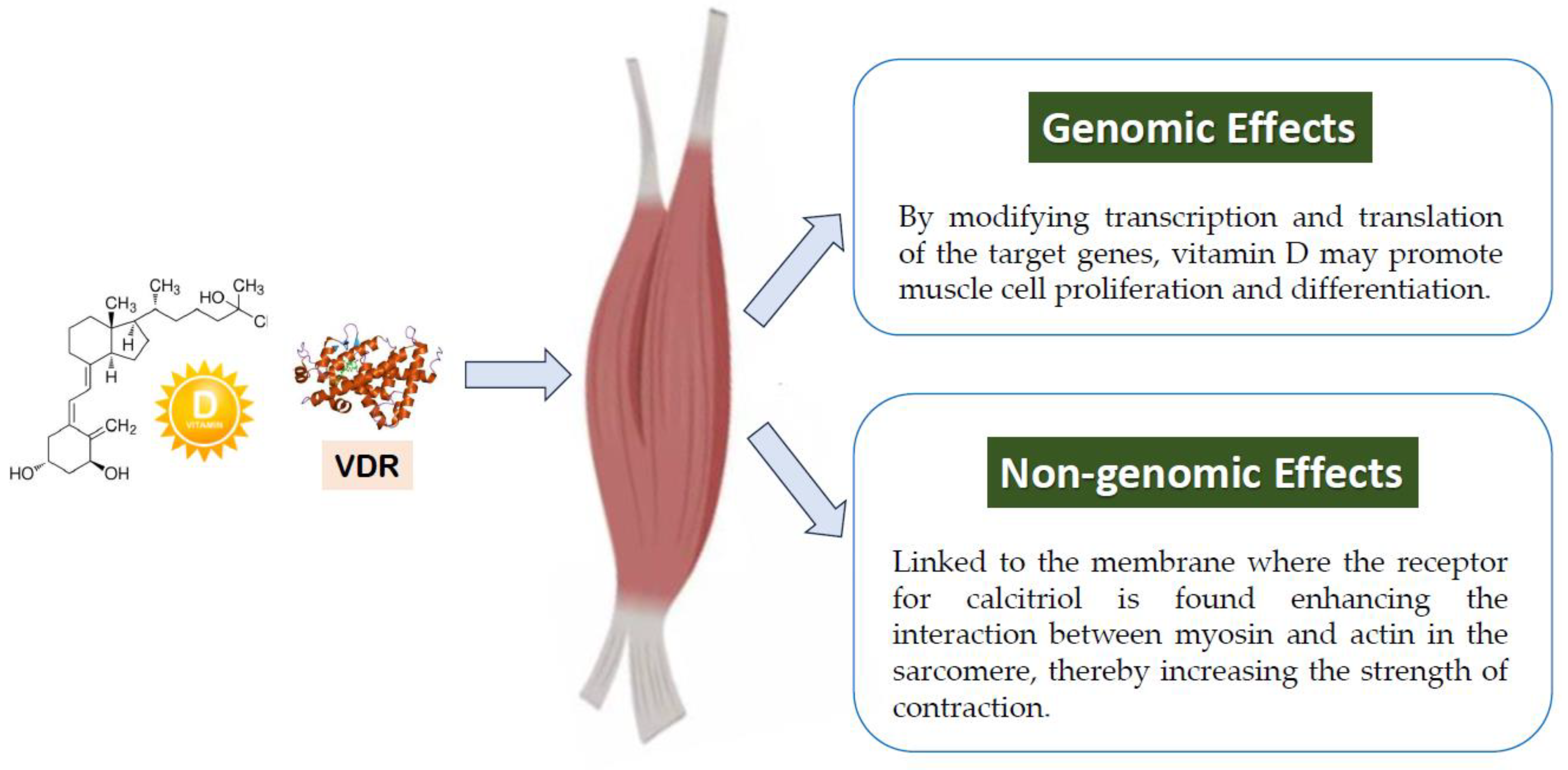
2.3.1. Muscle
2.3.2. Bone
2.3.3. Cardiovascular and Respiratory Systems
3. Magnesium in Athletes
3.1. The Importance of Magnesium in Cellular Function
3.2. Magnesium Deficiency in Athletes
3.3. Effects of Magnesium of Particular Interest for Athletes
3.3.1. Muscle
3.3.2. Bone
3.3.3. Cardiovascular System
3.3.4. Respiratory System
4. Vitamin D and Magnesium Interactions
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bytomski, J.R. Fueling for Performance. Sports Health 2018, 10, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.T.; Erdman, K.A.; Burke, L.M. American College of Sports Medicine Joint Position Statement. Nutrition and Athletic Performance. Med. Sci. Sports Exerc. 2016, 48, 543–568. [Google Scholar] [CrossRef]
- Rawson, E.S.; Miles, M.P.; Larson-Meyer, D.E. Dietary Supplements for Health, Adaptation, and Recovery in Athletes. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 188–199. [Google Scholar] [CrossRef]
- Charoenngam, N.; Holick, M.F. Immunologic Effects of Vitamin D on Human Health and Disease. Nutrients 2020, 12, 2097. [Google Scholar] [CrossRef]
- Pike, J.W.; Christakos, S. Biology and Mechanisms of Action of the Vitamin D Hormone. Endocrinol. Metab. Clin. N. Am. 2017, 46, 815–843. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, L.J.; Farruggia, M.; Veronese, N.; Barbagallo, M. Vitamin D Sources, Metabolism, and Deficiency: Available Compounds and Guidelines for Its Treatment. Metabolites 2021, 11, 255. [Google Scholar] [CrossRef] [PubMed]
- Bauer, P.; Henni, S.; Dorr, O.; Bauer, T.; Hamm, C.W.; Most, A. High prevalence of vitamin D insufficiency in professional handball athletes. Phys. Sportsmed. 2019, 47, 71–77. [Google Scholar] [CrossRef]
- Carswell, A.T.; Oliver, S.J.; Wentz, L.M.; Kashi, D.S.; Roberts, R.; Tang, J.C.Y.; Izard, R.M.; Jackson, S.; Allan, D.; Rhodes, L.E.; et al. Influence of Vitamin D Supplementation by Sunlight or Oral D3 on Exercise Performance. Med. Sci. Sports Exerc. 2018, 50, 2555–2564. [Google Scholar] [CrossRef]
- Rebolledo, B.J.; Bernard, J.A.; Werner, B.C.; Finlay, A.K.; Nwachukwu, B.U.; Dare, D.M.; Warren, R.F.; Rodeo, S.A. The Association of Vitamin D Status in Lower Extremity Muscle Strains and Core Muscle Injuries at the National Football League Combine. J. Arthrosc. Relat. Surg. 2018, 34, 1280–1285. [Google Scholar] [CrossRef]
- de la Puente Yague, M.; Collado Yurrita, L.; Ciudad Cabanas, M.J.; Cuadrado Cenzual, M.A. Role of Vitamin D in Athletes and Their Performance: Current Concepts and New Trends. Nutrients 2020, 12, 579. [Google Scholar] [CrossRef]
- Dominguez, L.J.; Veronese, N.; Barbagallo, M. Magnesium and the Hallmarks of Aging. Nutrients 2024, 16, 496. [Google Scholar] [CrossRef]
- Barbagallo, M.; Belvedere, M.; Dominguez, L.J. Magnesium homeostasis and aging. Magnes. Res. 2009, 22, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Kleczkowski, L.A.; Igamberdiev, A.U. Adenylate-driven equilibration of both ribo- and deoxyribonucleotides is under magnesium control: Quantification of the Mg2+-signal. J. Plant Physiol. 2025, 304, 154380. [Google Scholar] [CrossRef] [PubMed]
- Romani, A.M. Cellular magnesium homeostasis. Arch. Biochem. Biophys. 2011, 512, 1–23. [Google Scholar] [CrossRef]
- Littlefield, N.A.; Hass, B.S.; McGarrity, L.J.; Morris, S.M. Effect of magnesium on the growth and cell cycle of transformed and non-transformed epithelial rat liver cells in vitro. Cell Biol. Toxicol. 1991, 7, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Barbagallo, M.; Dominguez, L.J.; Galioto, A.; Ferlisi, A.; Cani, C.; Malfa, L.; Pineo, A.; Busardo, A.; Paolisso, G. Role of magnesium in insulin action, diabetes and cardio-metabolic syndrome X. Mol. Aspects Med. 2003, 24, 39–52. [Google Scholar] [CrossRef]
- Barbagallo, M.; Dominguez, L.J. Magnesium and type 2 diabetes. World J. Diabetes 2015, 6, 1152–1157. [Google Scholar] [CrossRef]
- Caspi, R.; Altman, T.; Dreher, K.; Fulcher, C.A.; Subhraveti, P.; Keseler, I.M.; Kothari, A.; Krummenacker, M.; Latendresse, M.; Mueller, L.A.; et al. The MetaCyc database of metabolic pathways and enzymes and the BioCyc collection of pathway/genome databases. Nucleic Acids Res. 2012, 40, D742–D753. [Google Scholar] [CrossRef]
- Dorup, I.; Clausen, T. Effects of magnesium and zinc deficiencies on growth and protein synthesis in skeletal muscle and the heart. Br. J. Nutr. 1991, 66, 493–504. [Google Scholar] [CrossRef]
- Gupta, R.K.; Benovic, J.L.; Rose, Z.B. The determination of the free magnesium level in the human red blood cell by 31P NMR. J. Biol. Chem. 1978, 253, 6172–6176. [Google Scholar] [CrossRef]
- Cameron, D.; Welch, A.A.; Adelnia, F.; Bergeron, C.M.; Reiter, D.A.; Dominguez, L.J.; Brennan, N.A.; Fishbein, K.W.; Spencer, R.G.; Ferrucci, L. Age and Muscle Function Are More Closely Associated With Intracellular Magnesium, as Assessed by (31)P Magnetic Resonance Spectroscopy, Than With Serum Magnesium. Front. Physiol. 2019, 10, 1454. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, L.J.; Barbagallo, M.; Lauretani, F.; Bandinelli, S.; Bos, A.; Corsi, A.M.; Simonsick, E.M.; Ferrucci, L. Magnesium and muscle performance in older persons: The InCHIANTI study. Am. J. Clin. Nutr. 2006, 84, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Potter, J.D.; Robertson, S.P.; Johnson, J.D. Magnesium and the regulation of muscle contraction. Fed. Proc. 1981, 40, 2653–2656. [Google Scholar]
- Dominguez, L.; Veronese, N.; Barbagallo, M. Magnesium and Hypertension in Old Age. Nutrients 2020, 13, 139. [Google Scholar] [CrossRef] [PubMed]
- Barbagallo, M.; Gupta, R.K.; Dominguez, L.J.; Resnick, L.M. Cellular ionic alterations with age: Relation to hypertension and diabetes. J. Am. Geriatr. Soc. 2000, 48, 1111–1116. [Google Scholar] [CrossRef]
- Dominguez, L.J.; Veronese, N.; Sabico, S.; Al-Daghri, N.M.; Barbagallo, M. Magnesium and Migraine. Nutrients 2025, 17, 725. [Google Scholar] [CrossRef]
- Newhouse, I.J.; Finstad, E.W. The effects of magnesium supplementation on exercise performance. Clin. J. Sport Med. 2000, 10, 195–200. [Google Scholar] [CrossRef]
- Lukaski, H.C. Magnesium, zinc, and chromium nutrition and athletic performance. Can. J. Appl. Physiol. 2001, 26 (Suppl. 1), S13–S22. [Google Scholar] [CrossRef]
- Nielsen, F.H.; Lukaski, H.C. Update on the relationship between magnesium and exercise. Magnes. Res. 2006, 19, 180–189. [Google Scholar]
- Rayssiguier, Y.; Guezennec, C.Y.; Durlach, J. New experimental and clinical data on the relationship between magnesium and sport. Magnes. Res. 1990, 3, 93–102. [Google Scholar]
- Veronese, N.; Berton, L.; Carraro, S.; Bolzetta, F.; De Rui, M.; Perissinotto, E.; Toffanello, E.D.; Bano, G.; Pizzato, S.; Miotto, F.; et al. Effect of oral magnesium supplementation on physical performance in healthy elderly women involved in a weekly exercise program: A randomized controlled trial. Am. J. Clin. Nutr. 2014, 100, 974–981. [Google Scholar] [CrossRef] [PubMed]
- Santos, D.A.; Matias, C.N.; Monteiro, C.P.; Silva, A.M.; Rocha, P.M.; Minderico, C.S.; Bettencourt Sardinha, L.; Laires, M.J. Magnesium intake is associated with strength performance in elite basketball, handball and volleyball players. Magnes. Res. 2011, 24, 215–219. [Google Scholar] [CrossRef]
- Matias, C.N.; Santos, D.A.; Monteiro, C.P.; Silva, A.M.; Raposo Mde, F.; Martins, F.; Sardinha, L.B.; Bicho, M.; Laires, M.J. Magnesium and strength in elite judo athletes according to intracellular water changes. Magnes. Res. 2010, 23, 138–141. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liang, Q.S.; Cheng, L.R.; Li, X.H.; Fu, W.; Dai, W.T.; Li, S.T. Magnesium sulfate enhances non-depolarizing muscle relaxant vecuronium action at adult muscle-type nicotinic acetylcholine receptor in vitro. Acta Pharmacol. Sin. 2011, 32, 1454–1459. [Google Scholar] [CrossRef] [PubMed]
- Serita, R.; Morisaki, H.; Tanaka, C.; Kosugi, S.; Sakuraba, S.; Takeda, J. Effects of magnesium sulfate on neuromuscular function and spontaneous breathing during sevoflurane and spinal anesthesia. J. Anesth. 2007, 21, 86–89. [Google Scholar] [CrossRef]
- Garrison, S.R.; Korownyk, C.S.; Kolber, M.R.; Allan, G.M.; Musini, V.M.; Sekhon, R.K.; Dugré, N. Magnesium for skeletal muscle cramps. Cochrane Database Syst. Rev. 2012, 2012, CD009402. [Google Scholar] [CrossRef]
- Dominguez, L.J.; Veronese, N.; Guerrero-Romero, F.; Barbagallo, M. Magnesium in Infectious Diseases in Older People. Nutrients 2021, 13, 180. [Google Scholar] [CrossRef]
- Dai, Q.; Zhu, X.; Manson, J.E.; Song, Y.; Li, X.; Franke, A.A.; Costello, R.B.; Rosanoff, A.; Nian, H.; Fan, L.; et al. Magnesium status and supplementation influence vitamin D status and metabolism: Results from a randomized trial. Am. J. Clin. Nutr. 2018, 108, 1249–1258. [Google Scholar] [CrossRef]
- Wolf, G. The discovery of vitamin D: The contribution of Adolf Windaus. J. Nutr. 2004, 134, 1299–1302. [Google Scholar] [CrossRef]
- Blunt, J.W.; Tanaka, Y.; DeLuca, H.F. Biological activity of 25-hydroxycholecalciferol, a metabolite of vitamin D3. Proc. Natl. Acad. Sci. USA 1968, 61, 1503–1506. [Google Scholar] [CrossRef]
- Ponchon, G.; Kennan, A.L.; DeLuca, H.F. “Activation” of vitamin D by the liver. J. Clin. Investig. 1969, 48, 2032–2037. [Google Scholar] [CrossRef]
- Norman, A.W.; Myrtle, J.F.; Midgett, R.J.; Nowicki, H.G.; Williams, V.; Popjak, G. 1,25-dihydroxycholecalciferol: Identification of the proposed active form of vitamin D3 in the intestine. Science 1971, 173, 51–54. [Google Scholar] [CrossRef]
- Holick, M.F.; Schnoes, H.K.; DeLuca, H.F. Identification of 1,25-dihydroxycholecalciferol, a form of vitamin D3 metabolically active in the intestine. Proc. Natl. Acad. Sci. USA 1971, 68, 803–804. [Google Scholar] [CrossRef] [PubMed]
- DeLuca, H.F. Overview of general physiologic features and functions of vitamin D. Am. J. Clin. Nutr. 2004, 80, 1689S–1696S. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Dattola, A.; Silvestri, M.; Bennardo, L.; Passante, M.; Scali, E.; Patruno, C.; Nistico, S.P. Role of Vitamins in Skin Health: A Systematic Review. Curr. Nutr. Rep. 2020, 9, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Webb, A.R.; Engelsen, O. Calculated ultraviolet exposure levels for a healthy vitamin D status. Photochem. Photobiol. 2006, 82, 1697–1703. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D: Importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. Am. J. Clin. Nutr. 2004, 79, 362–371. [Google Scholar] [CrossRef]
- Vieth, R. Vitamin D supplementation, 25-hydroxyvitamin D concentrations, and safety. Am. J. Clin. Nutr. 1999, 69, 842–856. [Google Scholar] [CrossRef]
- Binkley, N.; Novotny, R.; Krueger, D.; Kawahara, T.; Daida, Y.G.; Lensmeyer, G.; Hollis, B.W.; Drezner, M.K. Low vitamin D status despite abundant sun exposure. J. Clin. Endocrinol. Metab. 2007, 92, 2130–2135. [Google Scholar] [CrossRef]
- Haddad, J.G. Vitamin D--solar rays, the Milky Way, or both? N. Engl. J. Med. 1992, 326, 1213–1215. [Google Scholar] [CrossRef] [PubMed]
- Neville, J.J.; Palmieri, T.; Young, A.R. Physical Determinants of Vitamin D Photosynthesis: A Review. JBMR Plus 2021, 5, e10460. [Google Scholar] [CrossRef]
- Christakos, S.; Dhawan, P.; Verstuyf, A.; Verlinden, L.; Carmeliet, G. Vitamin D: Metabolism, Molecular Mechanism of Action, and Pleiotropic Effects. Physiol. Rev. 2016, 96, 365–408. [Google Scholar] [CrossRef]
- Smith, J.E.; Goodman, D.S. The turnover and transport of vitamin D and of a polar metabolite with the properties of 25-hydroxycholecalciferol in human plasma. J. Clin. Investig. 1971, 50, 2159–2167. [Google Scholar] [CrossRef] [PubMed]
- Takeyama, K.; Kitanaka, S.; Sato, T.; Kobori, M.; Yanagisawa, J.; Kato, S. 25-Hydroxyvitamin D3 1alpha-hydroxylase and vitamin D synthesis. Science 1997, 277, 1827–1830. [Google Scholar] [CrossRef] [PubMed]
- Haussler, M.R.; Norman, A.W. Chromosomal receptor for a vitamin D metabolite. Proc. Natl. Acad. Sci. USA 1969, 62, 155–162. [Google Scholar] [CrossRef]
- Baker, A.R.; McDonnell, D.P.; Hughes, M.; Crisp, T.M.; Mangelsdorf, D.J.; Haussler, M.R.; Pike, J.W.; Shine, J.; O’Malley, B.W. Cloning and expression of full-length cDNA encoding human vitamin D receptor. Proc. Natl. Acad. Sci. USA 1988, 85, 3294–3298. [Google Scholar] [CrossRef]
- McDonnell, D.P.; Mangelsdorf, D.J.; Pike, J.W.; Haussler, M.R.; O’Malley, B.W. Molecular cloning of complementary DNA encoding the avian receptor for vitamin D. Science 1987, 235, 1214–1217. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa, T.; Handa, Y.; Uematsu, Y.; Takeda, S.; Sekine, K.; Yoshihara, Y.; Kawakami, T.; Arioka, K.; Sato, H.; Uchiyama, Y.; et al. Mice lacking the vitamin D receptor exhibit impaired bone formation, uterine hypoplasia and growth retardation after weaning. Nat. Genet. 1997, 16, 391–396. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D: An ancient hormone. Exp. Dermatol. 2011, 20, 7–13. [Google Scholar] [CrossRef]
- Walters, M.R. Newly identified actions of the vitamin D endocrine system. Endocr. Rev. 1992, 13, 719–764. [Google Scholar] [CrossRef] [PubMed]
- Larson-Meyer, D.E.; Douglas, C.S.; Thomas, J.J.; Johnson, E.C.; Barcal, J.N.; Heller, J.E.; Hollis, B.W.; Halliday, T.M. Validation of a Vitamin D Specific Questionnaire to Determine Vitamin D Status in Athletes. Nutrients 2019, 11, 2732. [Google Scholar] [CrossRef] [PubMed]
- Sempos, C.T.; Heijboer, A.C.; Bikle, D.D.; Bollerslev, J.; Bouillon, R.; Brannon, P.M.; DeLuca, H.F.; Jones, G.; Munns, C.F.; Bilezikian, J.P.; et al. Vitamin D assays and the definition of hypovitaminosis D: Results from the First International Conference on Controversies in Vitamin D. Br. J. Clin. Pharmacol. 2018, 84, 2194–2207. [Google Scholar] [CrossRef] [PubMed]
- Giustina, A.; Adler, R.A.; Binkley, N.; Bouillon, R.; Ebeling, P.R.; Lazaretti-Castro, M.; Marcocci, C.; Rizzoli, R.; Sempos, C.T.; Bilezikian, J.P. Controversies in Vitamin D: Summary Statement From an International Conference. J. Clin. Endocrinol. Metab. 2019, 104, 234–240. [Google Scholar] [CrossRef]
- Mehran, N.; Schulz, B.M.; Neri, B.R.; Robertson, W.J.; Limpisvasti, O. Prevalence of Vitamin D Insufficiency in Professional Hockey Players. Orthop. J. Sports Med. 2016, 4, 2325967116677512. [Google Scholar] [CrossRef]
- Engelsen, O. The relationship between ultraviolet radiation exposure and vitamin D status. Nutrients 2010, 2, 482–495. [Google Scholar] [CrossRef]
- Lehtonen-Veromaa, M.; Mottonen, T.; Irjala, K.; Karkkainen, M.; Lamberg-Allardt, C.; Hakola, P.; Viikari, J. Vitamin D intake is low and hypovitaminosis D common in healthy 9- to 15-year-old Finnish girls. Eur. J. Clin. Nutr. 1999, 53, 746–751. [Google Scholar] [CrossRef]
- Bezuglov, E.; Tikhonova, A.; Zueva, A.; Khaitin, V.; Waskiewicz, Z.; Gerasimuk, D.; Zebrowska, A.; Rosemann, T.; Nikolaidis, P.; Knechtle, B. Prevalence and Treatment of Vitamin D Deficiency in Young Male Russian Soccer Players in Winter. Nutrients 2019, 11, 2405. [Google Scholar] [CrossRef]
- Barsan, M.; Chelaru, V.F.; Rajnoveanu, A.G.; Popa, S.L.; Socaciu, A.I.; Badulescu, A.V. Difference in Levels of Vitamin D between Indoor and Outdoor Athletes: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2023, 24, 7584. [Google Scholar] [CrossRef]
- Farrokhyar, F.; Tabasinejad, R.; Dao, D.; Peterson, D.; Ayeni, O.R.; Hadioonzadeh, R.; Bhandari, M. Prevalence of vitamin D inadequacy in athletes: A systematic-review and meta-analysis. Sports Med. 2015, 45, 365–378. [Google Scholar] [CrossRef]
- Ksiazek, A.; Zagrodna, A.; Slowinska-Lisowska, M. Vitamin D, Skeletal Muscle Function and Athletic Performance in Athletes-A Narrative Review. Nutrients 2019, 11, 1800. [Google Scholar] [CrossRef] [PubMed]
- Farrokhyar, F.; Sivakumar, G.; Savage, K.; Koziarz, A.; Jamshidi, S.; Ayeni, O.R.; Peterson, D.; Bhandari, M. Effects of Vitamin D Supplementation on Serum 25-Hydroxyvitamin D Concentrations and Physical Performance in Athletes: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Sports Med. 2017, 47, 2323–2339. [Google Scholar] [CrossRef] [PubMed]
- Langley, C.K.; Morse, C.I.; Buffey, A.J. The Prevalence of Low Vitamin D in Elite Para-Athletes: A Systematic Review. Sports Med. Open 2024, 10, 96. [Google Scholar] [CrossRef] [PubMed]
- Scragg, R.; Holdaway, I.; Jackson, R.; Lim, T. Plasma 25-hydroxyvitamin D3 and its relation to physical activity and other heart disease risk factors in the general population. Ann. Epidemiol. 1992, 2, 697–703. [Google Scholar] [CrossRef]
- Kluczynski, M.A.; Lamonte, M.J.; Mares, J.A.; Wactawski-Wende, J.; Smith, A.W.; Engelman, C.D.; Andrews, C.A.; Snetselaar, L.G.; Sarto, G.E.; Millen, A.E. Duration of physical activity and serum 25-hydroxyvitamin D status of postmenopausal women. Ann. Epidemiol. 2011, 21, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Brock, K.; Cant, R.; Clemson, L.; Mason, R.S.; Fraser, D.R. Effects of diet and exercise on plasma vitamin D (25(OH)D) levels in Vietnamese immigrant elderly in Sydney, Australia. J. Steroid Biochem. Mol. Biol. 2007, 103, 786–792. [Google Scholar] [CrossRef]
- Scragg, R.; Camargo, C.A., Jr. Frequency of leisure-time physical activity and serum 25-hydroxyvitamin D levels in the US population: Results from the Third National Health and Nutrition Examination Survey. Am. J. Epidemiol. 2008, 168, 577–586; discussion 587–591. [Google Scholar] [CrossRef]
- Sun, X.; Cao, Z.B.; Taniguchi, H.; Tanisawa, K.; Higuchi, M. Effect of an Acute Bout of Endurance Exercise on Serum 25(OH)D Concentrations in Young Adults. J. Clin. Endocrinol. Metab. 2017, 102, 3937–3944. [Google Scholar] [CrossRef]
- Barker, T.; Henriksen, V.T.; Martins, T.B.; Hill, H.R.; Kjeldsberg, C.R.; Schneider, E.D.; Dixon, B.M.; Weaver, L.K. Higher serum 25-hydroxyvitamin D concentrations associate with a faster recovery of skeletal muscle strength after muscular injury. Nutrients 2013, 5, 1253–1275. [Google Scholar] [CrossRef]
- Maimoun, L.; Manetta, J.; Couret, I.; Dupuy, A.M.; Mariano-Goulart, D.; Micallef, J.P.; Peruchon, E.; Rossi, M. The intensity level of physical exercise and the bone metabolism response. Int. J. Sports Med. 2006, 27, 105–111. [Google Scholar] [CrossRef]
- Maimoun, L.; Simar, D.; Caillaud, C.; Coste, O.; Barbotte, E.; Peruchon, E.; Rossi, M.; Mariano-Goulart, D. Response of calciotropic hormones and bone turnover to brisk walking according to age and fitness level. J. Sci. Med. Sport 2009, 12, 463–467. [Google Scholar] [CrossRef] [PubMed]
- Rosen, C.J.; Adams, J.S.; Bikle, D.D.; Black, D.M.; Demay, M.B.; Manson, J.E.; Murad, M.H.; Kovacs, C.S. The nonskeletal effects of vitamin D: An Endocrine Society scientific statement. Endocr. Rev. 2012, 33, 456–492. [Google Scholar] [CrossRef] [PubMed]
- Knechtle, B.; Nikolaidis, P.T. Vitamin D and Sport Performance. Nutrients 2020, 12, 841. [Google Scholar] [CrossRef]
- Wicinski, M.; Adamkiewicz, D.; Adamkiewicz, M.; Sniegocki, M.; Podhorecka, M.; Szychta, P.; Malinowski, B. Impact of Vitamin D on Physical Efficiency and Exercise Performance—A Review. Nutrients 2019, 11, 2826. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, H.A.; Borchers, M.; Gudat, F.; Duermueller, U.; Theiler, R.; Stahelin, H.B.; Dick, W. In situ detection of 1,25-dihydroxyvitamin D3 receptor in human skeletal muscle tissue. Histochem. J. 2001, 33, 19–24. [Google Scholar] [CrossRef]
- Wang, Y.; DeLuca, H.F. Is the vitamin d receptor found in muscle? Endocrinology 2011, 152, 354–363. [Google Scholar] [CrossRef]
- Srikuea, R.; Zhang, X.; Park-Sarge, O.K.; Esser, K.A. VDR and CYP27B1 are expressed in C2C12 cells and regenerating skeletal muscle: Potential role in suppression of myoblast proliferation. Am. J. Physiol. Cell Physiol. 2012, 303, C396–C405. [Google Scholar] [CrossRef]
- Ceglia, L. Vitamin D and skeletal muscle tissue and function. Mol. Asp. Med. 2008, 29, 407–414. [Google Scholar] [CrossRef]
- Koundourakis, N.E.; Avgoustinaki, P.D.; Malliaraki, N.; Margioris, A.N. Muscular effects of vitamin D in young athletes and non-athletes and in the elderly. Hormones 2016, 15, 471–488. [Google Scholar] [CrossRef]
- Oh, J.H.; Kim, S.H.; Kim, J.H.; Shin, Y.H.; Yoon, J.P.; Oh, C.H. The level of vitamin D in the serum correlates with fatty degeneration of the muscles of the rotator cuff. J. Bone Jt. Surg. Br. 2009, 91, 1587–1593. [Google Scholar] [CrossRef]
- Alliband, K.H.; Parr, T.; Jethwa, P.H.; Brameld, J.M. Active vitamin D increases myogenic differentiation in C2C12 cells via a vitamin D response element on the myogenin promoter. Front. Physiol. 2023, 14, 1322677. [Google Scholar] [CrossRef] [PubMed]
- Stratos, I.; Schleese, S.; Rinas, I.; Vollmar, B.; Mittlmeier, T. Effect of Calcitriol and Vitamin D Receptor Modulator 2 on Recovery of Injured Skeletal Muscle in Wistar Rats. Biomedicines 2023, 11, 2477. [Google Scholar] [CrossRef] [PubMed]
- Sist, M.; Zou, L.; Galloway, S.D.R.; Rodriguez-Sanchez, N. Effects of vitamin D supplementation on maximal strength and power in athletes: A systematic review and meta-analysis of randomized controlled trials. Front. Nutr. 2023, 10, 1163313. [Google Scholar] [CrossRef]
- Bergwitz, C.; Juppner, H. Regulation of phosphate homeostasis by PTH, vitamin D, and FGF23. Annu. Rev. Med. 2010, 61, 91–104. [Google Scholar] [CrossRef]
- Maroon, J.C.; Mathyssek, C.M.; Bost, J.W.; Amos, A.; Winkelman, R.; Yates, A.P.; Duca, M.A.; Norwig, J.A. Vitamin D profile in National Football League players. Am. J. Sports Med. 2015, 43, 1241–1245. [Google Scholar] [CrossRef]
- Silk, L.N.; Greene, D.A.; Baker, M.K.; Jander, C.B. The effect of calcium and vitamin D supplementation on bone health of male Jockeys. J. Sci. Med. Sport 2017, 20, 225–229. [Google Scholar] [CrossRef]
- Fredericson, M.; Jennings, F.; Beaulieu, C.; Matheson, G.O. Stress fractures in athletes. Top. Magn. Reson. Imaging 2006, 17, 309–325. [Google Scholar] [CrossRef] [PubMed]
- Schnackenburg, K.E.; Macdonald, H.M.; Ferber, R.; Wiley, J.P.; Boyd, S.K. Bone quality and muscle strength in female athletes with lower limb stress fractures. Med. Sci. Sports Exerc. 2011, 43, 2110–2119. [Google Scholar] [CrossRef]
- Kelsey, J.L.; Bachrach, L.K.; Procter-Gray, E.; Nieves, J.; Greendale, G.A.; Sowers, M.; Brown, B.W., Jr.; Matheson, K.A.; Crawford, S.L.; Cobb, K.L. Risk factors for stress fracture among young female cross-country runners. Med. Sci. Sports Exerc. 2007, 39, 1457–1463. [Google Scholar] [CrossRef] [PubMed]
- Bennell, K.L.; Malcolm, S.A.; Thomas, S.A.; Wark, J.D.; Brukner, P.D. The incidence and distribution of stress fractures in competitive track and field athletes. A twelve-month prospective study. Am. J. Sports Med. 1996, 24, 211–217. [Google Scholar] [CrossRef]
- Davey, T.; Lanham-New, S.A.; Shaw, A.M.; Hale, B.; Cobley, R.; Berry, J.L.; Roch, M.; Allsopp, A.J.; Fallowfield, J.L. Low serum 25-hydroxyvitamin D is associated with increased risk of stress fracture during Royal Marine recruit training. Osteoporos. Int. 2016, 27, 171–179. [Google Scholar] [CrossRef]
- Richards, T.; Wright, C. British Army recruits with low serum vitamin D take longer to recover from stress fractures. BMJ Mil. Health 2020, 166, 240–242. [Google Scholar] [CrossRef] [PubMed]
- Lappe, J.; Cullen, D.; Haynatzki, G.; Recker, R.; Ahlf, R.; Thompson, K. Calcium and vitamin d supplementation decreases incidence of stress fractures in female navy recruits. J. Bone Miner. Res. 2008, 23, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Dao, D.; Sodhi, S.; Tabasinejad, R.; Peterson, D.; Ayeni, O.R.; Bhandari, M.; Farrokhyar, F. Serum 25-Hydroxyvitamin D Levels and Stress Fractures in Military Personnel: A Systematic Review and Meta-analysis. Am. J. Sports Med. 2015, 43, 2064–2072. [Google Scholar] [CrossRef] [PubMed]
- Shimasaki, Y.; Nagao, M.; Miyamori, T.; Aoba, Y.; Fukushi, N.; Saita, Y.; Ikeda, H.; Kim, S.G.; Nozawa, M.; Kaneko, K.; et al. Evaluating the Risk of a Fifth Metatarsal Stress Fracture by Measuring the Serum 25-Hydroxyvitamin D Levels. Foot Ankle Int. 2016, 37, 307–311. [Google Scholar] [CrossRef]
- Nieves, J.W.; Melsop, K.; Curtis, M.; Kelsey, J.L.; Bachrach, L.K.; Greendale, G.; Sowers, M.F.; Sainani, K.L. Nutritional factors that influence change in bone density and stress fracture risk among young female cross-country runners. Randomized Control. Trial 2010, 2, 740–750, quiz 794. [Google Scholar] [CrossRef]
- Sejersen, C.; Volianitis, S.; Secher, N.H. The athlete’s heart: Allometric considerations on published papers and relation to cardiovascular variables. Eur. J. Appl. Physiol. 2024, 124, 1337–1346. [Google Scholar] [CrossRef]
- Thompson, B.; Waterhouse, M.; English, D.R.; McLeod, D.S.; Armstrong, B.K.; Baxter, C.; Duarte Romero, B.; Ebeling, P.R.; Hartel, G.; Kimlin, M.G.; et al. Vitamin D supplementation and major cardiovascular events: D-Health randomised controlled trial. BMJ 2023, 381, e075230. [Google Scholar] [CrossRef]
- de la Guia-Galipienso, F.; Martinez-Ferran, M.; Vallecillo, N.; Lavie, C.J.; Sanchis-Gomar, F.; Pareja-Galeano, H. Vitamin D and cardiovascular health. Clin. Nutr. 2021, 40, 2946–2957. [Google Scholar] [CrossRef]
- Allison, R.J.; Close, G.L.; Farooq, A.; Riding, N.R.; Salah, O.; Hamilton, B.; Wilson, M.G. Severely vitamin D-deficient athletes present smaller hearts than sufficient athletes. Eur. J. Prev. Cardiol. 2015, 22, 535–542. [Google Scholar] [CrossRef]
- Owens, D.J.; Allison, R.; Close, G.L. Vitamin D and the Athlete: Current Perspectives and New Challenges. Sports Med. 2018, 48, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Halliday, T.M.; Peterson, N.J.; Thomas, J.J.; Kleppinger, K.; Hollis, B.W.; Larson-Meyer, D.E. Vitamin D status relative to diet, lifestyle, injury, and illness in college athletes. Med. Sci. Sports Exerc. 2011, 43, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Sabetta, J.R.; DePetrillo, P.; Cipriani, R.J.; Smardin, J.; Burns, L.A.; Landry, M.L. Serum 25-hydroxyvitamin d and the incidence of acute viral respiratory tract infections in healthy adults. PLoS ONE 2010, 5, e11088. [Google Scholar] [CrossRef] [PubMed]
- He, C.S.; Aw Yong, X.H.; Walsh, N.P.; Gleeson, M. Is there an optimal vitamin D status for immunity in athletes and military personnel? Exerc. Immunol. Rev. 2016, 22, 42–64. [Google Scholar]
- Bergman, P.; Lindh, A.U.; Bjorkhem-Bergman, L.; Lindh, J.D. Vitamin D and Respiratory Tract Infections: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. PLoS ONE 2013, 8, e65835. [Google Scholar] [CrossRef]
- Urashima, M.; Segawa, T.; Okazaki, M.; Kurihara, M.; Wada, Y.; Ida, H. Randomized trial of vitamin D supplementation to prevent seasonal influenza A in schoolchildren. Am. J. Clin. Nutr. 2010, 91, 1255–1260. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, J.M.; Subramanian, S.; Laird, E.; Kenny, R.A. Editorial: Low population mortality from COVID-19 in countries south of latitude 35 degrees North supports vitamin D as a factor determining severity. Aliment. Pharmacol. Ther. 2020, 51, 1434–1437. [Google Scholar] [CrossRef]
- Martineau, A.R.; Jolliffe, D.A.; Hooper, R.L.; Greenberg, L.; Aloia, J.F.; Bergman, P.; Dubnov-Raz, G.; Esposito, S.; Ganmaa, D.; Ginde, A.A.; et al. Vitamin D supplementation to prevent acute respiratory tract infections: Systematic review and meta-analysis of individual participant data. BMJ 2017, 356, i6583. [Google Scholar] [CrossRef]
- Barbagallo, M.; Veronese, N.; Dominguez, L.J. Magnesium in Aging, Health and Diseases. Nutrients 2021, 13, 463. [Google Scholar] [CrossRef]
- Kleczkowski, L.A.; Igamberdiev, A.U. Magnesium and cell energetics: At the junction of metabolism of adenylate and non-adenylate nucleotides. J. Plant Physiol. 2023, 280, 153901. [Google Scholar] [CrossRef]
- Veronese, N.; Demurtas, J.; Pesolillo, G.; Celotto, S.; Barnini, T.; Calusi, G.; Caruso, M.G.; Notarnicola, M.; Reddavide, R.; Stubbs, B.; et al. Magnesium and health outcomes: An umbrella review of systematic reviews and meta-analyses of observational and intervention studies. Eur. J. Nutr. 2020, 59, 263–272. [Google Scholar] [CrossRef]
- Barbagallo, M.; Veronese, N.; Dominguez, L.J. Magnesium in Type 2 Diabetes Mellitus, Obesity, and Metabolic Syndrome. Nutrients 2022, 14, 714. [Google Scholar] [CrossRef] [PubMed]
- Feeney, K.A.; Hansen, L.L.; Putker, M.; Olivares-Yanez, C.; Day, J.; Eades, L.J.; Larrondo, L.F.; Hoyle, N.P.; O’Neill, J.S.; van Ooijen, G. Daily magnesium fluxes regulate cellular timekeeping and energy balance. Nature 2016, 532, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Veronese, N.; Dominguez, L.J.; Pizzol, D.; Demurtas, J.; Smith, L.; Barbagallo, M. Oral Magnesium Supplementation for Treating Glucose Metabolism Parameters in People with or at Risk of Diabetes: A Systematic Review and Meta-Analysis of Double-Blind Randomized Controlled Trials. Nutrients 2021, 13, 4074. [Google Scholar] [CrossRef]
- Hou, H.; Wang, L.; Fu, T.; Papasergi, M.; Yule, D.I.; Xia, H. Magnesium Acts as a Second Messenger in the Regulation of NMDA Receptor-Mediated CREB Signaling in Neurons. Mol. Neurobiol. 2020, 57, 2539–2550. [Google Scholar] [CrossRef] [PubMed]
- Pochwat, B.; Szewczyk, B.; Sowa-Kucma, M.; Siwek, A.; Doboszewska, U.; Piekoszewski, W.; Gruca, P.; Papp, M.; Nowak, G. Antidepressant-like activity of magnesium in the chronic mild stress model in rats: Alterations in the NMDA receptor subunits. Int. J. Neuropsychopharmacol. 2014, 17, 393–405. [Google Scholar] [CrossRef]
- Sun, J.; Lin, H.; He, G.; Lin, W.; Yang, J. Magnesium sulphate attenuate remifentanil-induced postoperative hyperalgesia via regulating tyrosine phosphorylation of the NR(2)B subunit of the NMDA receptor in the spinal cord. BMC Anesthesiol. 2017, 17, 30. [Google Scholar] [CrossRef]
- Li, F.Y.; Chaigne-Delalande, B.; Kanellopoulou, C.; Davis, J.C.; Matthews, H.F.; Douek, D.C.; Cohen, J.I.; Uzel, G.; Su, H.C.; Lenardo, M.J. Second messenger role for Mg2+ revealed by human T-cell immunodeficiency. Nature 2011, 475, 471–476. [Google Scholar] [CrossRef]
- Li, F.Y.; Chaigne-Delalande, B.; Su, H.; Uzel, G.; Matthews, H.; Lenardo, M.J. XMEN disease: A new primary immunodeficiency affecting Mg2+ regulation of immunity against Epstein-Barr virus. Blood 2014, 123, 2148–2152. [Google Scholar] [CrossRef]
- Chaigne-Delalande, B.; Li, F.Y.; O’Connor, G.M.; Lukacs, M.J.; Jiang, P.; Zheng, L.; Shatzer, A.; Biancalana, M.; Pittaluga, S.; Matthews, H.F.; et al. Mg2+ regulates cytotoxic functions of NK and CD8 T cells in chronic EBV infection through NKG2D. Science 2013, 341, 186–191. [Google Scholar] [CrossRef]
- Tur, J.; Chapalamadagu, K.C.; Manickam, R.; Cheng, F.; Tipparaju, S.M. Deletion of Kvbeta2 (AKR6) Attenuates Isoproterenol Induced Cardiac Injury with Links to Solute Carrier Transporter SLC41a3 and Circadian Clock Genes. Metabolites 2021, 11, 201. [Google Scholar] [CrossRef] [PubMed]
- Resnick, L.M.; Barbagallo, M.; Dominguez, L.J.; Veniero, J.M.; Nicholson, J.P.; Gupta, R.K. Relation of cellular potassium to other mineral ions in hypertension and diabetes. Hypertension 2001, 38, 709–712. [Google Scholar] [CrossRef] [PubMed]
- Saris, N.E.; Mervaala, E.; Karppanen, H.; Khawaja, J.A.; Lewenstam, A. Magnesium. An update on physiological, clinical and analytical aspects. Clin. Chim. Acta 2000, 294, 1–26. [Google Scholar] [CrossRef]
- Ford, E.S.; Mokdad, A.H. Dietary magnesium intake in a national sample of US adults. J. Nutr. 2003, 133, 2879–2882. [Google Scholar] [CrossRef]
- Mensink, G.B.; Fletcher, R.; Gurinovic, M.; Huybrechts, I.; Lafay, L.; Serra-Majem, L.; Szponar, L.; Tetens, I.; Verkaik-Kloosterman, J.; Baka, A.; et al. Mapping low intake of micronutrients across Europe. Br. J. Nutr. 2013, 110, 755–773. [Google Scholar] [CrossRef] [PubMed]
- King, D.E.; Mainous, A.G., 3rd; Geesey, M.E.; Woolson, R.F. Dietary magnesium and C-reactive protein levels. J. Am. Coll. Nutr. 2005, 24, 166–171. [Google Scholar] [CrossRef]
- Rosanoff, A.; Weaver, C.M.; Rude, R.K. Suboptimal magnesium status in the United States: Are the health consequences underestimated? Nutr. Rev. 2012, 70, 153–164. [Google Scholar] [CrossRef]
- Marriott, B.P.; Olsho, L.; Hadden, L.; Connor, P. Intake of added sugars and selected nutrients in the United States, National Health and Nutrition Examination Survey (NHANES) 2003-2006. Crit. Rev. Food Sci. Nutr. 2010, 50, 228–258. [Google Scholar] [CrossRef]
- Ye, L.; Zhang, C.; Duan, Q.; Shao, Y.; Zhou, J. Association of Magnesium Depletion Score With Cardiovascular Disease and Its Association With Longitudinal Mortality in Patients With Cardiovascular Disease. J. Am. Heart Assoc. 2023, 12, e030077. [Google Scholar] [CrossRef]
- Oost, L.J.; van der Heijden, A.; Vermeulen, E.A.; Bos, C.; Elders, P.J.M.; Slieker, R.C.; Kurstjens, S.; van Berkel, M.; Hoenderop, J.G.J.; Tack, C.J.; et al. Serum Magnesium Is Inversely Associated With Heart Failure, Atrial Fibrillation, and Microvascular Complications in Type 2 Diabetes. Diabetes Care 2021, 44, 1757–1765. [Google Scholar] [CrossRef]
- Grober, U.; Schmidt, J.; Kisters, K. Magnesium in Prevention and Therapy. Nutrients 2015, 7, 8199–8226. [Google Scholar] [CrossRef] [PubMed]
- Mooren, F.C.; Golf, S.W.; Volker, K. Effect of magnesium on granulocyte function and on the exercise induced inflammatory response. Magnes. Res. 2003, 16, 49–58. [Google Scholar]
- Beller, G.A.; Maher, J.T.; Hartley, L.H.; Bass, D.E.; Wacker, W.E. Changes in serum and sweat magnesium levels during work in the heat. Aviat. Space Environ. Med. 1975, 46, 709–712. [Google Scholar] [PubMed]
- Soria, M.; Gonzalez-Haro, C.; Lopez-Colon, J.L.; Llorente, M.T.; Escanero, J.F. Submaximal exercise intensities do not provoke variations in plasma magnesium concentration in well-trained euhydrated endurance athletes with no magnesium deficiency. Magnes. Res. 2011, 24, 36–44. [Google Scholar] [CrossRef]
- Siquier-Coll, J.; Bartolome, I.; Perez-Quintero, M.; Grijota, F.J.; Munoz, D.; Maynar-Marino, M. Effect of heat exposure and physical exercise until exhaustion in normothermic and hyperthermic conditions on serum, sweat and urinary concentrations of magnesium and phosphorus. J. Therm. Biol. 2019, 84, 176–184. [Google Scholar] [CrossRef]
- Chen, W.; Hammond-Bennett, A.; Hypnar, A.; Mason, S. Health-related physical fitness and physical activity in elementary school students. BMC Public. Health 2018, 18, 195. [Google Scholar] [CrossRef] [PubMed]
- Veronese, N.; Stubbs, B.; Ragusa, F.S.; Hajek, A.; Smith, L.; Barbagallo, M.; Dominguez, L.J.; Fontana, L.; Monastero, R.; Soysal, P.; et al. Physical activity and persistence of supra-threshold depressive symptoms in older adults: A ten-year cohort study. Psychiatry Res. 2024, 342, 116259. [Google Scholar] [CrossRef]
- Mikkelsen, K.; Stojanovska, L.; Polenakovic, M.; Bosevski, M.; Apostolopoulos, V. Exercise and mental health. Maturitas 2017, 106, 48–56. [Google Scholar] [CrossRef]
- Chow, L.S.; Gerszten, R.E.; Taylor, J.M.; Pedersen, B.K.; van Praag, H.; Trappe, S.; Febbraio, M.A.; Galis, Z.S.; Gao, Y.; Haus, J.M.; et al. Exerkines in health, resilience and disease. Nat. Rev. Endocrinol. 2022, 18, 273–289. [Google Scholar] [CrossRef]
- Piercy, K.L.; Troiano, R.P.; Ballard, R.M.; Carlson, S.A.; Fulton, J.E.; Galuska, D.A.; George, S.M.; Olson, R.D. The Physical Activity Guidelines for Americans. JAMA 2018, 320, 2020–2028. [Google Scholar] [CrossRef]
- Camera, D.M.; Smiles, W.J.; Hawley, J.A. Exercise-induced skeletal muscle signaling pathways and human athletic performance. Free Radic. Biol. Med. 2016, 98, 131–143. [Google Scholar] [CrossRef]
- Margolis, L.M.; Pasiakos, S.M. Optimizing intramuscular adaptations to aerobic exercise: Effects of carbohydrate restriction and protein supplementation on mitochondrial biogenesis. Adv. Nutr. 2013, 4, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Close, G.L.; Hamilton, D.L.; Philp, A.; Burke, L.M.; Morton, J.P. New strategies in sport nutrition to increase exercise performance. Free. Radic. Biol. Med. 2016, 98, 144–158. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, G.; Hamarsland, H.; Cumming, K.T.; Johansen, R.E.; Hulmi, J.J.; Borsheim, E.; Wiig, H.; Garthe, I.; Raastad, T. Vitamin C and E supplementation alters protein signalling after a strength training session, but not muscle growth during 10 weeks of training. J. Physiol. 2014, 592, 5391–5408. [Google Scholar] [CrossRef] [PubMed]
- Wardenaar, F.C.; Clark, N.; Stellingwerff, T.; Siegler, J.; Saunders, B.; Dolan, E.; Wilson, P.B.; Hawley, J.A.; Fuchs, C.J.; Aussieker, T.; et al. Summary of the 2024 Professionals in Nutrition for Exercise and Sport “10 Questions/10 Experts” Session-Hot Topics for the Paris Olympic Games. Int. J. Sport Nutr. Exerc. Metab. 2025, 35, 76–83. [Google Scholar] [CrossRef]
- Souza, A.C.R.; Vasconcelos, A.R.; Dias, D.D.; Komoni, G.; Name, J.J. The Integral Role of Magnesium in Muscle Integrity and Aging: A Comprehensive Review. Nutrients 2023, 15, 5127. [Google Scholar] [CrossRef]
- Mert, T.; Gunes, Y.; Guven, M.; Gunay, I.; Ozcengiz, D. Effects of calcium and magnesium on peripheral nerve conduction. Pol. J. Pharmacol. 2003, 55, 25–30. [Google Scholar]
- Petrovic, J.; Stanic, D.; Dmitrasinovic, G.; Plecas-Solarovic, B.; Ignjatovic, S.; Batinic, B.; Popovic, D.; Pesic, V. Magnesium Supplementation Diminishes Peripheral Blood Lymphocyte DNA Oxidative Damage in Athletes and Sedentary Young Man. Oxid. Med. Cell Longev. 2016, 2016, 2019643. [Google Scholar] [CrossRef]
- Nocella, C.; Cammisotto, V.; Pigozzi, F.; Borrione, P.; Fossati, C.; D’Amico, A.; Cangemi, R.; Peruzzi, M.; Gobbi, G.; Ettorre, E.; et al. Impairment between Oxidant and Antioxidant Systems: Short- and Long-term Implications for Athletes’ Health. Nutrients 2019, 11, 1353. [Google Scholar] [CrossRef]
- Zhang, Y.; Xun, P.; Wang, R.; Mao, L.; He, K. Can Magnesium Enhance Exercise Performance? Nutrients 2017, 9, 946. [Google Scholar] [CrossRef]
- Lukaski, H.C. Vitamin and mineral status: Effects on physical performance. Nutrition 2004, 20, 632–644. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Deminice, R.; Ozdemir, M.; Yoshihara, T.; Bomkamp, M.P.; Hyatt, H. Exercise-induced oxidative stress: Friend or foe? J. Sport Health Sci. 2020, 9, 415–425. [Google Scholar] [CrossRef]
- Negru, A.G.; Pastorcici, A.; Crisan, S.; Cismaru, G.; Popescu, F.G.; Luca, C.T. The Role of Hypomagnesemia in Cardiac Arrhythmias: A Clinical Perspective. Biomedicines 2022, 10, 2356. [Google Scholar] [CrossRef] [PubMed]
- Scherr, J.; Schuster, T.; Pressler, A.; Roeh, A.; Christle, J.; Wolfarth, B.; Halle, M. Repolarization perturbation and hypomagnesemia after extreme exercise. Med. Sci. Sports Exerc. 2012, 44, 1637–1643. [Google Scholar] [CrossRef] [PubMed]
- Bohl, C.H.; Volpe, S.L. Magnesium and exercise. Crit. Rev. Food Sci. Nutr. 2002, 42, 533–563. [Google Scholar] [CrossRef]
- Zalcman, I.; Guarita, H.V.; Juzwiak, C.R.; Crispim, C.A.; Antunes, H.K.; Edwards, B.; Tufik, S.; de Mello, M.T. Nutritional status of adventure racers. Nutrition 2007, 23, 404–411. [Google Scholar] [CrossRef]
- Noda, Y.; Iide, K.; Masuda, R.; Kishida, R.; Nagata, A.; Hirakawa, F.; Yoshimura, Y.; Imamura, H. Nutrient intake and blood iron status of male collegiate soccer players. Asia Pac. J. Clin. Nutr. 2009, 18, 344–350. [Google Scholar]
- Imamura, H.; Iide, K.; Yoshimura, Y.; Kumagai, K.; Oshikata, R.; Miyahara, K.; Oda, K.; Miyamoto, N.; Nakazawa, A. Nutrient intake, serum lipids and iron status of colligiate rugby players. J. Int. Soc. Sports Nutr. 2013, 10, 9. [Google Scholar] [CrossRef]
- Silva, M.R.; Paiva, T. Low energy availability and low body fat of female gymnasts before an international competition. Eur. J. Sport Sci. 2015, 15, 591–599. [Google Scholar] [CrossRef]
- Heaney, S.; O’Connor, H.; Gifford, J.; Naughton, G. Comparison of strategies for assessing nutritional adequacy in elite female athletes’ dietary intake. Int. J. Sport Nutr. Exerc. Metab. 2010, 20, 245–256. [Google Scholar] [CrossRef]
- Lukaski, H.C. Magnesium, zinc, and chromium nutriture and physical activity. Am. J. Clin. Nutr. 2000, 72, 585S–593S. [Google Scholar] [CrossRef]
- Volpe, S.L. Magnesium and the Athlete. Curr. Sports Med. Rep. 2015, 14, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, R.; Guo, S.; Tian, Q.; Zhang, S.; Guo, L.; Liu, T.; Wang, R. Lower serum magnesium concentration and higher 24-h urinary magnesium excretion despite higher dietary magnesium intake in athletes: A systematic review and meta-analysis. Food Sci. Hum. Wellness 2023, 12, 10. [Google Scholar] [CrossRef]
- Maeda, T.; Hamada, Y.; Funakoshi, S.; Hoshi, R.; Tsuji, M.; Narumi-Hyakutake, A.; Matsumoto, M.; Kakutani, Y.; Hatamoto, Y.; Yoshimura, E.; et al. Determination of Optimal Daily Magnesium Intake among Physically Active People: A Scoping Review. J. Nutr. Sci. Vitaminol. 2022, 68, 189–203. [Google Scholar] [CrossRef]
- Pollock, N.; Chakraverty, R.; Taylor, I.; Killer, S.C. An 8-year Analysis of Magnesium Status in Elite International Track & Field Athletes. J. Am. Coll. Nutr. 2020, 39, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Fiorentini, D.; Cappadone, C.; Farruggia, G.; Prata, C. Magnesium: Biochemistry, Nutrition, Detection, and Social Impact of Diseases Linked to Its Deficiency. Nutrients 2021, 13, 1136. [Google Scholar] [CrossRef]
- Reinhart, R.A. Magnesium metabolism. A review with special reference to the relationship between intracellular content and serum levels. Arch. Intern. Med. 1988, 148, 2415–2420. [Google Scholar] [CrossRef]
- Jahnen-Dechent, W.; Ketteler, M. Magnesium basics. Clin. Kidney J. 2012, 5, i3–i14. [Google Scholar] [CrossRef]
- Reddy, S.T.; Soman, S.S.; Yee, J. Magnesium Balance and Measurement. Adv. Chronic Kidney Dis. 2018, 25, 224–229. [Google Scholar] [CrossRef]
- Barbagallo, M.; Dominguez, L.J. Chapter 16-Magnesium, Oxidative Stress, and Aging Muscle. In Aging-Oxidative Stress and Dietary Antioxidants; Preedy, V.R., Ed.; Academic Press: Cambridge, MA, USA, 2014; Volume 1, pp. 157–166. [Google Scholar]
- Short, K.R.; Bigelow, M.L.; Kahl, J.; Singh, R.; Coenen-Schimke, J.; Raghavakaimal, S.; Nair, K.S. Decline in skeletal muscle mitochondrial function with aging in humans. Proc. Natl. Acad. Sci. USA 2005, 102, 5618–5623. [Google Scholar] [CrossRef]
- Barbagallo, M.; Dominguez, L.J. Magnesium and aging. Curr. Pharm. Des. 2010, 16, 832–839. [Google Scholar] [CrossRef] [PubMed]
- Brilla, L.R.; Giroux, M.S.; Taylor, A.; Knutzen, K.M. Magnesium-creatine supplementation effects on body water. Metabolism 2003, 52, 1136–1140. [Google Scholar] [CrossRef]
- Zajac, A.; Golas, A.; Chycki, J.; Halz, M.; Michalczyk, M.M. The Effects of Long-Term Magnesium Creatine Chelate Supplementation on Repeated Sprint Ability (RAST) in Elite Soccer Players. Nutrients 2020, 12, 2961. [Google Scholar] [CrossRef] [PubMed]
- Setaro, L.; Santos-Silva, P.R.; Nakano, E.Y.; Sales, C.H.; Nunes, N.; Greve, J.M.; Colli, C. Magnesium status and the physical performance of volleyball players: Effects of magnesium supplementation. J. Sports Sci. 2014, 32, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Golf, S.W.; Bender, S.; Gruttner, J. On the significance of magnesium in extreme physical stress. Cardiovasc. Drugs Ther. 1998, 12 (Suppl. 2), 197–202. [Google Scholar] [CrossRef]
- Peveler, W.W.; Palmer, T.G. Effect of magnesium lactate dihydrate and calcium lactate monohydrate on 20-km cycling time trial performance. J. Strength. Cond. Res. 2012, 26, 1149–1153. [Google Scholar] [CrossRef]
- Kass, L.S.; Skinner, P.; Poeira, F. A pilot study on the effects of magnesium supplementation with high and low habitual dietary magnesium intake on resting and recovery from aerobic and resistance exercise and systolic blood pressure. J. Sports Sci. Med. 2013, 12, 144–150. [Google Scholar]
- Finstad, E.W.; Newhouse, I.J.; Lukaski, H.C.; McAuliffe, J.E.; Stewart, C.R. The effects of magnesium supplementation on exercise performance. Med. Sci. Sports Exerc. 2001, 33, 493–498. [Google Scholar] [CrossRef]
- Steward, C.J.; Zhou, Y.; Keane, G.; Cook, M.D.; Liu, Y.; Cullen, T. One week of magnesium supplementation lowers IL-6, muscle soreness and increases post-exercise blood glucose in response to downhill running. Eur. J. Appl. Physiol. 2019, 119, 2617–2627. [Google Scholar] [CrossRef]
- Terblanche, S.; Noakes, T.D.; Dennis, S.C.; Marais, D.; Eckert, M. Failure of magnesium supplementation to influence marathon running performance or recovery in magnesium-replete subjects. Int. J. Sport Nutr. 1992, 2, 154–164. [Google Scholar] [CrossRef]
- Kanis, J.A.; Norton, N.; Harvey, N.C.; Jacobson, T.; Johansson, H.; Lorentzon, M.; McCloskey, E.V.; Willers, C.; Borgstrom, F. SCOPE 2021: A new scorecard for osteoporosis in Europe. Arch. Osteoporos. 2021, 16, 82. [Google Scholar] [CrossRef] [PubMed]
- Lorentzon, M.; Abrahamsen, B. Osteoporosis epidemiology using international cohorts. Curr. Opin. Rheumatol. 2022, 34, 280–288. [Google Scholar] [CrossRef]
- Rondanelli, M.; Faliva, M.A.; Barrile, G.C.; Cavioni, A.; Mansueto, F.; Mazzola, G.; Oberto, L.; Patelli, Z.; Pirola, M.; Tartara, A.; et al. Nutrition, Physical Activity, and Dietary Supplementation to Prevent Bone Mineral Density Loss: A Food Pyramid. Nutrients 2021, 14, 74. [Google Scholar] [CrossRef]
- Rude, R.K.; Gruber, H.E.; Norton, H.J.; Wei, L.Y.; Frausto, A.; Kilburn, J. Reduction of dietary magnesium by only 50% in the rat disrupts bone and mineral metabolism. Osteoporos. Int. 2006, 17, 1022–1032. [Google Scholar] [CrossRef]
- Rude, R.K.; Gruber, H.E.; Norton, H.J.; Wei, L.Y.; Frausto, A.; Mills, B.G. Bone loss induced by dietary magnesium reduction to 10% of the nutrient requirement in rats is associated with increased release of substance P and tumor necrosis factor-alpha. J. Nutr. 2004, 134, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Rude, R.K.; Singer, F.R.; Gruber, H.E. Skeletal and hormonal effects of magnesium deficiency. J. Am. Coll. Nutr. 2009, 28, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Luthringer, B.J.; Feyerabend, F.; Schilling, A.F.; Willumeit, R. Effects of extracellular magnesium on the differentiation and function of human osteoclasts. Acta Biomater. 2014, 10, 2843–2854. [Google Scholar] [CrossRef]
- Belluci, M.M.; Schoenmaker, T.; Rossa-Junior, C.; Orrico, S.R.; de Vries, T.J.; Everts, V. Magnesium deficiency results in an increased formation of osteoclasts. J. Nutr. Biochem. 2013, 24, 1488–1498. [Google Scholar] [CrossRef]
- Choi, S.; Kim, K.J.; Cheon, S.; Kim, E.M.; Kim, Y.A.; Park, C.; Kim, K.K. Biochemical activity of magnesium ions on human osteoblast migration. Biochem. Biophys. Res. Commun. 2020, 531, 588–594. [Google Scholar] [CrossRef]
- Wu, L.; Feyerabend, F.; Schilling, A.F.; Willumeit-Romer, R.; Luthringer, B.J.C. Effects of extracellular magnesium extract on the proliferation and differentiation of human osteoblasts and osteoclasts in coculture. Acta Biomater. 2015, 27, 294–304. [Google Scholar] [CrossRef]
- Orchard, T.S.; Larson, J.C.; Alghothani, N.; Bout-Tabaku, S.; Cauley, J.A.; Chen, Z.; LaCroix, A.Z.; Wactawski-Wende, J.; Jackson, R.D. Magnesium intake, bone mineral density, and fractures: Results from the Women’s Health Initiative Observational Study. Am. J. Clin. Nutr. 2014, 99, 926–933. [Google Scholar] [CrossRef] [PubMed]
- Tucker, K.L.; Hannan, M.T.; Chen, H.; Cupples, L.A.; Wilson, P.W.; Kiel, D.P. Potassium, magnesium, and fruit and vegetable intakes are associated with greater bone mineral density in elderly men and women. Am. J. Clin. Nutr. 1999, 69, 727–736. [Google Scholar] [CrossRef]
- Groenendijk, I.; van Delft, M.; Versloot, P.; van Loon, L.J.C.; de Groot, L. Impact of magnesium on bone health in older adults: A systematic review and meta-analysis. Bone 2022, 154, 116233. [Google Scholar] [CrossRef]
- Ryder, K.M.; Shorr, R.I.; Bush, A.J.; Kritchevsky, S.B.; Harris, T.; Stone, K.; Cauley, J.; Tylavsky, F.A. Magnesium intake from food and supplements is associated with bone mineral density in healthy older white subjects. J. Am. Geriatr. Soc. 2005, 53, 1875–1880. [Google Scholar] [CrossRef] [PubMed]
- Veronese, N.; Stubbs, B.; Solmi, M.; Noale, M.; Vaona, A.; Demurtas, J.; Maggi, S. Dietary magnesium intake and fracture risk: Data from a large prospective study. Br. J. Nutr. 2017, 117, 1570–1576. [Google Scholar] [CrossRef]
- Dominguez, L.J.; Veronese, N.; Ciriminna, S.; Perez-Albela, J.L.; Vasquez-Lopez, V.F.; Rodas-Regalado, S.; Di Bella, G.; Parisi, A.; Tagliaferri, F.; Barbagallo, M. Association between Serum Magnesium and Fractures: A Systematic Review and Meta-Analysis of Observational Studies. Nutrients 2023, 15, 1304. [Google Scholar] [CrossRef]
- Matias, C.N.; Santos, D.A.; Monteiro, C.P.; Vasco, A.M.; Baptista, F.; Sardinha, L.B.; Laires, M.J.; Silva, A.M. Magnesium intake mediates the association between bone mineral density and lean soft tissue in elite swimmers. Magnes. Res. 2012, 25, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Resnick, L.M. Magnesium in the pathophysiology and treatment of hypertension and diabetes mellitus: Where are we in 1997? Am. J. Hypertens. 1997, 10, 368–370. [Google Scholar] [CrossRef]
- Chiarello, D.I.; Marin, R.; Proverbio, F.; Coronado, P.; Toledo, F.; Salsoso, R.; Gutierrez, J.; Sobrevia, L. Mechanisms of the effect of magnesium salts in preeclampsia. Placenta 2018, 69, 134–139. [Google Scholar] [CrossRef]
- Dalton, L.M.; Ni Fhloinn, D.M.; Gaydadzhieva, G.T.; Mazurkiewicz, O.M.; Leeson, H.; Wright, C.P. Magnesium in pregnancy. Nutr. Rev. 2016, 74, 549–557. [Google Scholar] [CrossRef]
- Tangvoraphonkchai, K.; Davenport, A. Magnesium and Cardiovascular Disease. Adv. Chronic Kidney Dis. 2018, 25, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Mannuss, S.; Schuff-Werner, P.; Dreissiger, K.; Burstein, C. Inhibition of agonist-induced platelet aggregation by magnesium sulfate warrants its use as an alternative in vitro anticoagulant in pseudothrombocytopenia. Platelets 2020, 31, 680–684. [Google Scholar] [CrossRef]
- Zaslow, S.J.; Oliveira-Paula, G.H.; Chen, W. Magnesium and Vascular Calcification in Chronic Kidney Disease: Current Insights. Int. J. Mol. Sci. 2024, 25, 1155. [Google Scholar] [CrossRef]
- Levine, B.S.; Coburn, J.W. Magnesium, the mimic/antagonist of calcium. N. Engl. J. Med. 1984, 310, 1253–1255. [Google Scholar] [CrossRef]
- Nielsen, F.H. The Role of Dietary Magnesium in Cardiovascular Disease. Nutrients 2024, 16, 4223. [Google Scholar] [CrossRef]
- Celeski, M.; Di Gioia, G.; Nusca, A.; Segreti, A.; Squeo, M.R.; Lemme, E.; Mango, F.; Ferrera, A.; Ussia, G.P.; Grigioni, F. The Spectrum of Coronary Artery Disease in Elite Endurance Athletes-A Long-Standing Debate: State-of-the-Art Review. J. Clin. Med. 2024, 13, 5144. [Google Scholar] [CrossRef]
- Eisenberg, M.J. Magnesium deficiency and sudden death. Am. Heart J. 1992, 124, 544–549. [Google Scholar] [CrossRef]
- Maron, B.J. Sudden death in young athletes. N. Engl. J. Med. 2003, 349, 1064–1075. [Google Scholar] [CrossRef]
- Stendig-Lindberg, G. Sudden death of athletes: Is it due to long-term changes in serum magnesium, lipids and blood sugar? J. Basic. Clin. Physiol. Pharmacol. 1992, 3, 153–164. [Google Scholar] [CrossRef]
- Chadda, K.D.; Gupta, P.K.; Lichtenstein, E. Magnesium in cardiac arrhythmia. N. Engl. J. Med. 1972, 287, 1102. [Google Scholar] [CrossRef]
- Chiuve, S.E.; Korngold, E.C.; Januzzi, J.L., Jr.; Gantzer, M.L.; Albert, C.M. Plasma and dietary magnesium and risk of sudden cardiac death in women. Am. J. Clin. Nutr. 2011, 93, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Kieboom, B.C.; Niemeijer, M.N.; Leening, M.J.; van den Berg, M.E.; Franco, O.H.; Deckers, J.W.; Hofman, A.; Zietse, R.; Stricker, B.H.; Hoorn, E.J. Serum Magnesium and the Risk of Death From Coronary Heart Disease and Sudden Cardiac Death. J. Am. Heart Assoc. 2016, 5, 2707. [Google Scholar] [CrossRef]
- Ora, J.; De Marco, P.; Gabriele, M.; Cazzola, M.; Rogliani, P. Exercise-Induced Asthma: Managing Respiratory Issues in Athletes. J. Funct. Morphol. Kinesiol. 2024, 9, 15. [Google Scholar] [CrossRef]
- Kowal, A.; Panaszek, B.; Barg, W.; Obojski, A. The use of magnesium in bronchial asthma: A new approach to an old problem. Arch. Immunol. Ther. Exp. 2007, 55, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, M.N.; Malapati, B.R.; Gokani, R.; Patel, B.; Chatriwala, M. Serum Magnesium and Vitamin D Levels as Indicators of Asthma Severity. Pulm. Med. 2016, 2016, 1643717. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, L.J.; Barbagallo, M.; Di Lorenzo, G.; Drago, A.; Scola, S.; Morici, G.; Caruso, C. Bronchial reactivity and intracellular magnesium: A possible mechanism for the bronchodilating effects of magnesium in asthma. Clin. Sci. 1998, 95, 137–142. [Google Scholar] [CrossRef]
- Kelly, H.W. Magnesium sulfate for severe acute asthma in children. J. Pediatr. Pharmacol. Ther. 2003, 8, 40–45. [Google Scholar] [CrossRef]
- Song, W.J.; Chang, Y.S. Magnesium sulfate for acute asthma in adults: A systematic literature review. Asia Pac. Allergy 2012, 2, 76–85. [Google Scholar] [CrossRef]
- Hill, J.; Britton, J. Dose-response relationship and time-course of the effect of inhaled magnesium sulphate on airflow in normal and asthmatic subjects. Br. J. Clin. Pharmacol. 1995, 40, 539–544. [Google Scholar] [CrossRef]
- Gourgoulianis, K.I.; Chatziparasidis, G.; Chatziefthimiou, A.; Molyvdas, P.A. Magnesium as a relaxing factor of airway smooth muscles. J. Aerosol Med. 2001, 14, 301–307. [Google Scholar] [CrossRef]
- Cairns, C.B.; Kraft, M. Magnesium attenuates the neutrophil respiratory burst in adult asthmatic patients. Acad. Emerg. Med. 1996, 3, 1093–1097. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, S.; Goodacre, S. Intravenous and nebulised magnesium sulphate for acute asthma: Systematic review and meta-analysis. Emerg. Med. J. 2007, 24, 823–830. [Google Scholar] [CrossRef]
- Kokotajlo, S.; Degnan, L.; Meyers, R.; Siu, A.; Robinson, C. Use of intravenous magnesium sulfate for the treatment of an acute asthma exacerbation in pediatric patients. J. Pediatr. Pharmacol. Ther. 2014, 19, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.A.; Goodacre, S. Magnesium sulphate in the treatment of acute asthma: Evaluation of current practice in adult emergency departments. Emerg. Med. J. 2009, 26, 783–785. [Google Scholar] [CrossRef]
- Britton, J.; Pavord, I.; Richards, K.; Wisniewski, A.; Knox, A.; Lewis, S.; Tattersfield, A.; Weiss, S. Dietary magnesium, lung function, wheezing, and airway hyperreactivity in a random adult population sample. Lancet 1994, 344, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Powell, C.V.; Kolamunnage-Dona, R.; Lowe, J.; Boland, A.; Petrou, S.; Doull, I.; Hood, K.; Williamson, P.R.; MAGNETIC study group. MAGNEsium Trial In Children (MAGNETIC): A randomised, placebo-controlled trial and economic evaluation of nebulised magnesium sulphate in acute severe asthma in children. Health Technol. Assess. 2013, 17, v–vi, 1–216. [Google Scholar] [CrossRef]
- Liang, R.Y.; Wu, W.; Huang, J.; Jiang, S.P.; Lin, Y. Magnesium affects the cytokine secretion of CD4+ T lymphocytes in acute asthma. J. Asthma 2012, 49, 1012–1015. [Google Scholar] [CrossRef]
- Rovsing, A.H.; Savran, O.; Ulrik, C.S. Magnesium sulfate treatment for acute severe asthma in adults-a systematic review and meta-analysis. Front. Allergy 2023, 4, 1211949. [Google Scholar] [CrossRef]
- Deng, X.; Song, Y.; Manson, J.E.; Signorello, L.B.; Zhang, S.M.; Shrubsole, M.J.; Ness, R.M.; Seidner, D.L.; Dai, Q. Magnesium, vitamin D status and mortality: Results from US National Health and Nutrition Examination Survey (NHANES) 2001 to 2006 and NHANES III. BMC Med. 2013, 11, 187. [Google Scholar] [CrossRef]
- Anast, C.S.; Mohs, J.M.; Kaplan, S.L.; Burns, T.W. Evidence for parathyroid failure in magnesium deficiency. Science 1972, 177, 606–608. [Google Scholar] [CrossRef]
- Medalle, R.; Waterhouse, C.; Hahn, T.J. Vitamin D resistance in magnesium deficiency. Am. J. Clin. Nutr. 1976, 29, 854–858. [Google Scholar] [CrossRef]
- Rude, R.K.; Oldham, S.B.; Sharp, C.F., Jr.; Singer, F.R. Parathyroid hormone secretion in magnesium deficiency. J. Clin. Endocrinol. Metab. 1978, 47, 800–806. [Google Scholar] [CrossRef]
- Mutnuri, S.; Fernandez, I.; Kochar, T. Suppression of Parathyroid Hormone in a Patient with Severe Magnesium Depletion. Case Rep. Nephrol. 2016, 2016, 2608538. [Google Scholar] [CrossRef] [PubMed]
- Cheung, M.M.; DeLuccia, R.; Ramadoss, R.K.; Aljahdali, A.; Volpe, S.L.; Shewokis, P.A.; Sukumar, D. Low dietary magnesium intake alters vitamin D-parathyroid hormone relationship in adults who are overweight or obese. Nutr. Res. 2019, 69, 82–93. [Google Scholar] [CrossRef]
- Schwalfenberg, G.K.; Genuis, S.J. Vitamin D, Essential Minerals, and Toxic Elements: Exploring Interactions between Nutrients and Toxicants in Clinical Medicine. Sci. World J. 2015, 2015, 318595. [Google Scholar] [CrossRef] [PubMed]
- Pollock, N.; Dijkstra, P.; Chakraverty, R.; Hamilton, B. Low 25(OH) vitamin D concentrations in international UK track and field athletes. S. Afr. Sports Med. Assoc. 2012, 24, 5. [Google Scholar] [CrossRef]
- Brown, L.L.; Cohen, B.; Tabor, D.; Zappala, G.; Maruvada, P.; Coates, P.M. The vitamin D paradox in Black Americans: A systems-based approach to investigating clinical practice, research, and public health-expert panel meeting report. BMC Proc. 2018, 12, 6. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.A. Dietary Guidelines for Americans, 2020-2025. Workplace Health Saf. 2021, 69, 395. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on Dietary Reference Values for magnesium. EFSA J. 2015, 13, 4186. [Google Scholar] [CrossRef]

| Food | Serving | IU Per Serving |
|---|---|---|
| Cod liver oil | 1 tablespoon | 1360 |
| Trout (cooked) | 3 ounces | 645 |
| Salmon (cooked) | 3 ounces | 570 |
| Mushrooms (raw, exposed to UV light) | 1/2 cup | 366 |
| Sardines | 2 sardines | 46 |
| Egg | 1 large | 44 |
| Liver, beef (braised) | 3 ounces | 42 |
| Tuna fish (canned, drained) | 3 ounces | 40 |
| Cheese, cheddar, | 1 ounce | 12 |
| Mushrooms (raw) | 1/2 cup | 4 |
| Chicken breast (roasted) | 3 ounces | 4 |
| Beef (ground, lean, broiled) | 3 ounces | 1.7 |
| Metabolite | Chemical Formula | Site of Production | Enzyme |
|---|---|---|---|
| 7-Dehydrocholesterol | 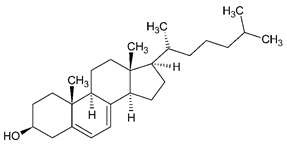 | Skin | Lathosterol oxidase |
| Vitamin D3 (cholecalciferol) |  | Skin or diet (animal origin) | CYP27A1 and CYP27B1 |
| Vitamin D2 (ergocalciferol) |  | Diet (vegetable origin) | |
| 25(OH)D (Calcifediol or calcidiol) | 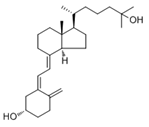 | Liver | Hydroxylases like CYP2R1 and CYP27A1 |
| 1,25 (OH)2D (Calcitriol) | 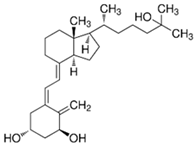 | Kidney | CYP27B1 |
| 24,25 (OH)2D | 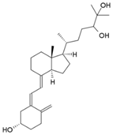 | Most tissues | CYP24A1 |
| Cellular Functions of Magnesium |
|---|
|
| Food | ↑ Bioavailability | ↓ Bioavailability |
|---|---|---|
|
|
|
| Magnesium Effects on Muscle |
|---|
|
| Magnesium Effects on Cardiovascular System |
|---|
|
| Magnesium | Vitamin D |
|---|---|
|
|
|
|
|
|
|
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dominguez, L.J.; Veronese, N.; Ragusa, F.S.; Baio, S.M.; Sgrò, F.; Russo, A.; Battaglia, G.; Bianco, A.; Barbagallo, M. The Importance of Vitamin D and Magnesium in Athletes. Nutrients 2025, 17, 1655. https://doi.org/10.3390/nu17101655
Dominguez LJ, Veronese N, Ragusa FS, Baio SM, Sgrò F, Russo A, Battaglia G, Bianco A, Barbagallo M. The Importance of Vitamin D and Magnesium in Athletes. Nutrients. 2025; 17(10):1655. https://doi.org/10.3390/nu17101655
Chicago/Turabian StyleDominguez, Ligia J., Nicola Veronese, Francesco Saverio Ragusa, Salvatore Maria Baio, Francesco Sgrò, Arcangelo Russo, Giuseppe Battaglia, Antonino Bianco, and Mario Barbagallo. 2025. "The Importance of Vitamin D and Magnesium in Athletes" Nutrients 17, no. 10: 1655. https://doi.org/10.3390/nu17101655
APA StyleDominguez, L. J., Veronese, N., Ragusa, F. S., Baio, S. M., Sgrò, F., Russo, A., Battaglia, G., Bianco, A., & Barbagallo, M. (2025). The Importance of Vitamin D and Magnesium in Athletes. Nutrients, 17(10), 1655. https://doi.org/10.3390/nu17101655










