Prevalence of Intolerance to Amines and Salicylates in Individuals with Atopic Dermatitis: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
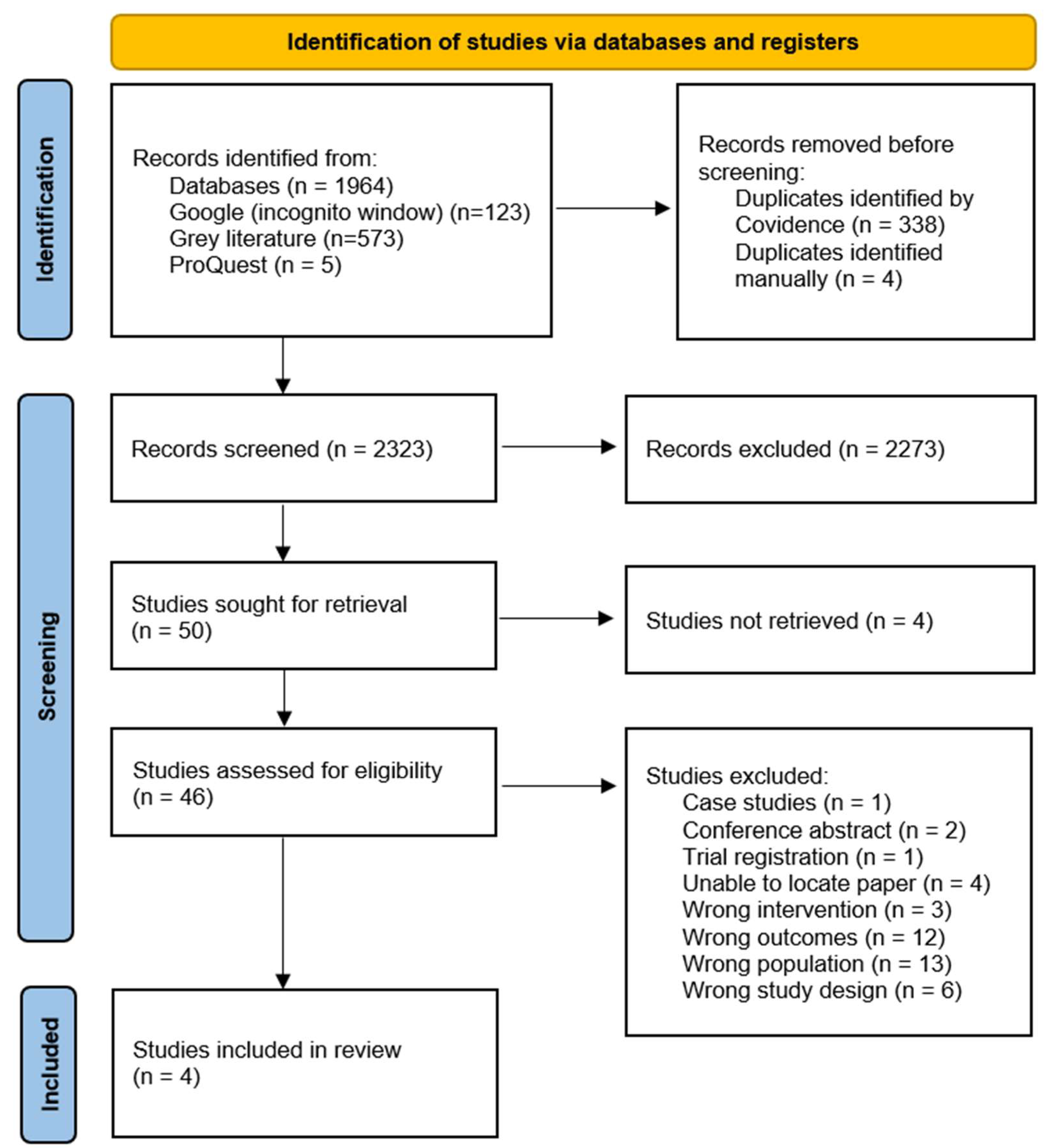
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction
2.4. Data Analysis
3. Results
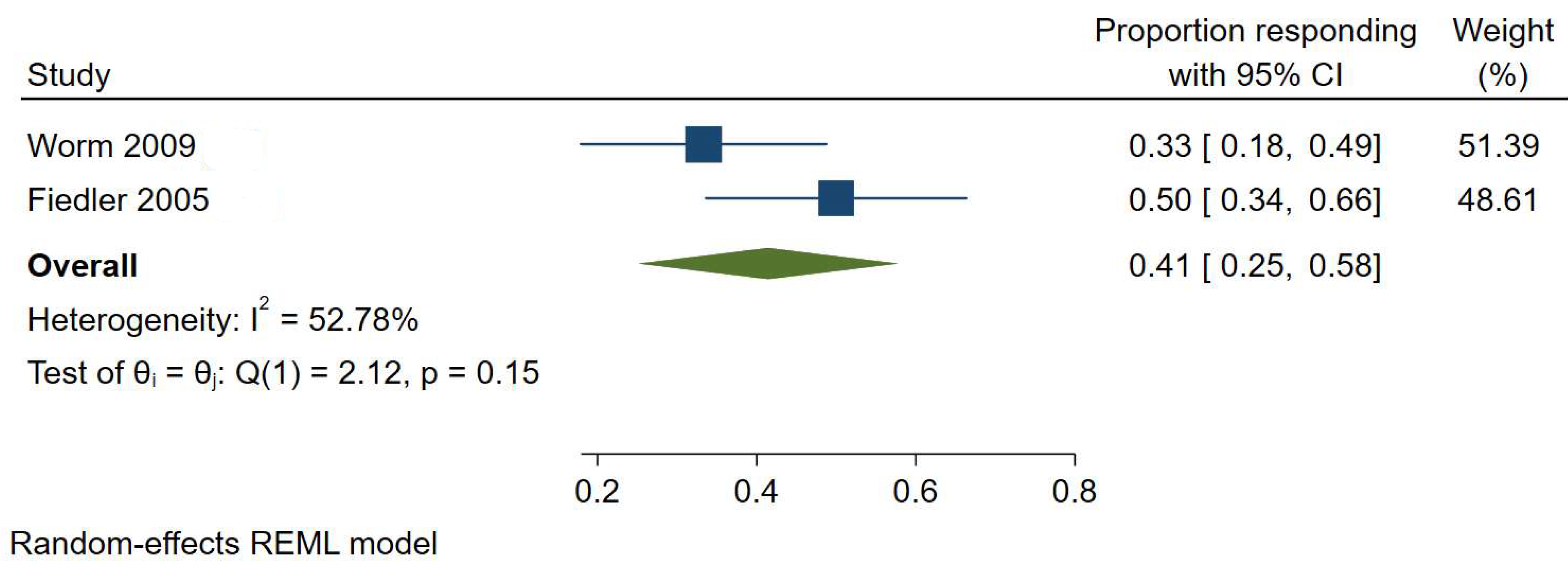
3.1. Histamine Intolerance
3.2. Amine Intolerance
3.3. Salicylate Intolerance
3.4. Other Adverse Reactions from Oral Challenge
3.5. Risk of Bias
4. Discussion
4.1. Main Findings and Their Significance
4.2. Potential Mechanisms for a Link Between Amine and Salicylate Intolerance and AD
4.3. Clinical Implications
4.4. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Oh, J.; Kim, S.; Kim, M.S.; Abate, Y.H.; Abd ElHafeez, S.; Abdelkader, A.; Abdi, P.; Abdulah, D.M.; Aboagye, R.G.; Abolhassani, H.; et al. Global, regional, and national burden of asthma and atopic dermatitis, 1990–2021, and projections to 2050: A systematic analysis of the Global Burden of Disease Study 2021. Lancet Respir. Med. 2025, 13, 425–446. [Google Scholar] [CrossRef]
- Jeskey, J.; Kurien, C.; Blunk, H.; Sehmi, K.; Areti, S.; Nguyen, D.; Hostoffer, R. Atopic Dermatitis: A Review of Diagnosis and Treatment. J. Pediatr. Pharmacol. Ther. 2024, 29, 587–603. [Google Scholar] [CrossRef] [PubMed]
- Wollenberg, A.; Christen-Zach, S.; Taieb, A.; Paul, C.; Thyssen, J.P.; de Bruin-Weller, M.; Vestergaard, C.; Seneschal, J.; Werfel, T.; Cork, M.J.; et al. ETFAD/EADV Eczema task force 2020 position paper on diagnosis and treatment of atopic dermatitis in adults and children. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 2717–2744. [Google Scholar] [CrossRef]
- Langan, S.M.; Thomas, K.S.; Williams, H.C. What is meant by a “flare” in atopic dermatitis? A systematic review and proposal. Arch. Dermatol. 2006, 142, 1190–1196. [Google Scholar] [CrossRef] [PubMed]
- Lonndahl, L.; Abdelhadi, S.; Holst, M.; Lonne-Rahm, S.B.; Nordlind, K.; Johansson, B. Psychological Stress and Atopic Dermatitis: A Focus Group Study. Ann. Dermatol. 2023, 35, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Alexander, H.; Paller, A.S.; Traidl-Hoffmann, C.; Beck, L.A.; De Benedetto, A.; Dhar, S.; Girolomoni, G.; Irvine, A.D.; Spuls, P.; Su, J.; et al. The role of bacterial skin infections in atopic dermatitis: Expert statement and review from the International Eczema Council Skin Infection Group. Br. J. Dermatol. 2020, 182, 1331–1342. [Google Scholar] [CrossRef]
- Chu, D.K.; Schneider, L.; Asiniwasis, R.N.; Boguniewicz, M.; De Benedetto, A.; Ellison, K.; Frazier, W.T.; Greenhawt, M.; Huynh, J.; Kim, E.; et al. Atopic dermatitis (eczema) guidelines: 2023 American Academy of Allergy, Asthma and Immunology/American College of Allergy, Asthma and Immunology Joint Task Force on Practice Parameters GRADE–and Institute of Medicine–based recommendations. Ann. Allergy Asthma Immunol. 2024, 132, 274–312. [Google Scholar] [CrossRef]
- Chua, G.T.; Mack, D.P.; Shaker, M.S.; Chan, E.S. Oral food immunotherapy in patients with atopic dermatitis. Ann. Allergy Asthma Immunol. 2024, 133, 278–283. [Google Scholar] [CrossRef]
- Christensen, M.O.; Barakji, Y.A.; Loft, N.; Khatib, C.M.; Egeberg, A.; Thomsen, S.F.; Silverberg, J.I.; Flohr, C.; Maul, J.T.; Schmid-Grendelmeier, P.; et al. Prevalence of and association between atopic dermatitis and food sensitivity, food allergy and challenge-proven food allergy: A systematic review and meta-analysis. J. Eur. Acad. Dermatol. Venereol. 2023, 37, 984–1003. [Google Scholar] [CrossRef]
- Worm, M.; Fiedler, E.M.; Dolle, S.; Schink, T.; Hemmer, W.; Jarisch, R.; Zuberbier, T. Exogenous histamine aggravates eczema in a subgroup of patients with atopic dermatitis. Acta Derm. Venereol. 2009, 89, 52–56. [Google Scholar] [CrossRef]
- Fiedler, E.M.; Forschner, K.; Focke, M.; Hemmer, W.; Jarisch, R.; Zuberbier, T.; Worm, M. Nutrition and eczema diseases—Importance of exogenously supplied histamine in patients with atopic dermatitis. Dermatol. Work Environ. 2005, 53, 93–96. [Google Scholar]
- Loblay, R.H.; Swain, A.R. Food Intolerance. Recent Adv. Clin. Nutr. 1986, 2, 169–177. [Google Scholar]
- Van Bever, H.P.; Docx, M.; Stevens, W.J. Food and food additives in severe atopic dermatitis. Allergy 1989, 44, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Tuck, C.J.; Biesiekierski, J.R.; Schmid-Grendelmeier, P.; Pohl, D. Food Intolerances. Nutrients 2019, 11, 1684. [Google Scholar] [CrossRef]
- Hrubisko, M.; Danis, R.; Huorka, M.; Wawruch, M. Histamine Intolerance-The More We Know the Less We Know. A Review. Nutrients 2021, 13, 2228. [Google Scholar] [CrossRef] [PubMed]
- Maleki-Yazdi, K.A.; Heen, A.F.; Zhao, I.X.; Guyatt, G.H.; Suzumura, E.A.; Makhdami, N.; Chen, L.; Winders, T.; Wheeler, K.E.; Wang, J.; et al. Values and Preferences of Patients and Caregivers Regarding Treatment of Atopic Dermatitis (Eczema): A Systematic Review. JAMA Dermatol. 2023, 159, 320–330. [Google Scholar] [CrossRef]
- Makkoukdji, N.; Gebbia, J.; Cruz, A.; Kleiner, G.; Bellodi-Schmidt, F.; Gans, M. A Survey of Parental Eczema Perceptions and its Relationship to Dietary Intake in South Florida. Ann. Allergy Asthma Immunol. 2024, 133, S99–S100. [Google Scholar] [CrossRef]
- Johnston, G.A.; Bilbao, R.M.; Graham-Brown, R.A. The use of dietary manipulation by parents of children with atopic dermatitis. Br. J. Dermatol. 2004, 150, 1186–1189. [Google Scholar] [CrossRef]
- Tsakok, T.; Marrs, T.; Mohsin, M.; Baron, S.; du Toit, G.; Till, S.; Flohr, C. Does atopic dermatitis cause food allergy? A systematic review. J. Allergy Clin. Immunol. 2016, 137, 1071–1078. [Google Scholar] [CrossRef]
- Ansotegui, I.J.; Melioli, G.; Canonica, G.W.; Caraballo, L.; Villa, E.; Ebisawa, M.; Passalacqua, G.; Savi, E.; Ebo, D.; Gomez, R.M.; et al. IgE allergy diagnostics and other relevant tests in allergy, a World Allergy Organization position paper. World Allergy Organ. J. 2020, 13, 100080. [Google Scholar] [CrossRef]
- Comas-Baste, O.; Sanchez-Perez, S.; Veciana-Nogues, M.T.; Latorre-Moratalla, M.; Vidal-Carou, M.D.C. Histamine Intolerance: The Current State of the Art. Biomolecules 2020, 10, 1181. [Google Scholar] [CrossRef] [PubMed]
- van Odijk, J.; Weisheit, A.; Arvidsson, M.; Miron, N.; Nwaru, B.; Ekerljung, L. The Use of DAO as a Marker for Histamine Intolerance: Measurements and Determinants in a Large Random Population-Based Survey. Nutrients 2023, 15, 2887. [Google Scholar] [CrossRef]
- de Weger, W.W.; Sprikkelman, A.B.; Herpertz, C.E.M.; van der Meulen, G.N.; Vonk, J.M.; Koppelman, G.H.; Kamps, A.W.A. Comparison of Double-Blind and Open Food Challenges for the Diagnosis of Food Allergy in Childhood: The ALDORADO Study. Allergy 2024, 80, 248–257. [Google Scholar] [CrossRef]
- Swain, A.R.; Loblay, R.H.; Soutter, V.L. Friendly Food: The Essential Guide to Managing Common Food Allergies and Intolerances, 2nd ed.; N.S.W.A.U. Royal Prince Alfred Hospital, Ed.; Murdoch Books: Crows Nest, NSW, Australia, 2019. [Google Scholar]
- Maintz, L.; Benfadal, S.; Allam, J.P.; Hagemann, T.; Fimmers, R.; Novak, N. Evidence for a reduced histamine degradation capacity in a subgroup of patients with atopic eczema. J. Allergy Clin. Immunol. 2006, 117, 1106–1112. [Google Scholar] [CrossRef] [PubMed]
- Schnedl, W.J.; Enko, D. Histamine Intolerance Originates in the Gut. Nutrients 2021, 13, 1262. [Google Scholar] [CrossRef]
- Keszycka, P.K.; Lange, E.; Gajewska, D. Effectiveness of Personalized Low Salicylate Diet in the Management of Salicylates Hypersensitive Patients: Interventional Study. Nutrients 2021, 13, 991. [Google Scholar] [CrossRef] [PubMed]
- Boyce, J.A.; Assa’ad, A.; Burks, A.W.; Jones, S.M.; Sampson, H.A.; Wood, R.A.; Plaut, M.; Cooper, S.F.; Fenton, M.J.; Arshad, S.H.; et al. Guidelines for the diagnosis and management of food allergy in the United States: Report of the NIAID-sponsored expert panel. J. Allergy Clin. Immunol. 2010, 126 (Suppl. 6), S1–S58. [Google Scholar] [CrossRef]
- Skypala, I. Adverse food reactions--an emerging issue for adults. J. Am. Diet. Assoc. 2011, 111, 1877–1891. [Google Scholar] [CrossRef]
- Skypala, I.J.; Williams, M.; Reeves, L.; Meyer, R.; Venter, C. Sensitivity to food additives, vaso-active amines and salicylates: A review of the evidence. Clin. Transl. Allergy 2015, 5, 34. [Google Scholar] [CrossRef]
- Gargano, D.; Appanna, R.; Santonicola, A.; De Bartolomeis, F.; Stellato, C.; Cianferoni, A.; Casolaro, V.; Iovino, P. Food Allergy and Intolerance: A Narrative Review on Nutritional Concerns. Nutrients 2021, 13, 1638. [Google Scholar] [CrossRef]
- Wantke, F.; Gotz, M.; Jarisch, R. The histamine-free diet. Der Hautarzt 1993, 44, 512–516. [Google Scholar]
- Lomer, M.C. Review article: The aetiology, diagnosis, mechanisms and clinical evidence for food intolerance. Aliment. Pharmacol. Ther. 2015, 41, 262–275. [Google Scholar] [CrossRef]
- Fulgoni, V.L., 3rd; Keast, D.R.; Lieberman, H.R. Trends in intake and sources of caffeine in the diets of US adults: 2001-2010. Am. J. Clin. Nutr. 2015, 101, 1081–1087. [Google Scholar] [CrossRef]
- Mitchell, D.C.; Knight, C.A.; Hockenberry, J.; Teplansky, R.; Hartman, T.J. Beverage caffeine intakes in the U.S. Food Chem. Toxicol. 2014, 63, 136–142. [Google Scholar] [CrossRef]
- Stevens, W.W.; Jerschow, E.; Baptist, A.P.; Borish, L.; Bosso, J.V.; Buchheit, K.M.; Cahill, K.N.; Campo, P.; Cho, S.H.; Keswani, A.; et al. The role of aspirin desensitization followed by oral aspirin therapy in managing patients with aspirin-exacerbated respiratory disease: A Work Group Report from the Rhinitis, Rhinosinusitis and Ocular Allergy Committee of the American Academy of Allergy, Asthma & Immunology. J. Allergy Clin. Immunol. 2021, 147, 827–844. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.E.; Skypala, I. Aspirin and salicylate in respiratory disease. Rhinology 2013, 51, 195–205. [Google Scholar] [CrossRef]
- Fisone, G.; Borgkvist, A.; Usiello, A. Caffeine as a psychomotor stimulant: Mechanism of action. Cell Mol. Life Sci. 2004, 61, 857–872. [Google Scholar] [CrossRef] [PubMed]
- Wojcik, W.; Lukasiewicz, M.; Puppel, K. Biogenic amines: Formation, action and toxicity—A review. J. Sci. Food Agric. 2021, 101, 2634–2640. [Google Scholar] [CrossRef]
- Feng, C.; Teuber, S.; Gershwin, M.E. Histamine (Scombroid) Fish Poisoning: A Comprehensive Review. Clin. Rev. Allergy Immunol. 2016, 50, 64–69. [Google Scholar] [CrossRef]
- Sanchez-Perez, S.; Comas-Baste, O.; Veciana-Nogues, M.T.; Latorre-Moratalla, M.L.; Vidal-Carou, M.C. Low-Histamine Diets: Is the Exclusion of Foods Justified by Their Histamine Content? Nutrients 2021, 13, 1395. [Google Scholar] [CrossRef]
- Chung, B.Y.; Park, S.Y.; Byun, Y.S.; Son, J.H.; Choi, Y.W.; Cho, Y.S.; Kim, H.O.; Park, C.W. Effect of Different Cooking Methods on Histamine Levels in Selected Foods. Ann. Dermatol. 2017, 29, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Laidlaw, T.M.; Levy, J.M. NSAID-ERD Syndrome: The New Hope from Prevention, Early Diagnosis, and New Therapeutic Targets. Curr. Allergy Asthma Rep. 2020, 20, 10. [Google Scholar] [CrossRef] [PubMed]
- Luskin, K.; Thakrar, H.; White, A. Nasal Polyposis and Aspirin-Exacerbated Respiratory Disease. Immunol. Allergy Clin. N. Am. 2020, 40, 329–343. [Google Scholar] [CrossRef]
- Cahill, K.N.; Bensko, J.C.; Boyce, J.A.; Laidlaw, T.M. Prostaglandin D(2): A dominant mediator of aspirin-exacerbated respiratory disease. J. Allergy Clin. Immunol. 2015, 135, 245–252. [Google Scholar] [CrossRef]
- Makowska, J.S.; Burney, P.; Jarvis, D.; Keil, T.; Tomassen, P.; Bislimovska, J.; Brozek, G.; Bachert, C.; Baelum, J.; Bindslev-Jensen, C.; et al. Respiratory hypersensitivity reactions to NSAIDs in Europe: The global allergy and asthma network (GA(2) LEN) survey. Allergy 2016, 71, 1603–1611. [Google Scholar] [CrossRef]
- Nguyen, T.; Cranswick, N.; Rosenbaum, J.; Gelbart, B.; Tosif, S. Chronic use of teething gel causing salicylate toxicity. J. Paediatr. Child. Health 2018, 54, 576–578. [Google Scholar] [CrossRef]
- Williams, G.D.; Kirk, E.P.; Wilson, C.J.; Meadows, C.A.; Chan, B.S. Salicylate intoxication from teething gel in infancy. Med. J. Aust. 2011, 194, 146–148. [Google Scholar] [CrossRef] [PubMed]
- Malakar, S.; Gibson, P.R.; Barrett, J.S.; Muir, J.G. Naturally occurring dietary salicylates: A closer look at common Australian foods. J. Food Compos. Anal. 2017, 57, 31–39. [Google Scholar] [CrossRef]
- Chiang, H.L.; Venter, C.; Syue, P.C.; Ku, K.L.; Wu, C.H. Which Fruits and Vegetables Should Be Excluded from a Low-Salicylate Diet? An Analysis of Salicylic Acid in Foodstuffs in Taiwan. Int. Arch. Allergy Immunol. 2018, 176, 198–204. [Google Scholar] [CrossRef]
- Baenkler, H.W. Salicylate intolerance: Pathophysiology, clinical spectrum, diagnosis and treatment. Dtsch. Arztebl. Int. 2008, 105, 137–142. [Google Scholar] [CrossRef]
- Tuck, C.J.; Malakar, S.; Barrett, J.S.; Muir, J.G.; Gibson, P.R. Naturally-occurring dietary salicylates in the genesis of functional gastrointestinal symptoms in patients with irritable bowel syndrome: Pilot study. JGH Open 2021, 5, 871–878. [Google Scholar] [CrossRef]
- Sommer, D.D.; Rotenberg, B.W.; Sowerby, L.J.; Lee, J.M.; Janjua, A.; Witterick, I.J.; Monteiro, E.; Gupta, M.K.; Au, M.; Nayan, S. A novel treatment adjunct for aspirin exacerbated respiratory disease: The low-salicylate diet: A multicenter randomized control crossover trial. Int. Forum Allergy Rhinol. 2016, 6, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Sowerby, L.J.; Patel, K.B.; Schmerk, C.; Rotenberg, B.W.; Rocha, T.; Sommer, D.D. Effect of low salicylate diet on clinical and inflammatory markers in patients with aspirin exacerbated respiratory disease—A randomized crossover trial. J. Otolaryngol. Head. Neck Surg. 2021, 50, 27. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Sampson, H.A.; Gerth van Wijk, R.; Bindslev-Jensen, C.; Sicherer, S.; Teuber, S.S.; Burks, A.W.; Dubois, A.E.; Beyer, K.; Eigenmann, P.A.; Spergel, J.M.; et al. Standardizing double-blind, placebo-controlled oral food challenges: American Academy of Allergy, Asthma & Immunology-European Academy of Allergy and Clinical Immunology PRACTALL consensus report. J. Allergy Clin. Immunol. 2012, 130, 1260–1274. [Google Scholar] [CrossRef]
- Kunz, B.; Oranje, A.P.; Labreze, L.; Stalder, J.F.; Ring, J.; Taieb, A. Clinical validation and guidelines for the SCORAD index: Consensus report of the European Task Force on Atopic Dermatitis. Dermatology 1997, 195, 10–19. [Google Scholar] [CrossRef]
- Munn, Z.; Moola, S.; Lisy, K.; Riitano, D.; Tufanaru, C. Chapter 5: Systematic Reviews of Prevalence and Incidence; JBI Manual for Evidence Synthesis; Aromataris, E., Lockwood, C., Porritt, K., Pilla, B., Jordan, Z., Eds.; Joanna Briggs Institute: Adelaide, South Australia, 2020. [Google Scholar]
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res. Synth. Methods 2021, 12, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Atkins, D.; Best, D.; Briss, P.A.; Eccles, M.; Falck-Ytter, Y.; Flottorp, S.; Guyatt, G.H.; Harbour, R.T.; Haugh, M.C.; Henry, D.; et al. Grading quality of evidence and strength of recommendations. BMJ 2004, 328, 1490. [Google Scholar] [CrossRef]
- Greenlees, N. Food Intolerance in Children with Eczema. Master’s Thesis, University of Sydney, Sydney, Australia, 1998. [Google Scholar]
- Steinman, H.A.; Potter, P.C. The precipitation of symptoms by common foods in children with atopic dermatitis. Allergy Proc. 1994, 15, 203–210. [Google Scholar] [CrossRef]
- Čelakovská, J.; Ettler, K.; Ettlerova, K.; Vaneckova, J. Food hypersensitivity in patients over 14 years of age suffering from atopic dermatitis. Indian. J. Dermatol. 2014, 59, 316. [Google Scholar] [CrossRef]
- Veien, N.K.; Hattel, T.; Justesen, O.; Norholm, A. Dermatoses in coffee drinkers. Dermatol. Clin. 1987, 40, 421–422. [Google Scholar]
- Reeves, B.C.; Deeks, J.J.; Higgins, J.P.; Shea, B.; Tugwell, P.; Wells, G.A. Cochrane Non-Randomized Studies of Interventions Methods Group. Including non-randomized studies on intervention effects. In Cochrane Handbook for Systematic Reviews of Interventions; Version 6.4 (Updated August 2023); Cochrane: London, UK, 2023; Chapter 24. [Google Scholar]
- Pike, M.G.; Carter, C.M.; Boulton, P.; Turner, M.W.; Soothill, J.F.; Atherton, D.J. Few food diets in the treatment of atopic eczema. Arch. Dis. Child. 1989, 64, 1691–1698. [Google Scholar] [CrossRef]
- Uenishi, T.; Sugiura, H.; Tanaka, T.; Uehara, M. Role of foods in irregular aggravation of skin lesions in children with atopic dermatitis. J. Dermatol. 2008, 35, 407–412. [Google Scholar] [CrossRef]
- Uenishi, T.; Sugiura, H.; Uehara, M. Role of foods in irregular aggravation of atopic dermatitis. J. Dermatol. 2003, 30, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Uenishi, T.; Sugiura, H.; Uehara, M. Involvement of food in irregular exacerbations of childhood atopic dermatitis. Ski. Sci. 2004, 3, 93–96. [Google Scholar]
- Falconieri, P.; Arcese, G.; Ziruolo, G.; Businco, L. Chocolate hypersensitivity is not common in children with atopic dermatitis. Eur. J. Pediat. Dermatol. 1994, 4, 29–32. [Google Scholar]
- Baggott, M.J.; Childs, E.; Hart, A.B.; de Bruin, E.; Palmer, A.A.; Wilkinson, J.E.; de Wit, H. Psychopharmacology of theobromine in healthy volunteers. Psychopharmacology 2013, 228, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.Y.C.; Lim, X.Y.; Yeo, J.H.H.; Lee, S.W.H.; Lai, N.M. The Health Effects of Chocolate and Cocoa: A Systematic Review. Nutrients 2021, 13, 2909. [Google Scholar] [CrossRef]
- Dala-Paula, B.M.; Deus, V.L.; Tavano, O.L.; Gloria, M.B.A. In vitro bioaccessibility of amino acids and bioactive amines in 70% cocoa dark chocolate: What you eat and what you get. Food Chem. 2021, 343, 128397. [Google Scholar] [CrossRef]
- Chafen, J.J.; Newberry, S.J.; Riedl, M.A.; Bravata, D.M.; Maglione, M.; Suttorp, M.J.; Sundaram, V.; Paige, N.M.; Towfigh, A.; Hulley, B.J.; et al. Diagnosing and managing common food allergies: A systematic review. JAMA 2010, 303, 1848–1856. [Google Scholar] [CrossRef]
- Lafon, I.; Lamperez, M.; Navarro, M.; Gastaminza, G.; Ferrer, M.; Tabar, A.I.; Gomez, S.; Agueros, M.; Garcia, B.E.; D’Amelio, C.M. Validation of novel recipes for masking peanuts in double-blind, placebo-controlled food challenges. Ann. Allergy Asthma Immunol. 2021, 127, 575–578. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, A.; Lee, K.E.; Patel, G.B.; Mathur, S.K.; Singh, A.M. Food allergy symptoms in adults and children using the Food Allergy Research & Education Patient Registry. J. Allergy Clin. Immunol. Pract. 2024, 12, 249–251.e1. [Google Scholar] [CrossRef]
- Zopf, Y.; Baenkler, H.W.; Silbermann, A.; Hahn, E.G.; Raithel, M. The differential diagnosis of food intolerance. Dtsch. Arztebl. Int. 2009, 106, 359–369. [Google Scholar] [CrossRef]
- Duelo, A.; Comas-Baste, O.; Sanchez-Perez, S.; Veciana-Nogues, M.T.; Ruiz-Casares, E.; Vidal-Carou, M.C.; Latorre-Moratalla, M.L. Pilot Study on the Prevalence of Diamine Oxidase Gene Variants in Patients with Symptoms of Histamine Intolerance. Nutrients 2024, 16, 1142. [Google Scholar] [CrossRef]
- Sanchez-Perez, S.; Comas-Baste, O.; Duelo, A.; Veciana-Nogues, M.T.; Berlanga, M.; Latorre-Moratalla, M.L.; Vidal-Carou, M.C. Intestinal Dysbiosis in Patients with Histamine Intolerance. Nutrients 2022, 14, 1774. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.K. Aspirin and salicylate: An old remedy with a new twist. Circulation 2000, 102, 2022–2023. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.J.; Majerus, P.W. The mechanism of the effect of aspirin on human platelets. I. Acetylation of a particulate fraction protein. J. Clin. Investig. 1975, 56, 624–632. [Google Scholar] [CrossRef]
- Marquette, M.; Tailor, B.V.; Calder, P.C.; Curtis, P.J.; Loke, Y.; Wilson, A.M. Urinary Leukotriene E4 as a Biomarker in NSAID-Exacerbated Respiratory Disease (N-ERD): A Systematic Review and Meta-analysis. Curr. Allergy Asthma Rep. 2022, 22, 209–229. [Google Scholar] [CrossRef]
- Wangberg, H.; White, A.A. Aspirin-exacerbated respiratory disease. Curr. Opin. Immunol. 2020, 66, 9–13. [Google Scholar] [CrossRef]
- Kawakami, T.; Ando, T.; Kimura, M.; Wilson, B.S.; Kawakami, Y. Mast cells in atopic dermatitis. Curr. Opin. Immunol. 2009, 21, 666–678. [Google Scholar] [CrossRef]
- Youngblood, B.; Schanin, J.; Tomasevic, N.; Kamboj, A. Atopic Dermatitis Skin Biopsies Have High Numbers of Activated Mast Cells that Are Inhibited by AK002 after Stimulation Ex Vivo. J. Allergy Clin. Immunol. 2020, 145, AB198. [Google Scholar] [CrossRef]
- Zhou, B.; Yue, X.; Liang, S.; Shang, S.; Xiang, L.; Zhou, K.; Li, L. Association between IL-25, IL-33 and atopic dermatitis: A systematic review and meta-analysis. Eur. J. Inflamm. 2023, 21, 1721727X231183670. [Google Scholar] [CrossRef]
- Imai, Y. Interleukin-33 in atopic dermatitis. J. Dermatol. Sci. 2019, 96, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.A.; Spergel, J.M. The atopic march: Critical evidence and clinical relevance. Ann. Allergy Asthma Immunol. 2018, 120, 131–137. [Google Scholar] [CrossRef]
- Wollenberg, A.; Kinberger, M.; Arents, B.; Aszodi, N.; Avila Valle, G.; Barbarot, S.; Bieber, T.; Brough, H.A.; Calzavara Pinton, P.; Christen-Zach, S.; et al. European guideline (EuroGuiDerm) on atopic eczema—Part II: Non-systemic treatments and treatment recommendations for special AE patient populations. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 1904–1926. [Google Scholar] [CrossRef]
- Sanchez-Perez, S.; Comas-Baste, O.; Rabell-Gonzalez, J.; Veciana-Nogues, M.T.; Latorre-Moratalla, M.L.; Vidal-Carou, M.C. Biogenic Amines in Plant-Origin Foods: Are They Frequently Underestimated in Low-Histamine Diets? Foods 2018, 7, 205. [Google Scholar] [CrossRef]
- Shulpekova, Y.O.; Nechaev, V.M.; Popova, I.R.; Deeva, T.A.; Kopylov, A.T.; Malsagova, K.A.; Kaysheva, A.L.; Ivashkin, V.T. Food Intolerance: The Role of Histamine. Nutrients 2021, 13, 3207. [Google Scholar] [CrossRef]
- Bessell, E. Nutritional Adequacy, Diet Quality and Calcium Intake on the Royal Prince Alfred Hospital Elimination Diet. University of Sydney. 2016. Available online: https://www.slhd.nsw.gov.au/rpa/allergy/research/students/2016/erica_bessell.pdf (accessed on 15 April 2025).
- Berry, M.J.; Adams, J.; Voutilainen, H.; Feustel, P.J.; Celestin, J.; Jarvinen, K.M. Impact of elimination diets on growth and nutritional status in children with multiple food allergies. Pediatr. Allergy Immunol. 2015, 26, 133–138. [Google Scholar] [CrossRef]
- Weisshaar, E.; Diepgen, T.L.; Bruckner, T.; Fartasch, M.; Kupfer, J.; Lob-Corzilius, T.; Ring, J.; Scheewe, S.; Scheidt, R.; Schmid-Ott, G.; et al. Itch intensity evaluated in the German Atopic Dermatitis Intervention Study (GADIS): Correlations with quality of life, coping behaviour and SCORAD severity in 823 children. Acta Derm. Venereol. 2008, 88, 234–239. [Google Scholar] [CrossRef]

| Source | Study Design | Country | Intervention | n, Total | n, Male | Age, Mean (SD), Range | Scoring Method | Chemical, Dose | n, Oral Challenge Responders | n, Diet Responders |
|---|---|---|---|---|---|---|---|---|---|---|
| Fiedler et al. [11]; 2005 | Single-arm pre–post intervention | Germany | Elimination diet then DBPCC | 36 | NR | NR | SCORAD | Histamine hydrochloride, 0.75 mg/kg and 1.5 mg/kg body weight | 11 | 18 |
| Loblay et al. [12]; 1986 | Single-arm pre–post intervention | Australia | Elimination diet then DBPCC | 110 | 40 | NR | Physician assessment | Acetylsalicylic acid, 300 mg Phenylethyl-amine, 4 mg, and Tyramine, 140 mg | 57 40 | 52 52 |
| Van Bever et al. [13]; 1989 | Single-arm pre–post intervention | Belgium | Elemental diet then DBPCC, both administered via nasogastric tube | 6 | NR | (5 mo–13.8 yo) | 0–4 point scale * Physician assessment | Tyramine, 20 mg Acetylsalicylic acid, 100 mg | 1 4 | 6 6 |
| Worm et al. [10]; 2009 | Non-randomised pre–post intervention | Germany | Elimination diet then DBPCC; healthy controls | 36 | 8 | 32 yo (+/−1.4 y) | SCORAD | Histamine hydrochloride, 0.75 mg/kg and 1.5 mg/kg body weight, capsule | 11 | 12 |
| Food Intolerance by Type | Challenge | Prevalence | Confidence Intervals | Heterogeneity | Certainty of Evidence |
|---|---|---|---|---|---|
| Histamine intolerance | Elimination diet | 41% | 95% CI, 25–58% | I2 = 52.78% | Low |
| Histamine intolerance | Oral challenge | 31% | 95% CI, 20–41% | I2 = 0.01% | Low |
| Amine intolerance | Oral challenge | 32% | 95% CI, 16–48% | I2 = 34.91% | Low |
| Salicylate intolerance | Oral challenge | 53% | 95% CI, 44–62% | I2 = 0.00% | Low |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fischer, K.; Jones, M.; O’Neill, H.M. Prevalence of Intolerance to Amines and Salicylates in Individuals with Atopic Dermatitis: A Systematic Review and Meta-Analysis. Nutrients 2025, 17, 1628. https://doi.org/10.3390/nu17101628
Fischer K, Jones M, O’Neill HM. Prevalence of Intolerance to Amines and Salicylates in Individuals with Atopic Dermatitis: A Systematic Review and Meta-Analysis. Nutrients. 2025; 17(10):1628. https://doi.org/10.3390/nu17101628
Chicago/Turabian StyleFischer, Karen, Mark Jones, and Hayley M. O’Neill. 2025. "Prevalence of Intolerance to Amines and Salicylates in Individuals with Atopic Dermatitis: A Systematic Review and Meta-Analysis" Nutrients 17, no. 10: 1628. https://doi.org/10.3390/nu17101628
APA StyleFischer, K., Jones, M., & O’Neill, H. M. (2025). Prevalence of Intolerance to Amines and Salicylates in Individuals with Atopic Dermatitis: A Systematic Review and Meta-Analysis. Nutrients, 17(10), 1628. https://doi.org/10.3390/nu17101628







