The Associations of Dietary Polyamines with Incident Type 2 Diabetes Mellitus: A Large Prospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Dietary Assessment
2.3. Outcome Assessment
2.4. Assessment of Other Covariates
2.5. Statistical Analyses
3. Results
3.1. Study Participants
3.2. The Association of Dietary Polyamines with Incident T2DM
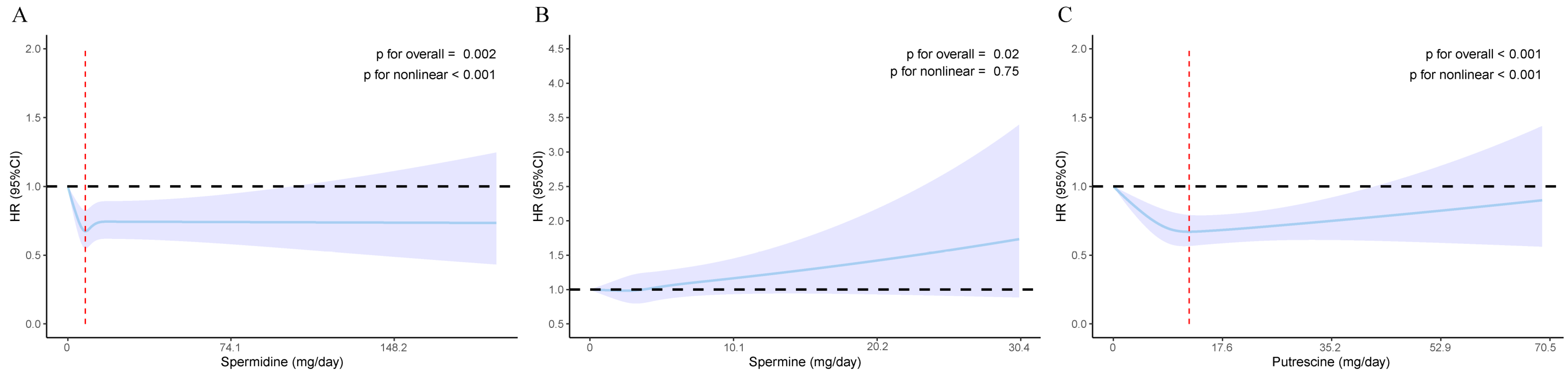
3.3. The Nonlinear Association of Dietary Polyamines with Incident T2DM
3.4. Stratified Analyses
3.5. Sensitivity Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| T2DM | type 2 diabetes mellitus |
| SD | standard deviation |
| IQR | interquartile range |
| HR | hazard ratio |
| CI | confidence interval |
| HbA1C | glycosylated hemoglobin A1c |
| HOMA-IR | homeostatic model assessment of insulin resistance |
| ROS | reactive oxygen species |
| Wnt | Wingless/Integrated |
References
- Magliano, D.J.; Boyko, E.J. IDF Diabetes Atlas [Internet], 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021. [Google Scholar]
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2017, 14, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Senesi, P.; Ferrulli, A.; Luzi, L.; Terruzzi, I. Diabetes Mellitus and Cardiovascular Diseases: Nutraceutical Interventions Related to Caloric Restriction. Int. J. Mol. Sci. 2021, 22, 7772. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Li, X. Advances in Research on Diabetes by Human Nutriomics. Int. J. Mol. Sci. 2019, 20, 5375. [Google Scholar] [CrossRef] [PubMed]
- Schuster, I.; Bernhardt, R. Interactions of natural polyamines with mammalian proteins. BioMol. Concepts 2011, 2, 79–94. [Google Scholar] [CrossRef]
- Muñoz-Esparza, N.C.; Latorre-Moratalla, M.L.; Comas-Basté, O.; Toro-Funes, N.; Veciana-Nogués, M.T.; Vidal-Carou, M.C. Polyamines in Food. Front. Nutr. 2019, 6, 108. [Google Scholar] [CrossRef]
- Tofalo, R.; Cocchi, S.; Suzzi, G. Polyamines and Gut Microbiota. Front. Nutr. 2019, 6, 16. [Google Scholar] [CrossRef]
- Sagar, N.A.; Tarafdar, S.; Agarwal, S.; Tarafdar, A.; Sharma, S. Polyamines: Functions, Metabolism, and Role in Human Disease Management. Med. Sci. 2021, 9, 44. [Google Scholar] [CrossRef]
- Moinard, C.; Cynober, L.; Debandt, J. Polyamines: Metabolism and implications in human diseases. Clin. Nutr. 2005, 24, 184–197. [Google Scholar] [CrossRef]
- Kalač, P. Health effects and occurrence of dietary polyamines. A review for the period 2005–mid 2013. Food Chem. 2014, 161, 27–39. [Google Scholar] [CrossRef]
- Atiya Ali, M.; Poortvliet, E.; Strömberg, R.; Yngve, A. Polyamines in foods: Development of a food database. Food Nutr. Res. 2017, 55, 5572. [Google Scholar] [CrossRef]
- Forouhi, N.G.; Misra, A.; Mohan, V.; Taylor, R.; Yancy, W. Dietary and nutritional approaches for prevention and management of type 2 diabetes. Br. Med. J. 2018, 361, k2234. [Google Scholar] [CrossRef] [PubMed]
- Esposito, K.; Giugliano, D. Mediterranean diet and type 2 diabetes. Diabetes/Metab. Res. Rev. 2014, 30, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Park, M.H.; Igarashi, K. Polyamines and Their Metabolites as Diagnostic Markers of Human Diseases. Biomol. Ther. 2013, 21, 1–9. [Google Scholar] [CrossRef]
- Amin, M.; Tang, S.; Shalamanova, L.; Taylor, R.L.; Wylie, S.; Abdullah, B.M.; Whitehead, K.A. Polyamine biomarkers as indicators of human disease. Biomarkers 2021, 26, 77–94. [Google Scholar] [CrossRef]
- Kulkarni, A.; Anderson, C.M.; Mirmira, R.G.; Tersey, S.A. Role of Polyamines and Hypusine in β Cells and Diabetes Pathogenesis. Metabolites 2022, 12, 344. [Google Scholar] [CrossRef]
- Jimenez Gutierrez, G.E.; Borbolla Jiménez, F.V.; Muñoz, L.G.; Tapia Guerrero, Y.S.; Murillo Melo, N.M.; Cristóbal-Luna, J.M.; Leyva Garcia, N.; Cordero-Martínez, J.; Magaña, J.J. The Molecular Role of Polyamines in Age-Related Diseases. An Update. Int. J. Mol. Sci. 2023, 24, 16469. [Google Scholar] [CrossRef]
- Kambis, T.N.; Tofilau, H.M.N.; Gawargi, F.I.; Chandra, S.; Mishra, P.K. Regulating Polyamine Metabolism by miRNAs in Diabetic Cardiomyopathy. Curr. Diabetes Rep. 2021, 21, 52. [Google Scholar] [CrossRef]
- Mastracci, T.L.; Robertson, M.A.; Mirmira, R.G.; Anderson, R.M. Polyamine biosynthesis is critical for growth and differentiation of the pancreas. Sci. Rep. 2015, 5, 13269. [Google Scholar] [CrossRef]
- Sjöholm, A.P.; Berggren, P.O.; Andersson, A. Polyamines in pancreatic islets of obese hyperglycemic (ob-ob) mice of different ages. Am. J. Physiol. Cell Physiol. 2001, 280, C317–C323. [Google Scholar] [CrossRef]
- Welsh, N. A role for polyamines in glucose-stimulated insulin-gene expression. Biochem. J. 1990, 271, 393–397. [Google Scholar] [CrossRef]
- Welsh, N.S.A. Polyamines and insulin production in isolated mouse pancreatic islets. Biochem. J. 1988, 252, 701–707. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Garcia, J.; Delpino-Rius, A.; Samarra, I.; Castellano-Castillo, D.; Muñoz-Garach, A.; Bernal-Lopez, M.; Queipo-Ortuño, M.; Cardona, F.; Ramos-Molina, B.; Tinahones, F. Type 2 Diabetes Is Associated with a Different Pattern of Serum Polyamines: A Case–Control Study from the PREDIMED-Plus Trial. J. Clin. Med. 2019, 8, 71. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, Y.; Li, S.; Liu, M.; Lu, Y.; He, M.; Sun, Z.; Ma, M.; Zheng, L. Non-linear associations of serum spermidine with type 2 diabetes mellitus and fasting plasma glucose: A cross-sectional study. Front. Nutr. 2024, 11, 1393552. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Ni, Y.; Wang, Z.; Tu, W.; Ni, L.; Zhuge, F.; Zheng, A.; Hu, L.; Zhao, Y.; Zheng, L.; et al. Spermidine improves gut barrier integrity and gut microbiota function in diet-induced obese mice. Gut Microbes 2020, 12, 1832857. [Google Scholar] [CrossRef] [PubMed]
- UK Biobank. UK Biobank: Protocol for a Large-Scale Prospective Epidemiological Resource [Internet]; UK Biobank, 2007; Available online: https://www.ukbiobank.ac.uk/media/3sbeknnz/ukbiobank_protocol.pdf (accessed on 1 January 2025).
- Maroto-Rodriguez, J.; Ortolá, R.; Carballo-Casla, A.; Iriarte-Campo, V.; Salinero-Fort, M.Á.; Rodríguez-Artalejo, F.; Sotos-Prieto, M. Association between a mediterranean lifestyle and Type 2 diabetes incidence: A prospective UK biobank study. Cardiovasc. Diabetol. 2023, 22, 271. [Google Scholar] [CrossRef]
- Liu, B.; Young, H.; Crowe, F.L.; Benson, V.S.; Spencer, E.A.; Key, T.J.; Appleby, P.N.; Beral, V. Development and evaluation of the Oxford WebQ, a low-cost, web-based method for assessment of previous 24 h dietary intakes in large-scale prospective studies. Public Health Nutr. 2011, 14, 1998–2005. [Google Scholar] [CrossRef]
- Piernas, C.; Perez-Cornago, A.; Gao, M.; Young, H.; Pollard, Z.; Mulligan, A.; Lentjes, M.; Carter, J.; Bradbury, K.; Key, T.J.; et al. Describing a new food group classification system for UK biobank: Analysis of food groups and sources of macro- and micronutrients in 208,200 participants. Eur. J. Nutr. 2021, 60, 2879–2890. [Google Scholar] [CrossRef]
- Gao, M.; Jebb, S.A.; Aveyard, P.; Ambrosini, G.L.; Perez-Cornago, A.; Papier, K.; Carter, J.; Piernas, C. Associations Between Dietary Patterns and Incident Type 2 Diabetes: Prospective Cohort Study of 120,343 UK Biobank Participants. Diabetes Care 2022, 45, 1315–1325. [Google Scholar] [CrossRef]
- Lockwood, D.H.; East, L.E. Studies of the Insulin-like Actions of Polyamines on Lipid and Glucose Metabolism in Adipose Tissue Cells. J. Biol. Chem. 1974, 249, 7717–7722. [Google Scholar] [CrossRef]
- Hougaard, D.M.N.J.; Larsson, L.I. Localization and biosynthesis of polyamines in insulin-producing cells. Biochem. J. 1986, 238, 43–47. [Google Scholar] [CrossRef]
- Sadasivan, S.K.; Vasamsetti, B.; Singh, J.; Marikunte, V.V.; Oommen, A.M.; Jagannath, M.R.; Pralhada Rao, R. Exogenous administration of spermine improves glucose utilization and decreases bodyweight in mice. Eur. J. Pharmacol. 2014, 729, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Méndez, J.D.; Balderas, F.L. Inhibition by l-arginine and spermidine of hemoglobin glycation and lipid peroxidation in rats with induced diabetes. Biomed. Pharmacother. 2006, 60, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Méndez, J.D.; Hernández, R.D.H. l-Arginine and polyamine administration protect β-cells against alloxan diabetogenic effect in Sprague–Dawley rats. Biomed. Pharmacother. 2005, 59, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Neuenschwander, M.; Stadelmaier, J.; Eble, J.; Grummich, K.; Szczerba, E.; Kiesswetter, E.; Schlesinger, S.; Schwingshackl, L. Substitution of animal-based with plant-based foods on cardiometabolic health and all-cause mortality: A systematic review and meta-analysis of prospective studies. BMC Med. 2023, 21, 404. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, J.; Li, S.; Zhang, X.; Guo, Z.; Hu, J.; Shao, X.; Song, N.; Zhao, Y.; Li, H.; et al. Exogenous spermine attenuates rat diabetic cardiomyopathy via suppressing ROS-p53 mediated downregulation of calcium-sensitive receptor. Redox Biol. 2020, 32, 101514. [Google Scholar] [CrossRef]
- Wang, Z. Polyamines instruct T-cell differentiation. Nat. Cell Biol. 2021, 23, 811. [Google Scholar] [CrossRef]
- Puleston, D.J.; Baixauli, F.; Sanin, D.E.; Edwards-Hicks, J.; Villa, M.; Kabat, A.M.; Kamiński, M.M.; Stanckzak, M.; Weiss, H.J.; Grzes, K.M.; et al. Polyamine metabolism is a central determinant of helper T cell lineage fidelity. Cell 2021, 184, 4186–4202.e4120. [Google Scholar] [CrossRef]
- Latour, Y.L.; Gobert, A.P.; Wilson, K.T. The role of polyamines in the regulation of macrophage polarization and function. Amino Acids 2019, 52, 151–160. [Google Scholar] [CrossRef]
- Mantri, A.; Köhlmoos, A.; Schelski, D.S.; Seel, W.; Stoffel-Wagner, B.; Krawitz, P.; Stehle, P.; Holst, J.J.; Weber, B.; Koban, L.; et al. Impact of Synbiotic Intake on Liver Metabolism in Metabolically Healthy Participants and Its Potential Preventive Effect on Metabolic-Dysfunction-Associated Fatty Liver Disease (MAFLD): A Randomized, Placebo-Controlled, Double-Blinded Clinical Trial. Nutrients 2024, 16, 1300. [Google Scholar] [CrossRef]
- Di Molfetta, I.V.; Bordoni, L.; Gabbianelli, R.; Sagratini, G.; Alessandroni, L. Vitamin D and Its Role on the Fatigue Mitigation: A Narrative Review. Nutrients 2024, 16, 221. [Google Scholar] [CrossRef]
- Garza, M.C.; Pérez-Calahorra, S.; Rodrigo-Carbó, C.; Sánchez-Calavera, M.A.; Jarauta, E.; Mateo-Gallego, R.; Gracia-Rubio, I.; Lamiquiz-Moneo, I. Effect of Aromatic Herbs and Spices Present in the Mediterranean Diet on the Glycemic Profile in Type 2 Diabetes Subjects: A Systematic Review and Meta-Analysis. Nutrients 2024, 16, 756. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, K.; Shiina, R.; Kashiwagi, K.; Igarashi, K. Decrease in Polyamines with Aging and Their Ingestion from Food and Drink. J. Biochem. 2006, 139, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Cipolla, B.G.; Havouis, R.; Moulinoux, J.P. Polyamine contents in current foods: A basis for polyamine reduced diet and a study of its long term observance and tolerance in prostate carcinoma patients. Amino Acids 2007, 33, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Kalač, P.; Křı́žek, M.; Pelikánová, T.; Langová, M.; Veškrna, P. Contents of polyamines in selected foods. Food Chem. 2005, 90, 561–564. [Google Scholar] [CrossRef]
- Bardócz, S.; Grant, G.; Brown, B.S.; Ralph, A.; Pusztai, A. Polyamines in food-implications for growth and health. Jnutrbiochem 1993, 4, 66–71. [Google Scholar]
- Romero, R.; Sánchez-Viñas, M.; Gázquez, D.; Bagur, M.G. Characterization of selected Spanish table wine samples according to their biogenic amine content from liquid chromatographic determination. J. Agric. Food Chem. 2002, 50, 4713–4717. [Google Scholar] [CrossRef]
- Eliassena, K.A.; Reistad, R.; Risøena, U.; Rønninga, H.F. Dietary polyamines. Food Chem. 2002, 78, 273–280. [Google Scholar] [CrossRef]
- Kiechl, S.; Pechlaner, R.; Willeit, P.; Notdurfter, M.; Paulweber, B.; Willeit, K.; Werner, P.; Ruckenstuhl, C.; Iglseder, B.; Weger, S.; et al. Higher spermidine intake is linked to lower mortality: A prospective population-based study. Am. J. Clin. Nutr. 2018, 108, 371–380. [Google Scholar] [CrossRef]
- Krausová, P.; Kalač, P.; Křížek, M.; Pelikánová, T. Content of biologically active polyamines in livers of cattle, pigs and chickens after animal slaughter. Meat Sci. 2006, 73, 640–644. [Google Scholar] [CrossRef]
- Nishibori, N.; Fujihara, S.; Akatuki, T. Amounts of polyamines in foods in Japan and intake by Japanese. Food Chem. 2007, 100, 491–497. [Google Scholar] [CrossRef]
- Muñoz-Esparza, N.C.; Costa-Catala, J.; Comas-Basté, O.; Toro-Funes, N.; Latorre-Moratalla, M.L.; Veciana-Nogués, M.T.; Vidal-Carou, M.C. Occurrence of Polyamines in Foods and the Influence of Cooking Processes. Foods 2021, 10, 1752. [Google Scholar] [CrossRef] [PubMed]
- Bardócz, S.; Duguid, T.J.; Brown, D.S.; Grant, G.; Pusztai, A.; White, A.; Ralph, A. The importance of dietary polyamines in cell regeneration and growth. Br. J. Nutr. 1995, 73, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Kalač, P.; Krausová, P. A review of dietary polyamines: Formation, implications for growth and health and occurrence in foods. Food Chem. 2004, 90, 219–230. [Google Scholar] [CrossRef]
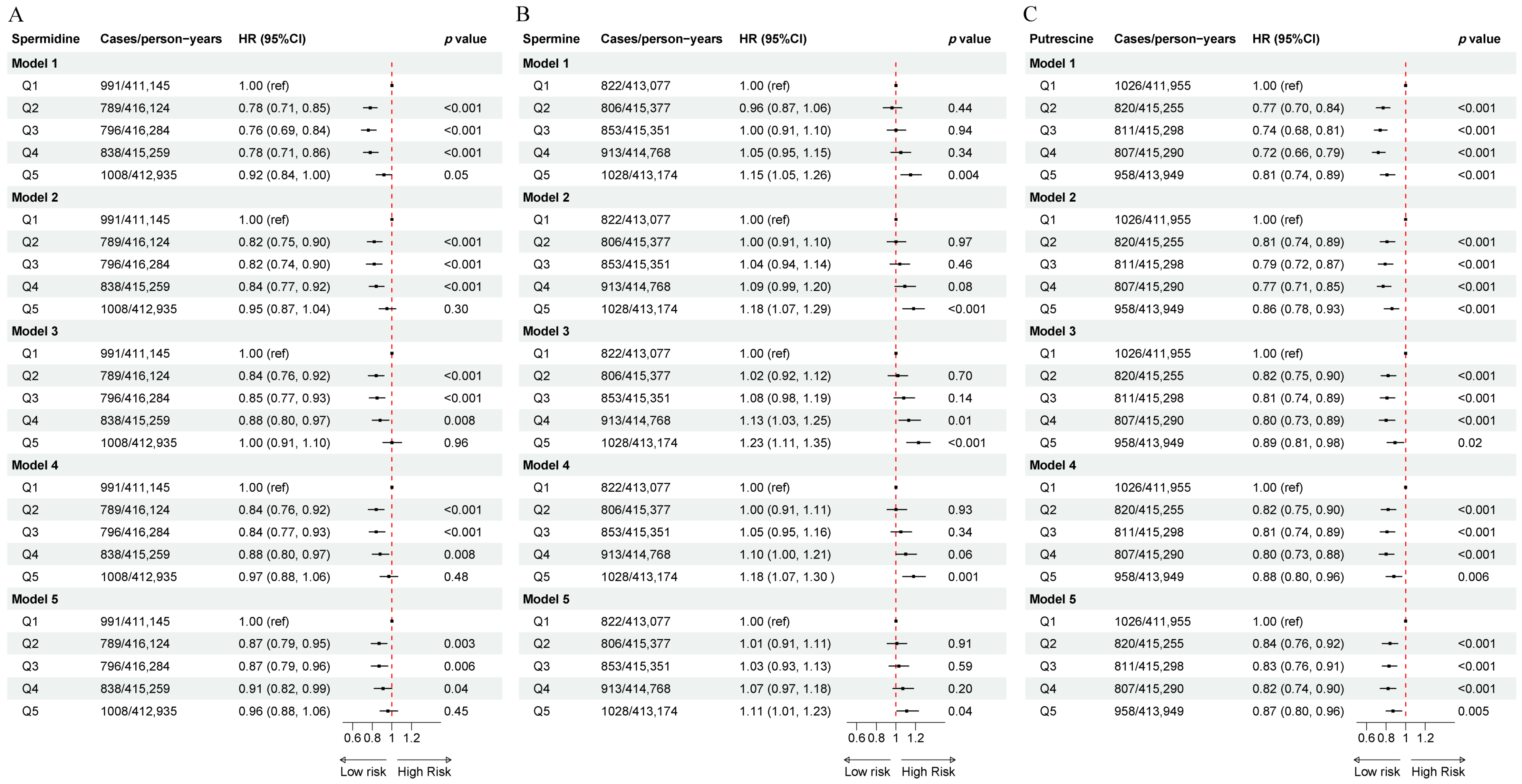
| Overall | Q1 (<6.2 mg/day) | Q2 (6.2~8.0 mg/day) | Q3 (8.0~9.8 mg/day) | Q4 (9.8~12.2 mg/day) | Q5 (>12.2 mg/day) | p Value | |
|---|---|---|---|---|---|---|---|
| n | 168,137 | 33,628 | 33,627 | 33,627 | 33,627 | 33,628 | |
| Follow-up, years, median [IQR] | 12.4 [11.8, 13.2] | 12.4 [11.8, 13.2] | 12.5 [11.9, 13.3] | 12.5 [11.9, 13.3] | 12.5 [11.9, 13.2] | 12.4 [11.8, 13.2] | <0.001 |
| Spermidine, mg/day | 10.5 ± 11.8 | 4.6 ± 1.2 | 7.2 ± 0.5 | 8.9 ± 0.5 | 10.9 ± 0.7 | 21.1 ± 23.1 | <0.001 |
| Incident T2DM, n (%) | <0.001 | ||||||
| No | 163,715 (97.4) | 32,637 (97.1) | 32,838 (97.7) | 32,831 (97.6) | 32,789 (97.5) | 32,620 (97.0) | |
| Yes | 4422 (2.6) | 991 (2.9) | 789 (2.3) | 796 (2.4) | 838 (2.5) | 1008 (3.0) | |
| Age | 55.8 ± 8.0 | 54.5 ± 8.0 | 55.3 ± 7.9 | 56.0 ± 7.9 | 56.5 ± 7.9 | 56.6 ± 7.9 | <0.001 |
| Sex, n (%) | <0.001 | ||||||
| Men | 77,117 (45.9) | 15,626 (46.5) | 15,107 (44.9) | 15,172 (45.1) | 15,156 (45.1) | 16,056 (47.7) | |
| Women | 91,020 (54.1) | 18,002 (53.5) | 18,520 (55.1) | 18,455 (54.9) | 18,471 (54.9) | 17,572 (52.3) | |
| Regions, n (%) | <0.001 | ||||||
| England | 136,222 (81.0) | 26,950 (80.1) | 27,143 (80.7) | 27,394 (81.5) | 27,522 (81.8) | 27,213 (80.9) | |
| Northern Ireland | 1146 (0.7) | 238 (0.7) | 240 (0.7) | 219 (0.7) | 233 (0.7) | 216 (0.6) | |
| Republic of Ireland | 1417 (0.8) | 290 (0.9) | 266 (0.8) | 288 (0.9) | 276 (0.8) | 297 (0.9) | |
| Scotland | 10,502 (6.2) | 2062 (6.1) | 2194 (6.5) | 2131 (6.3) | 2073 (6.2) | 2042 (6.1) | |
| Wales | 6198 (3.7) | 1160 (3.4) | 1234 (3.7) | 1262 (3.8) | 1300 (3.9) | 1242 (3.7) | |
| Elsewhere | 12,652 (7.5) | 2928 (8.7) | 2550 (7.6) | 2333 (6.9) | 2223 (6.6) | 2618 (7.8) | |
| Race/ethnicity, n (%) | <0.001 | ||||||
| White | 161,683 (96.2) | 31,761 (94.4) | 32,420 (96.4) | 32,628 (97.0) | 32,568 (96.9) | 32,306 (96.1) | |
| Non-white | 6454 (3.8) | 1867 (5.6) | 1207 (3.6) | 999 (3.0) | 1059 (3.1) | 1322 (3.9) | |
| Educational level, n (%) | <0.001 | ||||||
| Higher degree | 101,032 (60.1) | 18,256 (54.3) | 20,583 (61.2) | 21,206 (63.1) | 21,117 (62.8) | 19,870 (59.1) | |
| Any school degree | 47,138 (28.0) | 10,634 (31.6) | 9499 (28.2) | 8884 (26.4) | 8854 (26.3) | 9267 (27.6) | |
| Vocational qualifications | 7792 (4.6) | 1791 (5.3) | 1463 (4.4) | 1405 (4.2) | 1413 (4.2) | 1720 (5.1) | |
| Other | 12,175 (7.2) | 2947 (8.8) | 2082 (6.2) | 2132 (6.3) | 2243 (6.7) | 2771 (8.2) | |
| Townsend index, n (%) | <0.001 | ||||||
| Q1 | 33,641 (20.0) | 6058 (18.0) | 6735 (20.0) | 7003 (20.8) | 7082 (21.1) | 6763 (20.1) | |
| Q2 | 33,642 (20.0) | 6368 (18.9) | 6532 (19.4) | 6870 (20.4) | 6982 (20.8) | 6890 (20.5) | |
| Q3 | 33,607 (20.0) | 6504 (19.3) | 6632 (19.7) | 6825 (20.3) | 6793 (20.2) | 6853 (20.4) | |
| Q4 | 33,619 (20.0) | 6865 (20.4) | 6799 (20.2) | 6635 (19.7) | 6591 (19.6) | 6729 (20.0) | |
| Q5 | 33,628 (20.0) | 7833 (23.3) | 6929 (20.6) | 6294 (18.7) | 6179 (18.4) | 6393 (19.0) | |
| Physical activity, n (%) | <0.001 | ||||||
| Low | 29,989 (17.8) | 7129 (21.2) | 6462 (19.2) | 5883 (17.5) | 5592 (16.6) | 4923 (14.6) | |
| Moderate | 89,800 (53.4) | 17,479 (52.0) | 18,295 (54.4) | 18,537 (55.1) | 18,263 (54.3) | 17,226 (51.2) | |
| High | 48,348 (28.8) | 9020 (26.8) | 8870 (26.4) | 9207 (27.4) | 9772 (29.1) | 11,479 (34.1) | |
| Smoking status, n (%) | <0.001 | ||||||
| Never | 95,777 (57.0) | 18,539 (55.1) | 19,354 (57.6) | 19,580 (58.2) | 19,412 (57.7) | 18,892 (56.2) | |
| Current | 13,002 (7.7) | 3734 (11.1) | 2576 (7.7) | 2219 (6.6) | 2132 (6.3) | 2341 (7.0) | |
| Previous | 59,358 (35.3) | 11,355 (33.8) | 11,697 (34.8) | 11,828 (35.2) | 12,083 (35.9) | 12,395 (36.9) | |
| Alcohol consumption, n (%) | <0.001 | ||||||
| Never | 4920 (2.9) | 1112 (3.3) | 911 (2.7) | 878 (2.6) | 915 (2.7) | 1104 (3.3) | |
| Current | 158,481 (94.3) | 31,431 (93.5) | 31,835 (94.7) | 31,892 (94.8) | 31,880 (94.8) | 31,443 (93.5) | |
| Previous | 4736 (2.8) | 1085 (3.2) | 881 (2.6) | 857 (2.5) | 832 (2.5) | 1081 (3.2) | |
| Sleep duration, hours | 7.2 ± 1.0 | 7.1 ± 1.1 | 7.2 ± 1.0 | 7.2 ± 1.0 | 7.2 ± 1.0 | 7.1 ± 1.1 | <0.001 |
| Family history of diabetes, n (%) | <0.001 | ||||||
| No | 134,902 (80.2) | 26,653 (79.3) | 27,151 (80.7) | 27,077 (80.5) | 27,088 (80.6) | 26,933 (80.1) | |
| Yes | 33,235 (19.8) | 6975 (20.7) | 6476 (19.3) | 6550 (19.5) | 6539 (19.4) | 6695 (19.9) | |
| Hypertension, n (%) | <0.001 | ||||||
| No | 128,474 (76.4) | 25,959 (77.2) | 25,938 (77.1) | 25,922 (77.1) | 25,648 (76.3) | 25,007 (74.4) | |
| Yes | 39,663 (23.6) | 7669 (22.8) | 7689 (22.9) | 7705 (22.9) | 7979 (23.7) | 8621 (25.6) | |
| Cardiovascular disease, n (%) | <0.001 | ||||||
| No | 161,475 (96.0) | 32,353 (96.2) | 32,365 (96.2) | 32,333 (96.2) | 32,340 (96.2) | 32,084 (95.4) | |
| Yes | 6662 (4.0) | 1275 (3.8) | 1262 (3.8) | 1294 (3.8) | 1287 (3.8) | 1544 (4.6) | |
| Hyperlipidemia, n (%) | <0.001 | ||||||
| No | 145,054 (86.3) | 29,250 (87.0) | 29,198 (86.8) | 29,037 (86.4) | 29,037 (86.4) | 28,532 (84.8) | |
| Yes | 23,083 (13.7) | 4378 (13.0) | 4429 (13.2) | 4590 (13.6) | 4590 (13.6) | 5096 (15.2) | |
| BMI group †, n (%) | <0.001 | ||||||
| Underweight | 935 (0.6) | 157 (0.5) | 179 (0.5) | 211 (0.6) | 200 (0.6) | 188 (0.6) | |
| Healthy weight | 46,932 (27.9) | 8766 (26.1) | 9544 (28.4) | 9789 (29.1) | 9771 (29.1) | 9062 (26.9) | |
| Overweight | 66,137 (39.3) | 13,094 (38.9) | 13,516 (40.2) | 13,372 (39.8) | 13,268 (39.5) | 12,887 (38.3) | |
| Obese | 54,133 (32.2) | 11,611 (34.5) | 10,388 (30.9) | 10,255 (30.5) | 10,388 (30.9) | 11,491 (34.2) |
| Cases/Person-Years | Q1 (<6.2 mg/day) | Q2 (6.2~8.0 mg/day) | Q3 (8.0~9.8 mg/day) | Q4 (9.8~12.2 mg/day) | Q5 (>12.2 mg/day) | p for Interaction | |
|---|---|---|---|---|---|---|---|
| Age group | 0.13 | ||||||
| <60 | 2012/1,278,265 | 1.00 | 0.89 (0.78, 1.02) | 0.83 (0.72, 0.96) | 0.91 (0.79, 1.05) | 0.88 (0.77, 1.02) | |
| ≥60 | 2410/793,482 | 1.00 | 0.88 (0.77, 1.01) | 0.95 (0.83, 1.09) | 0.95 (0.83, 1.08) | 1.09 (0.95, 1.24) | |
| Sex | 0.72 | ||||||
| Men | 2670/942,905 | 1.00 | 0.91 (0.81, 1.03) | 0.89 (0.79, 1.01) | 0.96 (0.85, 1.09) | 1.03 (0.91, 1.16) | |
| Women | 1752/1,128,842 | 1.00 | 0.80 (0.69, 0.93) | 0.85 (0.73, 0.98) | 0.82 (0.71, 0.96) | 0.87 (0.75, 1.01) | |
| Smoking status | 0.67 | ||||||
| Never | 1976/1,188,596 | 1.00 | 0.86 (0.74, 0.99) | 0.85 (0.74, 0.98) | 0.84 (0.72, 0.97) | 0.88 (0.76, 1.02) | |
| Current | 511/157,194 | 1.00 | 0.78 (0.60, 1.03) | 0.81 (0.61, 1.07) | 1.08 (0.82, 1.42) | 1.05 (0.80, 1.39) | |
| Previous | 1935/725,956 | 1.00 | 0.90 (0.78, 1.04) | 0.92 (0.79, 1.06) | 0.95 (0.82, 1.09) | 1.04 (0.90, 1.20) | |
| Alcohol consumption | 0.55 | ||||||
| Never | 220/59,811 | 1.00 | 0.85 (0.56, 1.28) | 0.73 (0.46, 1.13) | 0.91 (0.60, 1.39) | 0.91 (0.60, 1.39) | |
| Current | 3962/1,955,145 | 1.00 | 0.87 (0.78, 0.96) | 0.88 (0.79, 0.97) | 0.89 (0.80, 0.98) | 0.97 (0.88, 1.07) | |
| Previous | 240/56,791 | 1.00 | 0.96 (0.64, 1.45) | 1.04 (0.68, 1.59) | 1.35 (0.90, 2.03) | 1.06 (0.70, 1.60) | |
| Townsend index | 0.07 | ||||||
| Q1 | 670/420,573 | 1.00 | 1.05 (0.82, 1.35) | 0.92 (0.71, 1.19) | 1.00 (0.77, 1.29) | 1.14 (0.88, 1.47) | |
| Q2 | 807/416,208 | 1.00 | 0.95 (0.80, 1.12) | 0.91 (0.77, 1.08) | 0.90 (0.76, 1.07) | 1.05 (0.89, 1.25) | |
| Q3 | 849/414,799 | 1.00 | 0.83 (0.66, 1.04) | 0.91 (0.73, 1.14) | 1.05 (0.84, 1.31) | 1.18 (0.95, 1.47) | |
| Q4 | 897/411,704 | 1.00 | 0.97 (0.79, 1.20) | 1.04 (0.84, 1.28) | 0.98 (0.79, 1.22) | 0.89 (0.72, 1.11) | |
| Q5 | 1199/408,462 | 1.00 | 0.75 (0.63, 0.89) | 0.73 (0.61, 0.87) | 0.80 (0.67, 0.96) | 0.80 (0.67, 0.96) | |
| Race/ethnicity | 0.51 | ||||||
| White | 4101/1,994,658 | 1.00 | 0.86 (0.78, 0.95) | 0.89 (0.80, 0.98) | 0.91 (0.82, 1.00) | 0.97 (0.88, 1.07) | |
| Non-white | 321/77,089 | 1.00 | 0.92 (0.67, 1.28) | 0.73 (0.50, 1.06) | 0.92 (0.65, 1.31) | 0.92 (0.64, 1.31) | |
| Physical activity | 0.63 | ||||||
| Low | 1118/367,879 | 1.00 | 0.91(0.76, 1.09) | 0.91 (0.75, 1.09) | 0.92 (0.77, 1.11) | 0.85 (0.70, 1.03) | |
| Moderate | 2208/1,109,518 | 1.00 | 0.85 (0.75, 0.98) | 0.90 (0.78, 1.03) | 0.89 (0.78, 1.03) | 0.99 (0.86, 1.14) | |
| High | 1096/594,350 | 1.00 | 0.84 (0.69, 1.03) | 0.80 (0.65, 0.98) | 0.91 (0.75, 1.11) | 1.00 (0.83, 1.21) | |
| Educational level | 0.03 | ||||||
| Higher degree | 2166/1,252,991 | 1.00 | 0.84 (0.73, 0.96) | 0.83 (0.72, 0.95) | 0.82 (0.72, 0.95) | 1.01 (0.88, 1.16) | |
| Any school degree | 1280/579,043 | 1.00 | 0.97 (0.81, 1.15) | 0.92 (0.77, 1.10) | 1.05 (0.88, 1.25) | 0.97 (0.81, 1.17) | |
| Vocational qualifications | 314/145,227 | 1.00 | 1.14 (0.80, 1.62) | 1.06 (0.73, 1.53) | 1.09 (0.75, 1.58) | 1.23 (0.86, 1.75) | |
| Other | 662/94,487 | 1.00 | 0.67 (0.52, 0.87) | 0.86 (0.68, 1.08) | 0.84 (0.66, 1.07) | 0.74 (0.58, 0.94) | |
| Family history of diabetes | 0.54 | ||||||
| No | 2932/1,666,274 | 1.00 | 0.86 (0.77, 0.97) | 0.89 (0.79, 1.00) | 0.88 (0.78, 0.99) | 0.99 (0.88, 1.11) | |
| Yes | 1490/405,473 | 1.00 | 0.87 (0.74, 1.02) | 0.84 (0.71, 0.99) | 0.95 (0.81, 1.12) | 0.92 (0.78, 1.09) | |
| Hypertension | 0.31 | ||||||
| No | 2203/159,4562 | 1.00 | 0.84 (0.74, 0.96) | 0.87 (0.76, 0.99) | 0.83 (0.73, 0.96) | 0.98 (0.86, 1.13) | |
| Yes | 2219/477,185 | 1.00 | 0.89 (0.78, 1.02) | 0.88 (0.77, 1.01) | 0.97 (0.85, 1.12) | 0.95 (0.83, 1.09) | |
| Cardiovascular disease | 0.32 | ||||||
| No | 3868/1,993,952 | 1.00 | 0.84 (0.76, 0.93) | 0.87 (0.79, 0.97) | 0.90 (0.81, 0.99) | 0.94 (0.84, 1.04) | |
| Yes | 554/77,795 | 1.00 | 1.07 (0.82, 1.40) | 0.87 (0.66, 1.16) | 0.96 (0.72, 1.27) | 1.18 (0.90, 1.53) | |
| Hyperlipidemia | 0.15 | ||||||
| No | 2930/1,797,116 | 1.00 | 0.81 (0.72, 0.91) | 0.84 (0.75, 0.94) | 0.85 (0.76, 0.95) | 0.87 (0.78, 0.98) | |
| Yes | 1492/274,631 | 1.00 | 1.00 (0.84, 1.18) | 0.97 (0.82, 1.15) | 1.04 (0.88, 1.23) | 1.17 (0.99, 1.38) | |
| BMI group † | 0.52 | ||||||
| Underweight/healthy weight | 338/597,623 | 1.00 | 0.66 (0.46, 0.94) | 0.78 (0.55, 1.10) | 0.78 (0.56, 1.11) | 0.95 (0.67, 1.35) | |
| Overweight | 1086/819,249 | 1.00 | 0.95 (0.78, 1.15) | 0.91 (0.75, 1.11) | 0.88 (0.72, 1.08) | 1.12 (0.92, 1.36) | |
| Obese | 2998/654,876 | 1.00 | 0.86 (0.77, 0.97) | 0.87 (0.78, 0.98) | 0.93 (0.83, 1.04) | 0.92 (0.81, 1.03) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Qian, M.; Liu, M.; He, M.; Li, F.-R.; Zheng, L. The Associations of Dietary Polyamines with Incident Type 2 Diabetes Mellitus: A Large Prospective Cohort Study. Nutrients 2025, 17, 186. https://doi.org/10.3390/nu17010186
Zhang X, Qian M, Liu M, He M, Li F-R, Zheng L. The Associations of Dietary Polyamines with Incident Type 2 Diabetes Mellitus: A Large Prospective Cohort Study. Nutrients. 2025; 17(1):186. https://doi.org/10.3390/nu17010186
Chicago/Turabian StyleZhang, Xiaohong, Mingxia Qian, Min Liu, Mengyao He, Fu-Rong Li, and Liqiang Zheng. 2025. "The Associations of Dietary Polyamines with Incident Type 2 Diabetes Mellitus: A Large Prospective Cohort Study" Nutrients 17, no. 1: 186. https://doi.org/10.3390/nu17010186
APA StyleZhang, X., Qian, M., Liu, M., He, M., Li, F.-R., & Zheng, L. (2025). The Associations of Dietary Polyamines with Incident Type 2 Diabetes Mellitus: A Large Prospective Cohort Study. Nutrients, 17(1), 186. https://doi.org/10.3390/nu17010186






