Assessing the Consumption of Ultra-Processed Foods in People with Diabetes: Is a Specific NOVA Questionnaire Always Necessary?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Design
2.3. EPIC Food Frequency Questionnaire (EPIC-FFQ)
2.4. NOVA Food Frequency Questionnaire (NOVA-FFQ)
2.5. Measurement of MPFs, PCIs, PFs, and UPFs
2.6. Sample Size and Statistical Analysis
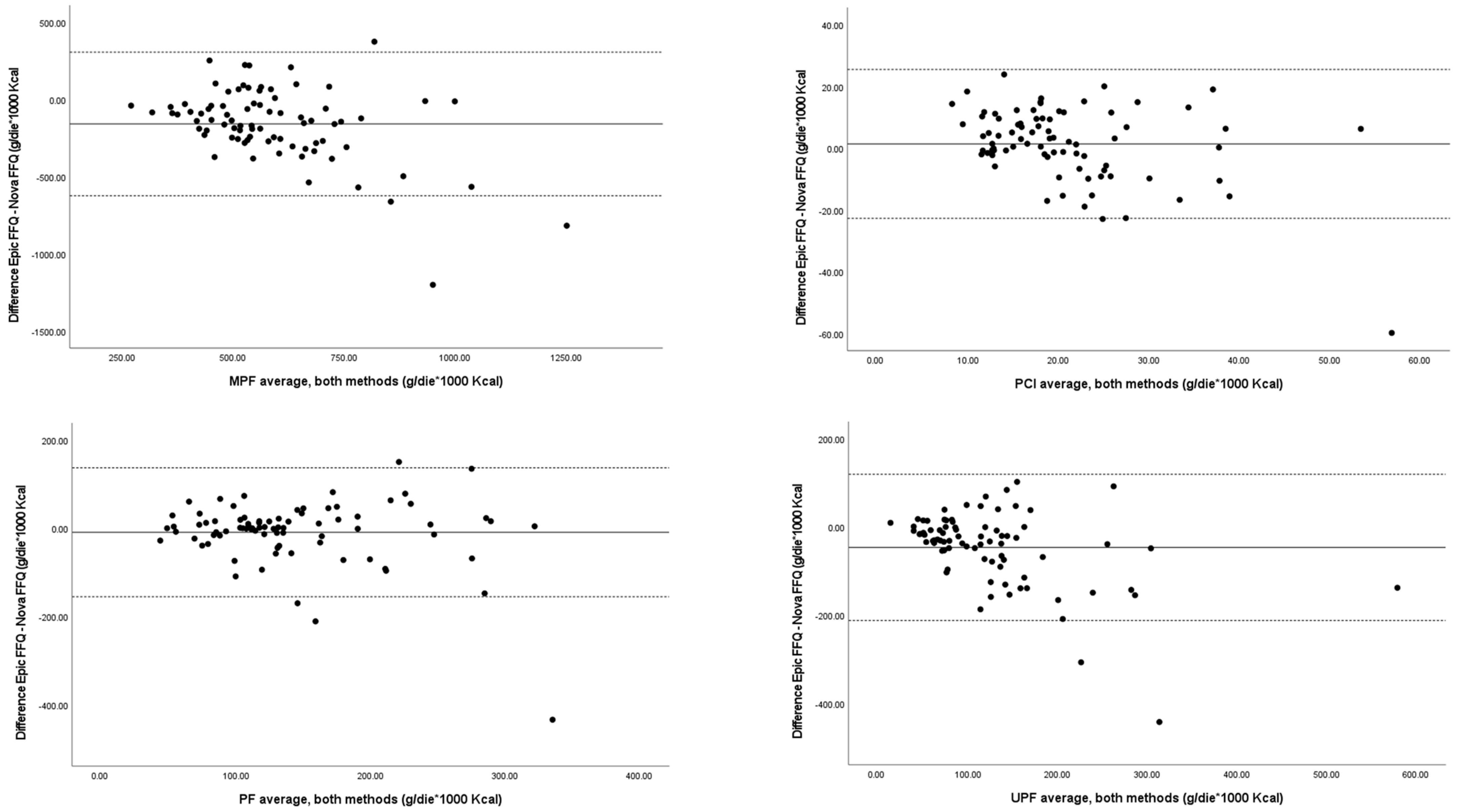
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Monteiro, C.A.; Cannon, G.; Levy, R.B.; Moubarac, J.C.; Louzada, M.L.C.; Rauber, F.; Khandpur, N.; Cediel, G.; Neri, D.; Martinez-Steele, E.; et al. Ultra-processed foods: What they are and how to identify them. Public Health Nutr. 2019, 22, 936–941. [Google Scholar] [CrossRef]
- Baker, P.; Friel, S. Food systems transformations, ultra-processed food markets and the nutrition transition in Asia. Glob. Health 2016, 12, 80. [Google Scholar] [CrossRef]
- Moubarac, J.C.; Batal, M.; Martins, A.P.B.; Claro, R.; Levy, R.B.; Cannon, G.; Monteiro, C. Processed and ultra-processed food products: Consumption trends in Canada from 1938 to 2011. Can. J. Diet. Pract. Res. 2014, 75, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Moubarac, J.-C.; Pan American Health Organization; World Health Organization. Ultra-Processed Food and Drink Products in Latin America: Trends, Impact on Obesity, Policy Implications. US, 1 January 2015. Available online: https://iris.paho.org/bitstream/handle/10665.2/7699/9789275118641_eng.pdf (accessed on 29 November 2024).
- Juul, F.; Hemmingsson, E. Trends in consumption of ultra-processed foods and obesity in Sweden between 1960 and 2010. Public Health Nutr. 2015, 18, 3096–3107. [Google Scholar] [CrossRef]
- Wang, L.; Martínez Steele, E.; Du, M.; Pomeranz, J.L.; O’Connor, L.E.; Herrick, K.A.; Luo, H.; Zhang, X.; Mozaffarian, D.; Zhang, F.F. Trends in Consumption of Ultra-processed Foods among US Youths Aged 2–19 Years, 1999–2018. JAMA 2021, 326, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Andrade, G.C.; Julia, C.; Deschamps, V.; Srour, B.; Hercberg, S.; Kesse-Guyot, E.; Allès, B.; Chazelas, E.; Deschasaux, M.; Touvier, M.; et al. Consumption of ultra-processed food and its association with sociodemographic characteristics and diet quality in a representative sample of French adults. Nutrients 2021, 13, 682. [Google Scholar] [CrossRef]
- Rauber, F.; Louzada, M.L.D.C.; Steele, E.M.; Millett, C.; Monteiro, C.A.; Levy, R.B. Ultra-processed food consumption and chronic non-communicable diseases-related dietary nutrient profile in the UK (2008–2014). Nutrients 2018, 10, 587. [Google Scholar] [CrossRef]
- Steele, E.M.; Baraldi, L.G.; Da Costa Louzada, M.L.; Moubarac, J.C.; Mozaffarian, D.; Monteiro, C.A. Ultra-processed foods and added sugars in the US diet: Evidence from a nationally representative cross-sectional study. BMJ Open 2016, 6, e009892. [Google Scholar] [CrossRef]
- Delpino, F.M.; Figueiredo, L.M.; Bielemann, R.M.; Da Silva, B.G.C.; Dos Santos, F.S.; Mintem, G.C.; Flores, T.R.; Arcêncio, R.A.; Nunes, B.P. Ultra-processed food and risk of type 2 diabetes: A systematic review and meta-analysis of longitudinal studies. Int. J. Epidemiol. 2022, 51, 1120–1141. [Google Scholar] [CrossRef]
- Suksatan, W.; Moradi, S.; Naeini, F.; Bagheri, R.; Mohammadi, H.; Talebi, S.; Mehrabani, S.; Hojjati Kermani, M.A.; Suzuki, K. Ultra-processed food consumption and adult mortality risk: A systematic review and dose–response meta-analysis of 207,291 participants. Nutrients 2022, 14, 174. [Google Scholar] [CrossRef] [PubMed]
- Askari, M.; Heshmati, J.; Shahinfar, H.; Tripathi, N.; Daneshzad, E. Ultra-processed food and the risk of overweight and obesity: A systematic review and meta-analysis of observational studies. Int. J. Obes. 2020, 44, 2080–2091. [Google Scholar] [CrossRef] [PubMed]
- Lane, M.M.; Davis, J.A.; Beattie, S.; Gómez-Donoso, C.; Loughman, A.; O’Neil, A.; Jacka, F.; Berk, M.; Page, R.; Marx, W.; et al. Ultraprocessed food and chronic non-communicable diseases: A systematic review and meta-analysis of 43 observational studies. Obes. Rev. 2021, 22, e13146. [Google Scholar] [CrossRef]
- Pagliai, G.; Dinu, M.; Madarena, M.P.; Bonaccio, M.; Iacoviello, L.; Sofi, F. Consumption of ultra-processed foods and health status: A systematic review and meta-Analysis. Br. J. Nutr. 2021, 125, 308–318. [Google Scholar] [CrossRef]
- Moradi, S.; Entezari, M.H.; Mohammadi, H.; Jayedi, A.; Lazaridi, A.V.; Kermani, M.A.H.; Miraghajani, M. Ultra-processed food consumption and adult obesity risk: A systematic review and dose-response meta-analysis. Crit. Rev. Food Sci. Nutr. 2022, 63, 249–260. [Google Scholar] [CrossRef]
- Srour, B.; Fezeu, L.K.; Kesse-Guyot, E.; Allès, B.; Méjean, C.; Andrianasolo, R.M.; Chazelas, E.; Deschasaux, M.; Hercberg, S.; Galan, P.; et al. Ultra-processed food intake and risk of cardiovascular disease: Prospective cohort study (NutriNet-Santé). BMJ 2019, 365, l1451. [Google Scholar] [CrossRef]
- Hercberg, S.; Castetbon, K.; Czernichow, S.; Malon, A.; Mejean, C.; Kesse, E.; Touvier, M.; Galan, P. The nutrinet-santé study: A web-based prospective study on the relationship between nutrition and health and determinants of dietary patterns and nutritional status. BMC Public Health 2010, 10, 242. [Google Scholar] [CrossRef]
- Ángel Martínez-González, M. The SUN cohort study (Seguimiento University of Navarra). Public Health Nutr. 2006, 9, 127–131. [Google Scholar] [CrossRef]
- Llavero-Valero, M.; Escalada-San Martín, J.; Martínez-González, M.A.; Basterra-Gortari, F.J.; de la Fuente-Arrillaga, C.; Bes-Rastrollo, M. Ultra-processed foods and type-2 diabetes risk in the SUN project: A prospective cohort study. Clin. Nutr. 2021, 40, 2817–2824. [Google Scholar] [CrossRef] [PubMed]
- Levy, R.B.; Rauber, F.; Chang, K.; Louzada, M.L.D.C.; Monteiro, C.A.; Millett, C.; Vamos, E.P. Ultra-processed food consumption and type 2 diabetes incidence: A prospective cohort study. Clin. Nutr. 2021, 40, 3608–3614. [Google Scholar] [CrossRef] [PubMed]
- Dinu, M.; Bonaccio, M.; Martini, D.; Madarena, M.P.; Vitale, M.; Pagliai, G.; Esposito, S.; Ferraris, C.; Guglielmetti, M.; Rosi, A.; et al. Reproducibility and validity of a food-frequency questionnaire (NFFQ) to assess food consumption based on the NOVA classification in adults. Int. J. Food Sci. Nutr. 2021, 72, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Pala, V.; Sieri, S.; Palli, D.; Salvini, S.; Berrino, F.; Bellegotti, M.; Frasca, G.; Tumino, R.; Sacerdote, C.; Fiorini, L.; et al. Diet in the Italian EPIC cohorts: Presentation of data and methodological issues. Tumori 2003, 89, 594–607. [Google Scholar] [CrossRef] [PubMed]
- Pisani, P.; Faggiano, F.; Krogh, V.; Palli, D.; Vineis, P.; Berrino, F. Relative validity and reproducibility of a food frequency dietary questionnaire for use in the Italian EPIC centres. Int. J. Epidemiol. 1997, 26, S152–S160. [Google Scholar] [CrossRef] [PubMed]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.S.; Oh, K.; Kim, H.C. Dietary assessment methods in epidemiologic studies. Epidemiol. Health 2014, 36, e2014009. [Google Scholar] [CrossRef] [PubMed]
| EPIC-FFQ | NOVA-FFQ | Difference (%) EPIC-FFQ vs. NOVA-FFQ | p-Value | |
|---|---|---|---|---|
| MPFs (g/1000 Kcal) | 512.2 ± 155.1 | 670.3 ± 248.1 | −24 | <0.001 |
| PCIs (g/1000 Kcal) | 21.5 ± 8.6 | 20.1 ± 12.9 | +1 | 0.309 |
| PFs (g/1000 Kcal) | 138.9 ± 70.0 | 147.9 ± 82.6 | +6 | 0.280 |
| UPFs (g/1000 Kcal) | 101.8 ± 74.0 | 148.0 ± 109.2 | −31 | <0.001 |
| ICC | p-Value | Grade of Consistency | |
|---|---|---|---|
| MPFs (g/1000 Kcal) | 0.510 | <0.001 | Moderate |
| PCIs (g/1000 Kcal) | 0.541 | <0.001 | Moderate |
| PFs (g/1000 Kcal) | 0.689 | <0.001 | Moderate |
| UPFs (g/1000 Kcal) | 0.741 | <0.001 | Moderate |
| EPIC-FFQ | NOVA-FFQ | p-Value | |
|---|---|---|---|
| Energy (Kcal/day) | 1635.1 ± 303.3 | 1754.9 ± 437.5 | 0.248 |
| Proteins (g) | 72.7 ± 14.7 | 75.4 ± 16.3 | 0.518 |
| Proteins (% of TE) | 17.9 ± 2.8 | 17.4 ± 2.2 | 0.459 |
| From animal food sources (g) | 50.82 ± 13.5 | 52.8 ± 12.9 | 0.576 |
| From animal food sources (% of TE) | 12.6 ± 3.3 | 12.3 ± 2.6 | 0.657 |
| From vegetable food sources (g) | 21.7 ± 6.2 | 22.4 ± 6.8 | 0.669 |
| From vegetable food sources (% of TE) | 5.3 ± 1.1 | 5.1 ± 1.2 | 0.626 |
| Total fat (g) | 68.4 ± 18.1 | 72.5 ± 21.5 | 0.450 |
| Total fat (% of TE) | 37.4 ± 5.2 | 37.2 ± 6.4 | 0.929 |
| SAFAs (g) | 21.1 ± 6.0 | 22.8 ± 7.0 | 0.343 |
| SAFAs (% of TE) | 11.6 ± 2.3 | 11.7 ± 2.2 | 0.847 |
| MUFAs (g) | 30.9 ± 8.6 | 32.6 ± 10.5 | 0.536 |
| MUFAs (% of TE) | 16.8 ± 2.6 | 16.7 ± 3.7 | 0.856 |
| PUFAs (g) | 10.3 ± 3.2 | 10.9 ± 3.8 | 0.497 |
| PUFAs (% of TE) | 5.6 ± 1.1 | 5.6 ± 1.4 | 0.924 |
| Cholesterol (mg/1000 Kcal) | 284.3 ± 75.6 | 295.7 ± 65.6 | 0.557 |
| Carbohydrates (g) | 189.9 ± 40.0 | 205.0 ± 53.1 | 0.243 |
| Carbohydrates (% of TE) | 43.7 ± 6.1 | 43.9 ± 6.1 | 0.912 |
| Soluble carbohydrates (g) | 68.1 ± 21.5 | 78.2 ± 33.5 | 0.193 |
| Soluble carbohydrates (% of TE) | 15.9 ± 5.2 | 16.8 ± 6.2 | 0.558 |
| Fiber (g) | 16.5 ± 3.8 | 17.7 ± 5.6 | 0.332 |
| Fiber (g/1000 Kcal) | 10.1 ± 1.9 | 10.1 ± 1.9 | 0.959 |
| Alcohol (g) | 2.27 ± 3.4 | 4.3 ± 11.2 | 0.366 |
| EPIC-FFQ | NOVA-FFQ | p-Value | |
|---|---|---|---|
| Age (years) | 62.5 ± 10.3 | 64.5 ± 10.1 | 0.474 |
| BMI (Kg/m2) | 31.2 ± 7.2 | 30.7 ± 6.4 | 0.797 |
| Waist circumference (cm) | 107.8 ± 19.9 | 107.1 ± 19.0 | 0.899 |
| Systolic blood pressure (mmHg) | 79.5 ± 9.0 | 81.3 ± 10.3 | 0.507 |
| Diastolic blood pressure (mmHg) | 85.0 ± 67.9 | 85.3 ± 69.0 | 0.988 |
| HbA1c (%) | 7.0 ± 1.0 | 7.1 ± 1.2 | 0.893 |
| Total cholesterol (mg/dL) | 158.7 ± 38.3 | 154.8 ± 37.2 | 0.711 |
| LDL-c (mg/dL) | 84.0 ± 34.0 | 80.8 ± 32.9 | 0.731 |
| Triglycerides (mg/dL) | 122.5 ± 49.0 | 106.8 ± 43.1 | 0.216 |
| HDL-c (mg/dL) | 48.7 ± 17.1 | 50.7 ± 17.1 | 0.668 |
| Creatinine (mg/dL) | 1.0 ± 0.3 | 1.2 ± 0.7 | 0.177 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Abbronzo, G.; Quaglia, C.; Di Costanzo, G.; Testa, R.; Giacco, R.; Riccardi, G.; Vaccaro, O.; Vitale, M. Assessing the Consumption of Ultra-Processed Foods in People with Diabetes: Is a Specific NOVA Questionnaire Always Necessary? Nutrients 2025, 17, 1. https://doi.org/10.3390/nu17010001
D’Abbronzo G, Quaglia C, Di Costanzo G, Testa R, Giacco R, Riccardi G, Vaccaro O, Vitale M. Assessing the Consumption of Ultra-Processed Foods in People with Diabetes: Is a Specific NOVA Questionnaire Always Necessary? Nutrients. 2025; 17(1):1. https://doi.org/10.3390/nu17010001
Chicago/Turabian StyleD’Abbronzo, Giovanna, Cinzia Quaglia, Giuseppe Di Costanzo, Roberta Testa, Rosalba Giacco, Gabriele Riccardi, Olga Vaccaro, and Marilena Vitale. 2025. "Assessing the Consumption of Ultra-Processed Foods in People with Diabetes: Is a Specific NOVA Questionnaire Always Necessary?" Nutrients 17, no. 1: 1. https://doi.org/10.3390/nu17010001
APA StyleD’Abbronzo, G., Quaglia, C., Di Costanzo, G., Testa, R., Giacco, R., Riccardi, G., Vaccaro, O., & Vitale, M. (2025). Assessing the Consumption of Ultra-Processed Foods in People with Diabetes: Is a Specific NOVA Questionnaire Always Necessary? Nutrients, 17(1), 1. https://doi.org/10.3390/nu17010001






