The Benefits of the Mediterranean Diet on Inflamm-Aging in Childhood Obesity
Abstract
1. Introduction
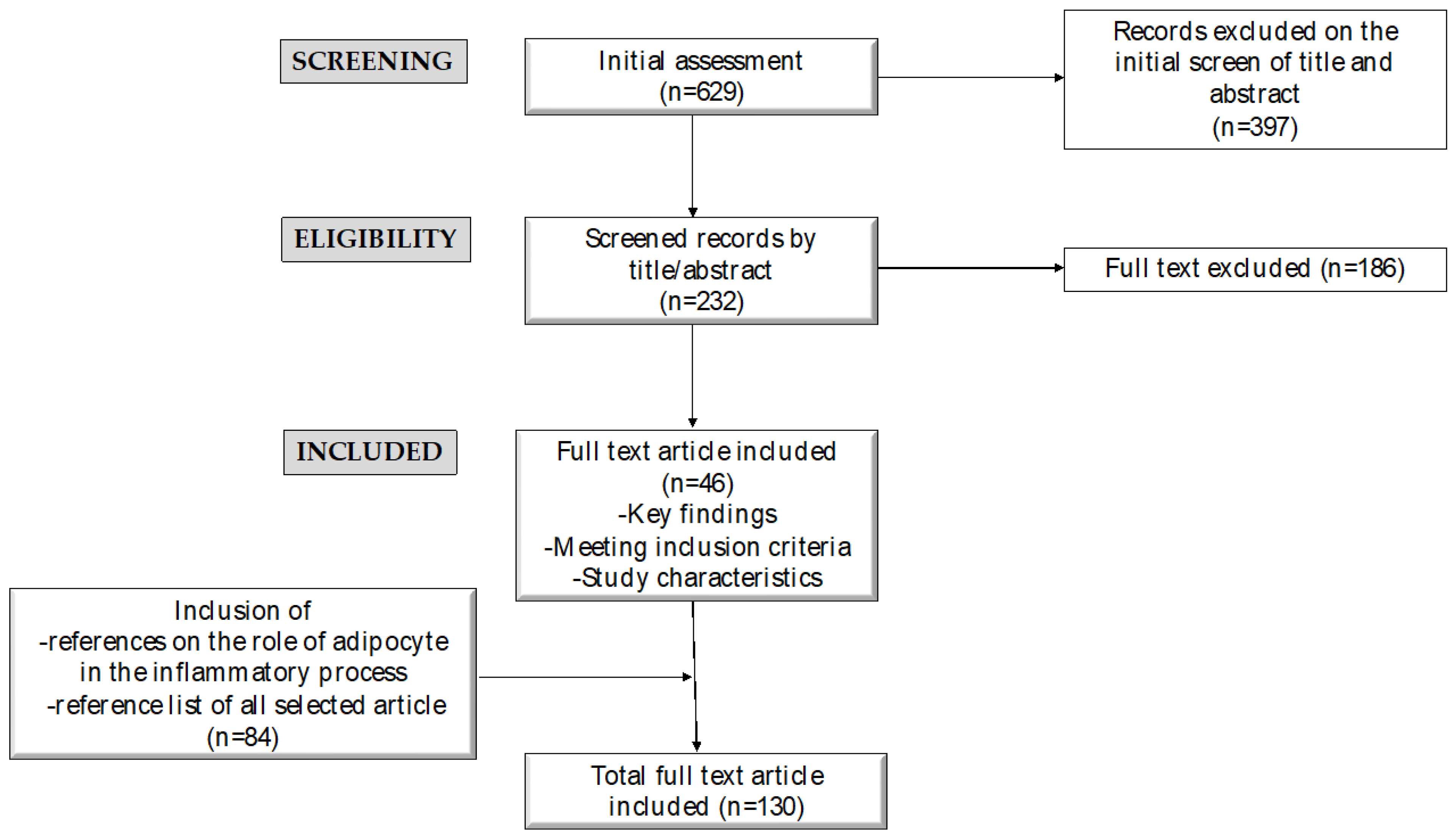
2. Methods
3. Relationship between Aging, Low-Grade Inflammation, and Pediatric Obesity-Related Complications
3.1. Aging and Chronic Low-Grade Inflammation
3.2. Inflammation and Its Role in Complications Related to Pediatric Obesity
4. Mediterranean Diet (MD) and Inflamm-Aging
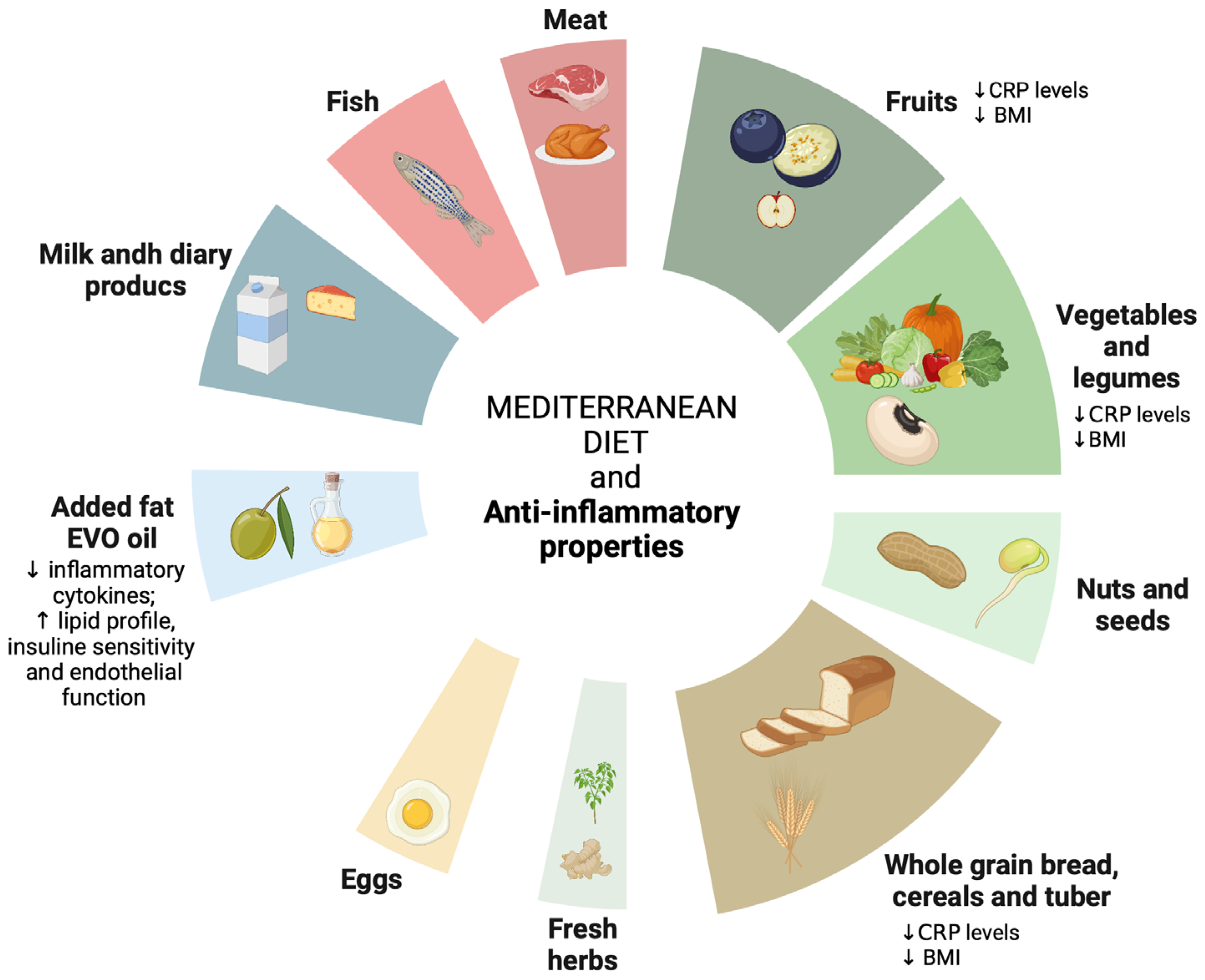
4.1. Anti-Inflammatory Properties of the Mediterranean Diet (MD)
| Sources | Foods | Metabolic Properties | References |
|---|---|---|---|
| PUFAs Omega-6 linoleic acid | Vegetable oil (grapeseed, wheatgerm, soya, corn, sunflower seed, sesame, rice, canola, peanut, almond); frying oil, walnuts, margarine, cod liver oil, and pork fat) |
| [80,97,98,99] |
| Omega-6 arachidonic acid | Beef, bone marrow, lard, chicken, and cod liver oil | ||
| Omega-3 alpha-linolenic acid (ALA) | Linseed, vegetable oil (canola, rapeseed, soya, wheatgerm, and palm), walnut, and seeds | ||
| EPA and DHA | Cod liver oil, mullet, salmon, mackerel, tuna, grouper, anchovies, and sardines | ||
| MUFAs | Vegetable oil (olive, almond, canola, rapeseed, macadamia, peanut, pecan nut, sesame, rice, and palm), cod liver oil, lard, beef tallow, pistachio nuts, and margarine |
| [98,100] |
| Polyphenols: phenolic acids, flavonoids (fisetin and quercetin), stilbenes, phenolic alcohols, and lignans | Fruits (grapes, berries, apples, cucumbers, strawberries, persimmons, and citrus fruit), vegetables (onions and tomatoes), cereals, olives, dry legumes, chocolate, beverage (tea, coffee, and red wine), and some spices |
| [75,76,101] |
| Vitamins | Vitamin C: grape, guava, peppers, orange, blackcurrant, nettle, parsley, lemon, tomatoes, kiwi, broccoli, and apricot ß-carotene: paprika, parsley, tomato, seaweed, apricot, carrot, basil, peppers, marjoram, mint, valerian, and vegetable oil |
| [98,102,103] |
| Vitamin E: vegetable oil (wheatgerm, corn, sunflower seed, almond, palm, rice, and mixed seeds) |
| [65,98,104] | |
| Vitamin B 12: beef, horse, sheep’ liver, lamb, and clams Vitamin B 6: wheat germ, corn and olive oil, yeast, pistachio nuts, and tempeh Folate: leavening agents, yeast, baker’s, active dry, chicken, and liver |
| [97,98] | |
| Vitamin D: cod liver oil, herring, salmon, catfish, and egg yolk |
| [98,105] | |
| Minerals (Ca, P, K, Fe, Mn, Zn, Selenium) | Calcium: basil, marjoram, thyme, roe, oregano, mint, milk, cow, shimmed, rosemary, cinnamon, and grana cheese Phosphorus: yeast, wheat bran, sea bass, wheat germ, gilthead bream, milk, eggs, and chicken Iron: liver, beef, veal, spleen, pork, poultry, fish, and legumes Magnesium: wheat bran, cocoa, coffee, grey mullet, roe, caviar, pine nuts, and almonds Potassium: mushrooms, leavening agents, seaweed, soya, tea, and flour |
| [98,106] |
| Zinc: mollusks, oyster, eastern, canned cheese made with cow milk, agar, and mushrooms |
| [97,98,106,107] | |
| Selenium: nuts, cod, beef, kidney, and tuna |
| [65,98,106] | |
| Soluble fiber | Figs, carrots, kiwifruit, nectarines, peaches, pears, melons, oranges, lettuce, and broccoli |
| [65,106] |
| Insoluble fiber | Figs, pears, broccoli, kiwifruit, carrots, oranges, and lettuce |
4.2. The Effectiveness of Mediterranean Diet Adherence in Children with Obesity
5. Limitations
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Saavedra, D.; Añé-Kourí, A.L.; Barzilai, N.; Caruso, C.; Cho, K.-H.; Fontana, L.; Franceschi, C.; Frasca, D.; Ledón, N.; Niedernhofer, L.J.; et al. Aging and Chronic Inflammation: Highlights from a Multidisciplinary Workshop. Immun. Ageing 2023, 20, 25. [Google Scholar] [CrossRef] [PubMed]
- Calcaterra, V.; Vinci, F.; Casari, G.; Pelizzo, G.; De Silvestri, A.; De Amici, M.; Albertini, R.; Regalbuto, C.; Montalbano, C.; Larizza, D.; et al. Evaluation of Allostatic Load as a Marker of Chronic Stress in Children and the Importance of Excess Weight. Front. Pediatr. 2019, 7, 335. [Google Scholar] [CrossRef] [PubMed]
- Sarni, R.O.S.; Kochi, C.; Suano-Souza, F.I. Childhood Obesity: An Ecological Perspective. J. Pediatr. 2022, 98, S38–S46. [Google Scholar] [CrossRef] [PubMed]
- Woolford, S.J.; Sidell, M.; Li, X.; Else, V.; Young, D.R.; Resnicow, K.; Koebnick, C. Changes in Body Mass Index among Children and Adolescents during the COVID-19 Pandemic. JAMA 2021, 326, 1434. [Google Scholar] [CrossRef] [PubMed]
- Jia, P. Obesogenic Environment and Childhood Obesity. Obes. Rev. 2021, 22, e13158. [Google Scholar] [CrossRef] [PubMed]
- González-Muniesa, P.; Mártinez-González, M.-A.; Hu, F.B.; Després, J.-P.; Matsuzawa, Y.; Loos, R.J.F.; Moreno, L.A.; Bray, G.A.; Martinez, J.A. Obesity. Nat. Rev. Dis. Primer 2017, 3, 17034. [Google Scholar] [CrossRef] [PubMed]
- Calcaterra, V.; Verduci, E.; Cena, H.; Magenes, V.C.; Todisco, C.F.; Tenuta, E.; Gregorio, C.; De Giuseppe, R.; Bosetti, A.; Di Profio, E.; et al. Polycystic Ovary Syndrome in Insulin-Resistant Adolescents with Obesity: The Role of Nutrition Therapy and Food Supplements as a Strategy to Protect Fertility. Nutrients 2021, 13, 1848. [Google Scholar] [CrossRef] [PubMed]
- Calcaterra, V.; Verduci, E.; Milanta, C.; Agostinelli, M.; Todisco, C.F.; Bona, F.; Dolor, J.; La Mendola, A.; Tosi, M.; Zuccotti, G. Micronutrient Deficiency in Children and Adolescents with Obesity—A Narrative Review. Children 2023, 10, 695. [Google Scholar] [CrossRef] [PubMed]
- Tussing-Humphreys, L.; Pustacioglu, C.; Nemeth, E.; Braunschweig, C. Rethinking Iron Regulation and Assessment in Iron Deficiency, Anemia of Chronic Disease, and Obesity: Introducing Hepcidin. J. Acad. Nutr. Diet. 2012, 112, 391–400. [Google Scholar] [CrossRef]
- Funtikova, A.N.; Navarro, E.; Bawaked, R.A.; Fíto, M.; Schröder, H. Impact of Diet on Cardiometabolic Health in Children and Adolescents. Nutr. J. 2015, 14, 118. [Google Scholar] [CrossRef]
- Gantenbein, K.V.; Kanaka-Gantenbein, C. Mediterranean Diet as an Antioxidant: The Impact on Metabolic Health and Overall Wellbeing. Nutrients 2021, 13, 1951. [Google Scholar] [CrossRef] [PubMed]
- Farràs, M.; Martinez-Gili, L.; Portune, K.; Arranz, S.; Frost, G.; Tondo, M.; Blanco-Vaca, F. Modulation of the Gut Microbiota by Olive Oil Phenolic Compounds: Implications for Lipid Metabolism, Immune System, and Obesity. Nutrients 2020, 12, 2200. [Google Scholar] [CrossRef]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-Related Inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.C.; Kunnumakkara, A.B.; Aggarwal, S.; Aggarwal, B.B. Inflammation, a Double-Edge Sword for Cancer and Other Age-Related Diseases. Front. Immunol. 2018, 9, 2160. [Google Scholar] [CrossRef] [PubMed]
- Colombini, B.; Dinu, M.; Murgo, E.; Lotti, S.; Tarquini, R.; Sofi, F.; Mazzoccoli, G. Ageing and Low-Level Chronic Inflammation: The Role of the Biological Clock. Antioxidants 2022, 11, 2228. [Google Scholar] [CrossRef]
- Soysal, P.; Arik, F.; Smith, L.; Jackson, S.E.; Isik, A.T. Inflammation, Frailty and Cardiovascular Disease. In Frailty and Cardiovascular Diseases; Veronese, N., Ed.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2020; Volume 1216, pp. 55–64. ISBN 978-3-030-33329-4. [Google Scholar]
- Lange, L.A.; Carlson, C.S.; Hindorff, L.A.; Lange, E.M.; Walston, J.; Durda, J.P.; Cushman, M.; Bis, J.C.; Zeng, D.; Lin, D.; et al. Association of Polymorphisms in the CRP Gene with Circulating C-Reactive Protein Levels and Cardiovascular Events. JAMA 2006, 296, 2703. [Google Scholar] [CrossRef]
- Fishman, D.; Faulds, G.; Jeffery, R.; Mohamed-Ali, V.; Yudkin, J.S.; Humphries, S.; Woo, P. The Effect of Novel Polymorphisms in the Interleukin-6 (IL-6) Gene on IL-6 Transcription and Plasma IL-6 Levels, and an Association with Systemic-Onset Juvenile Chronic Arthritis. J. Clin. Investig. 1998, 102, 1369–1376. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Liu, D.; Guo, H.; Wang, Y.; Bai, Y. Association between Polymorphism in the Promoter Region of Interleukin 6 (-174 G/C) and Risk of Alzheimer’s Disease: A Meta-Analysis. J. Neurol. 2012, 259, 414–419. [Google Scholar] [CrossRef]
- Testa, R.; Olivieri, F.; Bonfigli, A.R.; Sirolla, C.; Boemi, M.; Marchegiani, F.; Marra, M.; Cenerelli, S.; Antonicelli, R.; Dolci, A.; et al. Interleukin-6–174 G>C Polymorphism Affects the Association between IL-6 Plasma Levels and Insulin Resistance in Type 2 Diabetic Patients. Diabetes Res. Clin. Pract. 2006, 71, 299–305. [Google Scholar] [CrossRef]
- Ferrucci, L.; Fabbri, E. Inflammageing: Chronic Inflammation in Ageing, Cardiovascular Disease, and Frailty. Nat. Rev. Cardiol. 2018, 15, 505–522. [Google Scholar] [CrossRef]
- Fabian, M.R.; Sonenberg, N.; Filipowicz, W. Regulation of mRNA Translation and Stability by microRNAs. Annu. Rev. Biochem. 2010, 79, 351–379. [Google Scholar] [CrossRef]
- Hamann, L.; Szwed, M.; Mossakowska, M.; Chudek, J.; Puzianowska-Kuznicka, M. First Evidence for STING SNP R293Q Being Protective Regarding Obesity-Associated Cardiovascular Disease in Age-Advanced Subjects—A Cohort Study. Immun. Ageing 2020, 17, 7. [Google Scholar] [CrossRef]
- Noren Hooten, N.; Abdelmohsen, K.; Gorospe, M.; Ejiogu, N.; Zonderman, A.B.; Evans, M.K. microRNA Expression Patterns Reveal Differential Expression of Target Genes with Age. PLoS ONE 2010, 5, e10724. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.; Wu, Y.; Yu, S.; Yu, Y.; Lee, S.; Liu, C.; Hsieh, W.; Hwu, H.; Chen, P.; Jeng, S.; et al. Modulated Expression of Human Peripheral Blood Micro RNA s from Infancy to Adulthood and Its Role in Aging. Aging Cell 2014, 13, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Olivieri, F.; Spazzafumo, L.; Santini, G.; Lazzarini, R.; Albertini, M.C.; Rippo, M.R.; Galeazzi, R.; Abbatecola, A.M.; Marcheselli, F.; Monti, D.; et al. Age-Related Differences in the Expression of Circulating microRNAs: miR-21 as a New Circulating Marker of Inflammaging. Mech. Ageing Dev. 2012, 133, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yang, H.; Zhang, C.; Jing, Y.; Wang, C.; Liu, C.; Zhang, R.; Wang, J.; Zhang, J.; Zen, K.; et al. Investigation of MicroRNA Expression in Human Serum during the Aging Process. J. Gerontol. Ser. A 2015, 70, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Palmer, C.S.; Palchaudhuri, R.; Albargy, H.; Abdel-Mohsen, M.; Crowe, S.M. Exploiting Immune Cell Metabolic Machinery for Functional HIV Cure and the Prevention of Inflammaging. F1000Research 2018, 7, 125. [Google Scholar] [CrossRef] [PubMed]
- Heath, J.J.; Grant, M.D. The Immune Response Against Human Cytomegalovirus Links Cellular to Systemic Senescence. Cells 2020, 9, 766. [Google Scholar] [CrossRef] [PubMed]
- Rakoff-Nahoum, S.; Paglino, J.; Eslami-Varzaneh, F.; Edberg, S.; Medzhitov, R. Recognition of Commensal Microflora by Toll-Like Receptors Is Required for Intestinal Homeostasis. Cell 2004, 118, 229–241. [Google Scholar] [CrossRef]
- Biagi, E.; Franceschi, C.; Rampelli, S.; Severgnini, M.; Ostan, R.; Turroni, S.; Consolandi, C.; Quercia, S.; Scurti, M.; Monti, D.; et al. Gut Microbiota and Extreme Longevity. Curr. Biol. 2016, 26, 1480–1485. [Google Scholar] [CrossRef]
- Lukáčová, I.; Ambro, Ľ.; Dubayová, K.; Mareková, M. The Gut Microbiota, Its Relationship to the Immune System, and Possibilities of Its Modulation. Epidemiol. Mikrobiol. Imunol. Cas. Spol. Epidemiol. Mikrobiol. Ceske Lek. Spol. JE Purkyne 2023, 72, 40–53. [Google Scholar]
- Ruff, W.E.; Greiling, T.M.; Kriegel, M.A. Host–Microbiota Interactions in Immune-Mediated Diseases. Nat. Rev. Microbiol. 2020, 18, 521–538. [Google Scholar] [CrossRef] [PubMed]
- Santoro, A.; Ostan, R.; Candela, M.; Biagi, E.; Brigidi, P.; Capri, M.; Franceschi, C. Gut Microbiota Changes in the Extreme Decades of Human Life: A Focus on Centenarians. Cell. Mol. Life Sci. CMLS 2018, 75, 129–148. [Google Scholar] [CrossRef] [PubMed]
- Bibbò, S.; Ianiro, G.; Giorgio, V.; Scaldaferri, F.; Masucci, L.; Gasbarrini, A.; Cammarota, G. The Role of Diet on Gut Microbiota Composition. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 4742–4749. [Google Scholar] [PubMed]
- Mangiola, F.; Nicoletti, A.; Gasbarrini, A.; Ponziani, F.R. Gut Microbiota and Aging. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 7404–7413. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef] [PubMed]
- Martin-Gallausiaux, C.; Marinelli, L.; Blottière, H.M.; Larraufie, P.; Lapaque, N. SCFA: Mechanisms and Functional Importance in the Gut. Proc. Nutr. Soc. 2021, 80, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, J.; Lange, B.; Frick, J.-S.; Sauer, H.; Zimmermann, K.; Schwiertz, A.; Rusch, K.; Klosterhalfen, S.; Enck, P. A Vegan or Vegetarian Diet Substantially Alters the Human Colonic Faecal Microbiota. Eur. J. Clin. Nutr. 2012, 66, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Louis, P.; Flint, H.J. Formation of Propionate and Butyrate by the Human Colonic Microbiota. Environ. Microbiol. 2017, 19, 29–41. [Google Scholar] [CrossRef]
- He, J.; Zhang, P.; Shen, L.; Niu, L.; Tan, Y.; Chen, L.; Zhao, Y.; Bai, L.; Hao, X.; Li, X.; et al. Short-Chain Fatty Acids and Their Association with Signalling Pathways in Inflammation, Glucose and Lipid Metabolism. Int. J. Mol. Sci. 2020, 21, 6356. [Google Scholar] [CrossRef]
- Vaiserman, A.M.; Koliada, A.K.; Marotta, F. Gut Microbiota: A Player in Aging and a Target for Anti-Aging Intervention. Ageing Res. Rev. 2017, 35, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Man, A.L.; Bertelli, E.; Rentini, S.; Regoli, M.; Briars, G.; Marini, M.; Watson, A.J.M.; Nicoletti, C. Age-Associated Modifications of Intestinal Permeability and Innate Immunity in Human Small Intestine. Clin. Sci. 2015, 129, 515–527. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, B.C.; Chang, C.J. Chemistry and Biology of Reactive Oxygen Species in Signaling or Stress Responses. Nat. Chem. Biol. 2011, 7, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Sung, B. The Relationship between Inflammation and Cancer Is Analogous to That between Fuel and Fire. Oncology 2011, 25, 414–418. [Google Scholar] [PubMed]
- Karczewski, J.; Śledzińska, E.; Baturo, A.; Jończyk, I.; Maleszko, A.; Samborski, P.; Begier-Krasińska, B.; Dobrowolska, A. Obesity and Inflammation. Eur. Cytokine Netw. 2018, 29, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Schelbert, K.B. Comorbidities of Obesity. Prim. Care Clin. Off. Pract. 2009, 36, 271–285. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S.; Erbay, E. Nutrient Sensing and Inflammation in Metabolic Diseases. Nat. Rev. Immunol. 2008, 8, 923–934. [Google Scholar] [CrossRef] [PubMed]
- Trayhurn, P.; Wood, I.S. Adipokines: Inflammation and the Pleiotropic Role of White Adipose Tissue. Br. J. Nutr. 2004, 92, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Wozniak, S.E.; Gee, L.L.; Wachtel, M.S.; Frezza, E.E. Adipose Tissue: The New Endocrine Organ? A Review Article. Dig. Dis. Sci. 2009, 54, 1847–1856. [Google Scholar] [CrossRef]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W. Obesity Is Associated with Macrophage Accumulation in Adipose Tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef]
- Vandanmagsar, B.; Youm, Y.-H.; Ravussin, A.; Galgani, J.E.; Stadler, K.; Mynatt, R.L.; Ravussin, E.; Stephens, J.M.; Dixit, V.D. The NLRP3 Inflammasome Instigates Obesity-Induced Inflammation and Insulin Resistance. Nat. Med. 2011, 17, 179–188. [Google Scholar] [CrossRef]
- Fulop, T.; Witkowski, J.M.; Olivieri, F.; Larbi, A. The Integration of Inflammaging in Age-Related Diseases. Semin. Immunol. 2018, 40, 17–35. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, A.W. Obesity-Induced Inflammation: A Metabolic Dialogue in the Language of Inflammation. J. Intern. Med. 2007, 262, 408–414. [Google Scholar] [CrossRef]
- Marcus, C.; Danielsson, P.; Hagman, E. Pediatric Obesity—Long-term Consequences and Effect of Weight Loss. J. Intern. Med. 2022, 292, 870–891. [Google Scholar] [CrossRef] [PubMed]
- Versini, M.; Jeandel, P.-Y.; Rosenthal, E.; Shoenfeld, Y. Obesity in Autoimmune Diseases: Not a Passive Bystander. Autoimmun. Rev. 2014, 13, 981–1000. [Google Scholar] [CrossRef] [PubMed]
- Sayın, O.; Tokgöz, Y.; Arslan, N. Investigation of Adropin and Leptin Levels in Pediatric Obesity-Related Nonalcoholic Fatty Liver Disease. J. Pediatr. Endocrinol. Metab. 2014, 27, 479–484. [Google Scholar] [CrossRef]
- Newton, K.P.; Hou, J.; Crimmins, N.A.; Lavine, J.E.; Barlow, S.E.; Xanthakos, S.A.; Africa, J.; Behling, C.; Donithan, M.; Clark, J.M.; et al. Prevalence of Prediabetes and Type 2 Diabetes in Children with Nonalcoholic Fatty Liver Disease. JAMA Pediatr. 2016, 170, e161971. [Google Scholar] [CrossRef] [PubMed]
- Tomah, S.; Alkhouri, N.; Hamdy, O. Nonalcoholic Fatty Liver Disease and Type 2 Diabetes: Where Do Diabetologists Stand? Clin. Diabetes Endocrinol. 2020, 6, 9. [Google Scholar] [CrossRef]
- Pollock, N.K. Childhood Obesity, Bone Development, and Cardiometabolic Risk Factors. Mol. Cell. Endocrinol. 2015, 410, 52–63. [Google Scholar] [CrossRef]
- Weiss, S.T. Obesity: Insight into the Origins of Asthma. Nat. Immunol. 2005, 6, 537–539. [Google Scholar] [CrossRef]
- Bergström, A.; Melén, E. On Childhood Asthma, Obesity and Inflammation. Clin. Exp. Allergy 2012, 42, 5–7. [Google Scholar] [CrossRef] [PubMed]
- Verduci, E.; Bronsky, J.; Embleton, N.; Gerasimidis, K.; Indrio, F.; Köglmeier, J.; De Koning, B.; Lapillonne, A.; Moltu, S.J.; Norsa, L.; et al. Role of Dietary Factors, Food Habits, and Lifestyle in Childhood Obesity Development: A Position Paper from the European Society for Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2021, 72, 769–783. [Google Scholar] [CrossRef] [PubMed]
- Kiani, A.K.; Medori, M.C.; Bonetti, G.; Aquilanti, B.; Velluti, V.; Matera, G.; Iaconelli, A.; Stuppia, L.; Connelly, S.T.; Herbst, K.L.; et al. Modern Vision of the Mediterranean Diet. J. Prev. Med. Hyg. 2022, 63, E36. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Muscogiuri, G.; Frias-Toral, E.; Laudisio, D.; Pugliese, G.; Castellucci, B.; Garcia-Velasquez, E.; Savastano, S.; Colao, A. Nutrition and Immune System: From the Mediterranean Diet to Dietary Supplementary through the Microbiota. Crit. Rev. Food Sci. Nutr. 2021, 61, 3066–3090. [Google Scholar] [CrossRef] [PubMed]
- Bonaccio, M.; Cerletti, C.; Iacoviello, L.; De Gaetano, G. Mediterranean Diet and Low-Grade Subclinical Inflammation: The Moli-Sani Study. Endocr. Metab. Immune Disord.-Drug Targets 2015, 15, 18–24. [Google Scholar] [CrossRef]
- Soldati, L.; Di Renzo, L.; Jirillo, E.; Ascierto, P.A.; Marincola, F.M.; De Lorenzo, A. The Influence of Diet on Anti-Cancer Immune Responsiveness. J. Transl. Med. 2018, 16, 75. [Google Scholar] [CrossRef] [PubMed]
- Tosti, V.; Bertozzi, B.; Fontana, L. Health Benefits of the Mediterranean Diet: Metabolic and Molecular Mechanisms. J. Gerontol. Ser. A 2018, 73, 318–326. [Google Scholar] [CrossRef]
- Di Daniele, N.; Noce, A.; Vidiri, M.F.; Moriconi, E.; Marrone, G.; Annicchiarico-Petruzzelli, M.; D’Urso, G.; Tesauro, M.; Rovella, V.; De Lorenzo, A. Impact of Mediterranean Diet on Metabolic Syndrome, Cancer and Longevity. Oncotarget 2017, 8, 8947–8979. [Google Scholar] [CrossRef] [PubMed]
- Scarallo, L.; Lionetti, P. Dietary Management in Pediatric Patients with Crohn’s Disease. Nutrients 2021, 13, 1611. [Google Scholar] [CrossRef] [PubMed]
- Oddy, W.H.; Allen, K.L.; Trapp, G.S.A.; Ambrosini, G.L.; Black, L.J.; Huang, R.-C.; Rzehak, P.; Runions, K.C.; Pan, F.; Beilin, L.J.; et al. Dietary Patterns, Body Mass Index and Inflammation: Pathways to Depression and Mental Health Problems in Adolescents. Brain. Behav. Immun. 2018, 69, 428–439. [Google Scholar] [CrossRef]
- Cabral, M.; Araújo, J.; Lopes, C.; Ramos, E. Food Intake and High-Sensitivity C-Reactive Protein Levels in Adolescents. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 1067–1074. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, M.M.; Singer, M.R.; Moore, L.L. A Cross-Sectional Study of Food Group Intake and C-Reactive Protein among Children. Nutr. Metab. 2009, 6, 40. [Google Scholar] [CrossRef] [PubMed]
- Aparicio-Soto, M.; Sánchez-Hidalgo, M.; Rosillo, M.Á.; Castejón, M.L.; Alarcón-de-la-Lastra, C. Extra Virgin Olive Oil: A Key Functional Food for Prevention of Immune-Inflammatory Diseases. Food Funct. 2016, 7, 4492–4505. [Google Scholar] [CrossRef] [PubMed]
- Bonaccio, M.; Pounis, G.; Cerletti, C.; Donati, M.B.; Iacoviello, L.; De Gaetano, G.; on behalf of the MOLI-SANI Study Investigators Mediterranean Diet. Dietary Polyphenols and Low Grade Inflammation: Results from the MOLI-SANI Study. Br. J. Clin. Pharmacol. 2017, 83, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Parkinson, L.; Keast, R. Oleocanthal, a Phenolic Derived from Virgin Olive Oil: A Review of the Beneficial Effects on Inflammatory Disease. Int. J. Mol. Sci. 2014, 15, 12323–12334. [Google Scholar] [CrossRef] [PubMed]
- Franco, G.A.; Interdonato, L.; Cordaro, M.; Cuzzocrea, S.; Di Paola, R. Bioactive Compounds of the Mediterranean Diet as Nutritional Support to Fight Neurodegenerative Disease. Int. J. Mol. Sci. 2023, 24, 7318. [Google Scholar] [CrossRef]
- Martínez-González, M.A.; Salas-Salvadó, J.; Estruch, R.; Corella, D.; Fitó, M.; Ros, E. Benefits of the Mediterranean Diet: Insights from the PREDIMED Study. Prog. Cardiovasc. Dis. 2015, 58, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Morze, J.; Hoffmann, G. Mediterranean Diet and Health Status: Active Ingredients and Pharmacological Mechanisms. Br. J. Pharmacol. 2020, 177, 1241–1257. [Google Scholar] [CrossRef] [PubMed]
- Parikh, M.; Maddaford, T.G.; Austria, J.A.; Aliani, M.; Netticadan, T.; Pierce, G.N. Dietary Flaxseed as a Strategy for Improving Human Health. Nutrients 2019, 11, 1171. [Google Scholar] [CrossRef]
- Pauls, S.D.; Rodway, L.A.; Winter, T.; Taylor, C.G.; Zahradka, P.; Aukema, H.M. Anti-Inflammatory Effects of α-Linolenic Acid in M1-like Macrophages Are Associated with Enhanced Production of Oxylipins from α-Linolenic and Linoleic Acid. J. Nutr. Biochem. 2018, 57, 121–129. [Google Scholar] [CrossRef]
- Wu, D.; Lewis, E.D.; Pae, M.; Meydani, S.N. Nutritional Modulation of Immune Function: Analysis of Evidence, Mechanisms, and Clinical Relevance. Front. Immunol. 2019, 9, 3160. [Google Scholar] [CrossRef] [PubMed]
- Sureda, A.; Bibiloni, M.; Julibert, A.; Bouzas, C.; Argelich, E.; Llompart, I.; Pons, A.; Tur, J. Adherence to the Mediterranean Diet and Inflammatory Markers. Nutrients 2018, 10, 62. [Google Scholar] [CrossRef] [PubMed]
- Agostinis-Sobrinho, C.; Ramírez-Vélez, R.; García-Hermoso, A.; Rosário, R.; Moreira, C.; Lopes, L.; Martinkenas, A.; Mota, J.; Santos, R. The Combined Association of Adherence to Mediterranean Diet, Muscular and Cardiorespiratory Fitness on Low-Grade Inflammation in Adolescents: A Pooled Analysis. Eur. J. Nutr. 2019, 58, 2649–2656. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, K.; Ronca, D.; Michels, N.; Huybrechts, I.; Cuenca-Garcia, M.; Marcos, A.; Molnár, D.; Dallongeville, J.; Manios, Y.; Schaan, B.; et al. Does the Mediterranean Diet Protect against Stress-Induced Inflammatory Activation in European Adolescents? The HELENA Study. Nutrients 2018, 10, 1770. [Google Scholar] [CrossRef] [PubMed]
- Douros, K.; Thanopoulou, M.-I.; Boutopoulou, B.; Papadopoulou, A.; Papadimitriou, A.; Fretzayas, A.; Priftis, K.N. Adherence to the Mediterranean Diet and Inflammatory Markers in Children with Asthma. Allergol. Immunopathol. 2019, 47, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Rumawas, M.E.; Meigs, J.B.; Dwyer, J.T.; McKeown, N.M.; Jacques, P.F. Mediterranean-Style Dietary Pattern, Reduced Risk of Metabolic Syndrome Traits, and Incidence in the Framingham Offspring Cohort. Am. J. Clin. Nutr. 2009, 90, 1608–1614. [Google Scholar] [CrossRef] [PubMed]
- Muscogiuri, G.; Verde, L.; Sulu, C.; Katsiki, N.; Hassapidou, M.; Frias-Toral, E.; Cucalón, G.; Pazderska, A.; Yumuk, V.D.; Colao, A.; et al. Mediterranean Diet and Obesity-Related Disorders: What Is the Evidence? Curr. Obes. Rep. 2022, 11, 287–304. [Google Scholar] [CrossRef] [PubMed]
- Arpón, A.; Riezu-Boj, J.I.; Milagro, F.I.; Marti, A.; Razquin, C.; Martínez-González, M.A.; Corella, D.; Estruch, R.; Casas, R.; Fitó, M.; et al. Adherence to Mediterranean Diet Is Associated with Methylation Changes in Inflammation-Related Genes in Peripheral Blood Cells. J. Physiol. Biochem. 2016, 73, 445–455. [Google Scholar] [CrossRef]
- Marques-Rocha, J.L.; Milagro, F.I.; Mansego, M.L.; Zulet, M.A.; Bressan, J.; Martínez, J.A. Expression of Inflammation-Related miRNAs in White Blood Cells from Subjects with Metabolic Syndrome after 8 Wk of Following a Mediterranean Diet–Based Weight Loss Program. Nutrition 2016, 32, 48–55. [Google Scholar] [CrossRef]
- Selvakumar, P.; Badgeley, A.; Murphy, P.; Anwar, H.; Sharma, U.; Lawrence, K.; Lakshmikuttyamma, A. Flavonoids and Other Polyphenols Act as Epigenetic Modifiers in Breast Cancer. Nutrients 2020, 12, 761. [Google Scholar] [CrossRef]
- Ratovitski, E. Anticancer Natural Compounds as Epigenetic Modulators of Gene Expression. Curr. Genom. 2017, 18, 175–205. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, D.; Sharma, A.; Sak, K.; Tuli, H.S.; Buttar, H.S.; Bishayee, A. Fisetin: A Bioactive Phytochemical with Potential for Cancer Prevention and Pharmacotherapy. Life Sci. 2018, 194, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Liu, Y.; Wang, P.; Song, C.; Wang, K.; Dai, L.; Zhang, J.; Ye, H. The Effect of Quercetin Nanoparticle on Cervical Cancer Progression by Inducing Apoptosis, Autophagy and Anti-Proliferation via JAK2 Suppression. Biomed. Pharmacother. 2016, 82, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Turpin, W.; Dong, M.; Sasson, G.; Raygoza Garay, J.A.; Espin-Garcia, O.; Lee, S.-H.; Neustaeter, A.; Smith, M.I.; Leibovitzh, H.; Guttman, D.S.; et al. Mediterranean-Like Dietary Pattern Associations with Gut Microbiome Composition and Subclinical Gastrointestinal Inflammation. Gastroenterology 2022, 163, 685–698. [Google Scholar] [CrossRef] [PubMed]
- Beam, A.; Clinger, E.; Hao, L. Effect of Diet and Dietary Components on the Composition of the Gut Microbiota. Nutrients 2021, 13, 2795. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Muscogiuri, G.; Annunziata, G.; Laudisio, D.; Pugliese, G.; Salzano, C.; Colao, A.; Savastano, S. From Gut Microbiota Dysfunction to Obesity: Could Short-Chain Fatty Acids Stop This Dangerous Course? Hormones 2019, 18, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Database for Epidemiological Studies in Italy (Banca Dati Di Composizione Degli Alimenti per Studi Epidemiologici in Italia—BDA) Version 1. 2022. Available online: https://Www.Bda-Ieo.It/Wordpress/En/ (accessed on 10 February 2024).
- Gutierrez, S.; Svahn, S.L.; Johansson, M.E. Effects of Omega3 Fatty Acids on Immune Cells. Int. J. Mol. Sci. 2019, 20, 5028. [Google Scholar] [CrossRef] [PubMed]
- Ravaut, G.; Légiot, A.; Bergeron, K.-F.; Mounier, C. Monounsaturated Fatty Acids in Obesity-Related Inflammation. Int. J. Mol. Sci. 2020, 22, 330. [Google Scholar] [CrossRef] [PubMed]
- Goszcz, K.; Duthie, G.G.; Stewart, D.; Leslie, S.J.; Megson, I.L. Bioactive Polyphenols and Cardiovascular Disease: Chemical Antagonists, Pharmacological Agents or Xenobiotics That Drive an Adaptive Response? Br. J. Pharmacol. 2017, 174, 1209–1225. [Google Scholar] [CrossRef]
- Kim, H.; Han, S.N. Vitamin E: Regulatory Role on Gene and Protein Expression and Metabolomics Profiles. IUBMB Life 2019, 71, 442–455. [Google Scholar] [CrossRef]
- Wong, S.K.; Chin, K.-Y.; Ima-Nirwana, S. Vitamin C: A Review on Its Role in the Management of Metabolic Syndrome. Int. J. Med. Sci. 2020, 17, 1625–1638. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Han, S. The Role of Vitamin E in Immunity. Nutrients 2018, 10, 1614. [Google Scholar] [CrossRef] [PubMed]
- Gombart, A.F.; Pierre, A.; Maggini, S. A Review of Micronutrients and the Immune System–Working in Harmony to Reduce the Risk of Infection. Nutrients 2020, 12, 236. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Agriculture Agricultural Research Service. USDA FoodData Central. FoodData Central Version 10.0. Available online: https://Fdc.Nal.Usda.Gov/Fdc-App.Html#/ (accessed on 10 February 2024).
- Haase, H.; Rink, L. The Immune System and the Impact of Zinc during Aging. Immun. Ageing 2009, 6, 9. [Google Scholar] [CrossRef] [PubMed]
- Velázquez-López, L.; Santiago-Díaz, G.; Nava-Hernández, J.; Muñoz-Torres, A.V.; Medina-Bravo, P.; Torres-Tamayo, M. Mediterranean-Style Diet Reduces Metabolic Syndrome Components in Obese Children and Adolescents with Obesity. BMC Pediatr. 2014, 14, 175. [Google Scholar] [CrossRef] [PubMed]
- López-Gil, J.F.; García-Hermoso, A.; Sotos-Prieto, M.; Cavero-Redondo, I.; Martínez-Vizcaíno, V.; Kales, S.N. Mediterranean Diet-Based Interventions to Improve Anthropometric and Obesity Indicators in Children and Adolescents: A Systematic Review with Meta-Analysis of Randomized Controlled Trials. Adv. Nutr. 2023, 14, 858–869. [Google Scholar] [CrossRef] [PubMed]
- De La Torre-Moral, A.; Fàbregues, S.; Bach-Faig, A.; Fornieles-Deu, A.; Medina, F.X.; Aguilar-Martínez, A.; Sánchez-Carracedo, D. Family Meals, Conviviality, and the Mediterranean Diet among Families with Adolescents. Int. J. Environ. Res. Public Health 2021, 18, 2499. [Google Scholar] [CrossRef] [PubMed]
- García-Montero, C.; Fraile-Martínez, O.; Gómez-Lahoz, A.M.; Pekarek, L.; Castellanos, A.J.; Noguerales-Fraguas, F.; Coca, S.; Guijarro, L.G.; García-Honduvilla, N.; Asúnsolo, A.; et al. Nutritional Components in Western Diet Versus Mediterranean Diet at the Gut Microbiota–Immune System Interplay. Implications for Health and Disease. Nutrients 2021, 13, 699. [Google Scholar] [CrossRef] [PubMed]
- Hall, K.D.; Ayuketah, A.; Brychta, R.; Cai, H.; Cassimatis, T.; Chen, K.Y.; Chung, S.T.; Costa, E.; Courville, A.; Darcey, V.; et al. Ultra-Processed Diets Cause Excess Calorie Intake and Weight Gain: An Inpatient Randomized Controlled Trial of Ad Libitum Food Intake. Cell Metab. 2019, 30, 226. [Google Scholar] [CrossRef]
- Rauber, F.; Da Costa Louzada, M.L.; Steele, E.; Millett, C.; Monteiro, C.A.; Levy, R.B. Ultra-Processed Food Consumption and Chronic Non-Communicable Diseases-Related Dietary Nutrient Profile in the UK (2008–2014). Nutrients 2018, 10, 587. [Google Scholar] [CrossRef]
- Martínez Steele, E.; Monteiro, C. Association between Dietary Share of Ultra-Processed Foods and Urinary Concentrations of Phytoestrogens in the US. Nutrients 2017, 9, 209. [Google Scholar] [CrossRef] [PubMed]
- Kocaadam-Bozkurt, B.; Karaçil Ermumcu, M.Ş.; Erdoğan Gövez, N.; Bozkurt, O.; Akpinar, Ş.; Mengi Çelik, Ö.; Köksal, E.; Acar Tek, N. Association between Adherence to the Mediterranean Diet with Anthropometric Measurements and Nutritional Status in Adolescents. Nutr. Hosp. 2023, 40, 368. [Google Scholar] [CrossRef] [PubMed]
- Farajian, P.; Risvas, G.; Karasouli, K.; Pounis, G.D.; Kastorini, C.M.; Panagiotakos, D.B.; Zampelas, A. Very High Childhood Obesity Prevalence and Low Adherence Rates to the Mediterranean Diet in Greek Children: The GRECO Study. Atherosclerosis 2011, 217, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Notario-Barandiaran, L.; Valera-Gran, D.; Gonzalez-Palacios, S.; Garcia-de-la-Hera, M.; Fernández-Barrés, S.; Pereda-Pereda, E.; Fernández-Somoano, A.; Guxens, M.; Iñiguez, C.; Romaguera, D.; et al. High Adherence to a Mediterranean Diet at Age 4 Reduces Overweight, Obesity and Abdominal Obesity Incidence in Children at the Age of 8. Int. J. Obes. 2020, 44, 1906–1917. [Google Scholar] [CrossRef] [PubMed]
- Bacopoulou, F.; Landis, G.; Rentoumis, A.; Tsitsika, A.; Efthymiou, V. Mediterranean Diet Decreases Adolescent Waist Circumference. Eur. J. Clin. Investig. 2017, 47, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Velasquez, M.T. Altered Gut Microbiota: A Link between Diet and the Metabolic Syndrome. Metab. Syndr. Relat. Disord. 2018, 16, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Martino, F.; Puddu, P.E.; Lamacchia, F.; Colantoni, C.; Zanoni, C.; Barillà, F.; Martino, E.; Angelico, F. Mediterranean Diet and Physical Activity Impact on Metabolic Syndrome among Children and Adolescents from Southern Italy: Contribution from the Calabrian Sierras Community Study (CSCS). Int. J. Cardiol. 2016, 225, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Trichopoulou, A.; Costacou, T.; Bamia, C.; Trichopoulos, D. Adherence to a Mediterranean Diet and Survival in a Greek Population. N. Engl. J. Med. 2003, 348, 2599–2608. [Google Scholar] [CrossRef] [PubMed]
- Kanellopoulou, A.; Giannakopoulou, S.-P.; Notara, V.; Antonogeorgos, G.; Rojas-Gil, A.P.; Kornilaki, E.N.; Konstantinou, E.; Lagiou, A.; Panagiotakos, D.B. The Association between Adherence to the Mediterranean Diet and Childhood Obesity; the Role of Family Structure: Results from an Epidemiological Study in 1728 Greek Students. Nutr. Health 2021, 27, 39–47. [Google Scholar] [CrossRef]
- De Santi, M.; Callari, F.; Brandi, G.; Toscano, R.V.; Scarlata, L.; Amagliani, G.; Schiavano, G.F. Mediterranean Diet Adherence and Weight Status among Sicilian Middle School Adolescents. Int. J. Food Sci. Nutr. 2020, 71, 1010–1018. [Google Scholar] [CrossRef]
- Seral-Cortes, M.; Larruy-García, A.; De Miguel-Etayo, P.; Labayen, I.; Moreno, L.A. Mediterranean Diet and Genetic Determinants of Obesity and Metabolic Syndrome in European Children and Adolescents. Genes 2022, 13, 420. [Google Scholar] [CrossRef] [PubMed]
- Moreno, L.; González-Gross, M.; Kersting, M.; Molnár, D.; De Henauw, S.; Beghin, L.; Sjöström, M.; Hagströmer, M.; Manios, Y.; Gilbert, C.; et al. Assessing, Understanding and Modifying Nutritional Status, Eating Habits and Physical Activity in European Adolescents: The HELENA (Healthy Lifestyle in Europe by Nutrition in Adolescence) Study. Public Health Nutr. 2008, 11, 288–299. [Google Scholar] [CrossRef] [PubMed]
- Iaccarino Idelson, P.; Scalfi, L.; Valerio, G. Adherence to the Mediterranean Diet in Children and Adolescents: A Systematic Review. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 283–299. [Google Scholar] [CrossRef] [PubMed]
- Lassale, C.; Fitó, M.; Morales-Suárez-Varela, M.; Moya, A.; Gómez, S.F.; Schröder, H. Mediterranean Diet and Adiposity in Children and Adolescents: A Systematic Review. Obes. Rev. 2022, 23, e13381. [Google Scholar] [CrossRef] [PubMed]
- Yurtdaş, G.; Akbulut, G.; Baran, M.; Yılmaz, C. The Effects of Mediterranean Diet on Hepatic Steatosis, Oxidative Stress, and Inflammation in Adolescents with non-alcoholic Fatty Liver Disease: A Randomized Controlled Trial. Pediatr. Obes. 2022, 17, e12872. [Google Scholar] [CrossRef] [PubMed]
- Gregory, A.T.; Denniss, A.R. An Introduction to Writing Narrative and Systematic Reviews—Tasks, Tips and Traps for Aspiring Authors. Heart Lung Circ. 2018, 27, 893–898. [Google Scholar] [CrossRef]
- Naska, A.; Trichopoulou, A. Back to the Future: The Mediterranean Diet Paradigm. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 216–219. [Google Scholar] [CrossRef]
- Kafatos, A.; Diacatou, A.; Voukiklaris, G.; Nikolakakis, N.; Vlachonikolis, J.; Kounali, D.; Mamalakis, G.; Dontas, A. Heart Disease Risk-Factor Status and Dietary Changes in the Cretan Population over the Past 30 y: The Seven Countries Study. Am. J. Clin. Nutr. 1997, 65, 1882–1886. [Google Scholar] [CrossRef]

| Studies Evaluated | Study Design | Sample/Studies | Intervention and/or Study Design | MD Effects/Adherence in Pediatric Population |
|---|---|---|---|---|
| Velázquez-López et al. [119] | Open-label study | 49 children and adolescents | 24 children and adolescents were given the Mediterranean-style diet, while 25 were given the standard diet. Dietary calculations for children aged 3–10 and adolescents aged 10–18 were conducted using the Schofield equation. | Decrease in BMI, fat mass, blood glucose, total cholesterol, triglyceride, HDL- and LDL-cholesterol levels by following MD. |
| Kanellopoulou et al. [123] | Cross-section, population-based, observational study | 1728 primary-school students (46% males, aged 10–12). | Family structure, dietary habits, and lifestyle were evaluated using questionnaires. MD adherence was determined using the KIDMED score. Children’s BMI was assessed according to the International Obesity Task Force classification. | Higher MD adherence acts as a protective factor against childhood overweight/obesity, particularly among children living with their families. |
| Notario-Barandiaran et al. [117] | Cross-sectional study | 1801 and 1527 children who attended follow-up visits at age 4 and 8 years, respectively, | Dietary habits were evaluated at the age of 4 using a validated food frequency questionnaire. Adherence to MD was evaluated by rMED score. Children’s BMI was calculated according to the International Obesity Task Force classification. | High MD adherence at the age of 4 is linked to a reduced risk of overweight, obesity, and abdominal obesity by the age of 8. |
| Bacopoulou et al. [118] | Cross-sectional dietary intervention | 1610 adolescents (12–17 years) in 23 public high schools | Nutritional education, promotion of physical activity, and raising awareness about body image for adolescent participants, their parents, schoolteachers, and health staff. Dietary assessment was evaluated using the KIDMED score, while BP, BMI, WC, and WHtR were measured at baseline and after a 6-month school-based intervention. | Decrease in overweight and obesity, mean systolic and diastolic BP, WC, and WHtR by following MD. |
| De Santi et al. [124] | Cross-sectional study | 239 adolescent Italian students (119 boys and 120 girls, mean age: 12.1 ± 1.0) | Information on physical activity habits was gathered through a questionnaire. Adherence to the MD was assessed using the KIDMED score. Children’s BMI was determined based on the Cacciari classification. | Very low adherence to the MD among adolescents living in Mediterranean countries. association between MedDiet adherence, healthy behavior and normal weight status. |
| Seral-Cortes et al. [125] | Systematic-review | PubMed database was searched and only 1 study was included | Evaluated gene–MD interaction effects and its relationship with changes in body composition and metabolic parameters. | High adherence to the MD in individuals with a limited number of risk alleles was associated with a lower risk of adiposity and MetS. |
| López-Gil et al. [109] | Systematic review with meta-analysis | Four databases (PubMed, Scopus, Web of Science, and Cochrane Database of Systematic Reviews), and 15 studies were included | Assess the impact of Mediterranean diet-based interventions on anthropometric measurements and obesity indicators in children and adolescents. | Compared to the control group, the MD-based interventions showed small and significant reductions in BMI and significant reduction in the percentage of obesity MD-based interventions have a significant effect on reducing BMI. |
| Iaccarino et al. [127] | Systematic review | Several databases were systematically searched (PubMed, Scopus, Clinical Trials Results, Google Scholar and British Library Inside) and 58 studies published in the last 20 years were included | Compare MD adherence in children and adolescents with demographic and anthropometric variables (body composition, lifestyle, and diet adequacy). | 10 of 26 papers reported that higher adherence to the MD was associated with lower BMI values or prevalence of overweight. |
| Lassale et al. [128] | Systematic review | Medline database was searched and 55 article were included | Impact of MD adherence on adiposity markers and obesity in children and adolescents. | More than 50% of studies found no significant association between MD adherence and adiposity. |
| Yurtdaş et al. [129] | Single-blind, randomized, two-arm, parallel dietary intervention | 96 adolescents diagnosed with NAFLD (aged 11–18 years) were randomized to follow MD or conventional LFD (control diet) for 12 weeks. | Dietary status, anthropometric measurements, body composition, and biochemical parameters were assessed. Hepatic steatosis was diagnosed using ultrasonography. | Both MD and LFD decreased BMI, fat mass, hepatic steatosis, and insulin resistance, improved elevated transaminase levels, and had beneficial effects on inflammation and oxidative stress. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calcaterra, V.; Verduci, E.; Milanta, C.; Agostinelli, M.; Bona, F.; Croce, S.; Valsecchi, C.; Avanzini, M.A.; Zuccotti, G. The Benefits of the Mediterranean Diet on Inflamm-Aging in Childhood Obesity. Nutrients 2024, 16, 1286. https://doi.org/10.3390/nu16091286
Calcaterra V, Verduci E, Milanta C, Agostinelli M, Bona F, Croce S, Valsecchi C, Avanzini MA, Zuccotti G. The Benefits of the Mediterranean Diet on Inflamm-Aging in Childhood Obesity. Nutrients. 2024; 16(9):1286. https://doi.org/10.3390/nu16091286
Chicago/Turabian StyleCalcaterra, Valeria, Elvira Verduci, Chiara Milanta, Marta Agostinelli, Federica Bona, Stefania Croce, Chiara Valsecchi, Maria Antonietta Avanzini, and Gianvincenzo Zuccotti. 2024. "The Benefits of the Mediterranean Diet on Inflamm-Aging in Childhood Obesity" Nutrients 16, no. 9: 1286. https://doi.org/10.3390/nu16091286
APA StyleCalcaterra, V., Verduci, E., Milanta, C., Agostinelli, M., Bona, F., Croce, S., Valsecchi, C., Avanzini, M. A., & Zuccotti, G. (2024). The Benefits of the Mediterranean Diet on Inflamm-Aging in Childhood Obesity. Nutrients, 16(9), 1286. https://doi.org/10.3390/nu16091286








