Gut Microbiome—How Does Two-Month Consumption of Fiber-Enriched Rolls Change Microbiome in Patients Suffering from MASLD?
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Dietary Guidelines
2.3. Fecal Microbiota Sequenced Using 16S on Nanopore
2.4. Short-Chain Fatty Acids (SCFA) Analysis
2.5. Bioinformatic and Statistical Analysis
2.5.1. Taxonomic Assignment of 16S Reads
2.5.2. Statistical Analysis
3. Results
3.1. Patients’ Characteristics
3.2. Gut Microbiota-Derived Metabolites (SCFAs)
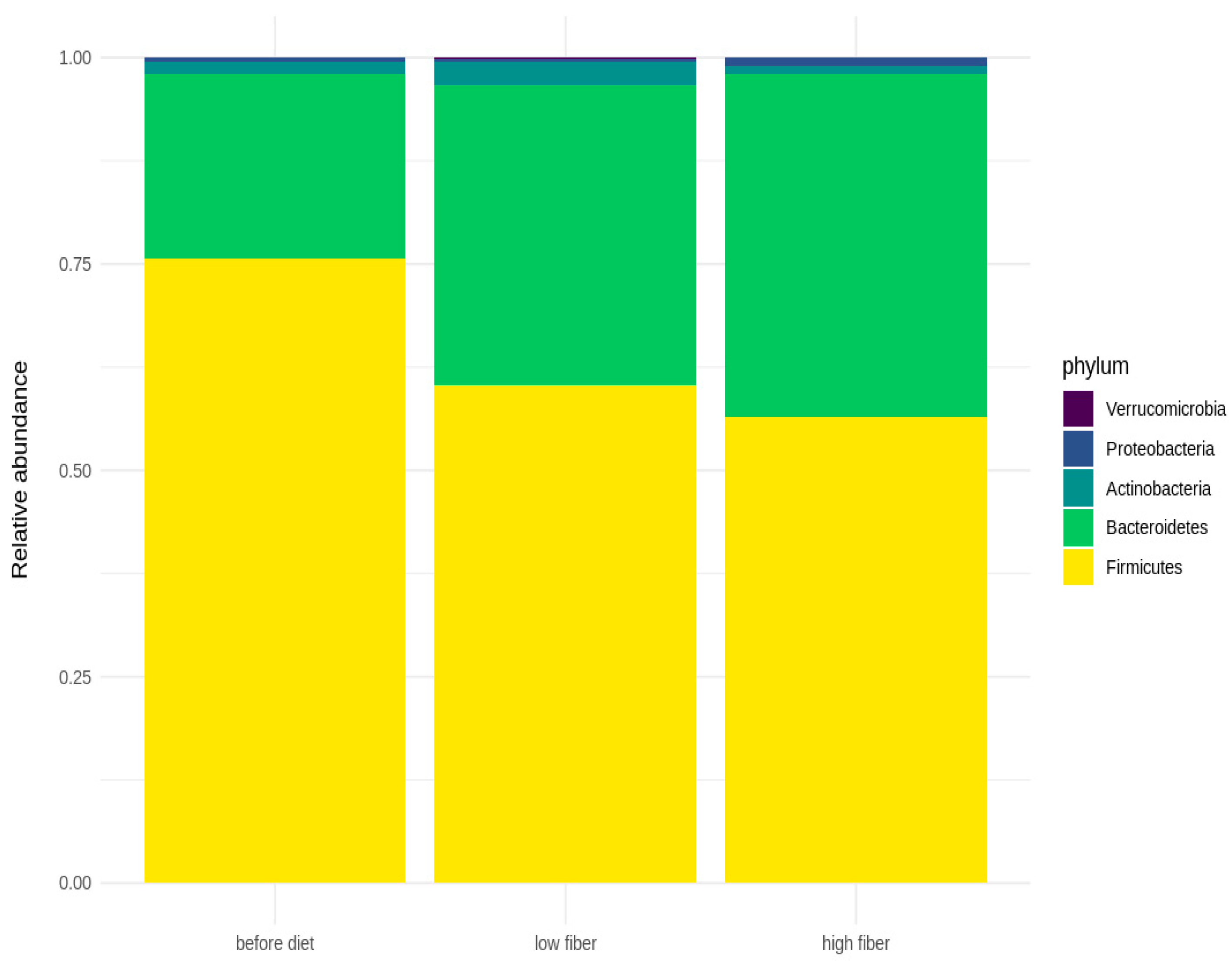
3.3. Microbial Diversity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krag, A.; Buti, M.; Lazarus, J.V.; Allen, A.M.; Bowman, J.; Burra, P.; Donnini, G.; Duseja, A.; El-Sayed, M.H.; Gastaldelli, A.; et al. Uniting to defeat steatotic liver disease: A global mission to promote healthy livers and healthy lives. J. Hepatol. 2023, 79, 1076–1078. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Golabi, P.; Paik, J.M.; Henry, A.; Van Dongen, C.; Henry, L. The global epidemiology of nonalcoholic fatty liver disease (MASLD) and nonalcoholic steatohepatitis (NASH): A systematic review. Hepatology 2023, 77, 1335–1347. [Google Scholar] [CrossRef]
- Mitra, S.; De, A.; Chowdhury, A. Epidemiology of non-alcoholic and alcoholic fatty liver diseases. Transl. Gastroenterol. Hepatol. 2020, 5, 16. [Google Scholar] [CrossRef]
- Friedman, S.L.; Neuschwander-Tetri, B.A.; Rinella, M.; Sanyal, A.J. Mechanisms of NAFLD development and therapeutic strategies. Nat. Med. 2018, 24, 908–922. [Google Scholar] [CrossRef] [PubMed]
- Yaskolka Meir, A.; Rinott, E.; Tsaban, G.; Zelicha, H.; Kaplan, A.; Rosen, P.; Shelef, I.; Youngster, I.; Shalev, A.; Blüher, M.; et al. Effect of green-Mediterranean diet on intrahepatic fat: The DIRECT PLUS randomised controlled trial. Gut 2021, 70, 2085–2095. [Google Scholar] [CrossRef]
- Li, T.; Zhang, Y.; Gong, C.; Wang, J.; Liu, B.; Shi, L.; Duan, J. Prevalence of malnutrition and analysis of related factors in elderly patients with COVID-19 in Wuhan, China. Eur. J Clin. Nutr. 2020, 74, 871–875. [Google Scholar] [CrossRef] [PubMed]
- Meroni, M.; Longo, M.; Rustichelli, A.; Dongiovanni, P. Nutrition and Genetics in NAFLD: The Perfect Binomium. Int. J Mol. Sci. 2020, 21, 2986. [Google Scholar] [CrossRef] [PubMed]
- Abenavoli, L.; Boccuto, L.; Federico, A.; Dallio, M.; Loguercio, C.; Di Renzo, L.; De Lorenzo, A. Diet and Non-Alcoholic Fatty Liver Disease: The Mediterranean Way. Int. J. Environ. Res. Public Health 2019, 16, 3011. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.; Nicolucci, A.C.; Virtanen, H.; Schick, A.; Meddings, J.; Reimer, R.A.; Huang, C. Effect of Prebiotic on Microbiota, Intestinal Permeability, and Glycemic Control in Children With Type 1 Diabetes. J. Clin. Endocrinol. Metab. 2019, 104, 4427–4440. [Google Scholar] [CrossRef] [PubMed]
- Ojo, O.; Ojo, O.O.; Zand, N.; Wang, X. The Effect of Dietary Fibre on Gut Microbiota, Lipid Profile, and Inflammatory Markers in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Nutrients 2021, 13, 1805. [Google Scholar] [CrossRef] [PubMed]
- Stachowska, E.; Portincasa, P.; Jamioł-Milc, D.; Maciejewska-Markiewicz, D.; Skonieczna-Żydecka, K. The Relationship between Prebiotic Supplementation and Anthropometric and Biochemical Parameters in Patients with NAFLD-A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2020, 12, 3460. [Google Scholar] [CrossRef] [PubMed]
- Hagströmer, M.; Oja, P.; Sjöström, M. The International Physical Activity Questionnaire (IPAQ): A study of concurrent and construct validity. Public Health Nutr. 2006, 9, 755–762. [Google Scholar] [CrossRef]
- Bach-Faig, A.; Berry, E.M.; Lairon, D.; Reguant, J.; Trichopoulou, A.; Dernini, S.; Medina, F.X.; Battino, M.; Belahsen, R.; Miranda, G.; et al. Mediterranean diet pyramid today. Science and cultural up-dates. Public Health Nutr. 2011, 14, 2274–2284. [Google Scholar] [CrossRef] [PubMed]
- Karlas, T.; Petroff, D.; Garnov, N.; Böhm, S.; Tenckhoff, H.; Wittekind, C.; Wiese, M.; Schiefke, I.; Linder, N.; Schaudinn, A.; et al. Non-invasive assessment of hepatic steatosis in patients with NAFLD using con-trolled attenuation parameter and 1H-MR spectroscopy. PLoS ONE 2014, 9, e91987. [Google Scholar] [CrossRef]
- Aparicio-Ugarriza, R.; Cuenca-García, M.; Gonzalez-Gross, M.; Julián, C.; Bel-Serrat, S.; Moreno, L.A.; Breidenassel, C.; Kersting, M.; Arouca, A.B.; Michels, N.; et al. Relative validation of the adapted Mediterranean Diet Score for Adolescents by comparison with nutritional biomarkers and nutrient and food in-takes: The Healthy Lifestyle in Europe by Nutrition in Adolescence (HELENA) study. Public Health Nutr. 2019, 22, 2381–2397. [Google Scholar] [CrossRef]
- Safari, Z.; Gérard, P. The links between the gut microbiome and non-alcoholic fatty liver disease (NAFLD). Cell Mol. Life Sci. 2019, 76, 1541–1558. [Google Scholar] [CrossRef]
- Jamioł-Milc, D.; Gudan, A.; Kaźmierczak-Siedlecka, K.; Hołowko-Ziółek, J.; Maciejewska-Markiewicz, D.; Janda-Milczarek, K.; Stachowska, E. Nutritional Support for Liver Diseases. Nutrients 2023, 15, 3640. [Google Scholar] [CrossRef]
- Semmler, G.; Datz, C.; Reiberger, T.; Trauner, M. Diet and exercise in NAFLD/NASH: Beyond the obvious. Liver Int. 2021, 41, 2249–2268. [Google Scholar] [CrossRef]
- Haigh, L.; Kirk, C.; El Gendy, K.; Gallacher, J.; Errington, L.; Mathers, J.C.; Anstee, Q.M. The effectiveness and acceptability of Mediterranean diet and calorie restriction in non-alcoholic fatty liver dis-ease (NAFLD): A systematic review and meta-analysis. Clin. Nutr. 2022, 41, 1913–1931. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Montes de Oca, A.; Julián, M.T.; Ramos, A.; Puig-Domingo, M.; Alonso, N. Microbiota, Fiber, and NAFLD: Is There Any Connection? Nutrients 2020, 12, 3100. [Google Scholar] [CrossRef]
- Holscher, H.D. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes 2017, 8, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Usuda, H.; Okamoto, T.; Wada, K. Leaky Gut: Effect of Dietary Fiber and Fats on Microbiome and Intestinal Barrier. Int. J. Mol. Sci. 2021, 22, 7613. [Google Scholar] [CrossRef] [PubMed]
- Kaźmierczak-Siedlecka, K.; Skonieczna-Żydecka, K.; Palma, J.; Sobocki, B.; Świerblewski, M.; Siedlecka-Kroplewska, K.; Kalinowski, L.; Połom, K. Microbiota-derived metabolites in colorectal cancer patients in preoperative period. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 1443–1449. [Google Scholar] [PubMed]
- Deng, M.; Qu, F.; Chen, L.; Liu, C.; Zhang, M.; Ren, F.; Guo, H.; Zhang, H.; Ge, S.; Wu, C.; et al. SCFAs alleviated steatosis and inflammation in mice with NASH induced by MCD. J. Endocrinol. 2020, 245, 425–437. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Yang, X.; Wu, Q.; Gong, Y.; Pang, N.; Ge, X.; Nagaratnam, N.; Jiang, P.; Zhou, M.; Hu, T.; et al. Butyrate Attenuates Hepatic Steatosis Induced by a High-Fat and Fiber-Deficient Diet via the Hepatic GPR41/43-CaMKII/HDAC1-CREB Pathway. Mol. Nutr. Food Res. 2023, 67, e2200597. [Google Scholar] [CrossRef] [PubMed]
- Rau, M.; Rehman, A.; Dittrich, M.; Groen, A.K.; Hermanns, H.M.; Seyfried, F.; Beyersdorf, N.; Dandekar, T.; Rosenstiel, P.; Geier, A. Fecal SCFAs and SCFA-producing bacteria in gut microbiome of human NAFLD as a putative link to systemic T-cell activation and advanced disease. United Eur. Gastroenterol. J. 2018, 6, 1496–1507. [Google Scholar] [CrossRef]
- Wu, M.Y.; Fan, J.G. Gut microbiome and nonalcoholic fatty liver disease. Hepatobiliary Pancreat. Dis. Int. 2023, 22, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Vitetta, L. Gut Microbiota Metabolites in NAFLD Pathogenesis and Therapeutic Implications. Int. J. Mol. Sci. 2020, 21, 5214. [Google Scholar] [CrossRef]
- Ji, Y.; Yin, Y.; Li, Z.; Zhang, W. Gut Microbiota-Derived Components and Metabolites in the Progression of Non-Alcoholic Fatty Liver Disease (NAFLD). Nutrients 2019, 11, 1712. [Google Scholar] [CrossRef]
- Hrncir, T.; Hrncirova, L.; Kverka, M.; Hromadka, R.; Machova, V.; Trckova, E.; Kostovcikova, K.; Kral-ickova, P.; Krejsek, J.; Tlaskalova-Hogenova, H. Gut Microbiota and NAFLD: Pathogenetic Mechanisms, Microbiota Signatures, and Therapeutic Interventions. Microorganisms 2021, 9, 957. [Google Scholar] [CrossRef]
- Ohtani, N.; Hara, E. Gut-liver axis-mediated mechanism of liver cancer: A special focus on the role of gut microbiota. Cancer Sci. 2021, 112, 4433–4443. [Google Scholar] [CrossRef] [PubMed]
- Beisner, J.; Filipe Rosa, L.; Kaden-Volynets, V.; Stolzer, I.; Günther, C.; Bischoff, S.C. Prebiotic Inulin and Sodium Butyrate Attenuate Obesity-Induced Intestinal Barrier Dysfunction by Induction of Antimicrobial Peptides. Front. Immunol. 2021, 12, 678360. [Google Scholar] [CrossRef]
- Behrouz, V.; Aryaeian, N.; Zahedi, M.J.; Jazayeri, S. Effects of probiotic and prebiotic supplementation on metabolic parameters, liver aminotransferases, and systemic inflammation in nonalcoholic fatty liver disease: A randomized clinical trial. J. Food Sci. 2020, 85, 3611–3617. [Google Scholar] [CrossRef] [PubMed]
- Leung, H.; Long, X.; Ni, Y.; Qian, L.; Nychas, E.; Siliceo, S.L.; Pohl, D.; Hanhineva, K.; Liu, Y.; Xu, A.; et al. Risk assessment with gut microbiome and metabolite markers in NAFLD development. Sci. Transl. Med. 2022, 14, eabk0855. [Google Scholar] [CrossRef] [PubMed]
- Vallianou, N.; Christodoulatos, G.S.; Karampela, I.; Tsilingiris, D.; Magkos, F.; Stratigou, T.; Kounatidis, D.; Dalamaga, M. Understanding the Role of the Gut Microbiome and Microbial Metabolites in Non-Alcoholic Fatty Liver Disease: Current Evidence and Perspectives. Biomolecules 2021, 12, 56. [Google Scholar] [CrossRef]
- Di Ciaula, A.; Bonfrate, L.; Portincasa, P. The role of microbiota in nonalcoholic fatty liver disease. Eur. J Clin. Investig. 2022, 52, e13768. [Google Scholar] [CrossRef]
- Marques, F.Z.; Nelson, E.; Chu, P.Y.; Horlock, D.; Fiedler, A.; Ziemann, M.; Tan, J.K.; Kuruppu, S.; Rajapakse, N.W.; El-Osta, A.; et al. High-Fiber Diet and Acetate Supplementation Change the Gut Microbiota and Prevent the Development of Hypertension and Heart Failure in Hypertensive Mice. Circulation 2017, 135, 964–977. [Google Scholar] [CrossRef]
- Jasirwan, C.O.M.; Muradi, A.; Hasan, I.; Simadibrata, M.; Rinaldi, I. Correlation of gut Firmicutes/Bacteroidetes ratio with fibrosis and steatosis stratified by body mass index in patients with non-alcoholic fatty liver disease. Biosci. Microbiota Food Health 2021, 40, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Huang, Y.; Xie, H.; Bai, H.; Lin, G.; Dong, Y.; Shi, D.; Wang, J.; Zhang, Q.; Zhang, Y.; et al. Impact of a low-carbohydrate and high-fiber diet on nonalcoholic fatty liver disease. Asia Pac. J. Clin. Nutr. 2020, 29, 483–490. [Google Scholar] [PubMed]
- Zhu, Y.; Yang, H.; Zhang, Y.; Rao, S.; Mo, Y.; Zhang, H.; Yang, W. Dietary fiber intake and non-alcoholic fatty liver disease: The mediating role of obesity. Front. Public Health 2022, 10, 1038435. [Google Scholar] [CrossRef]




| Parameters | Values (Min.–Max.) |
|---|---|
| BMI | 29.1 (22.2–35.7) |
| Body_weight | 87.4 (60.3–115.6) |
| Body fat | 29.5 (17.1–43.8) |
| Muscle_mass | 54.6 (39.5–76.7) |
| Fibroscan_CAP | 305.5 (242–400) |
| Fibroscan_elast | 5.65 (3.9–9.4) |
| ALT | 29 (11–136) |
| AST | 22 (11–52) |
| GGTP | 27.5 (12–70) |
| Total_Cholesterol | 205.6 (110–394.4) |
| LDL | 137 (43.5–282.2) |
| HDL | 47.15 (25–71.3) |
| Intervention with 12 g (LFIB) T1 vs. T3 | Intervention with 24 g (HFIB) T1 vs. T3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parameter Intervention | Median | IQR | Median | IQR | p | Median | IQR | Median | IQR | p |
| Fasting glucose [mg/dL] | 93 | 16.2 | 91 | 29.5 | 0.72 | 94.6 | 15.3 | 96.9 | 10.2 | 0.94 |
| Total cholesterol [mg/dL] | 191.4 | 53.2 | 179.9 | 27.1 | 0.18 | 221 | 59.6 | 197.3 | 47.7 | 0.01 |
| HDL [mg/dL] | 44 | 12.1 | 43.1 | 15.9 | 0.58 | 49.1 | 6 | 48.7 | 6.5 | 0.17 |
| LDL [mg/dL] | 125 | 54.5 | 111.9 | 42.1 | 0.21 | 148.9 | 38.9 | 127.7 | 44 | 0.05 |
| TG [mg/dL] | 153.3 | 94 | 129.3 | 92 | 0.11 | 168.2 | 154.2 | 162.3 | 111 | 0.28 |
| ALT [U/L] | 38 | 17 | 38 | 18 | 0.89 | 43 | 20 | 32 | 12 | 0.04 |
| AST [U/L] | 28 | 12 | 30 | 11 | 0.66 | 27 | 8 | 23 | 6 | 0.02 |
| GGTP [U/L] | 33 | 10 | 35 | 16 | 0.23 | 28 | 12 | 24 | 14 | 0.12 |
| Fasting insulin [uU/mL] | 19.1 | 20.3 | 16.1 | 13.9 | 0.66 | 36.8 | 87.2 | 37.6 | 31 | 0.18 |
| Age [years] | 47.5 | 12.3 | - | - | - | 47.5 | 14.5 | - | - | - |
| BMI [kg/m2] | 29.1 | 3.8 | 28.6 | 5.2 | 0.04 | 28.5 | 10.4 | 27.3 | 9.5 | 0.61 |
| Intervention with 12 g (LFIB) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| SCFA mol% | C 2:0 A,B | C 3:0 | C 4:0 n B,C | ||||||
| Visit | T1 | T2 | T3 | T1 | T2 | T3 | T1 | T2 | T3 |
| Median | 64.93 | 68.53 | 62.51 | 19.66 | 17.11 | 18.46 | 14.38 | 12.65 | 18.9 |
| IQR | 14.91 | 10.61 | 14.01 | 4.46 | 3.19 | 3.35 | 7.5 | 9.64 | 7.52 |
| Intervention with 24 g (HFIB) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| SCFA mol% | C 2:0 A,B | C 3:0 | C 4:0 n B*,C | ||||||
| Visit | T1 | T2 | T3 | T1 | T2 | T3 | T1 | T2 | T3 |
| Median | 60.52 | 63.12 | 56.1 | 20.39 | 19.08 | 20.18 | 14.79 | 12.61 | 15.76 |
| IQR | 9.62 | 10.89 | 9.84 | 4.52 | 4.51 | 2.71 | 4.69 | 7.74 | 7.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaźmierczak-Siedlecka, K.; Maciejewska-Markiewicz, D.; Sykulski, M.; Gruszczyńska, A.; Herman-Iżycka, J.; Wyleżoł, M.; Katarzyna Petriczko, K.; Palma, J.; Jakubczyk, K.; Janda-Milczarek, K.; et al. Gut Microbiome—How Does Two-Month Consumption of Fiber-Enriched Rolls Change Microbiome in Patients Suffering from MASLD? Nutrients 2024, 16, 1173. https://doi.org/10.3390/nu16081173
Kaźmierczak-Siedlecka K, Maciejewska-Markiewicz D, Sykulski M, Gruszczyńska A, Herman-Iżycka J, Wyleżoł M, Katarzyna Petriczko K, Palma J, Jakubczyk K, Janda-Milczarek K, et al. Gut Microbiome—How Does Two-Month Consumption of Fiber-Enriched Rolls Change Microbiome in Patients Suffering from MASLD? Nutrients. 2024; 16(8):1173. https://doi.org/10.3390/nu16081173
Chicago/Turabian StyleKaźmierczak-Siedlecka, Karolina, Dominika Maciejewska-Markiewicz, Maciej Sykulski, Agata Gruszczyńska, Julia Herman-Iżycka, Mariusz Wyleżoł, Karolina Katarzyna Petriczko, Joanna Palma, Karolina Jakubczyk, Katarzyna Janda-Milczarek, and et al. 2024. "Gut Microbiome—How Does Two-Month Consumption of Fiber-Enriched Rolls Change Microbiome in Patients Suffering from MASLD?" Nutrients 16, no. 8: 1173. https://doi.org/10.3390/nu16081173
APA StyleKaźmierczak-Siedlecka, K., Maciejewska-Markiewicz, D., Sykulski, M., Gruszczyńska, A., Herman-Iżycka, J., Wyleżoł, M., Katarzyna Petriczko, K., Palma, J., Jakubczyk, K., Janda-Milczarek, K., Skonieczna-Żydecka, K., & Stachowska, E. (2024). Gut Microbiome—How Does Two-Month Consumption of Fiber-Enriched Rolls Change Microbiome in Patients Suffering from MASLD? Nutrients, 16(8), 1173. https://doi.org/10.3390/nu16081173








