Impact of 1-Year Supplementation with High-Rich Docosahexaenoic Acid (DHA) on Clinical Variables and Inflammatory Biomarkers in Pediatric Cystic Fibrosis: A Randomized Double-Blind Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
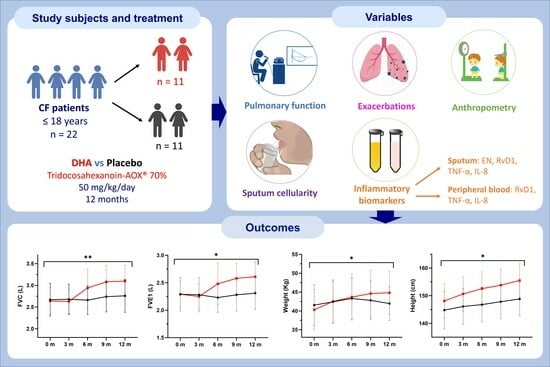
2.1. Study Design and Participants
2.2. Randomization and Intervention
2.3. Study Procedures
2.4. Study Variables
2.5. Study Endpoints
2.6. Statistical Analysis
3. Results
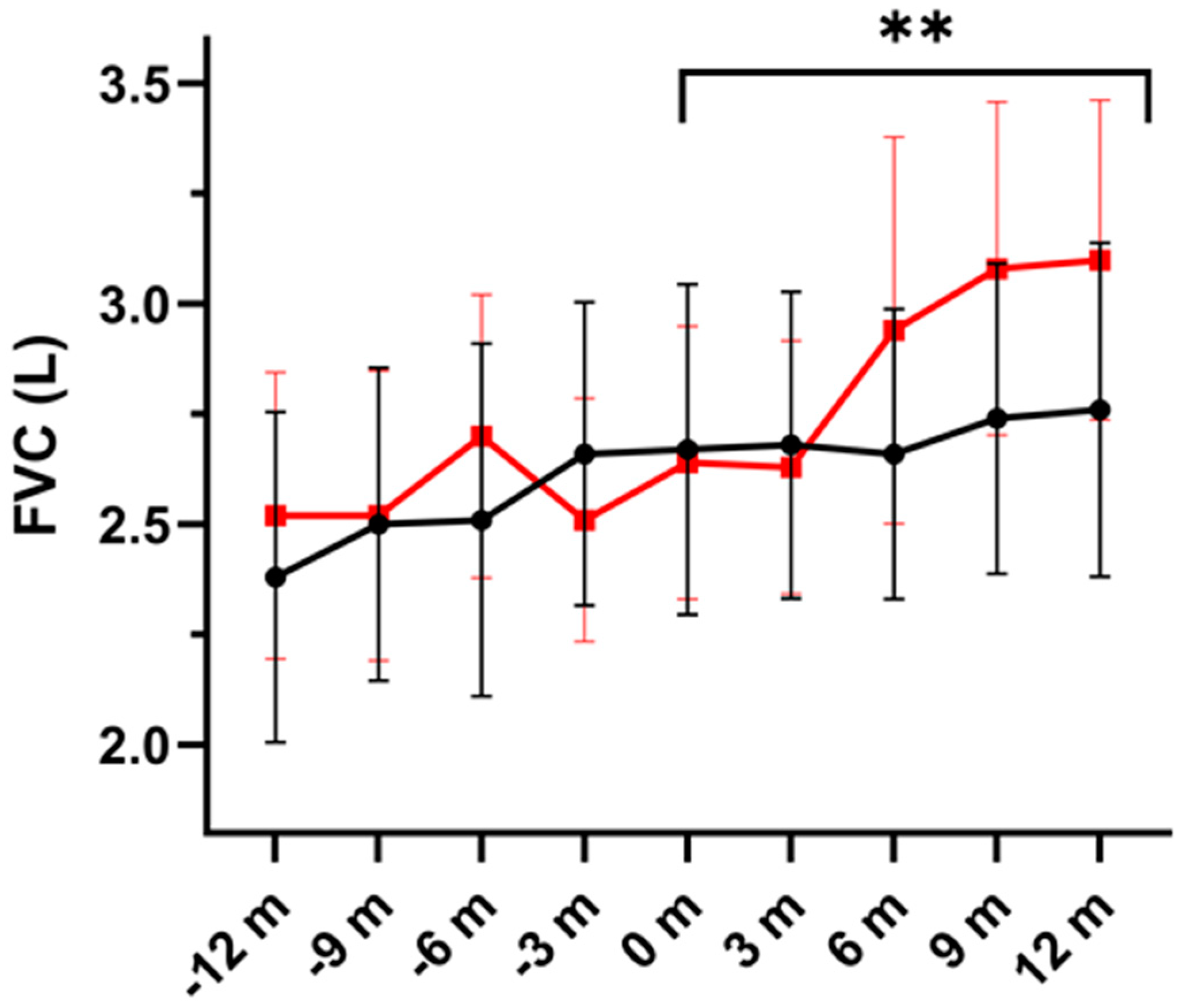
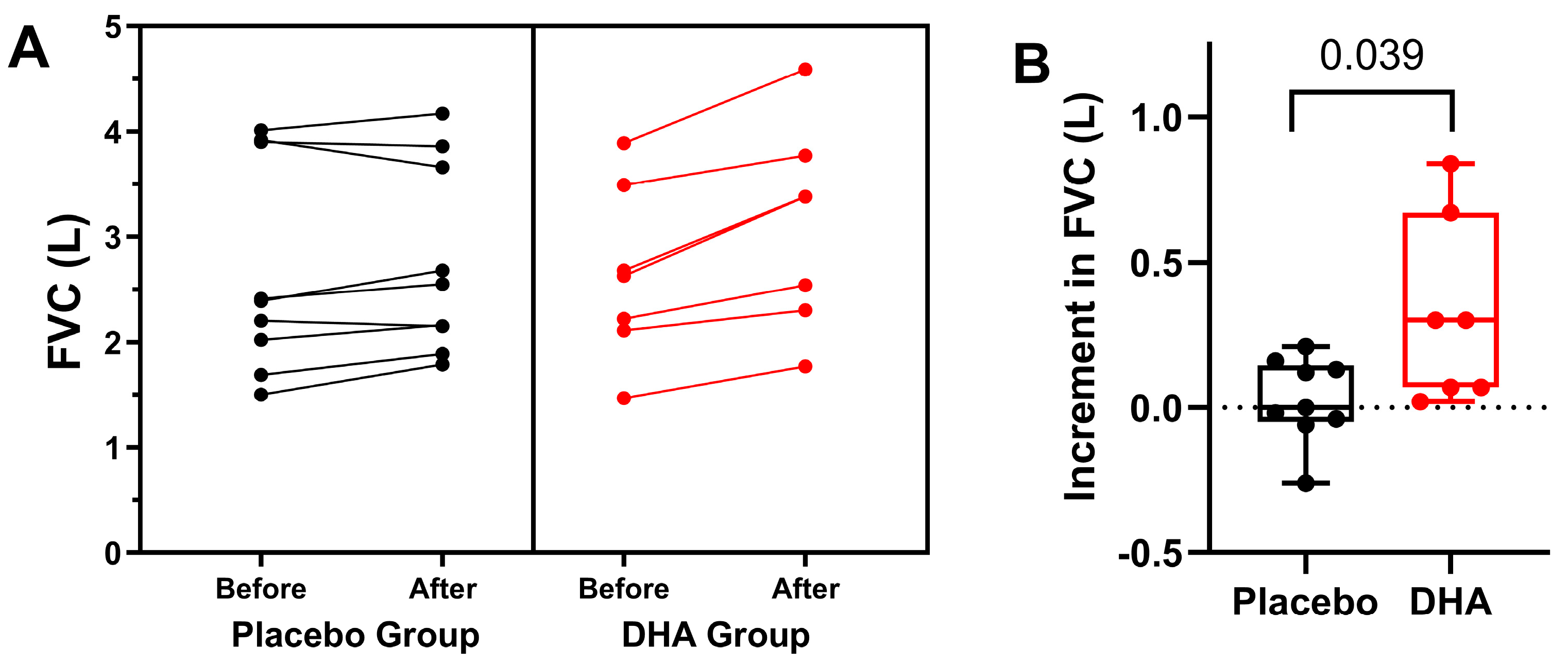
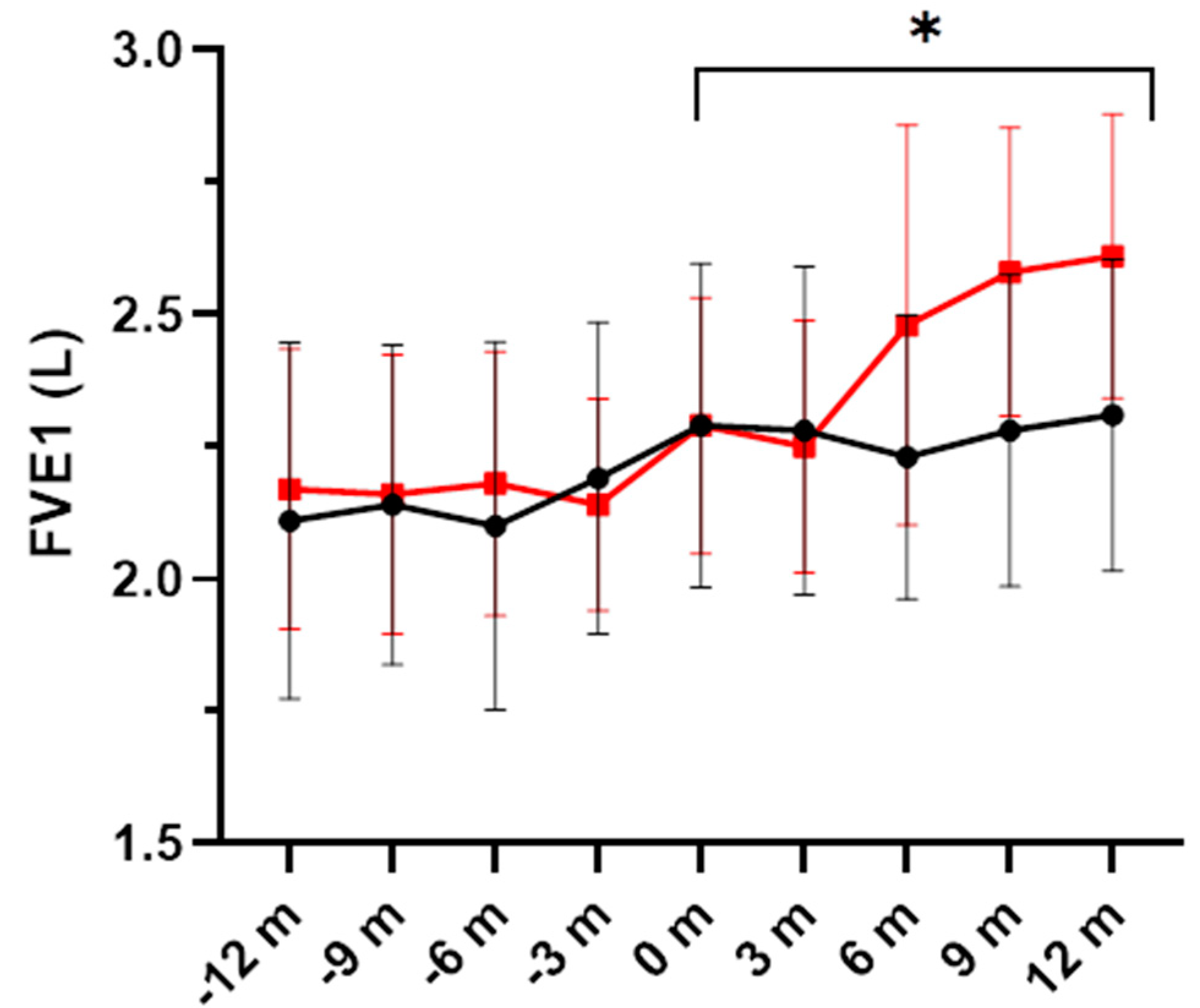
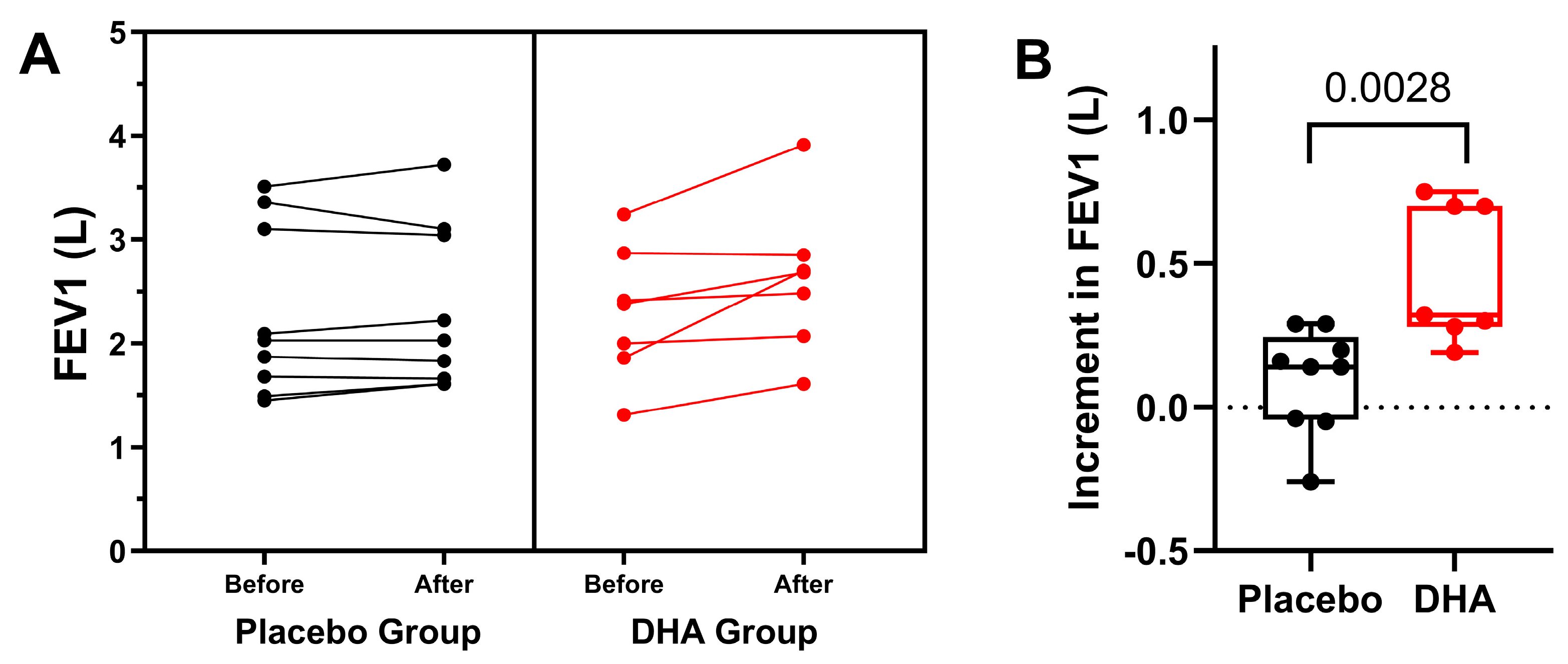
3.1. Pulmonary Function and Exacerbations
3.2. Sputum Cellularity
3.3. Inflammatory Biomarkers
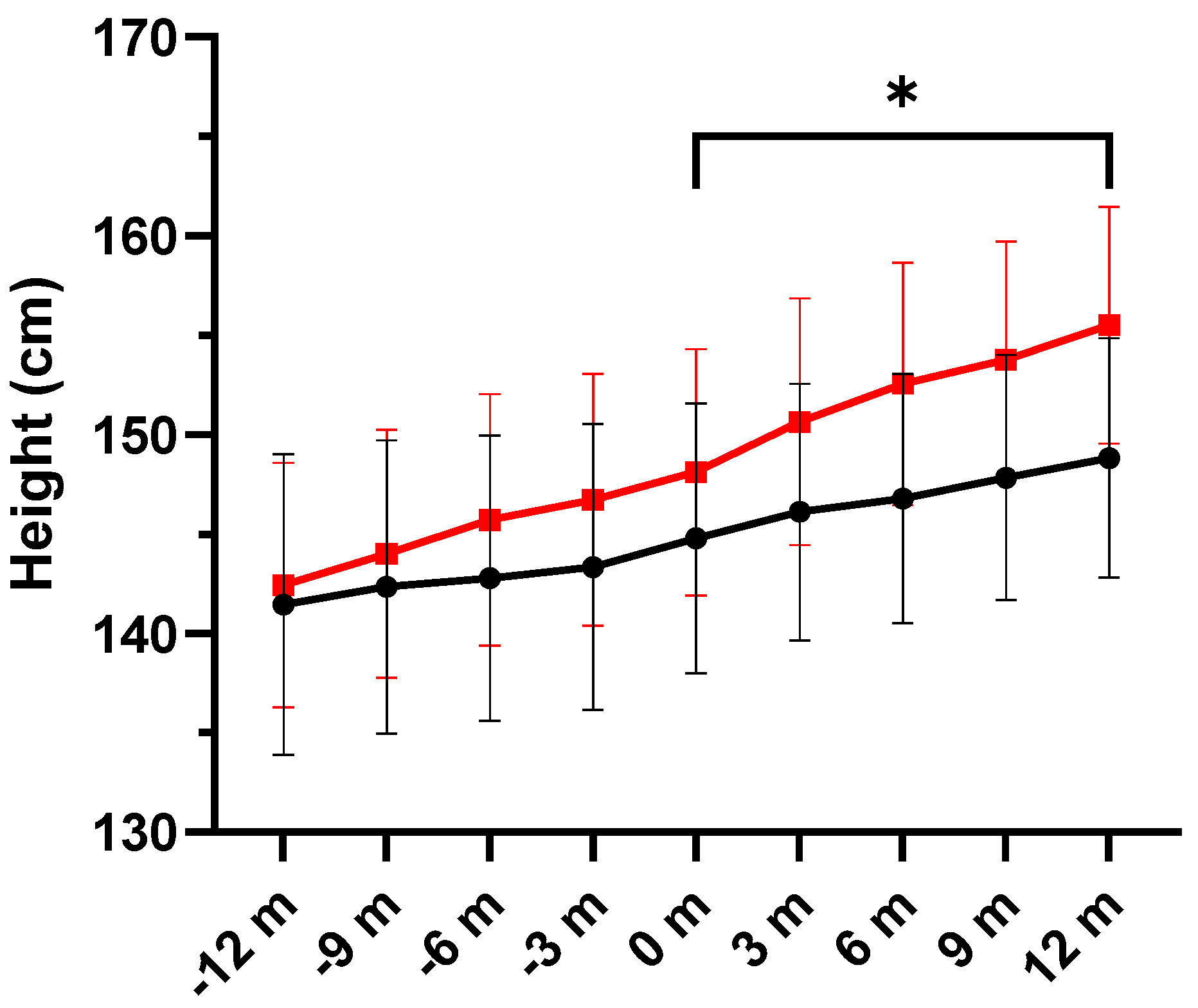
3.4. Anthropometric Variables
3.5. Adverse Events and Compliance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shrestha, N.; McCarron, A.; Rout-Pitt, N.; Donnelley, M.; Parsons, D.W.; Hryciw, D.H. Essential fatty acid deficiency in cystic fibrosis disease progression: Role of genotype and sex. Nutrients 2022, 14, 4666. [Google Scholar] [CrossRef] [PubMed]
- Wheelock, C.E.; Strandvik, B. Abnormal n-6 fatty acid metabolism in cystic fibrosis contributes to pulmonary symptoms. Prostaglandins Leukot. Essent. Fatty Acids 2020, 160, 102156. [Google Scholar] [CrossRef] [PubMed]
- Link, S.L.; Nayak, R.P. Review of rapid advances in cystic fibrosis. Mo. Med. 2020, 117, 548–554. [Google Scholar] [PubMed]
- Aldámiz-Echevarría, L.; Prieto, J.; Andrade, F.; Elorz, J.; Sojo, A.; Lage, S.; Sanjurjo, P.; Vázquez, C.; Rodríguez-Soriano, J. Persistence of essential fatty acid deficiency in cystic fibrosis despite nutritional therapy. Pediatr. Res. 2009, 66, 585–589. [Google Scholar] [CrossRef] [PubMed]
- Seegmiller, A.C. Abnormal unsaturated fatty acid metabolism in cystic fibrosis: Biochemical mechanisms and clinical implications. Int. J. Mol. Sci. 2014, 15, 16083–16099. [Google Scholar] [CrossRef]
- Van Biervliet, S.; Van Biervliet, J.P.; Robberecht, E.; Christophe, A. Docosahexaenoic acid trials in cystic fibrosis: A review of the rationale behind the clinical trials. J. Cyst. Fibros. 2005, 4, 27–34. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Strandvik, B. Fatty acid metabolism in cystic fibrosis. Prostaglandins Leukot. Essent. Fatty Acids 2010, 83, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Farrell, P.M.; Mischler, E.H.; Engle, M.J.; Brown, D.J.; Lau, S.M. Fatty acid abnormalities in cystic fibrosis. Pediatr. Res. 1985, 19, 104–109. [Google Scholar] [CrossRef]
- Vandebrouck, C.; Ferreira, T. Glued in lipids: Lipointoxica-tion in cystic fibrosis. EBioMedicine 2020, 61, 103038. [Google Scholar] [CrossRef]
- Basil, M.; Levy, B. Specialized pro-resolving mediators: Endogenous regulators of infection and inflammation. Nat. Rev. Immunol. 2016, 16, 51–67. [Google Scholar] [CrossRef]
- Olveira, G.; Olveira, C.; Acosta, E.; Espíldora, F.; Garrido-Sánchez, L.; García-Escobar, E.; Rojo-Martínez, G.; Gonzalo, M.; Soriguer, F. Fatty acid supplements improve respiratory, inflammatory and nutritional parameters in adults with cystic fibrosis. Arch. Bronconeumol. 2010, 46, 70–77. [Google Scholar] [CrossRef]
- Hanssens, L.; Thiébaut, I.; Lefèvre, N.; Malfroot, A.; Knoop, C.; Duchateau, J.; Casimir, G. The clinical benefits of long-term supplementation with omega-3 fatty acids in cystic fibrosis patients—A pilot study. Prostaglandins Leukot. Essent. Fatty Acids 2016, 108, 45–50. [Google Scholar] [CrossRef] [PubMed]
- De Vizia, B.; Raia, V.; Spano, C.; Pavlidis, C.; Coruzzo, A.; Alessio, M. Effect of an 8-month treatment with omega-3 fatty acids (eicosapentaenoic and docosahexaenoic) in patients with cystic fibrosis. JPEN J. Parenter. Enteral. Nutr. 2003, 27, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Oliver, C.; Watson, H. Omega-3 fatty acids for cystic fibrosis. Cochrane Database Syst. Rev. 2016, 2016, CD002201. [Google Scholar] [CrossRef] [PubMed]
- Watson, H.; Stackhouse, C. Omega-3 fatty acid supplementation for cystic fibrosis. Cochrane Database Syst. Rev. 2020, 4, CD002201. [Google Scholar] [CrossRef] [PubMed]
- Ayats-Vidal, R.; Bosque-García, M.; Cordobilla, B.; Asensio-De la Cruz, O.; García-González, M.; Castro-Marrero, J.; López-Rico, I.; Domingo, J.C. Changes of erythrocyte fatty acids after supplementation with highly concentrated docosahexaenoic acid (DHA) in pediatric cystic fibrosis: A randomized double-blind controlled trial. J. Clin. Med. 2023, 12, 3704. [Google Scholar] [CrossRef]
- Brudy Technology SL. Use of DHA for Treating a Pathology Associated with Cellular Oxidative Damage. European Patent EP 1962825 B1, 2 April 2014. [Google Scholar]
- Gasso, F.; Bogdanov, P.; Domingo, J.C. Docosahexaenoic acid improves endogenous antioxidant defense in ARPE-19 cells. Investig. Ophthalmol. Vis. Sci. 2008, 49, 5932. [Google Scholar]
- Lloyd-Still, J.D.; Powers, C.A.; Hoffman, D.R.; Boyd-Trull, K.; Lester, L.A.; Benisek, D.C.; Arterburn, L.-M. Bioavailability and safety of a high dose of docosahexaenoic acid triacylglycerol of algal origin in cystic fibrosis patients: A randomized, controlled study. Nutrition 2006, 22, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Pastor, Ó.; Guzmán-Lafuente, P.; Serna, J.; Muñoz-Hernández, M.; López Neyra, A.; García-Rozas, P.; García-Seisdedos, D.; Alcázar, A.; Lasunción, M.A.; Busto, R.; et al. A comprehensive evaluation of omega-3 fatty acid supplementation in cystic fibrosis patients using lipidomics. J. Nutr. Biochem. 2019, 63, 197–205. [Google Scholar] [CrossRef]
- López-Neyra, A.; Suárez, L.; Muñoz, M.; de Blas, A.; Ruiz de Valbuena, M.; Garriga, M.; Calvo, J.; Ribes, C.; Girón Moreno, R.; Máiz, L.; et al. Long-term docosahexaenoic acid (DHA) supplementation in cystic fibrosis patients: A randomized, multi-center, double-blind, placebo-controlled trial. Prostaglandins Leukot. Essent. Fatty Acids 2020, 162, 102186. [Google Scholar] [CrossRef]
- Belda, J. Sputum induction for the diagnosis and monitoring of respiratory disease. Arch. Bronconeumol. 2001, 37, 271–273. [Google Scholar] [CrossRef]
- Belda, J.; Giner, J.; Torrejón, M.; Casan, P. Procedimiento para la inducción del esputo para el estudio de la inflamación de las vías aéreas. In Manual SEPAR de Procedimientos. Pruebas Para el Estudio de la Inflación de las Vías Aéreas; Permanyer: Barcelona, Spain, 2004; pp. 11–12. [Google Scholar]
- Graham, B.L.; Steenbruggen, I.; Miller, M.R.; Barjaktarevic, I.Z.; Cooper, B.G.; Hall, G.L.; Hallstrand, T.S.; Kaminsky, D.A.; McCarthy, K.; McCormack, M.C.; et al. Standardization of Spirometry 2019 Update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am. J. Respir. Crit. Care Med. 2019, 200, e70–e88. [Google Scholar] [CrossRef] [PubMed]
- García-Río, F.; Calle, M.; Burgos, F.; Casan, P.; Del Campo, F.; Galdiz, J.B.; Giner, J.; González-Mangado, N.; Ortega, F.; Puente Maestu, L.; et al. Spirometry. Spanish Society of Pulmonology and Thoracic Surgery (SEPAR). Arch. Bronconeumol. 2013, 49, 388–401. [Google Scholar] [CrossRef] [PubMed]
- Roca, J.; Sanchis, J.; Agusti-Vidal, A.; Segarra, F.; Navajas, D.; Rodriguez-Roisin, R.; Casan, P.; Sans, S. Spirometric reference values from a Mediterranean population. Bull. Eur. Physiopathol. Respir. 1986, 22, 217–224. [Google Scholar]
- Sagel, S.D.; Wagner, B.D.; Anthony, M.M.; Emmett, P.; Zemanick, E.T. Sputum biomarkers of inflammation and lung function decline in children with cystic fibrosis. Am. J. Respir. Crit. Care Med. 2012, 186, 857–865. [Google Scholar] [CrossRef]
- Sohouli, M.H.; Magalhães, E.I.D.S.; Ghahramani, S.; Nasresfahani, M.; Ezoddin, N.; Sharifi, P.; Rohani, P. Impact of omega-3 supplementation on children and adolescents patients with cystic fibrosis: A systematic review and meta-analysis of randomized-controlled trials. Pediatr. Pulmonol. 2023, 58, 2219–2228. [Google Scholar] [CrossRef]
- Leggieri, E.; De Biase, R.V.; Savi, D.; Zullo, S.; Halili, I.; Quattrucci, S. Clinical effects of diet supplementation with DHA in pediatric patients suffering from cystic fibrosis. Minerva Pediatr. 2013, 65, 389–398. [Google Scholar]
- Lawrence, R.; Sorrell, T. Eicosapentaenoic acid in cystic fibrosis: Evidence of a pathogenetic role for leukotriene B4. Lancet 1993, 342, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Alicandro, G.; Faelli, N.; Gagliardini, R.; Santini, B.; Magazzù, G.; Biffi, A.; Risé, P.; Galli, C.; Tirelli, A.S.; Loi, S.; et al. A randomized placebo-controlled study on high-dose oral algal docosahexaenoic acid supplementation in children with cystic fibrosis. Prostaglandins Leukot. Essent. Fatty Acids 2013, 88, 163–169. [Google Scholar] [CrossRef]
- Van Biervliet, S.; Devos, M.; Delhaye, T.; Van Biervliet, J.P.; Robberecht, E.; Christophe, A. Oral DHA supplementation in DeltaF508 homozygous cystic fibrosis patients. Prostaglandins Leukot. Essent. Fatty Acids 2008, 78, 109–115. [Google Scholar] [CrossRef]
- Laval, J.; Ralhan, A.; Hartl, D. Neutrophils in cystic fibrosis. Biol. Chem. 2016, 397, 485–496. [Google Scholar] [CrossRef]
- Teopompi, E.; Risé, P.; Pisi, R.; Buccellati, C.; Aiello, M.; Pisi, G.; Tripodi, C.; Fainardi, V.; Clini, E.; Chetta, A.; et al. Arachidonic acid and docosahexaenoic acid metabolites in the airways of adults with cystic fibrosis: Effect of docosahexaenoic acid supplementation. Front. Pharmacol. 2019, 10, 938. [Google Scholar] [CrossRef]
- Eickmeier, O.; Fussbroich, D.; Mueller, K.; Serve, F.; Smaczny, C.; Zielen, S.; Schubert, R. Pro-resolving lipid mediator resolvin D1 serves as a marker of lung disease in cystic fibrosis. PLoS ONE 2017, 12, e0171249. [Google Scholar] [CrossRef] [PubMed]
- Briottet, M.; Shum, M.; Urbach, V. The role of specialized pro-resolving mediators in cystic fibrosis airways disease. Front. Pharmacol. 2020, 11, 1290. [Google Scholar] [CrossRef] [PubMed]
- Recchiuti, A.; Mattoscio, D.; Isopi, E. Roles, actions, and therapeutic potential of specialized pro-resolving lipid mediators for the treatment of inflammation in cystic fibrosis. Front. Pharmacol. 2019, 10, 252. [Google Scholar] [CrossRef] [PubMed]
- Tabary, O.; Zahm, J.M.; Hinnrasky, J.; Couetil, J.P.; Cornillet, P.; Guenounou, M.; Gaillard, D.; Puchelle, E.; Jacquot, J. Selective up-regulation of chemokine IL-8 expression in cystic fibrosis bronchial gland cells in vivo and in vitro. Am. J. Pathol. 1998, 153, 921–930. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Hou, Y.; Sun, F.; Yang, Z.; Li, C. Dysregulated chemokine signaling in cystic fibrosis lung disease: A potential therapeutic target. Curr. Drug Targets 2016, 17, 1535–1544. [Google Scholar] [CrossRef] [PubMed]
- Karpati, F.; Hjelte, F.L.; Wretlind, B. TNF-alpha and IL-8 in consecutive sputum samples from cystic fibrosis patients during antibiotic treatment. Scand. J. Infect. Dis. 2000, 32, 75–79. [Google Scholar] [PubMed]
- Duvall, M.G.; Levy, B.D. DHA- and EPA-derived resolvins, protectins, and maresins in airway inflammation. Eur. J. Pharmacol. 2016, 785, 144–155. [Google Scholar] [CrossRef]
- Serhan, C.N.; Krishnamoorthy, S.; Recchiuti, A.; Chiang, N. Novel anti-inflammatory--pro-resolving mediators and their receptors. Curr. Top. Med. Chem. 2011, 11, 629–647. [Google Scholar] [CrossRef]
- Levy, B.D.; Serhan, C.N. Resolution of acute inflammation in the lung. Annu. Rev. Physiol. 2014, 76, 467–492. [Google Scholar] [CrossRef] [PubMed]



| Weight, kg | Dose, mg | Number of Capsules | Daily Intakes * |
|---|---|---|---|
| ≥13 to 17 | 700 | 2 | One or two |
| ≥18 to 24 | 1050 | 3 | One or two |
| ≥25 to 30 | 1400 | 4 | Two (2 capsules–2 capsules) |
| ≥31 to 36 | 1750 | 5 | Two (2 capsules–3 capsules) |
| ≥37 to 43 | 2100 | 6 | Two (3 capsules–3 capsules) |
| ≥44 to 49 | 2450 | 7 | Three (3 capsules–1 capsule–3 capsules) |
| ≥50 | 2800 | 8 | Three (3 capsules–2 capsule–3 capsules) |
| Variables | Patients with Cystic Fibrosis (n = 22) | ||
|---|---|---|---|
| DHA Group (n = 11) | Placebo Group (n = 11) | p Value | |
| Gender, male, n (%) | 7 (63.6) | 3 (23.3) | 0.160 |
| Age, years, mean (SD) | 10.9 (3.2) | 12.5 (3.8) | 0.381 |
| Weight, kg, mean (SD) | 39.7 (17.4) | 43.4 (14.6) | 0.803 |
| Height, cm, mean (SD) | 145.2 (20.2) | 149.3 (19.0) | 0.743 |
| Body mass index, kg/m2, mean (SD) | 17.9 (2.9) | 19.3 (2.4) | 0.501 |
| Mutations, n (%) * | |||
| Severe/severe | 7 (63.6) | 5 (45.5) | 0.668 |
| Severe/mild | 3 (27.3) | 4 (36.4) | 1.0 |
| Mild/mild | 1 (9.1) | 2 (18.2) | 1.0 |
| Genotype, n (%) | |||
| ΔF508/ΔF508 | 3 (27.3) | 1 (9.1) | 0.580 |
| ΔF508del/other | 5 (45.5) | 7 (63.6) | 0.668 |
| Other/other | 3 (27.3) | 3 (27.3) | 1.0 |
| Pancreatic insufficiency, n (%) | 7 (63.6) | 5 (45.5) | 0.668 |
| Chronic S. aureus infection, n (%) | 3 (27.3) | 2 (18.2) | 1.0 |
| Chronic P. aeruginosa infection, n (%) | 0 | 1 (9.1) | 1.0 |
| FEV1, %, mean (SD) | 92.36 (9.8) | 97.39 (15.5) | 0.373 |
| Diagnosis by neonatal screening, n (%) | 11 (100) | 10 (90.9) | >0.999 |
| Exercise (≥ 3 times/week), n (%) | 8 (72.7) | 6 (54.5) | 0.659 |
| CF-related diabetes, n (%) | 0 | 0 | 1.0 |
| CF-related liver disease, n (%) | 0 | 0 | 1.0 |
| Allergic bronchopulmonary aspergillosis (ABPA), n (%) | 0 | 0 | 1.0 |
| Usual medication, n (%) | |||
| Nebulized hypertonic saline | 7 (63.6) | 9 (81.8) | 0.635 |
| Nebulized dornase alfa | 4 (36.4) | 8 (72.7) | 0.198 |
| Nebulized sodium bicarbonate | 5 (45.5) | 4 (36.4) | >0.999 |
| Azithromycin 3 times a week | 1 (9.1) | 2 (18.2) | >0.999 |
| Inhaled corticosteroids | 4 (36.4) | 6 (54.5) | 0.670 |
| Multivitamins | 11 (100) | 11 (100) | 1.0 |
| Pancreatic enzymes | 7 (63.6) | 5 (45.5) | 0.688 |
| Variables | Baseline | 3 Months | 6 Months | 9 Months | 12 Months | p Value |
|---|---|---|---|---|---|---|
| FVC, L, | ||||||
| Placebo | 2.67 ± 0.99 | 2.60 ± 0.92 | 2.66 ± 0.87 | 2.74 ± 0.93 | 2.76 ± 1.0 | 0.004 |
| DHA | 2.64 ± 0.82 | 2.63 ± 0.76 | 2.94 ± 1.16 | 3.08 ± 1.0 | 3.10 ± 0.96 | |
| FVC, % | ||||||
| Placebo | 98.55 ± 14.69 | 97.88 ± 11.01 | 96.44 ± 8.54 | 96.01 ± 31.86 | 96.84 ± 14.69 | 0.317 |
| DHA | 91.71 ± 10.09 | 87.99 ± 11.96 | 86.55 ± 5.90 | 93.81 ± 6.36 | 94.38 ± 7.44 | |
| FEV1, L | ||||||
| Placebo | 2.29 ± 0.81 | 2.28 ± 0.82 | 2.23 ± 0.71 | 2.28 ± 0.78 | 2.31 ± 0.78 | 0.044 |
| DHA | 2.29 ± 0.64 | 2.25 ± 0.63 | 2.48 ± 1.00 | 2.58 ± 0.72 | 2.61 ± 0.71 | |
| FEV1, % | ||||||
| Placebo | 98.55 ± 16.92 | 95.44 ± 12.63 | 95.61 ± 10.86 | 93.96 ± 6.32 | 94.63 ± 19.96 | 0.429 |
| DHA | 93.28 ± 11.57 | 87.55 ± 7.58 | 89.68 ± 6.46 | 93.96 ± 6.32 | 93.44 ± 8.74 | |
| FEF25-75, L | ||||||
| Placebo | 2.50 ± 0.62 | 2.42 ± 0.84 | 3.07 ± 1.15 | 2.45 ± 1.13 | 2.81 ± 1.22 | 0.476 |
| DHA | 2.66 ± 0.61 | 2.78 ± 0.80 | 3.26 ± 0.94 | 3.23 ± 0.77 | 3.68 ± 2.06 | |
| FEF25-75, % | ||||||
| Placebo | 93.33 ± 22.86 | 86.11 ± 22.84 | 93.33 ± 18.26 | 82.04 ± 25.83 | 85.75 ± 20.53 | 0.493 |
| DHA | 92.71 ± 15.53 | 85.45 ± 12.43 | 91.35 ± 9.70 | 92.04 ± 12.08 | 87.69 ± 17.22 |
| Placebo Group | DHA Group | p Value | |||||
|---|---|---|---|---|---|---|---|
| Baseline | 6 Months | 12 Months | Baseline | 6 Months | 12 Months | ||
| Neutrophils, % | 56.70 ± 24.20 | 72.12 ± 13.09 | 61.62 ± 19.09 | 78.16 ± 6.28 | 74.76 ± 18.90 | 78.13 ± 6.21 | 0.385 |
| Eosinophils, % | 1.62 ± 0.19 | 1.01 ± 0.55 | 1.65 ± 2.10 | 4.50 ± 3.81 | 2.76 ± 1.51 | 3.33 ± 2.75 | 0.679 |
| Lymphocytes, % | 6.90 ± 4.03 | 6.93 ± 5.94 | 24.35 ± 31.45 | 5.53 ± 3.78 | 6.73 ± 1.11 | 3.27 ± 0.86 | 0.342 |
| Macrophages, % | 27.75 ± 24.47 | 15.52 ± 13.25 | 23.20 ± 24.24 | 3.86 ± 1.79 | 13.63 ± 8.86 | 8.97 ± 28.26 | 0.328 |
| Variables | Placebo Group | DHA Group | p Value | ||
|---|---|---|---|---|---|
| Baseline | 12 Months | Baseline | 12 Months | ||
| Sputum samples | |||||
| NE, µg/L | 49.38 ± 53.21 | 79.50 ± 125.82 | 16.80 ± 10.42 | 49.60 ± 61.67 | 0.675 |
| RvD1, pg/mL | 521.29 ± 92.52 | 502.24 ± 73.49 | 481.87 ± 63.14 | 467.34 ± 60.43 | 0.298 |
| IL-8, pg/mL * | 9310.8 (6285.2–14,825.6) | 8285.9 (5796.4–8861.2) | 6449.4 (1719.4–21,146) | 5756.6 (5556.7–7535.8) | 0.248 |
| TNF-α, pg/mL * | 4.03 (3.56–5.21) | 3.69 (3.28–3.85) | 4.09 (3.91–7.45) | 3.71 (3.34–4.68) | 0.699 |
| Serum samples | |||||
| RvD1, pg/mL | 556.80 ± 157.91 | 567.95 ± 147.80 | 483.30 ± 157.91 | 474.86 ± 127.91 | 0.386 |
| IL-8, pg/mL * | 19.25 (5.47–45.01) | 9.78 (3.97–44.84) | 4.85 (3.60–6.40) | 8.64 (3.89–38.19) | 0.068 |
| TNF-α, pg/mL * | 8.37 (7.33–9.47) | 7.18 (6.34–7.62) | 8.42 (7.57–9.24) | 6.88 (6.14–7.38) | 1.0 |
| Fecal samples | |||||
| Fecal calprotectin, µg/g | 66.22 ± 65.94 | 41.78 ± 37.36 | 140.92 ± 102.79 | 96.40 ± 87.69 | 0.293 |
| Variables | Study Period | p Value | ||||
|---|---|---|---|---|---|---|
| Baseline | 3 Months | 6 Months | 9 Months | 12 Months | ||
| Weight, kg | ||||||
| Placebo | 41.55 ± 14.39 | 42.42 ± 14.76 | 43.30 ± 14.69 | 42.81 ± 13.60 | 41.96 ± 11.87 | 0.034 |
| DHA | 40.30 ± 14.26 | 42.51 ± 15.36 | 43.67 ± 16.16 | 44.61 ± 16.42 | 44.83 ± 15.25 | |
| Height, cm | ||||||
| Placebo | 144.78 ± 17.95 | 146.10 ± 17.06 | 146.79 ± 16.58 | 147.83 ± 16.32 | 148.82 ± 15.93 | 0.026 |
| DHA | 148.11 ± 16.38 | 150.64 ± 16.42 | 152.54 ± 16.16 | 153.78 ± 15.68 | 155.50 ± 15.79 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayats-Vidal, R.; Bosque-García, M.; Cordobilla, B.; Asensio-De la Cruz, O.; García-González, M.; Loureda-Pérez, S.; Fernández-López, E.; Robert-Barriocanal, E.; Valiente-Planas, A.; Domingo, J.C. Impact of 1-Year Supplementation with High-Rich Docosahexaenoic Acid (DHA) on Clinical Variables and Inflammatory Biomarkers in Pediatric Cystic Fibrosis: A Randomized Double-Blind Controlled Trial. Nutrients 2024, 16, 970. https://doi.org/10.3390/nu16070970
Ayats-Vidal R, Bosque-García M, Cordobilla B, Asensio-De la Cruz O, García-González M, Loureda-Pérez S, Fernández-López E, Robert-Barriocanal E, Valiente-Planas A, Domingo JC. Impact of 1-Year Supplementation with High-Rich Docosahexaenoic Acid (DHA) on Clinical Variables and Inflammatory Biomarkers in Pediatric Cystic Fibrosis: A Randomized Double-Blind Controlled Trial. Nutrients. 2024; 16(7):970. https://doi.org/10.3390/nu16070970
Chicago/Turabian StyleAyats-Vidal, Roser, Montserrat Bosque-García, Begoña Cordobilla, Oscar Asensio-De la Cruz, Miguel García-González, Susana Loureda-Pérez, Elena Fernández-López, Eva Robert-Barriocanal, Andrea Valiente-Planas, and Joan Carles Domingo. 2024. "Impact of 1-Year Supplementation with High-Rich Docosahexaenoic Acid (DHA) on Clinical Variables and Inflammatory Biomarkers in Pediatric Cystic Fibrosis: A Randomized Double-Blind Controlled Trial" Nutrients 16, no. 7: 970. https://doi.org/10.3390/nu16070970
APA StyleAyats-Vidal, R., Bosque-García, M., Cordobilla, B., Asensio-De la Cruz, O., García-González, M., Loureda-Pérez, S., Fernández-López, E., Robert-Barriocanal, E., Valiente-Planas, A., & Domingo, J. C. (2024). Impact of 1-Year Supplementation with High-Rich Docosahexaenoic Acid (DHA) on Clinical Variables and Inflammatory Biomarkers in Pediatric Cystic Fibrosis: A Randomized Double-Blind Controlled Trial. Nutrients, 16(7), 970. https://doi.org/10.3390/nu16070970