Association of Gut Microbiota-Related Metabolites and Type 2 Diabetes in Two Puerto Rican Cohorts
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Assessment of Plasma Metabolites
2.3. Assessment of Type 2 Diabetes
2.4. Assessment of Cardiometabolic Markers
2.5. Covariate Assessment
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Cardiometabolic Risk Factors
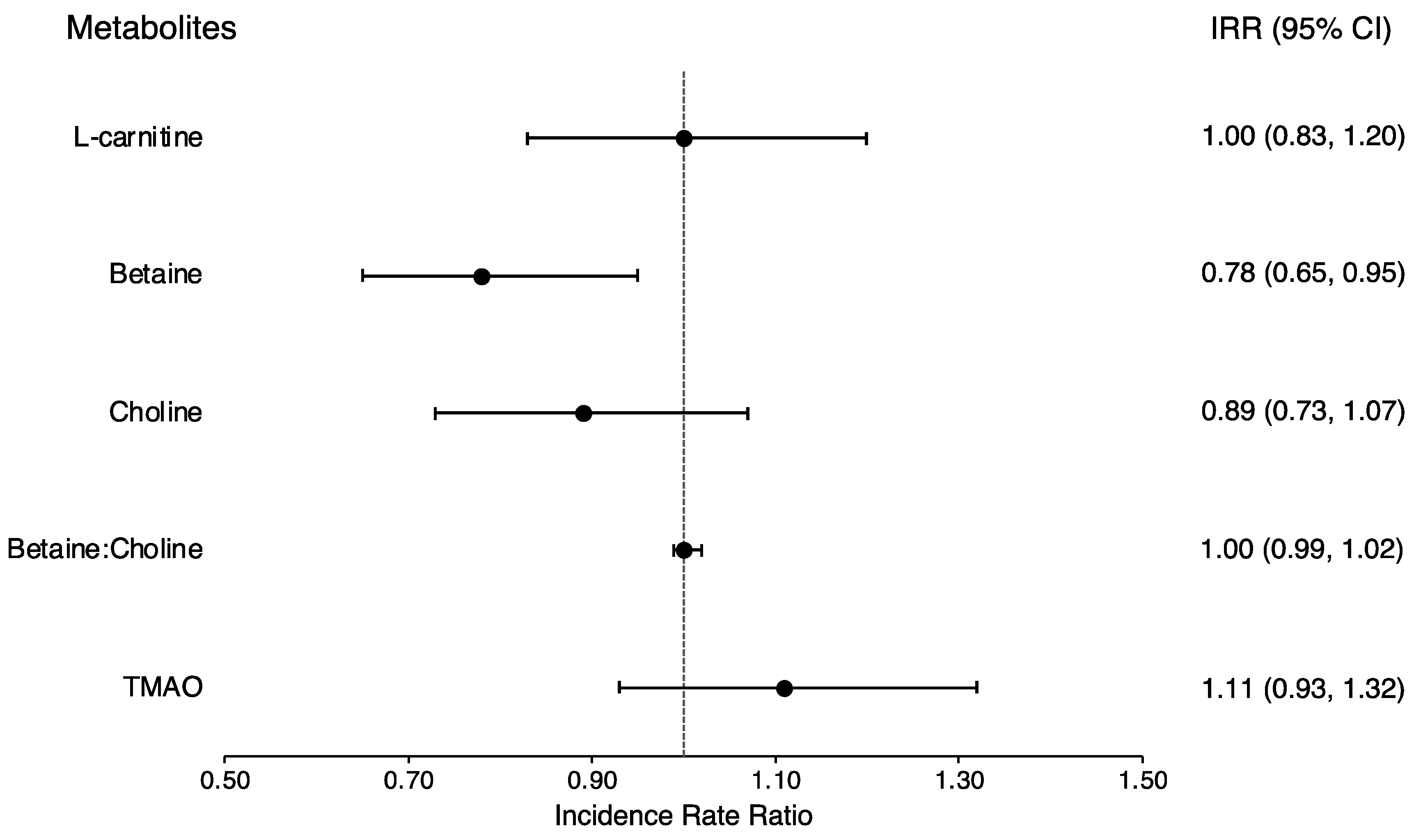
3.3. Prevalent and Incident Type 2 Diabetes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Clemente, J.C.; Ursell, L.K.; Parfrey, L.W.; Knight, R. The Impact of the Gut Microbiota on Human Health: An Integrative View. Cell 2012, 148, 1258–1270. [Google Scholar] [CrossRef]
- Qin, J.; Li, Y.; Cai, Z.; Li, S.; Zhu, J.; Zhang, F.; Liang, S.; Zhang, W.; Guan, Y.; Shen, D.; et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012, 490, 55–60. [Google Scholar] [CrossRef]
- Karlsson, F.H.; Tremaroli, V.; Nookaew, I.; Bergström, G.; Behre, C.J.; Fagerberg, B.; Nielsen, J.; Bäckhed, F. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature 2013, 498, 99–103. [Google Scholar] [CrossRef]
- Sciuto, M.; Catanzaro, R. Composition of gut microbiota and its correlations with neurological, intestinal, cardiovascular and metabolic diseases. Acta Microbiol. Immunol. Hung. 2023, 70, 259–271. [Google Scholar] [CrossRef] [PubMed]
- GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1736–1788. [Google Scholar] [CrossRef] [PubMed]
- GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Sonnenburg, J.L.; Bäckhed, F. Diet–microbiota interactions as moderators of human metabolism. Nature 2016, 535, 56–64. [Google Scholar] [CrossRef]
- Valdes, A.M.; Walter, J.; Segal, E.; Spector, T.D. Role of the gut microbiota in nutrition and health. BMJ 2018, 361, k2179. [Google Scholar] [CrossRef] [PubMed]
- Kasubuchi, M.; Hasegawa, S.; Hiramatsu, T.; Ichimura, A.; Kimura, I. Dietary Gut Microbial Metabolites, Short-chain Fatty Acids, and Host Metabolic Regulation. Nutrients 2015, 7, 2839–2849. [Google Scholar] [CrossRef]
- Knudsen, K.E.B. Microbial degradation of whole-grain complex carbohydrates and impact on short-chain fatty acids and health. Adv. Nutr. 2015, 6, 206–213. [Google Scholar] [CrossRef]
- De Vos, W.M.; Tilg, H.; Van Hul, M.; Cani, P.D. Gut microbiome and health: Mechanistic insights. Gut 2022, 71, 1020–1032. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; DuGar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.-M.; et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011, 472, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef]
- Tang, W.H.W.; Wang, Z.; Levison, B.S.; Koeth, R.A.; Britt, E.B.; Fu, X.; Wu, Y.; Hazen, S.L. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N. Engl. J. Med. 2013, 368, 1575–1584. [Google Scholar] [CrossRef]
- Gomez, S.; Blumer, V.; Rodriguez, F. Unique Cardiovascular Disease Risk Factors in Hispanic Individuals. Curr. Cardiovasc. Risk Rep. 2022, 16, 53–61. [Google Scholar] [CrossRef]
- US Department of Health and Human Services. Hispanic/Latino Health|Office of Minority Health. HHS.gov. 2024. Available online: https://minorityhealth.hhs.gov/hispaniclatino-health (accessed on 9 January 2024).
- National Diabetes Statistics Report|Diabetes|CDC. 2023. Available online: https://www.cdc.gov/diabetes/data/statistics-report/index.html (accessed on 23 February 2024).
- Daviglus, M.L.; Talavera, G.A.; Avilés-Santa, M.L.; Allison, M.; Cai, J.; Criqui, M.H.; Gellman, M.; Giachello, A.L.; Gouskova, N.; Kaplan, R.C.; et al. Prevalence of Major Cardiovascular Risk Factors and Cardiovascular Diseases among Hispanic/Latino Individuals of Diverse Backgrounds in the United States. JAMA 2012, 308, 1775–1784. [Google Scholar] [CrossRef]
- Koyama, A.K.; Bullard, K.M.; Onufrak, S.; Xu, F.; Saelee, R.; Miyamoto, Y.; Pavkov, M.E. Risk Factors Amenable to Primary Prevention of Type 2 Diabetes Among Disaggregated Racial and Ethnic Subgroups in the U.S. Diabetes Care 2023, 46, 2112–2119. [Google Scholar] [CrossRef] [PubMed]
- Tucker, K.L.; Mattei, J.; Noel, S.E.; Collado, B.M.; Mendez, J.; Nelson, J.; Griffith, J.; Ordovas, J.M.; Falcon, L.M. The Boston Puerto Rican Health Study, a longitudinal cohort study on health disparities in Puerto Rican adults: Challenges and opportunities. BMC Public Health 2010, 10, 107. [Google Scholar] [CrossRef]
- Pérez, C.M.; Muñoz, F.; Andriankaja, O.M.; Ritchie, C.S.; Martínez, S.; Vergara, J.; Vivaldi, J.; López, L.; Campos, M.; Joshipura, K.J. Cross-sectional associations of impaired glucose metabolism measures with bleeding on probing and periodontitis. J. Clin. Periodontol. 2017, 44, 142–149. [Google Scholar] [CrossRef]
- Evans, A.M.; DeHaven, C.D.; Barrett, T.; Mitchell, M.; Milgram, E. Integrated, Nontargeted Ultrahigh Performance Liquid Chromatography/Electrospray Ionization Tandem Mass Spectrometry Platform for the Identification and Relative Quantification of the Small-Molecule Complement of Biological Systems. Anal. Chem. 2009, 81, 6656–6667. [Google Scholar] [CrossRef]
- Gao, X.; Randell, E.; Tian, Y.; Zhou, H.; Sun, G. Low serum choline and high serum betaine levels are associated with favorable components of metabolic syndrome in Newfoundland population. J. Diabetes Complicat. 2019, 33, 107398. [Google Scholar] [CrossRef]
- Konstantinova, S.V.; Tell, G.S.; Vollset, S.E.; Nygård, O.; Bleie, Ø.; Ueland, P.M. Divergent associations of plasma choline and betaine with components of metabolic syndrome in middle age and elderly men and women. J. Nutr. 2008, 138, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Bhupathiraju, S.N.; Lichtenstein, A.H.; Dawson-Hughes, B.; Tucker, K.L. Adherence Index Based on the AHA 2006 Diet and Lifestyle Recommendations Is Associated with Select Cardiovascular Disease Risk Factors in Older Puerto Ricans. J. Nutr. 2011, 141, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Tucker, K.L.; Bianchi, L.A.; Maras, J.; Bermudez, O.I. Adaptation of a food frequency questionnaire to assess diets of Puerto Rican and non-Hispanic adults. Am. J. Epidemiol. 1998, 148, 507–518. [Google Scholar] [CrossRef]
- Balduzzi, S.; Rücker, G.; Schwarzer, G. How to perform a meta-analysis with R: A practical tutorial. Evid. Based Ment. Health 2019, 22, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Tang, W.H.W.; Buffa, J.A.; Fu, X.; Britt, E.B.; Koeth, R.A.; Levison, B.S.; Fan, Y.; Wu, Y.; Hazen, S.L. Prognostic value of choline and betaine depends on intestinal microbiota-generated metabolite trimethylamine-N-oxide. Eur. Heart J. 2014, 35, 904–910. [Google Scholar] [CrossRef]
- Lever, M.; George, P.M.; Slow, S.; Bellamy, D.; Young, J.M.; Ho, M.; McEntyre, C.J.; Elmslie, J.L.; Atkinson, W.; Molyneux, S.L.; et al. Betaine and Trimethylamine-N-Oxide as Predictors of Cardiovascular Outcomes Show Different Patterns in Diabetes Mellitus: An Observational Study. PLoS ONE 2014, 9, e114969. [Google Scholar] [CrossRef]
- Senthong, V.; Li, X.S.; Hudec, T.; Coughlin, J.; Wu, Y.; Levison, B.; Wang, Z.; Hazen, S.L.; Tang, W.W. Plasma Trimethylamine N-Oxide, a Gut Microbe–Generated Phosphatidylcholine Metabolite, Is Associated with Atherosclerotic Burden. J. Am. Coll. Cardiol. 2016, 67, 2620–2628. [Google Scholar] [CrossRef]
- Brown, J.M.; Hazen, S.L. Microbial modulation of cardiovascular disease. Nat. Rev. Microbiol. 2018, 16, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Nagata, C.; Wada, K.; Tamura, T.; Konishi, K.; Kawachi, T.; Tsuji, M.; Nakamura, K. Choline and Betaine Intakes Are Not Associated with Cardiovascular Disease Mortality Risk in Japanese Men and Women. J. Nutr. 2015, 145, 1787–1792. [Google Scholar] [CrossRef] [PubMed]
- Meyer, K.A.; Shea, J.W. Dietary Choline and Betaine and Risk of CVD: A Systematic Review and Meta-Analysis of Prospective Studies. Nutrients 2017, 9, 711. [Google Scholar] [CrossRef] [PubMed]
- Lever, M.; George, P.M.; Elmslie, J.L.; Atkinson, W.; Slow, S.; Molyneux, S.L.; Troughton, R.W.; Richards, A.M.; Frampton, C.M.; Chambers, S.T. Betaine and secondary events in an acute coronary syndrome cohort. PLoS ONE 2012, 7, e37883. [Google Scholar] [CrossRef]
- Lemaitre, R.N.; Jensen, P.N.; Wang, Z.; Fretts, A.M.; McKnight, B.; Nemet, I.; Biggs, M.L.; Sotoodehnia, N.; Otto, M.C.d.O.; Psaty, B.M.; et al. Association of Trimethylamine N-Oxide and Related Metabolites in Plasma and Incident Type 2 Diabetes: The Cardiovascular Health Study. JAMA Netw. Open 2021, 4, e2122844. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Wang, Y.; Sun, G. High dietary choline and betaine intake is associated with low insulin resistance in the Newfoundland population. Nutrition 2017, 33, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.; Wong, E.Y.; Tran, H.N.; Tran, R.J.C.; Cao, D.X. The glycemic, cholesterol, and weight effects of L-carnitine in diabetes: A systematic review and meta-analysis of randomized controlled trials. Diabetes Epidemiol. Manag. 2023, 10, 100122. [Google Scholar] [CrossRef]
- Bene, J.; Hadzsiev, K.; Melegh, B. Role of carnitine and its derivatives in the development and management of type 2 diabetes. Nutr. Diabetes 2018, 8, 8. [Google Scholar] [CrossRef]
- Karalis, D.T.; Karalis, T.; Karalis, S.; Kleisiari, A.S. L-Carnitine as a Diet Supplement in Patients with Type II Diabetes. Cureus 2020, 12, e7982. [Google Scholar] [CrossRef]
- Dambrova, M.; Latkovskis, G.; Kuka, J.; Strele, I.; Konrade, I.; Grinberga, S.; Hartmane, D.; Pugovics, O.; Erglis, A.; Liepinsh, E. Diabetes is Associated with Higher Trimethylamine N-oxide Plasma Levels. Exp. Clin. Endocrinol. Diabetes Off. J. Ger. Soc. Endocrinol. Ger. Diabetes Assoc. 2016, 124, 251–256. [Google Scholar] [CrossRef]
- Zhuang, R.; Ge, X.; Han, L.; Yu, P.; Gong, X.; Meng, Q.; Zhang, Y.; Fan, H.; Zheng, L.; Liu, Z.; et al. Gut microbe–generated metabolite trimethylamine N-oxide and the risk of diabetes: A systematic review and dose-response meta-analysis. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2019, 20, 883–894. [Google Scholar] [CrossRef] [PubMed]
- Shan, Z.; Sun, T.; Huang, H.; Chen, S.; Chen, L.; Luo, C.; Yang, W.; Yang, X.; Yao, P.; Cheng, J.; et al. Association between microbiota-dependent metabolite trimethylamine-N-oxide and type 2 diabetes. Am. J. Clin. Nutr. 2017, 106, 888–894. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-Y.; Chen, S.; Lu, X.-T.; Fang, A.-P.; Chen, Y.-M.; Huang, R.-Z.; Lin, X.-L.; Huang, Z.-H.; Ma, J.-F.; Huang, B.-X.; et al. Serum trimethylamine-N-oxide is associated with incident type 2 diabetes in middle-aged and older adults: A prospective cohort study. J. Transl. Med. 2022, 20, 374. [Google Scholar] [CrossRef] [PubMed]
- Papandreou, C.; Bulló, M.; Zheng, Y.; Ruiz-Canela, M.; Yu, E.; Guasch-Ferré, M.; Toledo, E.; Clish, C.; Corella, D.; Estruch, R.; et al. Plasma trimethylamine-N-oxide and related metabolites are associated with type 2 diabetes risk in the Prevención con Dieta Mediterránea (PREDIMED) trial. Am. J. Clin. Nutr. 2018, 108, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Svingen, G.F.; Schartum-Hansen, H.; Pedersen, E.R.; Ueland, P.M.; Tell, G.S.; Mellgren, G.; Njølstad, P.R.; Seifert, R.; Strand, E.; Karlsson, T.; et al. Prospective Associations of Systemic and Urinary Choline Metabolites with Incident Type 2 Diabetes. Clin. Chem. 2016, 62, 755–765. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Yuzefpolskaya, M.; Nandakumar, R.; Colombo, P.C.; Demmer, R.T. Plasma Trimethylamine-N-oxide and impaired glucose regulation: Results from The Oral Infections, Glucose Intolerance and Insulin Resistance Study (ORIGINS). PLoS ONE 2020, 15, e0227482. [Google Scholar] [CrossRef] [PubMed]
- McEntyre, C.J.; Lever, M.; Chambers, S.T.; George, P.M.; Slow, S.; Elmslie, J.L.; Florkowski, C.M.; Lunt, H.; Krebs, J.D. Variation of betaine, N,N-dimethylglycine, choline, glycerophosphorylcholine, taurine and trimethylamine-N-oxide in the plasma and urine of overweight people with type 2 diabetes over a two-year period. Ann. Clin. Biochem. 2015, 52 Pt 3, 352–360. [Google Scholar] [CrossRef]
- Garcia, E.; Osté, M.C.J.; Bennett, D.W.; Jeyarajah, E.J.; Shalaurova, I.; Gruppen, E.G.; Hazen, S.L.; Otvos, J.D.; Bakker, S.J.L.; Dullaart, R.P.; et al. High Betaine, a Trimethylamine N-Oxide Related Metabolite, Is Prospectively Associated with Low Future Risk of Type 2 Diabetes Mellitus in the PREVEND Study. J. Clin. Med. 2019, 8, 1813. [Google Scholar] [CrossRef]
- Morze, J.; Wittenbecher, C.; Schwingshackl, L.; Danielewicz, A.; Rynkiewicz, A.; Hu, F.B.; Guasch-Ferré, M. Metabolomics and Type 2 Diabetes Risk: An Updated Systematic Review and Meta-analysis of Prospective Cohort Studies. Diabetes Care 2022, 45, 1013–1024. [Google Scholar] [CrossRef]
- Guo, F.; Qiu, X.; Zhu, Y.; Tan, Z.; Li, Z.; Ouyang, D. Association between plasma betaine levels and dysglycemia in patients with coronary artery disease. Biosci. Rep. 2020, 40, BSR20200676. [Google Scholar] [CrossRef]
- Lever, M.; Slow, S. The clinical significance of betaine, an osmolyte with a key role in methyl group metabolism. Clin. Biochem. 2010, 43, 732–744. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Zhang, P.; Luo, J.; Shen, L.; Zhang, S.; Gu, H.; He, J.; Wang, L.; Zhao, X.; Gan, M.; et al. Dietary betaine prevents obesity through gut microbiota-drived microRNA-378a family. Gut Microbes 2021, 13, 1862612. [Google Scholar] [CrossRef] [PubMed]
- Ejaz, A.; Martinez-Guino, L.; Goldfine, A.B.; Ribas-Aulinas, F.; De Nigris, V.; Ribó, S.; Gonzalez-Franquesa, A.; Garcia-Roves, P.M.; Li, E.; Dreyfuss, J.M.; et al. Dietary Betaine Supplementation Increases Fgf21 Levels to Improve Glucose Homeostasis and Reduce Hepatic Lipid Accumulation in Mice. Diabetes 2016, 65, 902–912. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Shen, L.; Tan, Z.; Zhang, P.; Zhao, X.; Xu, Y.; Gan, M.; Yang, Q.; Ma, J.; Jiang, A.; et al. Betaine Supplementation Enhances Lipid Metabolism and Improves Insulin Resistance in Mice Fed a High-Fat Diet. Nutrients 2018, 10, 131. [Google Scholar] [CrossRef] [PubMed]
| BPRHS (n = 670) | SOALS (n = 999) | |
|---|---|---|
| Mean (SD) or n (%) | Mean (SD) or n (%) | |
| Age, years | 57.2 (7.41) | 50.7 (6.77) |
| Female, n (%) | 502 (74.9) | 729 (73.0) |
| Total income, USD | 18,003 (18,221) | - |
| <20,000, n (%) | - | 543 (54.4) |
| 20,000–49,999, n (%) | - | 338 (33.8) |
| ≥50,000, n (%) | - | 118 (11.8) |
| Education, n (%) | ||
| No schooling—7th to 8th grade | 336 (50.2) | 113 (11.3) |
| 9th–12th grade | 237 (35.4) | 439 (43.9) |
| Some college or more | 97 (14.5) | 447 (44.7) |
| Smoking status, n (%) | ||
| Never | 315 (47.0) | 639 (64.0) |
| Past | 204 (30.5) | 179 (17.9) |
| Current | 151 (22.5) | 181 (18.1) |
| Alcohol consumption status, n (%) | ||
| Never | 203 (30.3) | 442 (44.2) |
| Past | 197 (29.4) | 113 (11.3) |
| Current | 270 (40.3) | 444 (44.4) |
| Multivitamin use, n (%) | 134 (20.0) | - |
| Lipid-lowering medication use, n (%) | 295 (44.0) | 85 (8.51) |
| Hypertension medication use, n (%) | 376 (56.1) | 267 (26.73) |
| BMI, kg/m2 | 32.2 (6.67) | 33.3 (6.17) |
| Waist circumference, cm | 102 (14.9) | 106 (13.98) |
| LDL cholesterol, mg/dL | 108 (34.4) | 123 (32.7) |
| HDL cholesterol, mg/dL | 45.2 (12.4) | 48.1 (13.1) |
| Triglycerides a, mg/dL | 162 (112) | 149 (83.7) |
| Glucose, mg/dL | 120 (50.2) | 95.8 (20.2) |
| Hemoglobin A1c, % | 7.00 (1.78) | 5.80 (0.62) |
| HOMA-IR | 6.06 (9.99) | 2.62 (1.83) |
| Insulin a, mcU/mL | 18.8 (26.2) | 10.8 (6.83) |
| C-reactive protein, mg/L | 6.36 (8.83) | 5.92 (6.32) |
| Systolic blood pressure, mmHg | 136 (18.8) | 129 (17.1) |
| Diastolic blood pressure, mmHg | 81.5 (10.7) | 80.9 (9.67) |
| Physical activity score | 31.4 (4.40) | 22.0 (39.7) |
| Alcohol, g/d | 4.05 (15.4) | 2.36 (5.82) |
| AHA diet score | 8.70 (2.04) | - |
| Psychosocial stress score | 23.4 (9.67) | - |
| Language acculturation score | 22.6 (21.2) | - |
| L-Carnitine | Betaine | Choline | Betaine:Choline | TMAO | |
|---|---|---|---|---|---|
| Glycemia | |||||
| HOMA-IR | 0.05 (−0.03; 0.14) | −0.14 (−0.23; −0.05) | −0.01 (−0.11; 0.08) | −0.003 (−0.01; 0.004) | 0.04 (−0.05; 0.13) |
| Insulin, mcU/mL | 0.14 (−0.10; 0.37) | −0.27 (−0.51; −0.03) | 0.01 (−0.24; 0.26) | −0.01 (−0.02; 0.01) | 0.13 (−0.10; 0.36) |
| Glucose, mg/dL | −0.68 (−1.29; −0.07) | −0.97 (−1.59; −0.34) | 0.46 (−0.20; 1.12) | −0.01 (−0.06; 0.03) | 0.83 (0.22; 1.44) |
| HbA1c, % | −0.03 (−0.05; −0.01) | −0.02 (−0.04; −0.01) | 0.01 (−0.01; 0.03) | 0.001 (−0.001; 0.002) | 0.01 (−0.01; 0.03) |
| Dyslipidemia and Inflammation | |||||
| HDL-C, mg/dL | −0.04 (−0.24; 0.16) | 0.08 (−0.13; 0.28) | −0.13 (−0.35; 0.09) | −0.01 (−0.03; 0.002) | −0.16 (−0.36; 0.05) |
| LDL-C, mg/dL | −0.10 (−0.80; 0.60) | 0.58 (−0.12; 1.28) | −0.63 (−1.38; 0.13) | −0.02 (−0.07; 0.04) | −0.44 (−1.15; 0.26) |
| Triglycerides, mg/dL | 1.09 (−0.59; 2.77) | 0.09 (−1.65; 1.82) | 1.40 (−0.42; 3.21) | −0.06 (−0.19; 0.07) | 3.52 (1.83; 5.20) |
| CRP, mg/L | −0.10 (−0.27; 0.08) | 0.04 (−0.14; 0.22) | 0.01 (−0.18; 0.20) | 0.01 (−0.04; 0.06) * | 0.07 (−0.10; 0.25) |
| Anthropometrics | |||||
| Waist, cm | 0.13 (−0.14; 0.41) | −0.02 (−0.29; 0.26) | 0.07 (−0.23; 0.36) | −0.01 (−0.03; 0.01) | 0.12 (−0.15; 0.39) |
| Weight, kg | 0.09 (−0.13; 0.31) | 0.17 (−0.05; 0.39) | −0.04 (−0.27; 0.20) | −0.07 (−0.19; 0.05) * | −0.20 (−0.42; 0.03) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sawicki, C.M.; Pacheco, L.S.; Rivas-Tumanyan, S.; Cao, Z.; Haslam, D.E.; Liang, L.; Tucker, K.L.; Joshipura, K.; Bhupathiraju, S.N. Association of Gut Microbiota-Related Metabolites and Type 2 Diabetes in Two Puerto Rican Cohorts. Nutrients 2024, 16, 959. https://doi.org/10.3390/nu16070959
Sawicki CM, Pacheco LS, Rivas-Tumanyan S, Cao Z, Haslam DE, Liang L, Tucker KL, Joshipura K, Bhupathiraju SN. Association of Gut Microbiota-Related Metabolites and Type 2 Diabetes in Two Puerto Rican Cohorts. Nutrients. 2024; 16(7):959. https://doi.org/10.3390/nu16070959
Chicago/Turabian StyleSawicki, Caleigh M., Lorena S. Pacheco, Sona Rivas-Tumanyan, Zheyi Cao, Danielle E. Haslam, Liming Liang, Katherine L. Tucker, Kaumudi Joshipura, and Shilpa N. Bhupathiraju. 2024. "Association of Gut Microbiota-Related Metabolites and Type 2 Diabetes in Two Puerto Rican Cohorts" Nutrients 16, no. 7: 959. https://doi.org/10.3390/nu16070959
APA StyleSawicki, C. M., Pacheco, L. S., Rivas-Tumanyan, S., Cao, Z., Haslam, D. E., Liang, L., Tucker, K. L., Joshipura, K., & Bhupathiraju, S. N. (2024). Association of Gut Microbiota-Related Metabolites and Type 2 Diabetes in Two Puerto Rican Cohorts. Nutrients, 16(7), 959. https://doi.org/10.3390/nu16070959






