Effects of Soluble Dextrin Fiber from Potato Starch on Body Weight and Associated Gut Dysbiosis Are Evident in Western Diet-Fed Mice but Not in Overweight/Obese Children
Abstract
1. Introduction
2. Materials and Methods
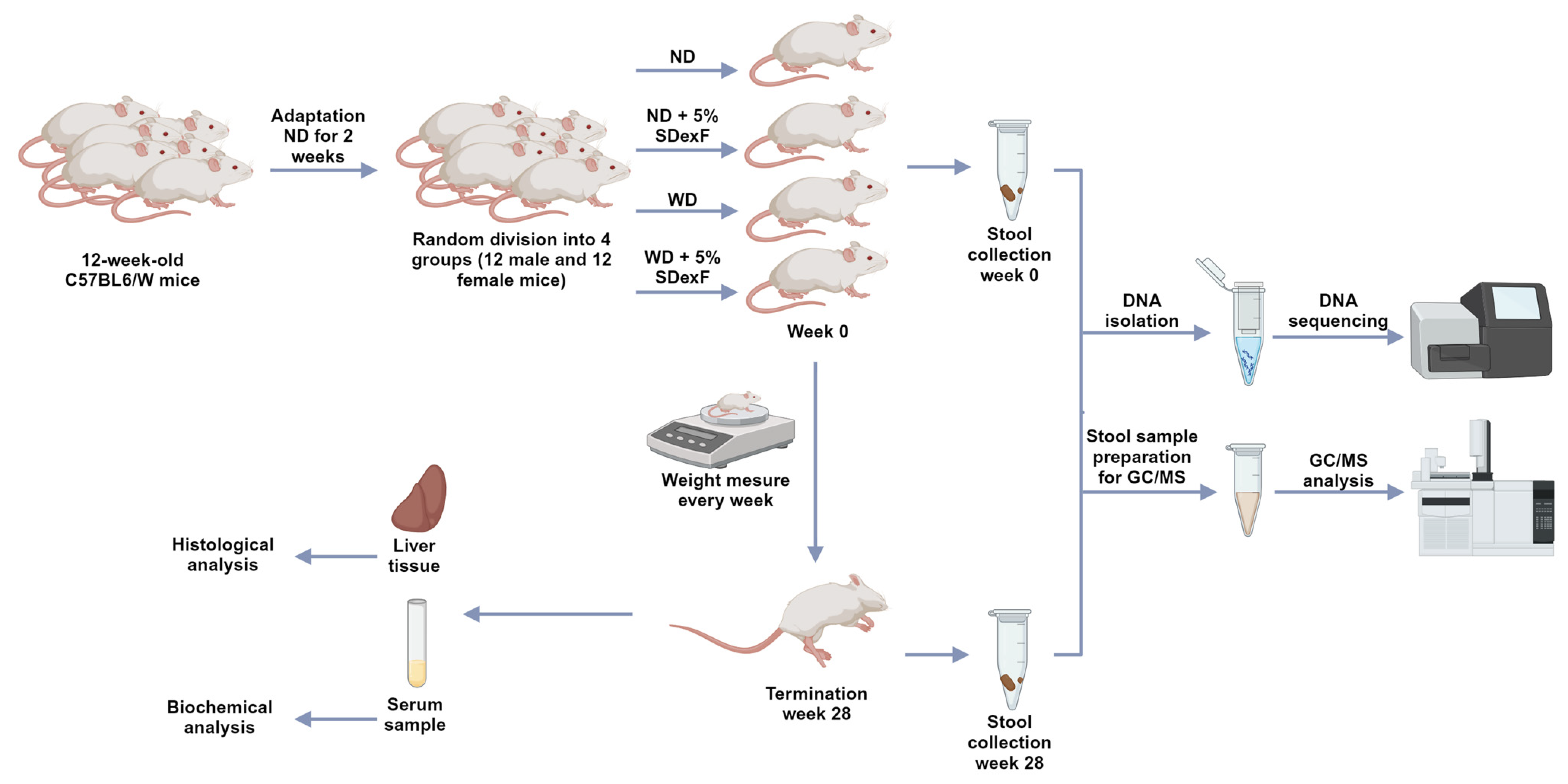
2.1. Animal Study
2.2. Serum Biochemical Measurements
2.3. Histological Analysis
2.4. Human Study
2.5. 16s-rRNA-Seq Metagenomics and Metabolomics Procedures
2.6. Statistical Methods
3. Results
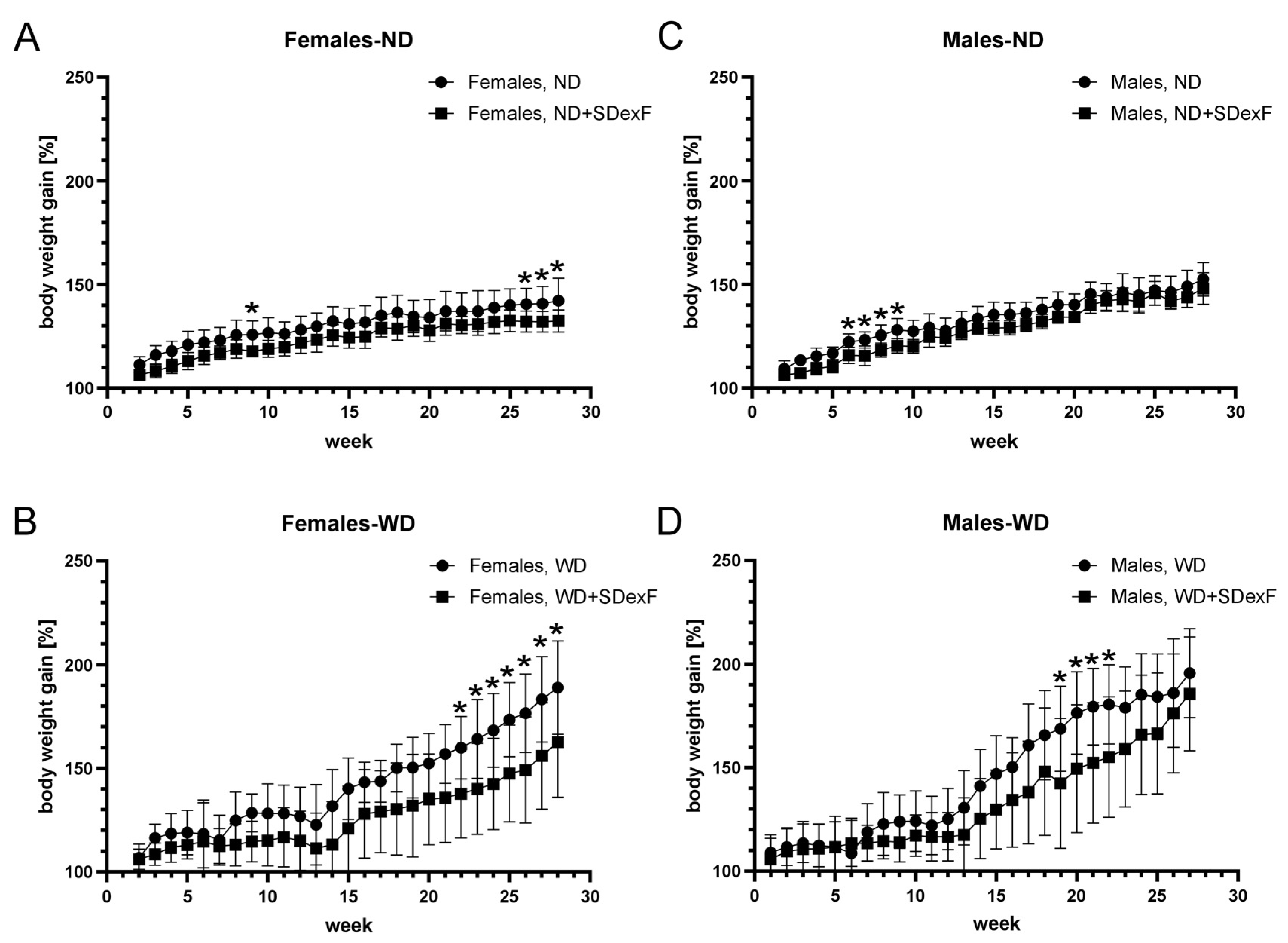
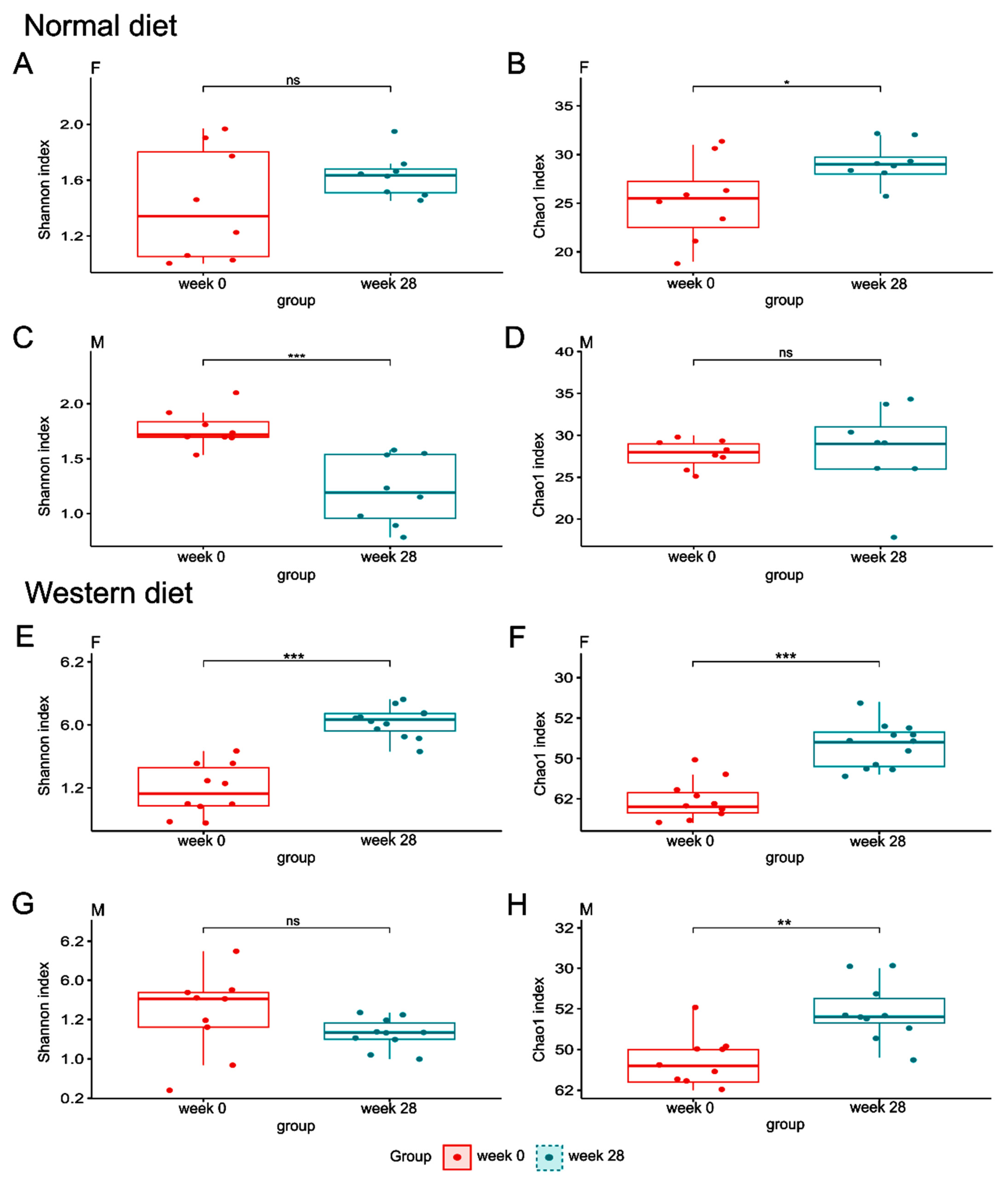
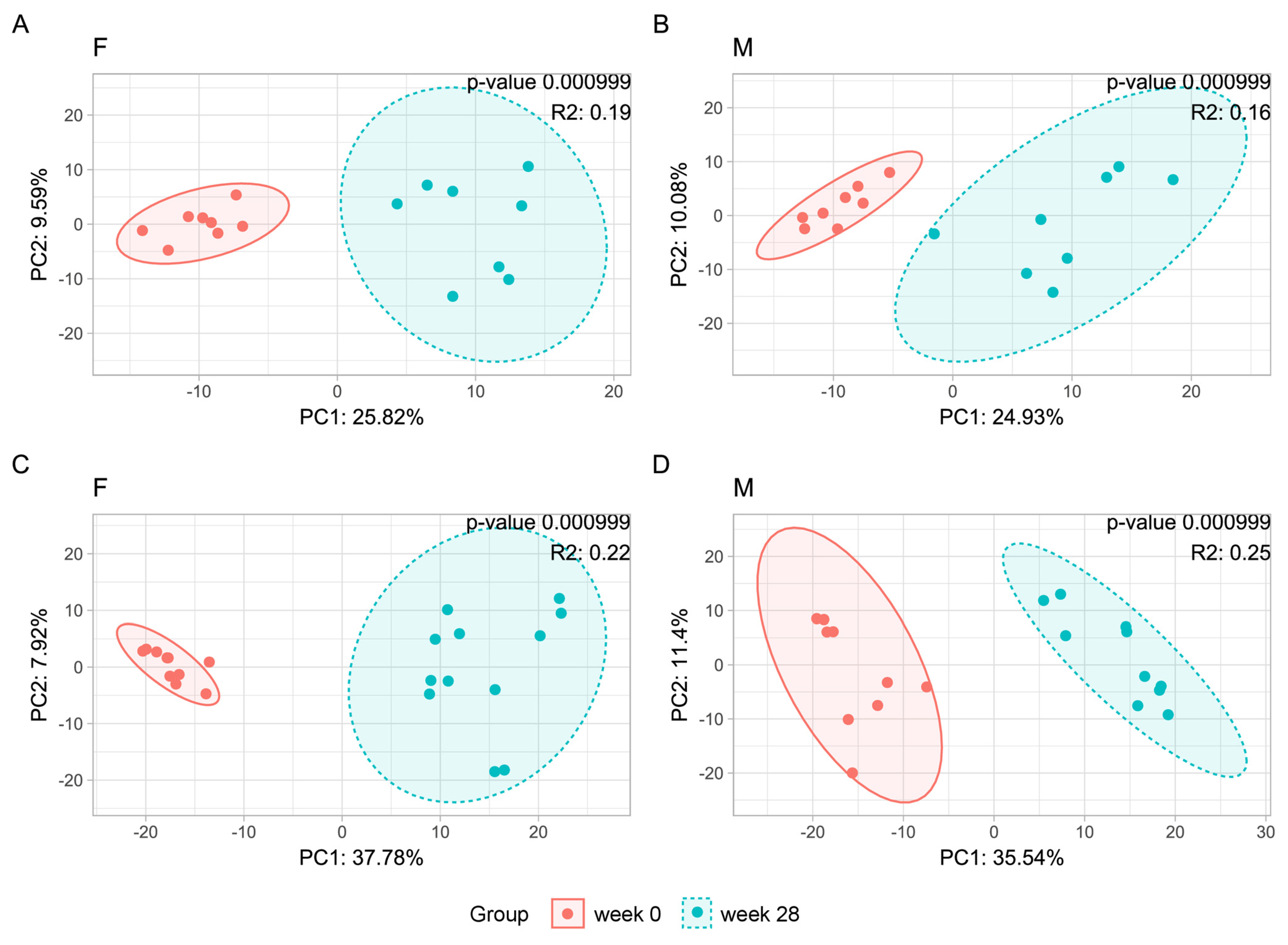
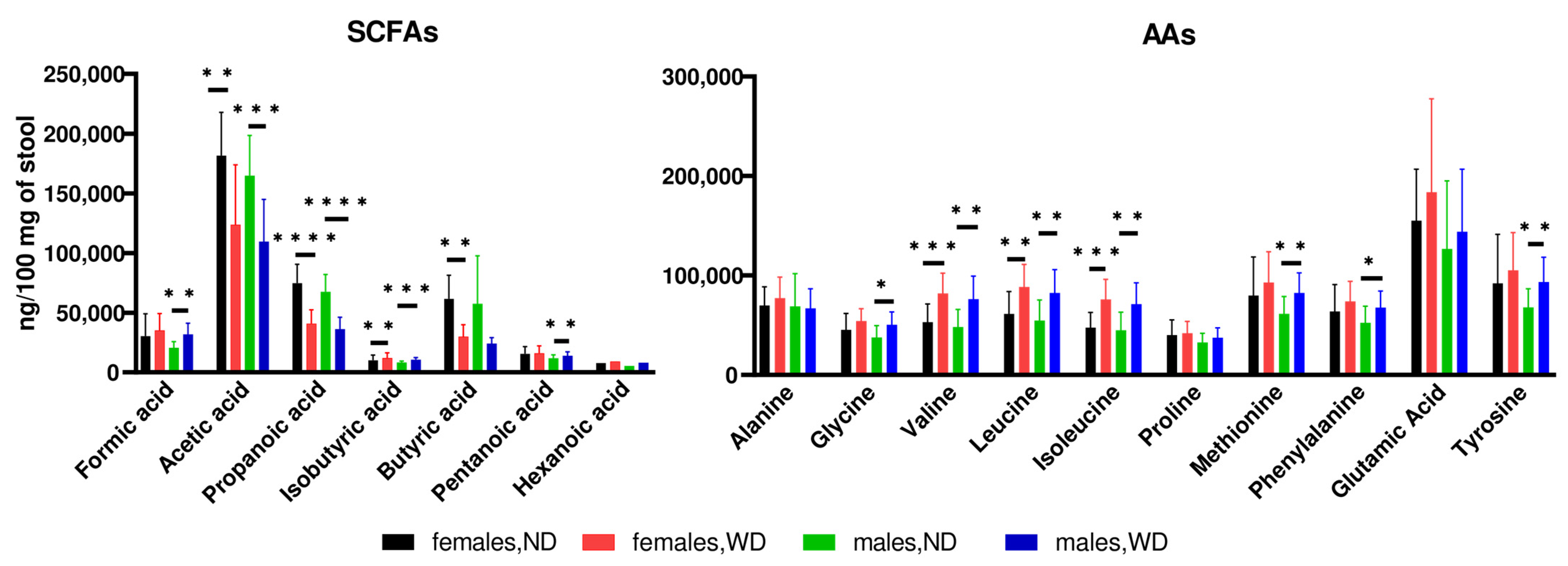
3.1. Animal Study
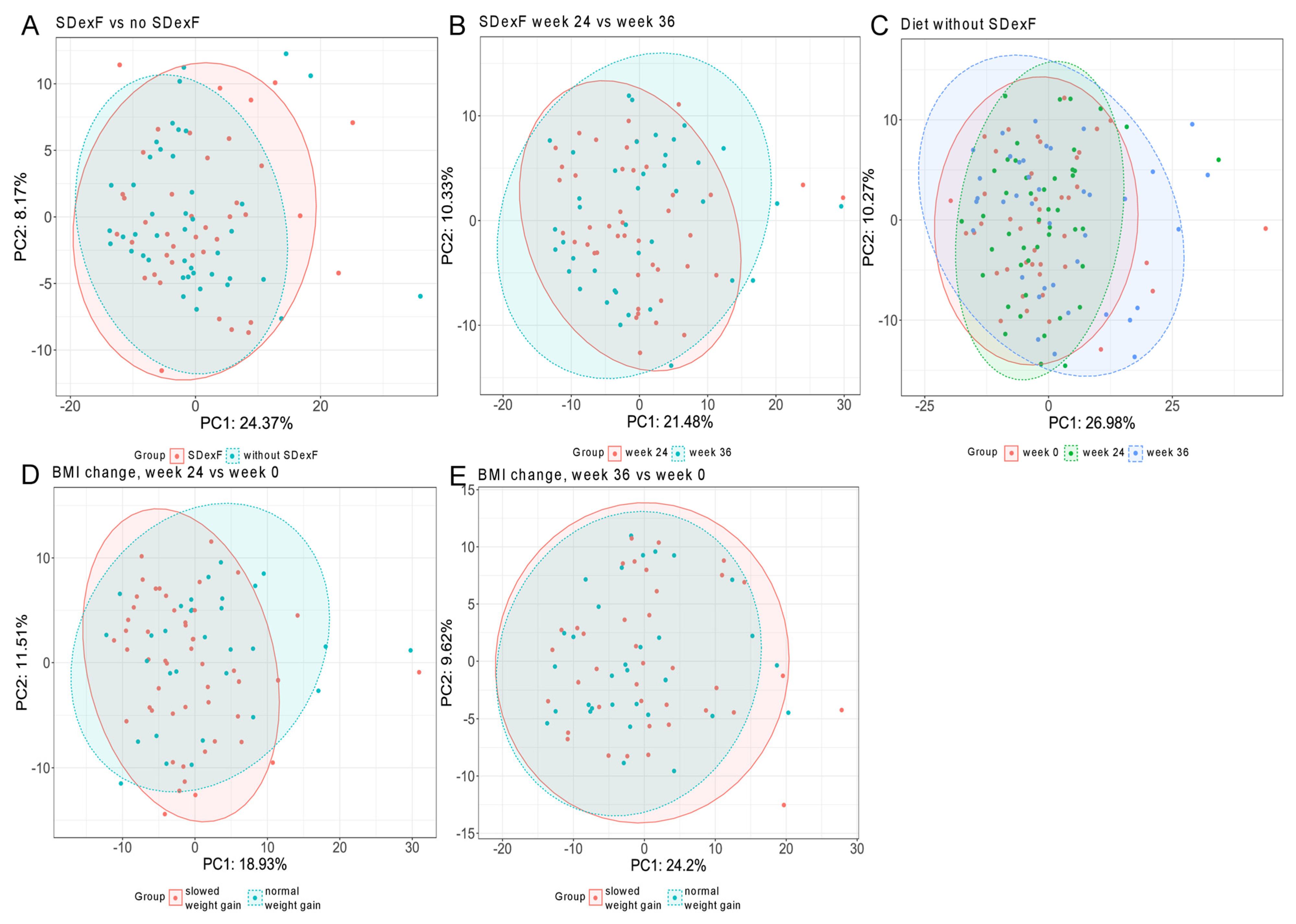
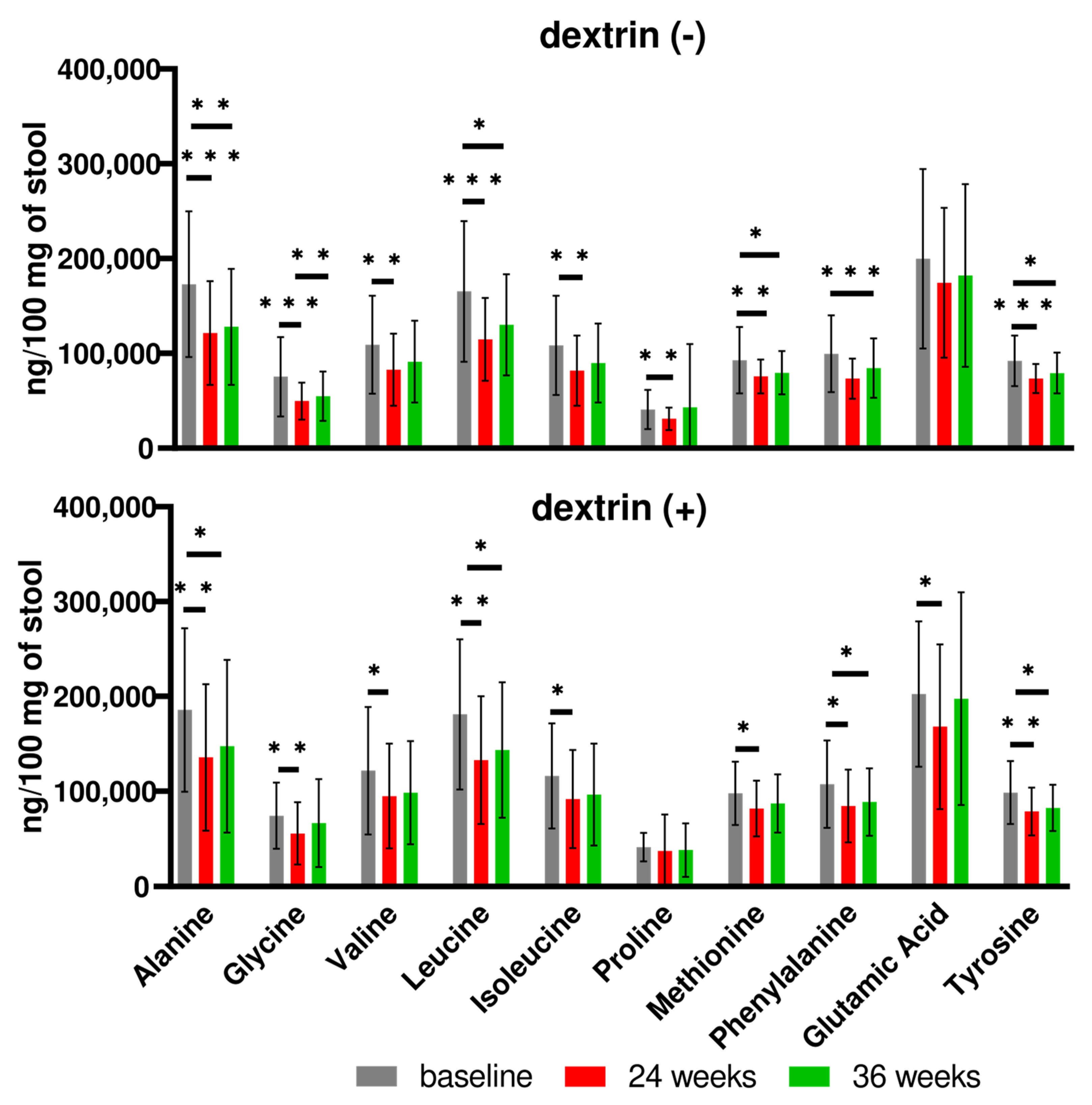
3.2. Human Study
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What Is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.C.; Hoffmann, C.; Mota, J.F. The Human Gut Microbiota: Metabolism and Perspective in Obesity. Gut Microbes 2018, 9, 308–325. [Google Scholar] [CrossRef] [PubMed]
- Heiss, C.N.; Olofsson, L.E. Gut Microbiota-Dependent Modulation of Energy Metabolism. J. Innate Immun. 2018, 10, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 10 July 2023).
- Włodarczyk, M.; Nowicka, G. Obesity, DNA Damage, and Development of Obesity-Related Diseases. Int. J. Mol. Sci. 2019, 20, 1146. [Google Scholar] [CrossRef] [PubMed]
- Rakhra, V.; Galappaththy, S.L.; Bulchandani, S.; Cabandugama, P.K. Obesity and the Western Diet: How We Got Here. Mo. Med. 2020, 117, 536–538. [Google Scholar] [PubMed]
- Kopp, W. How Western Diet And Lifestyle Drive The Pandemic of Obesity And Civilization Diseases. Diabetes Metab. Syndr. Obes. 2019, 12, 2221–2236. [Google Scholar] [CrossRef]
- Cerdó, T.; García-Santos, J.A.; Bermúdez, M.G.; Campoy, C. The Role of Probiotics and Prebiotics in the Prevention and Treatment of Obesity. Nutrients 2019, 11, 635. [Google Scholar] [CrossRef] [PubMed]
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef]
- Ghosh, S.; Pramanik, S. Structural Diversity, Functional Aspects and Future Therapeutic Applications of Human Gut Microbiome. Arch. Microbiol. 2021, 203, 5281–5308. [Google Scholar] [CrossRef]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Human Gut Microbes Associated with Obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef]
- Tsai, Y.-L.; Lin, T.-L.; Chang, C.-J.; Wu, T.-R.; Lai, W.-F.; Lu, C.-C.; Lai, H.-C. Probiotics, Prebiotics and Amelioration of Diseases. J. Biomed. Sci. 2019, 26, 3. [Google Scholar] [CrossRef]
- Brownawell, A.M.; Caers, W.; Gibson, G.R.; Kendall, C.W.C.; Lewis, K.D.; Ringel, Y.; Slavin, J.L. Prebiotics and the Health Benefits of Fiber: Current Regulatory Status, Future Research, and Goals1,2. J. Nutr. 2012, 142, 962–974. [Google Scholar] [CrossRef]
- Jochym, K.; Kapusniak, J.; Barczynska, R.; Sliżewska, K. New Starch Preparations Resistant to Enzymatic Digestion. J. Sci. Food Agric. 2012, 92, 886–891. [Google Scholar] [CrossRef]
- Włodarczyk, M.; Śliżewska, K.; Barczyńska, R.; Kapuśniak, J. Effects of Resistant Dextrin from Potato Starch on the Growth Dynamics of Selected Co-Cultured Strains of Gastrointestinal Bacteria and the Activity of Fecal Enzymes. Nutrients 2022, 14, 2158. [Google Scholar] [CrossRef]
- Barczynska, R.; Kapusniak, J.; Litwin, M.; Slizewska, K.; Szalecki, M. Dextrins from Maize Starch as Substances Activating the Growth of Bacteroidetes and Actinobacteria Simultaneously Inhibiting the Growth of Firmicutes, Responsible for the Occurrence of Obesity. Plant Foods Hum. Nutr. 2016, 71, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Śliżewska, K.; Libudzisz, Z.; Barczyńska, R.; Kapuśniak, J.; Zduńczyk, Z.; Juśkiewicz, J. Dietary Resistant Dextrins Positively Modulate Fecal and Cecal Microbiota Composition in Young Rats. Acta Biochim. Pol. 2015, 62, 677–681. [Google Scholar] [CrossRef] [PubMed]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.-C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and Validation of a Histological Scoring System for Nonalcoholic Fatty Liver Disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef]
- Kapusniak Jochym, K.; Wojcik, M.; Wrobel, K.; Rosicka-Kaczmarek, J.; Kapusniak, J. Assessment of Physicochemical and Thermal Properties of Soluble Dextrin Fiber from Potato Starch for Use in Fruit Mousses. J. Sci. Food Agric. 2021, 101, 4125–4133. [Google Scholar] [CrossRef]
- Kulecka, M.; Paziewska, A.; Zeber-Lubecka, N.; Ambrozkiewicz, F.; Kopczynski, M.; Kuklinska, U.; Pysniak, K.; Gajewska, M.; Mikula, M.; Ostrowski, J. Prolonged Transfer of Feces from the Lean Mice Modulates Gut Microbiota in Obese Mice. Nutr. Metab. 2016, 13, 57. [Google Scholar] [CrossRef] [PubMed]
- Zeber-Lubecka, N.; Kulecka, M.; Ambrozkiewicz, F.; Paziewska, A.; Goryca, K.; Karczmarski, J.; Rubel, T.; Wojtowicz, W.; Mlynarz, P.; Marczak, L.; et al. Limited Prolonged Effects of Rifaximin Treatment on Irritable Bowel Syndrome-Related Differences in the Fecal Microbiome and Metabolome. Gut Microbes 2016, 7, 397–413. [Google Scholar] [CrossRef]
- Kulecka, M.; Fraczek, B.; Balabas, A.; Czarnowski, P.; Zeber-Lubecka, N.; Zapala, B.; Baginska, K.; Glowienka, M.; Szot, M.; Skorko, M.; et al. Characteristics of the Gut Microbiome in Esports Players Compared with Those in Physical Education Students and Professional Athletes. Front. Nutr. 2022, 9, 1092846. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing Mothur: Open-Source, Platform-Independent, Community-Supported Software for Describing and Comparing Microbial Communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA Ribosomal RNA Gene Database Project: Improved Data Processing and Web-Based Tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, T.C.; Ma, K.H.; Chao, A. iNEXT: An R package for rarefaction and extrapolation of species diversity (Hill numbers). Methods Ecol. Evol. 2016, 7, 1451–1456. [Google Scholar] [CrossRef]
- lmerTest-Package: LmerTest: Tests in Linear Mixed Effects Models in lmerTest: Tests in Linear Mixed Effects Models. Available online: https://rdrr.io/cran/lmerTest/man/lmerTest-package.html (accessed on 20 June 2023).
- Dixon, P. VEGAN, A Package of R Functions for Community Ecology. J. Veg. Sci. 2003, 14, 927–930. [Google Scholar] [CrossRef]
- Zhou, H.; He, K.; Chen, J.; Zhang, X. LinDA: Linear Models for Differential Abundance Analysis of Microbiome Compositional Data. Genome Biol. 2022, 23, 95. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef]
- Gill, V.J.S.; Soni, S.; Shringarpure, M.; Anusheel; Bhardwaj, S.; Yadav, N.K.; Patel, A.; Patel, A. Gut Microbiota Interventions for the Management of Obesity: A Literature Review. Cureus 2022, 14, e29317. [Google Scholar] [CrossRef]
- Slavin, J. Fiber and Prebiotics: Mechanisms and Health Benefits. Nutrients 2013, 5, 1417–1435. [Google Scholar] [CrossRef]
- Hu, F.; Niu, Y.; Xu, X.; Hu, Q.; Su, Q.; Zhang, H. Resistant Dextrin Improves High-Fat-High-Fructose Diet Induced Insulin Resistance. Nutr. Metab. 2020, 17, 36. [Google Scholar] [CrossRef] [PubMed]
- Valcheva, R.; Hotte, N.; Gillevet, P.; Sikaroodi, M.; Thiessen, A.; Madsen, K.L. Soluble Dextrin Fibers Alter the Intestinal Microbiota and Reduce Proinflammatory Cytokine Secretion in Male IL-10-Deficient Mice. J. Nutr. 2015, 145, 2060–2066. [Google Scholar] [CrossRef]
- Cani, P.D. Human Gut Microbiome: Hopes, Threats and Promises. Gut 2018, 67, 1716–1725. [Google Scholar] [CrossRef] [PubMed]
- Roberfroid, M.; Gibson, G.R.; Hoyles, L.; McCartney, A.L.; Rastall, R.; Rowland, I.; Wolvers, D.; Watzl, B.; Szajewska, H.; Stahl, B.; et al. Prebiotic Effects: Metabolic and Health Benefits. Br. J. Nutr. 2010, 104 (Suppl. 2), S1–S63. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, M.; Pang, X.; Zhao, Y.; Wang, L.; Zhao, L. Structural Resilience of the Gut Microbiota in Adult Mice under High-Fat Dietary Perturbations. ISME J. 2012, 6, 1848–1857. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet Rapidly and Reproducibly Alters the Human Gut Microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef]
- Gibson, G.R.; Beatty, E.R.; Wang, X.; Cummings, J.H. Selective Stimulation of Bifidobacteria in the Human Colon by Oligofructose and Inulin. Gastroenterology 1995, 108, 975–982. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Gupta, A.K. Applications of Inulin and Oligofructose in Health and Nutrition. J. Biosci. 2002, 27, 703–714. [Google Scholar] [CrossRef]
- Zhu, Q.; Qi, N.; Shen, L.; Lo, C.C.; Xu, M.; Duan, Q.; Ollberding, N.J.; Wu, Z.; Hui, D.Y.; Tso, P.; et al. Sexual Dimorphism in Lipid Metabolism and Gut Microbiota in Mice Fed a High-Fat Diet. Nutrients 2023, 15, 2175. [Google Scholar] [CrossRef]
- Peng, C.; Xu, X.; Li, Y.; Li, X.; Yang, X.; Chen, H.; Zhu, Y.; Lu, N.; He, C. Sex-Specific Association between the Gut Microbiome and High-Fat Diet-Induced Metabolic Disorders in Mice. Biol. Sex. Differ. 2020, 11, 5. [Google Scholar] [CrossRef]
- Jiang, J.; Fu, Y.; Tang, A.; Gao, X.; Zhang, D.; Shen, Y.; Mou, T.; Hu, S.; Gao, J.; Lai, J. Sex difference in prebiotics on gut and blood–brain barrier dysfunction underlying stress-induced anxiety and depression. CNS Neurosci. Ther. 2023, 29, 115–128. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, D.; Zheng, Y.; Tu, Y.; Xu, Q.; Jiang, H.; Li, C.; Zhao, L.; Li, Y.; Zheng, H.; et al. Sex-Dependent Effects on the Gut Microbiota and Host Metabolome in Type 1 Diabetic Mice. Biochim. Biophys. Acta Mol. Basis Dis. 2021, 1867, 166266. [Google Scholar] [CrossRef]
- Solah, V.A.; Kerr, D.A.; Hunt, W.J.; Johnson, S.K.; Boushey, C.J.; Delp, E.J.; Meng, X.; Gahler, R.J.; James, A.P.; Mukhtar, A.S.; et al. Effect of Fibre Supplementation on Body Weight and Composition, Frequency of Eating and Dietary Choice in Overweight Individuals. Nutrients 2017, 9, 149. [Google Scholar] [CrossRef]
- Namazi, N.; Larijani, B.; Azadbakht, L. Are Isolated and Complex Fiber Supplements Good Choices for Weight Management? A Systematic Review. Arch. Iran. Med. 2017, 20, 704–713. [Google Scholar]
- Hugenholtz, F.; de Vos, W.M. Mouse Models for Human Intestinal Microbiota Research: A Critical Evaluation. Cell. Mol. Life Sci. 2018, 75, 149–160. [Google Scholar] [CrossRef]




| Feeding | Sex | Score | Steatosis | Lobular Inflammation | Hepatocellular Ballooning | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 | 0 | 1 | 2 | |||
| ND | F | N= | 12 | 0 | 0 | 0 | 10 | 2 | 0 | 0 | 12 | 0 | 0 |
| M | N= | 12 | 0 | 0 | 0 | 9 | 3 | 0 | 0 | 12 | 0 | 0 | |
| ND + SDexF | F | N= | 12 | 0 | 0 | 0 | 7 | 5 | 0 | 0 | 12 | 0 | 0 |
| M | N= | 12 | 0 | 0 | 0 | 8 | 4 | 0 | 0 | 12 | 0 | 0 | |
| WD | F | N= | 0 | 1 | 6 | 5 | 1 | 3 | 7 | 1 | 6 | 6 | 0 |
| M | N= | 0 | 0 | 2 | 10 | 0 | 3 | 8 | 0 | 5 | 7 | 0 | |
| WD + SDexF | F | N= | 0 | 5 | 4 | 3 | 3 | 4 | 6 | 0 | 2 | 10 | 0 |
| M | N= | 0 | 3 | 2 | 7 | 5 | 7 | 0 | 0 | 7 | 5 | 0 | |
| Prebiotic Group (n = 39) | Control Group (n = 42) | p Value | |
|---|---|---|---|
| Age (years), mean (SD) | 8.6 (1.19) | 8.3 (1.18) | 0.13 (Mann–Whitney U test) |
| Height (cm), mean (SD) | 141.4 (10.8) | 137.6 (9.3) | 0.1 (Student’s t test) |
| Weight (kg), mean (SD) | 49.8 (11.6) | 46.5 (11.8) | 0.15 (Mann–Whitney U test) |
| BMI (kg/m2), mean (SD) | 24.6 (3.4) | 24.3 (4.1) | 0.43 (Mann–Whitney U test) |
| BMI z-score, mean (SD) | 3.0 (0.8) | 3.1 (1.5) | 0.47 (Mann–Whitney U test) |
| Boys, n (%) | 23 (54.8%) | 19 (45.2%) | 0.22 (Chi-squared test) |
| Girls, n (%) | 16 (41.0%) | 23 (59.0%) | 0.22 (Chi-squared test) |
| Changes in Body Weight Gain | Prebiotic Group | Control Group | ||
|---|---|---|---|---|
| After 6 Months N = 39 | After 9 Months N = 36 | After 6 Months N = 42 | After 9 Months N = 40 | |
| Slowed, n (%) | 25 (64.1%) | 20 (55.6%) | 25 (59.5%) | 22 (55%) |
| Unchanged or gained, n (%) | 14 (35.9%) | 16 (44.4%) | 17 (30.5%) | 18 (45%) |
| Pair-Wise Comparison | Shannon Index | Chao Index |
|---|---|---|
| Adjusted p Value (Mean Values) | Adjusted p Value (Mean Values) | |
| SDexF-supplemented group vs. control group at baseline | 0.76 | 0.91 |
| Comparison of stool samples collected at week 24 | ||
| SDexF-supplemented group, sample 2 vs. baseline | 0.70 | 0.44 |
| Control group, sample 2 vs. baseline | 0.31 | 0.46 |
| SDexF-supplemented group, with vs. without slowed weight gain at week 24 | 0.96 (2.39 vs. 2.35) | 0.98 (55.7 vs. 54.1) |
| Control group, with vs. without slowed weight gain at week 24 | 0.034 (2.52 vs. 2.34) | 0.11 (57.3 vs. 53.0) |
| SDexF-supplemented group vs. control group, both with slowed weight gain at week 24 | 0.081 (2.39 vs. 2.52) | 0.42 (55.7 vs. 57.3) |
| SDexF-supplemented group vs. control group, both without slowed weight gain at week 24 | 0.71 (2.35 vs. 2.34) | 0.46 (54.1 vs. 53) |
| Comparison of stool samples collected at week 36 | ||
| SDexF-supplemented group, sample 3 vs. baseline | 0.84 | 0.22 |
| Control group, sample 3 vs. baseline | 0.49 | 0.53 |
| SDexF-supplemented group, with vs. without slowed weight gain at week 36 | 0.77 (2.39 vs. 2.38) | 0.67 (56.8 vs. 57.8) |
| Control group, with vs. without slowed weight gain at week 36 | 0.25 (2.51 vs. 2.42) | 0.73 (56.2 vs. 57.8) |
| SDexF-supplemented group vs. control group, both with slowed weight gain at week 36 | 0.39 (2.39 vs. 2.51) | 0.72 (56.8 vs. 56.2) |
| SDexF-supplemented group vs. control group, both without slowed weight gain at week 36 | 0.56 (2.38 vs. 2.42) | 0.79 (55.3 vs. 57.8) |
| Pair-Wise Comparison | Genus (Phylum) | Fold Change; Adjusted p Value (Mean Normalized Counts Group 1, Group 2) |
|---|---|---|
| SDexF-supplemented group vs. control group at baseline | None | (-) |
| Comparison of stool samples collected at week 24 | ||
| SDexF-supplemented group, sample 2 vs. baseline | Genus, Escherichia-Shigella (phylum, Proteobacteria) | 3.53; 0.0701 (162, 42.3) |
| Control group, sample 2 vs. baseline | Genus, Erysipelotrichaceae UCG-003 (phylum, Firmicutes) | 0.37; 0.0036 (36.4, 101) |
| SDexF-supplemented group, with vs. without slowed weight gain at week 24 | None | (-) |
| Control group, with vs. without slowed weight gain at week 24 | Genus, Streptococcus (phylum, Firmicutes) | 7.5; 0.0013 (17.5, 2.0) |
| SDexF-supplemented group vs. control group, both with slowed weight gain at week 24 | Genus, Lachnoclostridium (phylum, Firmicutes) Genus, Butyricicoccus (phylum, Firmicutes) Genus, Erysipelotrichaceae UCG-003 (phylum, Firmicutes) | 3.52; 0.0211 (57.4, 15.6) 2.58; 0.0591 (62.4, 23.7) 2.36; 0.0853 (82.5, 34.3) |
| SDexF-supplemented group vs. control group, both without slowed weight gain at week 24 | Genus, Streptococcus (phylum, Firmicutes) | 7.99; 0.0062 (19.0, 2.0) |
| Comparison of stool samples collected at week 36 | ||
| SDexF-supplemented group, sample 3 vs. baseline | Genus, Escherichia-Shigella (phylum, Proteobacteria) | 3.61; 0.0679 (167, 42.3) |
| Control group, sample 3 vs. baseline | Genus, Escherichia-Shigella (phylum, Proteobacteria) | 4.59; 0.0039 (207, 62.4) |
| SDexF-supplemented group, with vs. without slowed weight gain at week 36 | Genus, Lachnoclostridium (phylum Firmicutes) | 0.71; 0.0378 (67.2, 95.6) |
| Control group, with vs. without slowed weight gain at week 36 | None | (-) |
| SDexF-supplemented group vs. control group, both with slowed weight gain at week 36 | None | (-) |
| SDexF-supplemented group vs. control group, both without slowed weight gain at week 36 | None | (-) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czarnowski, P.; Bałabas, A.; Kułaga, Z.; Kulecka, M.; Goryca, K.; Pyśniak, K.; Unrug-Bielawska, K.; Kluska, A.; Bagińska-Drabiuk, K.; Głowienka-Stodolak, M.; et al. Effects of Soluble Dextrin Fiber from Potato Starch on Body Weight and Associated Gut Dysbiosis Are Evident in Western Diet-Fed Mice but Not in Overweight/Obese Children. Nutrients 2024, 16, 917. https://doi.org/10.3390/nu16070917
Czarnowski P, Bałabas A, Kułaga Z, Kulecka M, Goryca K, Pyśniak K, Unrug-Bielawska K, Kluska A, Bagińska-Drabiuk K, Głowienka-Stodolak M, et al. Effects of Soluble Dextrin Fiber from Potato Starch on Body Weight and Associated Gut Dysbiosis Are Evident in Western Diet-Fed Mice but Not in Overweight/Obese Children. Nutrients. 2024; 16(7):917. https://doi.org/10.3390/nu16070917
Chicago/Turabian StyleCzarnowski, Paweł, Aneta Bałabas, Zbigniew Kułaga, Maria Kulecka, Krzysztof Goryca, Kazimiera Pyśniak, Katarzyna Unrug-Bielawska, Anna Kluska, Katarzyna Bagińska-Drabiuk, Maria Głowienka-Stodolak, and et al. 2024. "Effects of Soluble Dextrin Fiber from Potato Starch on Body Weight and Associated Gut Dysbiosis Are Evident in Western Diet-Fed Mice but Not in Overweight/Obese Children" Nutrients 16, no. 7: 917. https://doi.org/10.3390/nu16070917
APA StyleCzarnowski, P., Bałabas, A., Kułaga, Z., Kulecka, M., Goryca, K., Pyśniak, K., Unrug-Bielawska, K., Kluska, A., Bagińska-Drabiuk, K., Głowienka-Stodolak, M., Piątkowska, M., Dąbrowska, M., Żeber-Lubecka, N., Wierzbicka-Rucińska, A., Kotowska, A., Więckowski, S., Mikula, M., Kapuśniak, J., Socha, P., & Ostrowski, J. (2024). Effects of Soluble Dextrin Fiber from Potato Starch on Body Weight and Associated Gut Dysbiosis Are Evident in Western Diet-Fed Mice but Not in Overweight/Obese Children. Nutrients, 16(7), 917. https://doi.org/10.3390/nu16070917









