Association between Socioecological Status, Nutrient Intake, and Cancer Screening Behaviors in Adults Aged 40 and Over: Insights from the Eighth Korea National Health and Nutrition Examination Survey (KNHANES, 2019)
Abstract
1. Introduction
2. Methods
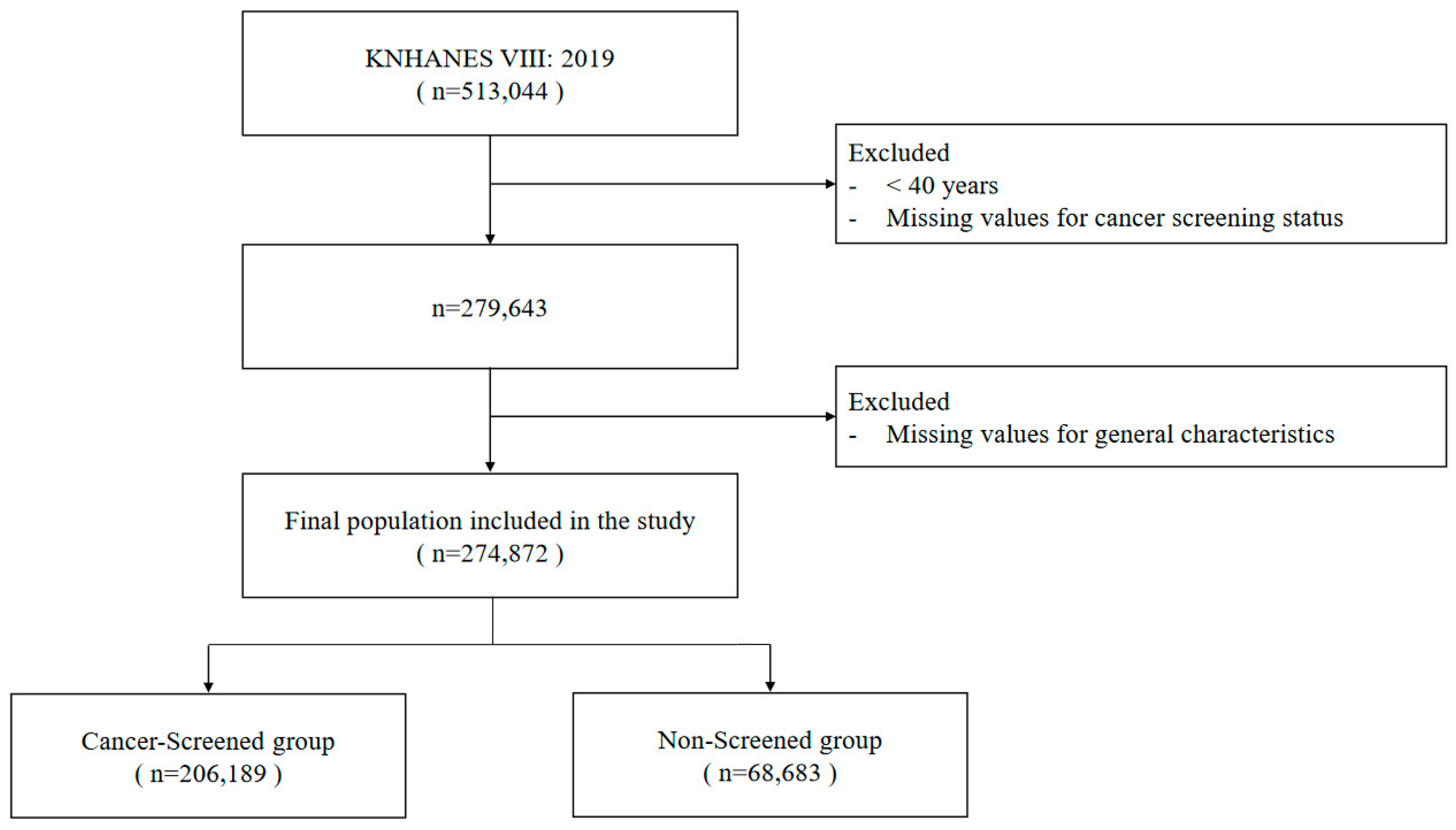
2.1. Data Source and Research Objectives
2.2. Variable Descriptions
2.2.1. Dependent Variable
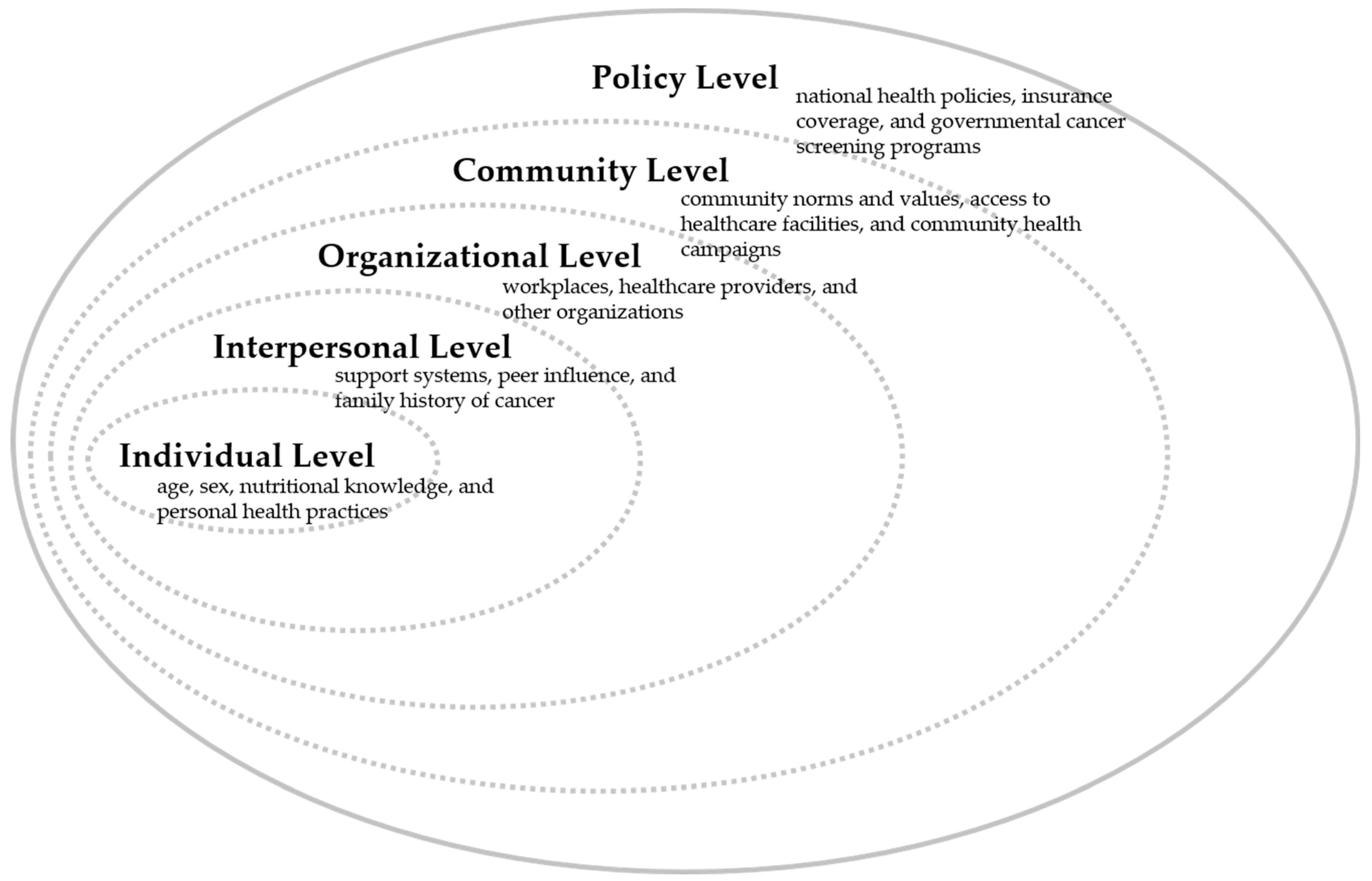
2.2.2. Socio-Demographic Variables
2.2.3. Lifestyle and Health-Related Variables
2.3. Dietary Intake Assessment
2.4. Statistical Analysis
3. Results
3.1. Characteristics of the Study Participants
3.2. Cancer Screening in Relation to Lifestyle and Health Status
3.3. Variation in Cancer Screening Utilization Based on Income Bracket
3.4. Determinants of Cancer Screening Utilization Stratified by Income Levels
3.5. Nutrient Intake Analysis Based on Cancer Screening Status
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef]
- Han, K.T.; Jun, J.K.; Im, J.S. National Cancer Control Plan of the Korea: Current Status and the Fourth Plan (2021–2025). J. Prev. Med. Public Health 2023, 56, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.J.; Jung, K.W.; Bang, S.H.; Choi, S.H.; Park, E.H.; Yun, E.H.; Kim, H.J.; Kong, H.J.; Im, J.S.; Seo, H.G.; et al. Cancer Statistics in Korea: Incidence, Mortality, Survival, and Prevalence in 2020. Cancer Res. Treat. 2023, 55, 385–399. [Google Scholar] [CrossRef]
- Kweon, S.S. Updates on Cancer Epidemiology in Korea, 2018. Chonnam Med. J. 2018, 54, 90–100. [Google Scholar] [CrossRef][Green Version]
- The National Cancer Information Center. The National Cancer Statistics 2023; National Cancer Information Center: Goyang, Republic of Korea, 2023. Available online: https://www.cancer.go.kr/ (accessed on 12 August 2023).
- Jung, K.W.; Kang, M.J.; Park, E.H.; Yun, E.H.; Kim, H.J.; Kong, H.J.; Im, J.S.; Seo, H.G. Prediction of Cancer Incidence and Mortality in Korea, 2023. Cancer Res. Treat. 2023, 55, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Song, J.H.; Lee, Y.; Heo, J.; Son, S.Y.; Hur, H.; Han, S.U. Secondary Primary Cancer after Primary Gastric Cancer: Literature Review and Big Data Analysis Using the Health Insurance Review and Assessment Service (HIRA) Database of Republic of Korea. Cancers 2022, 14, 6165. [Google Scholar] [CrossRef]
- Park, S.H.; Kang, M.J.; Yun, E.H.; Jung, K.W. Epidemiology of gastric cancer in Korea: Trends in incidence and survival based on korea central cancer registry data (1999–2019). J. Gastric Cancer 2022, 22, 160–168. [Google Scholar] [CrossRef] [PubMed]
- The Information Committee of the Korean Gastric Cancer Association. Korean gastric cancer association-led nationwide survey on surgically treated gastric cancers in 2019. J. Gastric Cancer 2021, 21, 221–235. [Google Scholar] [CrossRef]
- Hamashima, C. Update version of the japanese guidelines for gastric cancer screening. Jpn. J. Clin. Oncol. 2018, 48, 673–683. [Google Scholar] [CrossRef]
- Bae, S.; Yi, B.K. Development of eClaim system for private indemnity health insurance in South Korea: Compatibility and interoperability. Health Inform. J. 2022, 28, 14604582211071019. [Google Scholar] [CrossRef]
- Park, B.; Kim, Y.; Lee, J.; Lee, N.; Jang, S.H. Sex Difference and Smoking Effect of Lung Cancer Incidence in Asian Population. Cancers 2020, 13, 113. [Google Scholar] [CrossRef]
- Myong, J.-P.; Shin, J.-Y.; Kim, S.-J. Factors Associated with Participation in Colorectal Cancer Screening in Korea: The Fourth Korean National Health and Nutrition Examination Survey (KNHANES IV). Int. J. Color. Dis. 2012, 27, 1061–1069. [Google Scholar] [CrossRef]
- Hahm, M.-I.; Choi, K.S.; Lee, H.-Y.; Jun, J.K.; Oh, D.; Park, E.-C. Who Participates in the Gastric Cancer Screening and on-Time Rescreening in the National Cancer Screening Program? A Population-Based Study in Korea. Cancer Sci. 2011, 102, 2241–2247. [Google Scholar] [CrossRef]
- Korous, K.M.; Farr, D.E.; Brooks, E.; Tuuhetaufa, F.; Rogers, C.R. Economic Pressure and Intention to Complete Colorectal Cancer Screening: A Cross-Sectional Analysis among U.S. Men. Am. J. Men’s Health 2022, 16, 15579883221125571. [Google Scholar] [CrossRef]
- Jung, M. National Cancer Screening Programs and evidence-based healthcare policy in South Korea. Health Policy 2015, 119, 26–32. [Google Scholar] [CrossRef]
- Suh, M.; Choi, K.S.; Park, B.; Lee, Y.Y.; Jun, J.K.; Lee, D.H.; Kim, Y. Trends in Cancer Screening Rates among Korean Men and Women: Results of the Korean National Cancer Screening Survey, 2004–2013. Cancer Res. Treat. 2016, 48, 1–10. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kang, H.T. Association between Socioeconomic Status and Cancer Screening in Koreans over 40 Years in Age Based on the 2010–2012 Korean National Health and Nutrition Examination Survey. Korean J. Fam. Med. 2016, 37, 287–292. [Google Scholar] [CrossRef]
- Kim, S.; Kwon, S.; Subramanian, S.V. Has the National Cancer Screening Program reduced income inequalities in screening attendance in South Korea? Cancer Causes Control 2015, 26, 1617–1625. [Google Scholar] [CrossRef] [PubMed]
- Yun, E.H.; Hong, S.; Her, E.Y.; Park, B.; Suh, M.; Choi, K.S.; Jun, J.K. Trends in Participation Rates of the National Cancer Screening Program among Cancer Survivors in Korea. Cancers 2020, 13, 81. [Google Scholar] [CrossRef] [PubMed]
- Le Bonniec, A.; Sun, S.; Andrin, A.; Dima, A.L.; Letrilliart, L. Barriers and Facilitators to Participation in Health Screening: An Umbrella Review Across Conditions. Prev. Sci. 2022, 23, 1115–1142. [Google Scholar] [CrossRef] [PubMed]
- Rajaguru, V.; Kim, T.H.; Shin, J.; Lee, S.G. Income Disparities in Cancer Screening: A Cross-Sectional Study of the Korean National Health and Nutrition Examination Survey, 2013–2019. Front. Public Health 2022, 10, 820643. [Google Scholar] [CrossRef] [PubMed]
- Ishii, K.; Tabuchi, T.; Iso, H. Trends in socioeconomic inequalities in cervical, breast, and colorectal cancer screening participation among women in Japan, 2010–2019. Cancer Epidemiol. 2023, 84, 102353. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, H.; Liu, B.; Yu, J.; Gao, Y. Socioeconomic status index is an independent determinant of breast cancer screening practices: Evidence from Eastern China. PLoS ONE 2022, 17, e0279107. [Google Scholar] [CrossRef] [PubMed]
- Stuart, G.W.; Chamberlain, J.A.; Milne, R.L. Socio-economic and ethnocultural influences on geographical disparities in breast cancer screening participation in Victoria, Australia. Front. Oncol. 2022, 12, 980879. [Google Scholar] [CrossRef] [PubMed]
- Walsh, B.; Silles, M.; O’Neill, C. The importance of socio-economic variables in cancer screening participation: A comparison between population-based and opportunistic screening in the EU-15. Health Policy 2011, 101, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Korous, K.M.; Cuevas, A.G.; Chahoud, J.; Ogbonnaya, U.C.; Brooks, E.; Rogers, C.R. Examining the relationship between household wealth and colorectal cancer screening behaviors among U.S. men aged 45–75. SSM Popul. Health 2022, 19, 101222. [Google Scholar] [CrossRef] [PubMed]
- Han, M.A.; Choi, K.S.; Jun, J.K.; Kim, Y.; Park, E.C.; Lee, H.Y. Factors associated with the intention to have colorectal cancer screening in Korean adults. Eur. J. Cancer Care 2011, 20, 475–482. [Google Scholar] [CrossRef]
- Kwak, M.S.; Park, E.C.; Bang, J.Y.; Sung, N.Y.; Lee, J.Y.; Choi, K.S. Factors associated with cancer screening participation, Korea. J. Prev. Med. Public Health 2005, 38, 473–481. [Google Scholar]
- Tworoger, S.S.; Hecht, J.L.; Giovannucci, E.; Hankinson, S.E. Intake of folate and related nutrients in relation to risk of epithelial ovarian cancer. Am. J. Epidemiol. 2006, 163, 1101–1111. [Google Scholar] [CrossRef]
- Aune, D.; Giovannucci, E.; Boffetta, P.; Fadnes, L.T.; Keum, N.; Norat, T.; Greenwood, D.C.; Riboli, E.; Vatten, L.J.; Tonstad, S. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality—A systematic review and dose-response meta-analysis of prospective studies. Int. J. Epidemiol. 2017, 46, 1029–1056. [Google Scholar] [CrossRef] [PubMed]
- Aune, D.; Keum, N.; Giovannucci, E.; Fadnes, L.T.; Boffetta, P.; Greenwood, D.C.; Tonstad, S.; Vatten, L.J.; Riboli, E.; Norat, T. Dietary intake and blood concentrations of antioxidants and the risk of cardiovascular disease, total cancer, and all-cause mortality: A systematic review and dose-response meta-analysis of prospective studies. Am. J. Clin. Nutr. 2018, 108, 1069–1091. [Google Scholar] [CrossRef] [PubMed]
- Gang, B.G.; Shin, J.S.; Lee, J.; Lee, Y.J.; Cho, H.W.; Kim, M.R.; Kang, K.; Koh, W.; Kim, E.J.; Park, Y.; et al. Association Between Acupuncture and Knee Surgery for Osteoarthritis: A Korean, Nationwide, Matched, Retrospective Cohort Study. Front. Med. 2020, 7, 524628. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Kim, H.; Jun, T.J.; Kim, Y.H. Association of dietary sodium intake with impaired fasting glucose in adult cancer survivors: A population-based cross-sectional study. PLoS ONE 2023, 18, e0286346. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.N.; Lee, J.S.; Shim, J.S.; Yoon, M.O.; Lee, H.S. Estimated dietary vitamin D intake and major vitamin D food sources of Koreans: Based on the Korea National Health and Nutrition Examination Survey 2016–2019. Nutr. Res. Pract. 2023, 17, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.; Lee, Y.; Park, H.; Song, K. Gender and age group differences in nutrition intake and dietary quality of Korean adults eating alone: Based on Korean National Health and Nutrition Examination Survey Data, 2013–2016. Nutr. Res. Pract. 2021, 15, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Nelder, J.A.; Wedderburn, R.W.M. Generalized Linear Models. J. R. Stat. Soc. Ser. A 1972, 135, 370–384. [Google Scholar] [CrossRef]
- Kim, Y.H.; Lee, J.S.; Park, J.H. Association between bone mineral density and knee osteoarthritis in Koreans: The Fourth and Fifth Korea National Health and Nutrition Examination Surveys. Osteoarthr. Cartil. 2018, 26, 1511–1517. [Google Scholar] [CrossRef]
- Weng, X.; Tan, Y.; Fei, Q.; Yao, H.; Fu, Y.; Wu, X.; Zeng, H.; Yang, Z.; Zeng, Z.; Liang, H.; et al. Association between mixed exposure of phthalates and cognitive function among the U.S. elderly from NHANES 2011–2014: Three statistical models. Sci. Total Environ. 2022, 828, 154362. [Google Scholar] [CrossRef]
- Bai, J.; Ma, Y.; Zhao, Y.; Yang, D.; Mubarik, S.; Yu, C. Mixed exposure to phenol, parabens, pesticides, and phthalates and insulin resistance in NHANES: A mixture approach. Sci. Total Environ. 2022, 851, 158218. [Google Scholar] [CrossRef]
- Colditz, G.A.; Wei, E.K. Preventability of cancer: The relative contributions of biologic and social and physical environmental determinants of cancer mortality. Annu. Rev. Public Health 2012, 33, 137–156. [Google Scholar] [CrossRef] [PubMed]
- Le Bonniec, A.; Meade, O.; Fredrix, M.; Morrissey, E.; O’Carroll, R.E.; Murphy, P.J.; Murphy, A.W.; Mc Sharry, J. Exploring non-participation in colorectal cancer screening: A systematic review of qualitative studies. Soc. Sci. Med. 2023, 329, 116022. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Center. Korea National Cancer Screening Survey 2018; National Cancer Center: Goyang, Republic of Korea, 2018; Available online: http://www.ncc.re.kr/prBoardView1.ncc?nwsId=4335 (accessed on 12 August 2023).
- Allen, C.; Waters, E.A.; Hamilton, J.G.; Vu, M.; Gabriel, J.; Roberts, M.C. Multifactorial causal beliefs and colorectal cancer screening: A structural equation modeling investigation. J. Health Psychol. 2022, 27, 2463–2477. [Google Scholar] [CrossRef] [PubMed]
- Layne, T.M.; Agarwal, P.; Rapkin, B.D.; Jandorf, L.H.; Bickell, N.A. Cancer beliefs and screening behaviors: The impact of neighborhood and other social determinants of health. Front. Oncol. 2023, 13, 1072259. [Google Scholar] [CrossRef] [PubMed]
- Goding Sauer, A.; Siegel, R.L.; Jemal, A.; Fedewa, S.A. Current Prevalence of Major Cancer Risk Factors and Screening Test Use in the United States: Disparities by Education and Race/Ethnicity. Cancer Epidemiol. Biomark. Prev. 2019, 28, 629–642. [Google Scholar] [CrossRef] [PubMed]
- Sabatino, S.A.; Coates, R.J.; Uhler, R.J.; Breen, N.; Tangka, F.; Shaw, K.M. Disparities in mammography use among US women aged 40–64 years, by race, ethnicity, income, and health insurance status, 1993 and 2005. Med Care. 2008, 46, 692–700. [Google Scholar] [CrossRef]
- Hjerkind, K.V.; Ellingjord-Dale, M.; Johansson, A.L.V.; Aase, H.S.; Hoff, S.R.; Hofvind, S.; Fagerheim, S.; Dos-Santos-Silva, I.; Ursin, G. Volumetric Mammographic Density, Age-Related Decline, and Breast Cancer Risk Factors in a National Breast Cancer Screening Program. Cancer Epidemiol. Biomark. Prev. 2018, 27, 1065–1074. [Google Scholar] [CrossRef] [PubMed]
- Friedenreich, C.M.; Orenstein, M.R. Physical activity and cancer prevention: Etiologic evidence and biological mechanisms. J. Nutr. 2002, 132, 3456S–3464S. [Google Scholar] [CrossRef]
- Shapiro, J.A.; Seeff, L.C.; Nadel, M.R. Colorectal cancer-screening tests and associated health behaviors. Am. J. Prev. Med. 2001, 21, 132–137. [Google Scholar] [CrossRef]
- Tessaro, I.; Mangone, C.; Parkar, I.; Pawar, V. Knowledge, barriers, and predictors of colorectal cancer screening in an Appalachian church population. Prev. Chronic Dis. 2006, 3, A123. [Google Scholar]
- Janz, N.K.; Wren, P.A.; Schottenfeld, D.; Guire, K.E. Colorectal cancer screening attitudes and behavior: A population-based study. Prev. Med. 2003, 37, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Seo, H.; Choe, S.; Jeong, S.Y.; Park, J.W.; Suh, M.; Shin, A.; Choi, K.S. Intentions to undergo primary screening with colonoscopy under the National Cancer Screening Program in Korea. PLoS ONE 2021, 16, e0247252. [Google Scholar] [CrossRef] [PubMed]
- Scaglioni, G.; Chiereghin, A.; Bazzani, C.; Mezzetti, F.; Cavazza, N. Psychosocial Predictors of Colorectal Cancer Screening Intention: An Experiment on the Invitation Letter. Int. J. Behav. Med. 2022, 30, 867–877. [Google Scholar] [CrossRef] [PubMed]
- Korous, K.M.; Ogbonnaya, U.C.; De Vera, M.A.; Brooks, E.; Moore, J.X.; Rogers, C.R. Perceived economic pressure and colorectal cancer-related perceptions among U.S. males (aged 45–75). Cancer Causes Control 2023, 34, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Bandi, P.; Minihan, A.K.; Siegel, R.L.; Islami, F.; Nargis, N.; Jemal, A.; Fedewa, S.A. Updated Review of Major Cancer Risk Factors and Screening Test Use in the United States in 2018 and 2019, with a Focus on Smoking Cessation. Cancer Epidemiol. Biomark. Prev. 2021, 30, 1287–1299. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.R.; Choe, Y.R. Health-promoting behaviors among middle-aged breast cancer survivors compared with matched non-cancer controls: A KNHANES VI-VII (2013–2018) study. Medicine 2023, 102, e34065. [Google Scholar] [CrossRef] [PubMed]
- Sutkowska, E.; Stanek, A.; Madziarska, K.; Jakubiak, G.K.; Sokołowski, J.; Madziarski, M.; Sutkowska-Stępień, K.; Biernat, K.; Mazurek, J.; Borowkow-Bulek, A.; et al. Physical Activity Modifies the Severity of COVID-19 in Hospitalized Patients-Observational Study. J. Clin. Med. 2023, 12, 4046. [Google Scholar] [CrossRef] [PubMed]
- McTiernan, A.; Friedenreich, C.M.; Katzmarzyk, P.T.; Powell, K.E.; Macko, R.; Buchner, D.; Pescatello, L.S.; Bloodgood, B.; Tennant, B.; Vaux-Bjerke, A.; et al. Physical Activity in Cancer Prevention and Survival: A Systematic Review. Med. Sci. Sports Exerc. 2019, 51, 1252–1261. [Google Scholar] [CrossRef]
- Suh, M.; Song, S.; Cho, H.N.; Park, B.; Jun, J.K.; Choi, E.; Kim, Y.; Choi, K.S. Trends in Participation Rates for the National Cancer Screening Program in Korea, 2002–2012. Cancer Res. Treat. 2017, 49, 798–806. [Google Scholar] [CrossRef]
- Le Bonniec, A.; Mas, S.; Préau, M.; Cousson-Gélie, F. Understanding barriers and facilitators to participation in colorectal cancer screening: A French qualitative study. J. Health Psychol. 2021, 26, 2260–2277. [Google Scholar] [CrossRef]
- Bradley, C.J.; Simon, K.; Winkfield, K.; Moy, B. Enhancing Health Equity through Cancer Health Economics Research. J. Natl. Cancer Inst. Monogr. 2022, 2022, 74–78. [Google Scholar] [CrossRef]
- Ahmed, N.U.; Chowdhury, M.A.B.; Rodriguez, A.; Azim, S.I.; Taskin, T.; Ahmed, S. Disparities in Compliance with Colorectal Cancer Screening: Evidence from Two US National Surveys. Asian Pac. J. Cancer Prev. 2023, 24, 1173–1180. [Google Scholar] [CrossRef] [PubMed]
- Fawns-Ritchie, C.; Miller, C.B.; van der Pol, M.; Douglas, E.; Bell, D.; O’Carroll, R.E.; Deary, I.J. Psychological correlates of free colorectal cancer screening uptake in a Scottish sample: A cross-sectional observational study. BMJ Open 2022, 12, e042210. [Google Scholar] [CrossRef] [PubMed]
- Moss, J.L.; Leach, K.; Stoltzfus, K.C.; Granzow, M.; Reiter, P.L.; Onega, T.; Klesges, L.M.; Ruffin, M.T. 4th. Multilevel Associations with Cancer Screening among Women in Rural, Segregated Communities within the Northeastern USA: A Mixed-Methods Study. J. Cancer Educ. 2022, 37, 1982–1992. [Google Scholar] [CrossRef] [PubMed]
- Weller, D.P.; Patnick, J.; McIntosh, H.M.; Dietrich, A.J. Uptake in cancer screening programmes. Lancet Oncol. 2009, 10, 693–699. [Google Scholar] [CrossRef]
- Alcaraz, K.I.; Wiedt, T.L.; Daniels, E.C.; Yabroff, K.R.; Guerra, C.E.; Wender, R.C. Understanding and addressing social determinants to advance cancer health equity in the United States: A blueprint for practice, research, and policy. CA Cancer J. Clin. 2020, 70, 31–46. [Google Scholar] [CrossRef]
| Cancer Screening | |||
|---|---|---|---|
| Variables | Yes (n = 206,189) | No (n = 68,683) | p-Value |
| Age (yrs.) | 58.40 ± 11.37 | 60.33 ± 12.47 | <0.0001 (1) |
| Age (yrs.) | <0.0001 (2) | ||
| 40–49 | 56,565 (76.94%) | 16,951 (23.06%) | |
| 50–64 | 85,047 (76.73%) | 25,794 (23.27%) | |
| ≥65 | 64,577 (71.34%) | 25,938 (28.66%) | |
| Sex (n (%)) | <0.0001 | ||
| Male | 88,527 (72.75%) | 33,163 (27.25%) | |
| Female | 117,662 (76.81%) | 35,520 (23.19%) | |
| Marital status (n (%)) | <0.0001 | ||
| With spouse | 172,149 (77.12%) | 51,084 (22.88%) | |
| Divorced | 29,033 (68.28%) | 13,487 (31.72%) | |
| Unmarried | 5007 (54.91%) | 4112 (45.09%) | |
| Employed (n (%)) | <0.0001 | ||
| Yes | 128,883 (76.73%) | 39,086 (23.27%) | |
| No | 77,306 (72.31%) | 29,597 (27.69%) | |
| Region (n (%)) | 0.0014 | ||
| Urban | 136,156 (74.81%) | 45,837 (25.19%) | |
| Rural | 70,033 (75.40%) | 22,846 (24.60%) | |
| Education level (n (%)) | <0.0001 | ||
| ≤Elementary school | 40,800 (68.56%) | 18,709 (31.44%) | |
| Middle school | 24,613 (76.82%) | 7426 (23.18%) | |
| High school | 68,519 (75.17%) | 22,634 (24.83%) | |
| ≥College | 72,257 (78.39%) | 19,914 (21.61%) | |
| Family income level (n (%)) | <0.0001 | ||
| Low | 35,092 (67.10%) | 17,209 (32.90%) | |
| Middle low | 51,652 (72.26%) | 19,832 (27.74%) | |
| Middle high | 51,810 (75.42%) | 16,881 (24.58%) | |
| High | 67,635 (82.09%) | 14,761 (17.91%) | |
| Health insurance (n (%)) | <0.0001 | ||
| National health (local) | 55,944 (70.54%) | 23,363 (29.46%) | |
| National health (employer) | 145,895 (78.04%) | 41,065 (21.96%) | |
| Medicare | 4350 (50.55%) | 4255 (49.45%) | |
| Private insurance (n (%)) | <0.0001 | ||
| Yes | 170,541 (77.92%) | 48,314 (22.08%) | |
| No | 35,648 (63.64%) | 20,369 (36.36%) | |
| Cancer Screening | |||
|---|---|---|---|
| Variables | Yes (n = 206,189) | No (n = 68,683) | p-Value |
| Height (cm) | 162.49 ± 8.88 | 162.13 ± 9.55 | <0.0001 (1) |
| Weight (kg) | 63.34 ± 11.23 | 63.96 ± 12.53 | <0.0001 |
| Waist circumference (cm) | 84.58 ± 9.29 | 85.95 ± 10.14 | <0.0001 |
| BMI (kg/m2) | 23.90 ± 3.12 | 24.24 ± 3.73 | <0.0001 |
| BMI (kg/m2) | <0.0001 (2) | ||
| Underweight | 4849 (62.16%) | 2952 (37.84%) | |
| Normal | 80,450 (77.83%) | 22,919 (22.17%) | |
| Overweight | 54,639 (76.73%) | 16,570 (23.27%) | |
| Obesity | 66,251 (71.63%) | 26,242 (28.37%) | |
| Cancer Screening | |||
|---|---|---|---|
| Variables | Yes (n = 206,189) | No (n = 68,683) | p-Value (1) |
| Self-reported health status (n (%)) | <0.0001 | ||
| Good | 60,669 (75.57%) | 19,617 (24.43%) | |
| Moderate | 110,607 (76.30%) | 34,358 (23.70%) | |
| Poor | 34,913 (70.36%) | 14,708 (29.64%) | |
| Stress (n (%)) | 0.2226 | ||
| Rarely | 162,093 (74.96%) | 54,137 (25.04%) | |
| Often | 44,096 (75.20%) | 14,546 (24.80%) | |
| Heavy alcohol drinking (n (%)) | <0.0001 | ||
| Yes | 20,009 (70.21%) | 8490 (29.79%) | |
| No | 186,180 (75.57%) | 60,193 (24.43%) | |
| Current smoking (n (%)) | <0.0001 | ||
| Yes | 26,944 (67.59%) | 12,918 (32.41%) | |
| No | 179,245 (76.27%) | 55,765 (23.73%) | |
| Walking (n (%)) | <0.0001 | ||
| <5 days/w | 118,212 (72.69%) | 44,406 (27.31%) | |
| ≥5 days/w | 87,977 (78.37%) | 24,277 (35.35%) | |
| Physical activity (n (%)) | <0.0001 | ||
| Yes | 54,369 (82.15%) | 11,817 (17.85%) | |
| No | 151,820 (72.75%) | 56,866 (27.25%) | |
| History of cancer (n (%)) | <0.0001 | ||
| Yes | 17,212 (76.64%) | 5247 (23.36%) | |
| No | 188,977 (74.87%) | 63,436 (25.13%) | |
| Multiple primary cancer (n (%)) | <0.0001 | ||
| 0 | 188,977 (74.87%) | 63,436 (25.13%) | |
| 1 | 16,324 (76.09%) | 5130 (23.91%) | |
| 2 | 888 (90.61%) | 92 (9.39%) | |
| ≥3 | 0 (0.00%) | 25 (100.00%) | |
| Pre-existing comorbidities (n (%)) | <0.0001 | ||
| 0 | 90,484 (74.45%) | 31,059 (25.55%) | |
| 1 | 55,393 (74.34%) | 19,123 (25.66%) | |
| 2 | 33,111 (77.79%) | 9456 (22.21%) | |
| ≥3 | 27,201 (75.05%) | 9045 (24.95%) | |
| CCI scores (n (%)) | <0.0001 | ||
| 0 | 160,504 (75.39%) | 52,384 (24.61%) | |
| 1 | 31,727 (74.20%) | 11,033 (25.80%) | |
| ≥2 | 13,958 (72.61%) | 5266 (27.39%) | |
| Cancer Screening | |||
|---|---|---|---|
| Variables | Yes (n = 206,189) | No (n = 68,683) | p-Value (1) |
| Eating breakfast (n (%)) | <0.0001 | ||
| 5–7 times/w | 158,359 (75.84%) | 50,437 (24.16%) | |
| 3–4 times/w | 15,531 (76.00%) | 4904 (24.00%) | |
| 1–2 times/w | 15,552 (74.79%) | 5241 (25.21%) | |
| none | 16,747 (67.40%) | 8101 (32.60%) | |
| Eating out (n (%)) | <0.0001 | ||
| ≥1 times/d | 44,737 (75.93%) | 14,178 (24.07%) | |
| 1–6 times/w | 107,526 (76.82%) | 32,450 (23.18%) | |
| <1 time/w | 53,926 (70.97%) | 22,055 (29.03%) | |
| Diet therapy | <0.0001 | ||
| Yes | 64,137 (80.44%) | 15,592 (19.56%) | |
| No | 142,052 (72.79%) | 53,091 (27.21%) | |
| Eating dietary supplements in a year (n (%)) | <0.0001 | ||
| Yes | 140,931 (78.76%) | 38,013 (21.24%) | |
| No | 65,258 (68.03%) | 30,670 (31.97%) | |
| Household Income | ||||||
|---|---|---|---|---|---|---|
| Variables | Total (n = 274,872) | Lowest (n = 52,301) | Lower Middle (n = 71,484) | Upper Middle (n = 68,691) | Highest (n = 82,396) | p-Value (1) |
| Cancer screening (CS) (n (%)) | <0.0001 | |||||
| Yes | 206,189 | 35,092 | 51,652 | 51,810 | 67,635 | |
| (75.01%) | (67.10%) | (72.26%) | (75.42%) | (82.09%) | ||
| No | 68,683 | 17,209 | 19,832 | 16,881 | 14,761 | |
| (24.99%) | (32.90%) | (27.74%) | (24.58%) | (17.91%) | ||
| Self-pay CS (n (%)) | <0.0001 | |||||
| Yes | 30,676 | 3749 | 6770 | 7904 | 12,253 | |
| (11.16%) | (7.17%) | (9.47%) | (11.51%) | (14.87%) | ||
| No | 175,513 | 31,343 | 44,882 | 43,906 | 55,382 | |
| (63.85%) | (59.93%) | (62.79%) | (63.92%) | (67.21%) | ||
| NA (2) | 68,683 | 17,209 | 19,832 | 16,881 | 14,761 | |
| (24.99%) | (32.90%) | (27.74%) | (24.58%) | (17.91%) | ||
| Partial self-pay CS (n (%)) | <0.0001 | |||||
| Yes | 95,115 | 11,486 | 24,825 | 26,831 | 31,973 | |
| (34.60%) | (21.96%) | (34.73%) | (39.06%) | (38.80%) | ||
| No | 111,074 | 23,606 | 26,827 | 24,979 | 35,662 | |
| (40.41%) | (45.13%) | (37.53%) | (36.36%) | (43.28%) | ||
| NA | 68,683 | 17,209 | 19,832 | 16,881 | 14,761 | |
| (24.99%) | (32.90%) | (27.74%) | (24.58%) | (17.91%) | ||
| National health insurance CS (n (%)) | <0.0001 | |||||
| Yes | 132,086 | 25,955 | 35,045 | 33,004 | 38,082 | |
| (48.05%) | (49.63%) | (49.02%) | (48.05%) | (46.22%) | ||
| No | 74,103 | 9137 | 16,607 | 18,806 | 29,553 | |
| (26.96%) | (17.47%) | (23.23%) | (27.38%) | (35.87%) | ||
| NA | 68,683 | 17,209 | 19,832 | 16,881 | 14,761 | |
| (24.99%) | (32.90%) | (27.74%) | (24.58%) | (17.91%) | ||
| Free CS (n (%)) | <0.0001 | |||||
| Yes | 972 | 385 | 324 | 65 | 198 | |
| (0.35%) | (0.74%) | (0.45%) | (0.09%) | (0.24%) | ||
| No | 205,217 | 34,707 | 51,328 | 51,745 | 67,437 | |
| (74.66%) | (66.36%) | (71.80%) | (75.33%) | (81.84%) | ||
| NA | 68,683 | 17,209 | 19,832 | 16,881 | 14,761 | |
| (24.99%) | (32.90%) | (27.74%) | (24.58%) | (17.91%) | ||
| Household Income Level | p-Value | |
|---|---|---|
| Variables | Q1 (Lowest) | |
| Adjusted OR (1) (95% CI) | ||
| Age | ||
| 40–49 | 1 | |
| 50–64 | 1.865 (1.725–2.017) | <0.0001 |
| ≥65 | 1.637 (1.527–1.756) | <0.0001 |
| Sex | ||
| Male | 1 | |
| Female | 1.383 (1.333–1.436) | <0.0001 |
| Marital status | ||
| With spouse | 4.019 (3.694–4.372) | <0.0001 |
| Divorced | 1.973 (1.809–2.153) | 0.5629 |
| Unmarried | 1 | |
| Employed | ||
| Yes | 1 | |
| No | 1.444 (1.387–1.502) | <0.0001 |
| Region | ||
| Urban | 1 | |
| Rural | 1.176 (1.133–1.221) | <0.0001 |
| Education level | ||
| ≤Elementary school | 1 | |
| Middle school | 1.826 (1.721–1.938) | <0.0001 |
| High school | 0.969 (0.921–1.020) | <0.0001 |
| ≥College | 1.006 (0.931–1.088) | <0.0001 |
| Health insurance | ||
| National health (local) | 2.059 (1.948–2.177) | <0.0001 |
| National health (employer) | 2.664 (2.528–2.808) | <0.0001 |
| Medicare | 1 | |
| Private insurance | ||
| Yes | 2.839 (2.720–2.962) | <0.0001 |
| No | 1 | |
| BMI (kg/m2) | ||
| Underweight | 1 | |
| Normal | 3.921 (3.505–4.386) | <0.0001 |
| Overweight | 5.197 (4.635–5.827) | <0.0001 |
| Obesity | 3.606 (3.223–4.034) | <0.0001 |
| Self-reported health status | ||
| Good | 1 | |
| Moderate | 1.176 (1.117–1.238) | <0.0001 |
| Poor | 0.856 (0.809–0.906) | <0.0001 |
| Stress | ||
| Rarely | 1 | |
| Often | 1.061 (1.014–1.111) | 0.0109 |
| Heavy alcohol drinking | ||
| Yes | 0.669 (0.620–0.723) | <0.0001 |
| No | 1 | |
| Current smoking | ||
| Yes | 0.598 (0.565–0.632) | <0.0001 |
| No | 1 | |
| Walking | ||
| <5 days/w | 1 | |
| ≥5 days/w | 1.369 (1.317–1.423) | <0.0001 |
| Physical activity (moderate intensity) | ||
| Yes | 1.444 (1.362–1.532) | <0.0001 |
| No | 1 | |
| History of cancer | ||
| Yes | 1.054 (0.987–1.126) | 0.1164 |
| No | 1 | |
| Multiple primary cancer | ||
| 0 | 1 | |
| 1 | 1.104 (1.032–1.181) | <0.0001 |
| 2 | 0.333 (0.242–0.458) | <0.0001 |
| Pre-existing comorbidities | ||
| 0 | 1 | |
| 1 | 1.238 (1.177–1.302) | 0.0012 |
| 2 | 1.356 (1.282–1.434) | <0.0001 |
| ≥3 | 1.144 (1.083–0.209) | 0.0943 |
| CCI scores | ||
| 0 | 1 | |
| 1 | 0.931 (0.891–0.972) | 0.8127 |
| ≥2 | 0.877 (0.820–0.937) | 0.0043 |
| Eating breakfast | ||
| 5–7 times/w | 1 | |
| 3–4 times/w | 0.445 (0.404–0.489) | <0.0001 |
| 1–2 times/w | 0.587 (0.533–0.647) | 0.0102 |
| none | 0.676 (0.619–0.738) | 0.2321 |
| Eating out | ||
| ≥1 times/d | 1 | |
| 1–6 times/w | 1.404 (0.298–1.520) | <0.0001 |
| <1 time/w | 1.086 (1.004–1.176) | 0.0004 |
| Diet therapy | ||
| Yes | 1.741 (1.666–1.820) | <0.0001 |
| No | 1 | |
| Eating dietary supplements in a year | ||
| Yes | 2.267 (2.183–2.354) | <0.0001 |
| No | 1 |
| Cancer Screening | |||
|---|---|---|---|
| Variables | Yes (n = 206,189) | No (n = 68,683) | p-Value (1) |
| Energy (Kcal) (2) | 1912.01 ± 1.46 | 1867.22 ± 2.54 | <0.0001 |
| Carbohydrate (g) | 287.57 ± 0.14 | 288.49 ± 0.24 | <0.0001 |
| Protein (g) | 70.61 ± 0.04 | 68.79 ± 0.07 | <0.0001 |
| Fat (g) | 43.54 ± 0.04 | 42.08 ± 0.07 | <0.0001 |
| Saturated fat (g) | 13.14 ± 0.02 | 12.81 ± 0.03 | <0.0001 |
| Cholesterol (mg) | 242.46 ± 0.37 | 223.76 ± 0.64 | <0.0001 |
| Fiber (g) | 29.60 ± 0.02 | 28.29 ± 0.04 | <0.0001 |
| Sugar (g) | 61.75 ± 0.07 | 57.33 ± 0.13 | <0.0001 |
| Vitamin A (µg RAE) | 441.47 ± 0.91 | 392.57 ± 1.58 | <0.0001 |
| Vitamin B1 (mg) | 1.17 ± 0.00 | 1.19 ± 0.00 | <0.0001 |
| Vitamin B2 (mg) | 1.55 ± 0.00 | 1.49 ± 0.00 | <0.0001 |
| Niacin (mg) | 12.24 ± 0.01 | 12.30 ± 0.02 | 0.0033 |
| Vitamin C (mg) | 74.06 ± 0.16 | 68.00 ± 0.28 | <0.0001 |
| Calcium (mg) | 539.93 ± 0.58 | 505.96 ± 1.01 | <0.0001 |
| Phosphorus (mg) | 1110.14 ± 0.56 | 1077.29 ± 0.98 | <0.0001 |
| Sodium (mg) | 3473.83 ± 3.13 | 3440.66 ± 5.44 | <0.0001 |
| Potassium (mg) | 3028.63 ± 2.12 | 2901.56 ± 3.68 | <0.0001 |
| Iron (mg) | 10.34 ± 0.01 | 10.04 ± 0.02 | <0.0001 |
| Energy distribution | |||
| % Carbohydrate | 63.96 ± 0.02 | 64.87 ± 0.04 | <0.0001 |
| % Protein | 15.45 ± 0.01 | 15.06 ± 0.02 | <0.0001 |
| % Fat | 20.59 ± 0.02 | 20.07 ± 0.03 | <0.0001 |
| Cancer Screening | |||
|---|---|---|---|
| Variables | Yes (n = 85,047) | No (n = 25,794) | p-Value (3) |
| Carbohydrate (4) | 2.29 ± 0.00 | 2.31 ± 0.01 | 0.0001 |
| Protein | 1.36 ± 0.00 | 1.31 ± 0.00 | <0.0001 |
| Cholesterol | 0.87 ± 0.00 | 0.75 ± 0.00 | <0.0001 |
| Fiber | 1.33 ± 0.00 | 1.32 ± 0.00 | 0.0099 |
| Vitamin A | 0.71 ± 0.00 | 0.68 ± 0.00 | <0.0001 |
| Vitamin B1 | 1.08 ± 0.00 | 1.10 ± 0.00 | <0.0001 |
| Vitamin B2 | 1.23 ± 0.00 | 1.19 ± 0.00 | <0.0001 |
| Niacin | 0.86 ± 0.00 | 0.85 ± 0.00 | 0.6006 |
| Vitamin C | 0.80 ± 0.00 | 0.75 ± 0.01 | <0.0001 |
| Calcium | 0.73 ± 0.00 | 0.73 ± 0.00 | 0.3697 |
| Phosphorus | 1.67 ± 0.00 | 1.62 ± 0.00 | <0.0001 |
| Sodium | 2.42 ± 0.00 | 2.39 ± 0.01 | 0.0003 |
| Potassium | 0.93 ± 0.00 | 0.90 ± 0.002 | <0.0001 |
| Iron | 1.22 ± 0.00 | 1.22 ± 0.00 | 0.1218 |
| MAR | 1.25 ± 0.00 | 1.22 ± 0.00 | <0.0001 |
| Cancer Screening | |||
|---|---|---|---|
| Variables | Yes (n = 85,047) | No (n = 25,794) | p-Value (2) |
| Carbohydrate (3) | 2.26 ± 0.00 | 2.28 ± 0.00 | <0.0001 |
| Protein | 1.32 ± 0.00 | 1.27 ± 0.00 | <0.0001 |
| Cholesterol | 0.83 ± 0.00 | 0.73 ± 0.00 | <0.0001 |
| Fiber | 1.31 ± 0.00 | 1.31 ± 0.00 | 0.3398 |
| Vitamin A | 0.69 ± 0.00 | 0.67 ± 0.00 | <0.0001 |
| Vitamin B1 | 1.06 ± 0.00 | 1.05 ± 0.00 | 0.0019 |
| Vitamin B2 | 1.20 ± 0.00 | 1.17 ± 0.00 | <0.0001 |
| Niacin | 0.84 ± 0.00 | 0.84 ± 0.00 | 0.6840 |
| Vitamin C | 0.78 ± 0.00 | 0.74 ± 0.00 | <0.0001 |
| Calcium | 0.72 ± 0.00 | 0.73 ± 0.00 | 0.0014 |
| Phosphorus | 1.63 ± 0.00 | 1.59 ± 0.00 | <0.0001 |
| Sodium | 2.37 ± 0.00 | 2.32 ± 0.01 | <0.0001 |
| Potassium | 0.91 ± 0.00 | 0.88 ± 0.00 | <0.0001 |
| Iron | 1.20 ± 0.00 | 1.18 ± 0.00 | <0.0001 |
| Cancer Screening | p-Value (3) | Cancer Screening | p-Value (4) | |||
|---|---|---|---|---|---|---|
| Variables | Yes (n = 85,047) | No (n = 25,794) | Yes (n = 85,047) | No (n = 25,794) | ||
| Adjusted OR (3) (95% CI) | Adjusted OR (4) (95% CI) | |||||
| MAR (1) | ||||||
| 4 (Highest) | 1.145 (1.117–1.173) | 1 | <0.0001 | 1.092 (1.065–1.119) | 1 | <0.0001 |
| INQ (2) | ||||||
| 4 (Highest) | 1.179 (1.150–1.209) | 1 | <0.0001 | 1.125 (1.097–1.153) | 1 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, S.; Choi, Y.-J. Association between Socioecological Status, Nutrient Intake, and Cancer Screening Behaviors in Adults Aged 40 and Over: Insights from the Eighth Korea National Health and Nutrition Examination Survey (KNHANES, 2019). Nutrients 2024, 16, 1048. https://doi.org/10.3390/nu16071048
Jeong S, Choi Y-J. Association between Socioecological Status, Nutrient Intake, and Cancer Screening Behaviors in Adults Aged 40 and Over: Insights from the Eighth Korea National Health and Nutrition Examination Survey (KNHANES, 2019). Nutrients. 2024; 16(7):1048. https://doi.org/10.3390/nu16071048
Chicago/Turabian StyleJeong, Seungpil, and Yean-Jung Choi. 2024. "Association between Socioecological Status, Nutrient Intake, and Cancer Screening Behaviors in Adults Aged 40 and Over: Insights from the Eighth Korea National Health and Nutrition Examination Survey (KNHANES, 2019)" Nutrients 16, no. 7: 1048. https://doi.org/10.3390/nu16071048
APA StyleJeong, S., & Choi, Y.-J. (2024). Association between Socioecological Status, Nutrient Intake, and Cancer Screening Behaviors in Adults Aged 40 and Over: Insights from the Eighth Korea National Health and Nutrition Examination Survey (KNHANES, 2019). Nutrients, 16(7), 1048. https://doi.org/10.3390/nu16071048






