Long-Term Changes to the Microbiome, Blood Lipid Profiles and IL-6 in Female and Male Swedish Patients in Response to Bariatric Roux-en-Y Gastric Bypass
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Patients
2.3. Surgical Techniques
2.4. Material Sampling and Biomarkers
2.5. DNA Extraction, PCR Amplification, and 16S rRNA Gene Sequencing
2.6. Bioinformatics and Sequence Analysis
2.7. Statistics and Calculations
3. Results
3.1. Surgery Outcome
3.1.1. Effect on Body Weight and Quality of Life
3.1.2. Blood Parameters
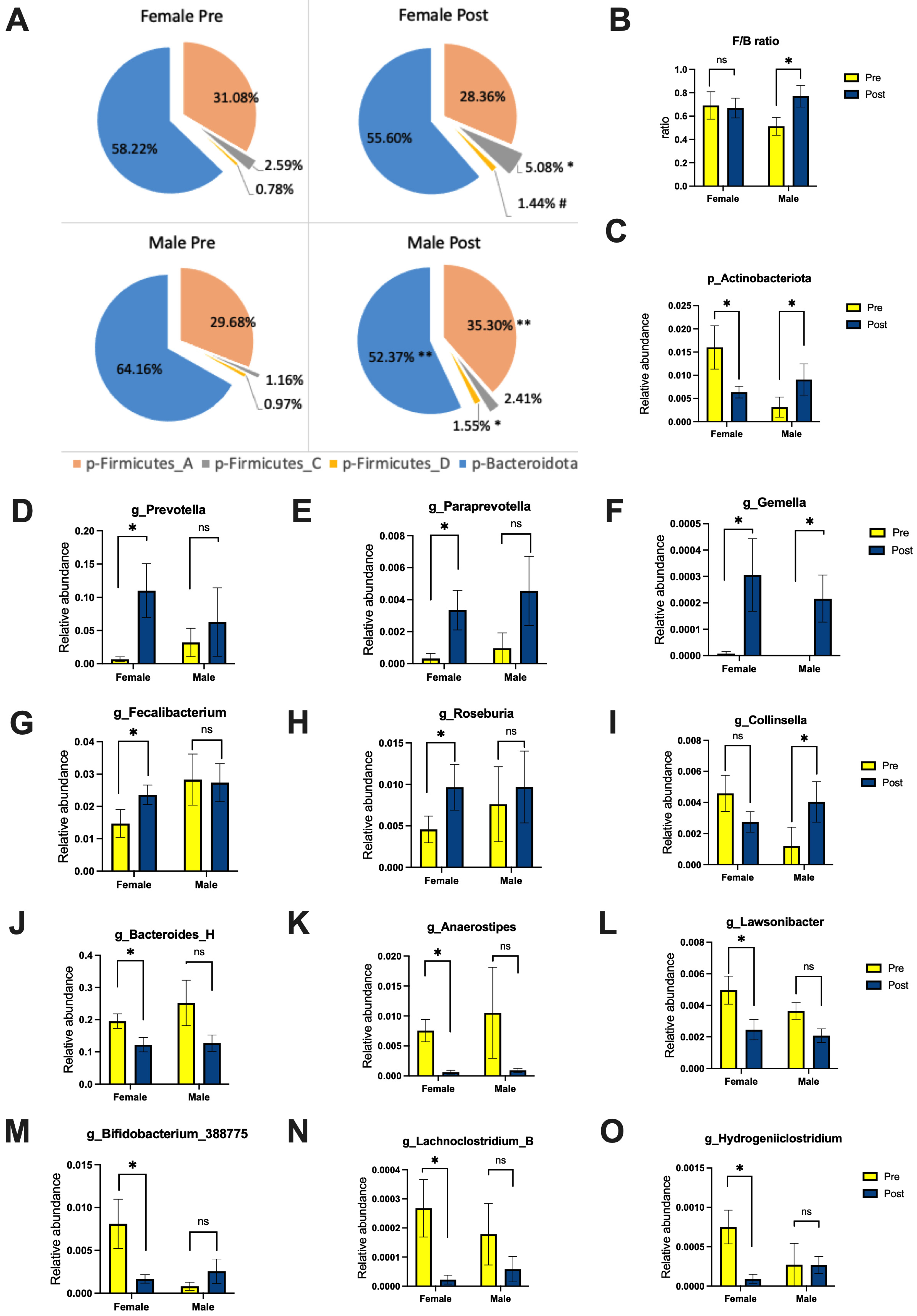
3.1.3. Gut Microbiome
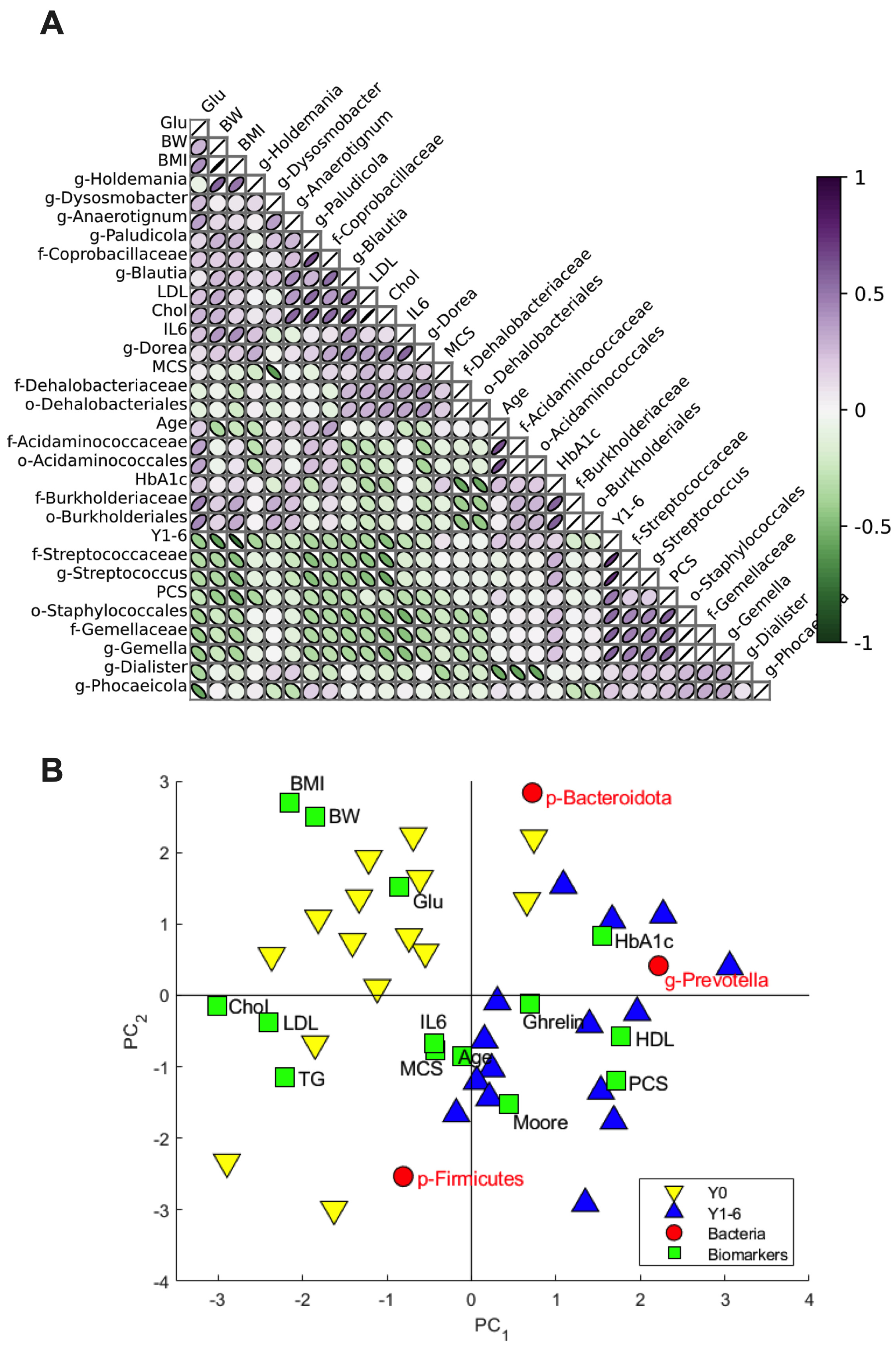
3.2. Relation of Microbial Taxa to Biomarkers
4. Discussion
RYGB Effects on Gut Microbiota
5. Conclusions
6. Limitation of Study
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martinez, K.B.; Leone, V.; Chang, E.B. Western diets, gut dysbiosis, and metabolic diseases: Are they linked? Gut Microbes 2017, 8, 130–142. [Google Scholar] [CrossRef]
- Shaw, J.E.; Sicree, R.A.; Zimmet, P.Z. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res. Clin. Pract. 2010, 87, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Rogers, R.; Eagle, T.F.; Sheetz, A.; Woodward, A.; Leibowitz, R.; Song, M.; Sylvester, R.; Corriveau, N.; Kline-Rogers, E.; Jiang, Q.; et al. The Relationship between Childhood Obesity, Low Socioeconomic Status, and Race/Ethnicity: Lessons from Massachusetts. Child. Obes. 2015, 11, 691–695. [Google Scholar] [CrossRef] [PubMed]
- Moschonis, G.; Trakman, G.L. Overweight and Obesity: The Interplay of Eating Habits and Physical Activity. Nutrients 2023, 15, 2896. [Google Scholar] [CrossRef] [PubMed]
- Olbers, T.; Björkman, S.; Lindroos, A.; Maleckas, A.; Lönn, L.; Sjöström, L.; Lönroth, H. Body composition, dietary intake, and energy expenditure after laparoscopic Roux-en-Y gastric bypass and laparoscopic vertical banded gastroplasty: A randomized clinical trial. Ann. Surg. 2006, 244, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Mantziari, S.; Dayer, A.; Duvoisin, C.; Demartines, N.; Allemann, P.; Calmes, J.M.; Favre, L.; Fournier, P.; Suter, M. Long-Term Weight Loss, Metabolic Outcomes, and Quality of Life at 10 Years After Roux-en-Y Gastric Bypass Are Independent of Patients’ Age at Baseline. Obes. Surg. 2020, 4, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, G.; Samson, S.L.; Sun, Y. Ghrelin: Much more than a hunger hormone. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Moorehead, M.K.; Ardelt-Gattinger, E.; Lechner, H.; Oria, H.E. The validation of the Moorehead-Ardelt Quality of Life Questionnaire II. Obes. Surg. 2003, 13, 684–692. [Google Scholar] [CrossRef]
- Welbourn, R.; Hollyman, M.; Kinsman, R.; Dixon, J.; Liem, R.; Ottosson, J.; Ramos, A.; Våge, V.; Al-Sabah, S.; Brown, W.; et al. Bariatric Surgery Worldwide: Baseline Demographic Description and One-Year Outcomes from the Fourth IFSO Global Registry Report 2018. Obes. Surg. 2019, 29, 782–795. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Microbial ecology: Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef]
- Shendure, J.; Ji, H. Next-generation DNA sequencing. Nat. Biotechnol. 2008, 26, 1135–1145. [Google Scholar] [CrossRef]
- Isolauri, E.; Sütas, Y.; Kankaanpää, P.; Arvilommi, H.; Salminen, S. Probiotics: Effects on immunity. Am. J. Clin. Nutr. 2001, 73, 444–450. [Google Scholar] [CrossRef]
- Bäckhed, F.; Fraser, C.M.; Ringel, Y.; Sanders, M.E.; Sartor, R.B.; Sherman, P.M.; Versalovic, J.; Young, V.; Finlay, B.B. Defining a healthy human gut microbiome: Current concepts, future directions, and clinical applications. Cell Host Microbe 2012, 12, 611–622. [Google Scholar] [CrossRef]
- Mathew, O.P.; Ranganna, K.; Milton, S.G. Involvement of the Antioxidant Effect and Anti-inflammatory Response in Butyrate-Inhibited Vascular Smooth Muscle Cell Proliferation. Pharmaceuticals 2014, 7, 1008–1027. [Google Scholar] [CrossRef] [PubMed]
- Jakobsdottir, G.; Xu, J.; Molin, G.; Ahrné, S.; Nyman, M. High-fat diet reduces the formation of butyrate, but increases succinate, inflammation, liver fat and cholesterol in rats, while dietary fibre counteracts these effects. PLoS ONE 2013, 8, e80476. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, S.; Watanabe, N.; Imaoka, A.; Okabe, Y. Preventive effects of Bifidobacterium- and Lactobacillus-fermented milk on the development of inflammatory bowel disease in senescence-accelerated mouse P1/Yit strain mice. Digestion 2001, 64, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Aoun, A.; Darwish, F.; Hamod, N. The Influence of the Gut Microbiome on Obesity in Adults and the Role of Probiotics, Prebiotics, and Synbiotics for Weight Loss. Prev. Nutr. Food Sci. 2020, 25, 113–123. [Google Scholar] [CrossRef]
- John, G.K.; Mullin, G.E. The Gut Microbiome and Obesity. Curr. Oncol. Rep. 2016, 18, 45. [Google Scholar] [CrossRef] [PubMed]
- Haro, C.; Rangel-Zúñiga, O.A.; Alcalá-Díaz, J.F.; Gómez-Delgado, F.; Pérez-Martínez, P.; Delgado-Lista, J.; Quintana-Navarro, G.M.; Landa, B.B.; Navas-Cortés, J.A.; Tena-Sempere, M.; et al. Intestinal Microbiota Is Influenced by Gender and Body Mass Index. PLoS ONE 2016, 11, e0154090. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; DiBaise, J.K.; Zuccolo, A.; Kudrna, D.; Braidotti, M.; Yu, Y.; Parameswaran, P.; Crowell, M.D.; Wing, R.; Rittmann, B.E.; et al. Human gut microbiota in obesity and after gastric bypass. Proc. Natl. Acad. Sci. USA 2009, 106, 2365–2370. [Google Scholar] [CrossRef] [PubMed]
- Palleja, A.; Kashani, A.; Allin, K.H.; Nielsen, T.; Zhang, C.; Li, Y.; Brach, T.; Liang, S.; Feng, Q.; Jørgensen, N.B.; et al. Roux-en-Y gastric bypass surgery of morbidly obese patients induces swift and persistent changes of the individual gut microbiota. Genome Med. 2016, 8, 67. [Google Scholar] [CrossRef] [PubMed]
- Sanmiguel, C.; Gupta, A.; Mayer, E.A. Gut Microbiome and Obesity: A Plausible Explanation for Obesity. Curr. Obes. Rep. 2015, 4, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Campanello, M.; Lindskog, S.; Zilling, T. Linear and circular stapled gastrojejunal anastomoses in Roux-en-Y gastric bypass: Stomal diameter at onset and at long-term follow-up. ANZ J. Surg. 2022, 92, 2896–2900. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, M.; Karlsson, J.; Ware, J.E., Jr. The Swedish SF-36 Health Survey-I. Evaluation of data quality, scaling assumptions, reliability and construct validity across general populations in Sweden. Soc. Sci. Med. 1995, 41, 1349–1358. [Google Scholar] [CrossRef]
- Kozich, J.J.; Westcott, S.L.; Baxter, N.T.; Highlander, S.K.; Schloss, P.D. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl. Environ. Microbiol. 2013, 79, 5112–5120. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Schmieder, R.; Edwards, R. Quality control and preprocessing of metagenomic datasets. Bioinformatics 2011, 27, 863–864. [Google Scholar] [CrossRef]
- McDonald, D.; Price, M.N.; Goodrich, J.; Nawrocki, E.P.; DeSantis, T.Z.; Probst, A.; Andersen, G.L.; Knight, R.; Hugenholtz, P. An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. ISME J. 2012, 6, 610–618. [Google Scholar] [CrossRef]
- Chakraborti, C.K. New-found link between microbiota and obesity. World J. Gastrointest. Pathophysiol. 2015, 6, 110–119. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, H.; Lu, M.; Cai, J.; Lu, B.; Luo, C.; Dai, M. Habitual Diet Pattern Associations with Gut Microbiome Diversity and Composition: Results from a Chinese Adult Cohort. Nutrients 2022, 14, 2639. [Google Scholar] [CrossRef]
- Asnicar, F.; Berry, S.E.; Valdes, A.M.; Nguyen, L.H.; Piccinno, G.; Drew, D.A.; Leeming, E.; Gibson, R.; Le Roy, C.; Khatib, H.A.; et al. Microbiome connections with host metabolism and habitual diet from 1,098 deeply phenotyped individuals. Nat. Med. 2021, 27, 321–332. [Google Scholar] [CrossRef]
- Mohebali, N.; Ekat, K.; Kreikemeyer, B.; Breitrück, A. Barrier Protection and Recovery Effects of Gut Commensal Bacteria on Differentiated Intestinal Epithelial Cells In Vitro. Nutrients 2020, 12, 2251. [Google Scholar] [CrossRef]
- Kovatcheva-Datchary, P.; Nilsson, A.; Akrami, R.; Lee, Y.S.; De Vadder, F.; Arora, T.; Hallen, A.; Martens, E.; Björck, I.; Bäckhed, F. Dietary Fiber-Induced Improvement in Glucose Metabolism Is Associated with Increased Abundance of Prevotella. Cell Metab. 2015, 22, 971–982. [Google Scholar] [CrossRef]
- Patra, A.K.; Yu, Z. Genomic Insights into the Distribution of Peptidases and Proteolytic Capacity among Prevotella and Paraprevotella Species. Microbiol. Spectr. 2022, 10, e0218521. [Google Scholar] [CrossRef] [PubMed]
- Lange, J.; Königsrainer, A. Malnutrition as a Complication of Bariatric Surgery—A Clear and Present Danger? Visc. Med. 2019, 35, 305–311. [Google Scholar] [CrossRef]
- Gomez-Arango, L.F.; Barrett, H.L.; Wilkinson, S.A.; Callaway, L.K.; McIntyre, H.D.; Morrison, M.; Dekker Nitert, M. Low dietary fiber intake increases Collinsella abundance in the gut microbiota of overweight and obese pregnant women. Gut Microbes 2018, 9, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Tanner, A.C.; Sonis, A.L.; Lif Holgerson, P.; Starr, J.R.; Nunez, Y.; Kressirer, C.A.; Paster, B.J.; Johansson, I. White-spot lesions and gingivitis microbiotas in orthodontic patients. J. Dent. Res. 2012, 91, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Lau, E.; Belda, E.; Picq, P.; Carvalho, D.; Ferreira-Magalhães, M.; Silva, M.M.; Barroso, I.; Correia, F.; Vaz, C.P.; Miranda, I.; et al. Gut microbiota changes after metabolic surgery in adult diabetic patients with mild obesity: A randomised controlled trial. Diabetol. Metab. Syndr. 2021, 13, 56. [Google Scholar] [CrossRef]

| Before Surgery | After Surgery | |||||
|---|---|---|---|---|---|---|
| Blood Markers, Units | Female | Male | Total | Female | Male | Total |
| Alb, mmol/L | 38.27 ± 0.98 | 40.00 ± 2.68 | 38.73 ± 0.98 | 37.00 ± 1.018 | 39.50 ± 2.67 | 37.67 ± 1.02 |
| Fe, mmol/L | 14.82 ± 1.60 | 16.75 ± 3.09 | 15.33 ± 1.39 | 14.46 ± 1.19 | 23.25 ± 2.93 | 16.80 ± 1.52 |
| Lipid metabolism: | ||||||
| Chol, mmol/L | 5.17 ± 0.35 | 6.53 ± 0.71 | 5.53 ± 0.35 | 4.10 ± 0.25 ** | 4.95 ± 0.30 * | 4.33 ± 0.22 *** |
| Tg, mmol/L | 1.17 ± 0.21 | 1.25 ± 0.22 | 1.19 ± 0.16 | 0.81 ± 0.11 # | 1.30 ± 0.23 | 0.94 ± 0.11 # |
| HDL, mmol/L | 1.17 ± 0.10 | 1.08 ± 0.10 | 1.15 ± 0.08 | 1.55 ± 0.11 ** | 1.55 ± 0.30 * | 1.55 ± 0.11 *** |
| LDL, mmol/L | 3.16 ± 0.19 | 4.68 ± 0.61 | 3.57 ± 0.27 | 2.68 ± 0.25 | 3.40 ± 0.29 ** | 2.87 ± 0.21 ** |
| LDL/HDL, ratio | 2.90 ± 0.31 | 4.42 ± 0.60 | 3.31 ± 0.32 | 1.80 ± 0.18 *** | 2.36 ± 0.33 *** | 1.95 ± 0.17 **** |
| Glucose metabolism: | ||||||
| Glu, mmol/L | 5.67 ± 0.42 | 5.35 ± 1.55 | 5.59 ± 0.31 | 5.15 ± 0.30 * | 4.70 ± 0.18 * | 5.03 ± 0.26 ** |
| HbA1c, mmol/mol | 38.73 ± 19.10 | 30.28 ± 15.13 | 38.00 ± 1.94 | 39.7 ± 11.99 | 34.20 ± 2.86 | 37.87 ± 2.61 |
| Appetite regulation: | ||||||
| Ghrelin, pg/mL | 623.36 ± 106.23 | 665.5 ± 49.59 | 634.60 ± 77.95 | 801.82 ± 124.17 ** | 809.75 ± 42.89 | 803.93 ± 90.45 *** |
| Inflammation: | ||||||
| IL-6, pg/mL | 4.21 ± 0.89 | 2.89 ± 0.92 | 3.69 ± 0.74 | 2.43 ± 0.62 ** | 2.64 ± 1.24 | 2.48 ± 0.65 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prykhodko, O.; Burleigh, S.; Campanello, M.; Iresjö, B.-M.; Zilling, T.; Ljungh, Å.; Smedh, U.; Hållenius, F.F. Long-Term Changes to the Microbiome, Blood Lipid Profiles and IL-6 in Female and Male Swedish Patients in Response to Bariatric Roux-en-Y Gastric Bypass. Nutrients 2024, 16, 498. https://doi.org/10.3390/nu16040498
Prykhodko O, Burleigh S, Campanello M, Iresjö B-M, Zilling T, Ljungh Å, Smedh U, Hållenius FF. Long-Term Changes to the Microbiome, Blood Lipid Profiles and IL-6 in Female and Male Swedish Patients in Response to Bariatric Roux-en-Y Gastric Bypass. Nutrients. 2024; 16(4):498. https://doi.org/10.3390/nu16040498
Chicago/Turabian StylePrykhodko, Olena, Stephen Burleigh, Magnus Campanello, Britt-Marie Iresjö, Thomas Zilling, Åsa Ljungh, Ulrika Smedh, and Frida Fåk Hållenius. 2024. "Long-Term Changes to the Microbiome, Blood Lipid Profiles and IL-6 in Female and Male Swedish Patients in Response to Bariatric Roux-en-Y Gastric Bypass" Nutrients 16, no. 4: 498. https://doi.org/10.3390/nu16040498
APA StylePrykhodko, O., Burleigh, S., Campanello, M., Iresjö, B.-M., Zilling, T., Ljungh, Å., Smedh, U., & Hållenius, F. F. (2024). Long-Term Changes to the Microbiome, Blood Lipid Profiles and IL-6 in Female and Male Swedish Patients in Response to Bariatric Roux-en-Y Gastric Bypass. Nutrients, 16(4), 498. https://doi.org/10.3390/nu16040498





