Nutritional and Microbial Strategies for Treating Acne, Alopecia, and Atopic Dermatitis
Abstract
1. Introduction
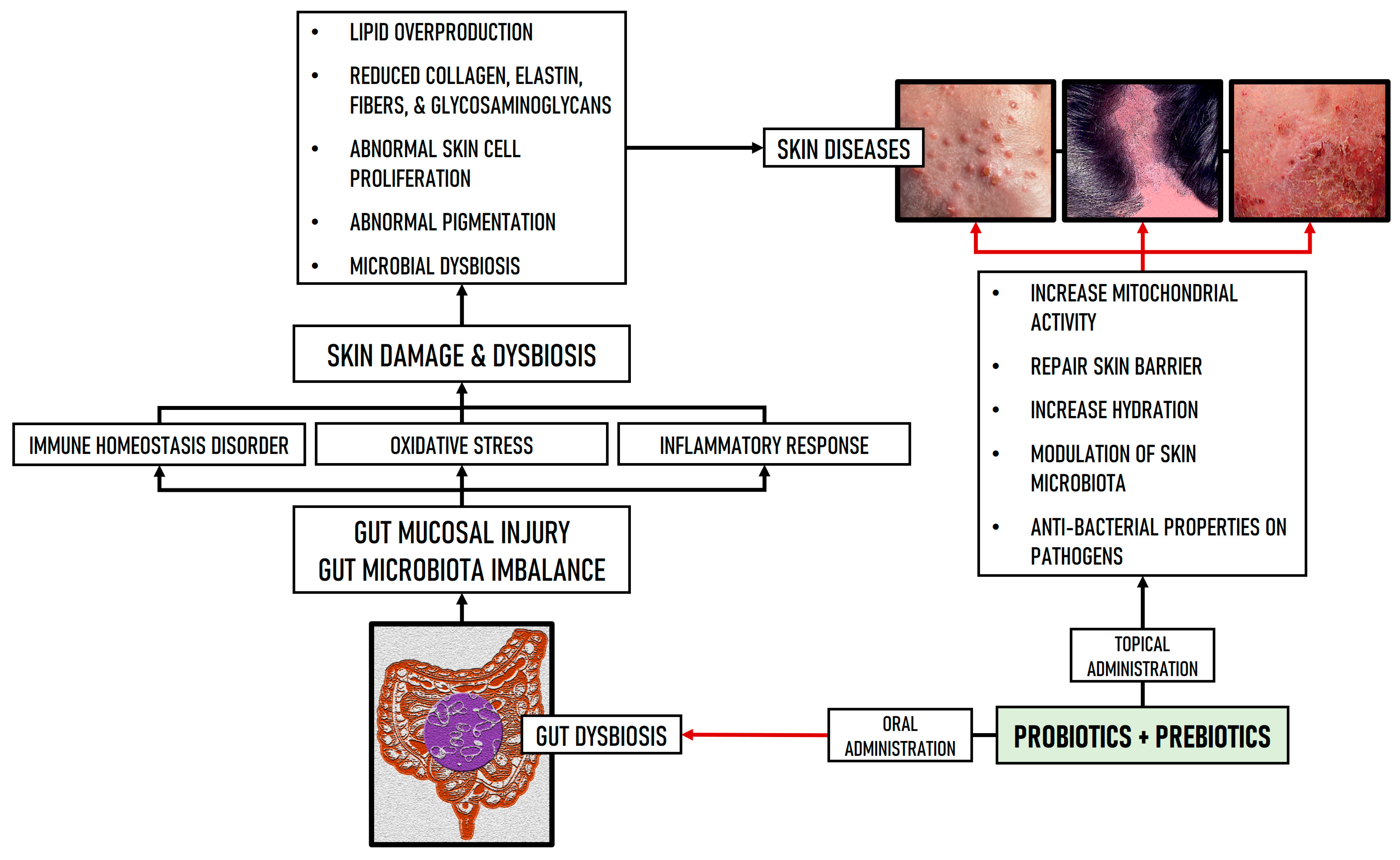
2. Interactions Between GM, Nutrients, and Skin Health
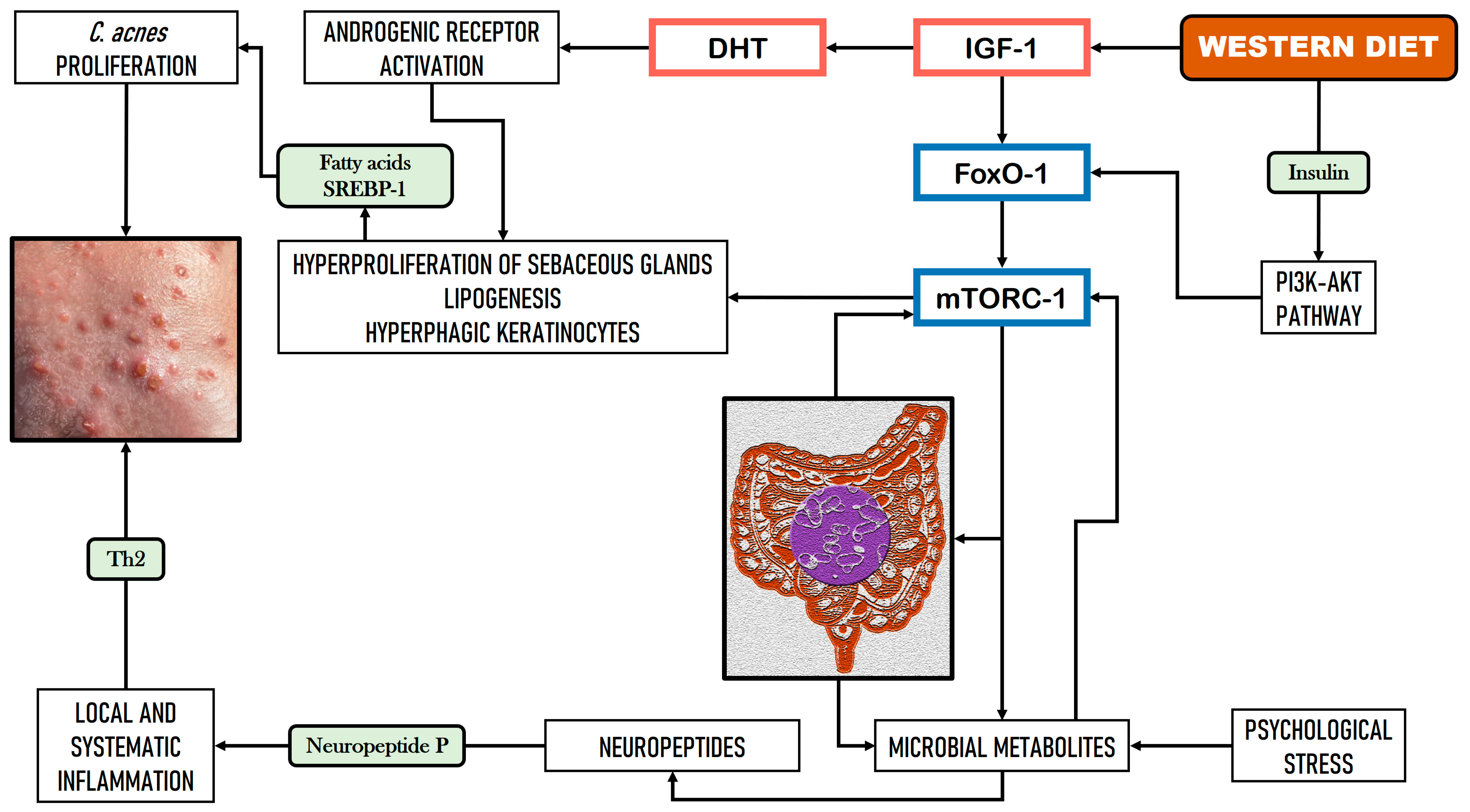
2.1. Implications of GM and Dietary Factors in Acne Vulgaris
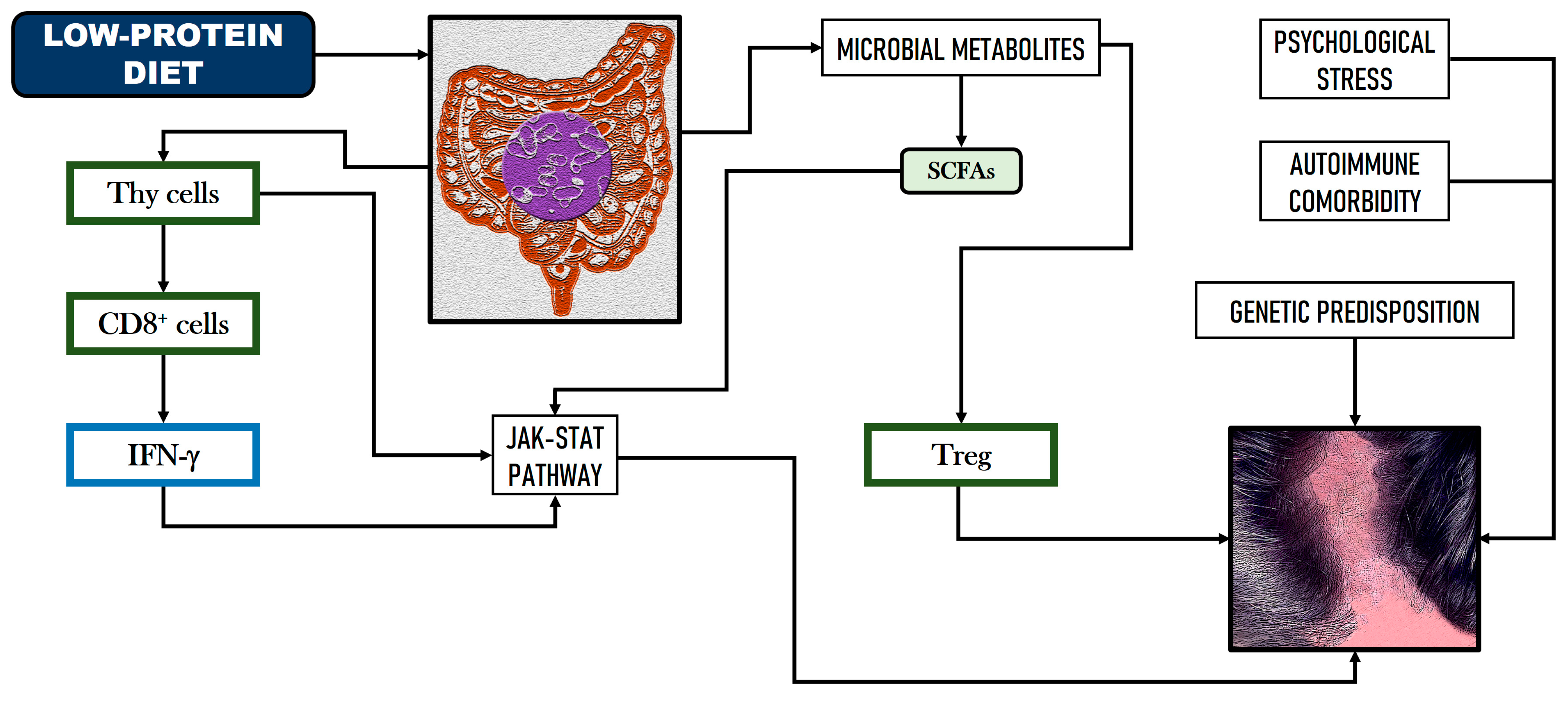
2.2. Implications of GM and Dietary Factors in Alopecia
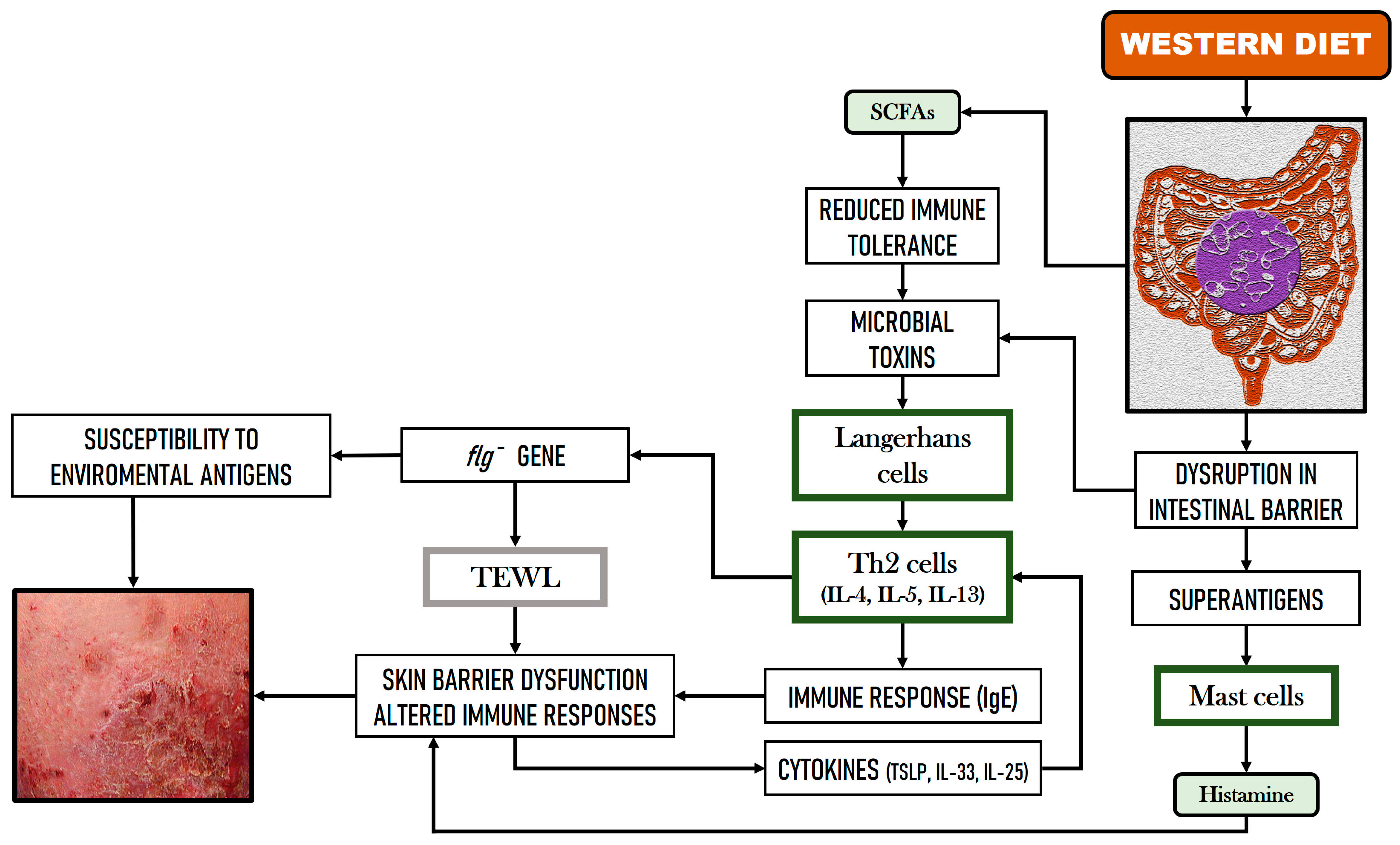
2.3. Implications of GM and Dietary Factors in Atopic Dermatitis (AD)
3. Influence of Healthy Diets on Acne, Alopecia, and Atopic Dermatitis
3.1. Vegetarian Diets
3.2. Mediterranean Diet
4. Microbial Therapeutic Tools for Skin Diseases
4.1. Impact of Microbial Therapeutics on Acne
4.2. Impact of Microbial Therapeutics on Alopecia
4.3. Impact of Microbial Therapeutics on Atopic Dermatitis
5. Discussion
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lal, M.K.; Sharma, E.; Tiwari, R.K.; Devi, R.; Mishra, U.N.; Thakur, R.; Gupta, R.; Dey, A.; Lal, P.; Sahu, S.K.; et al. Nutrient-mediated perception and signalling in human metabolism: A perspective of nutrigenomics. Int. J. Mol. Sci. 2022, 23, 11305. [Google Scholar] [CrossRef] [PubMed]
- Borrego-Ruiz, A.; Borrego, J.J. Human gut microbiome, diet, and mental disorders. Int. Microbiol. 2024, 27, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Flint, H.J. The impact of nutrition on the human microbiome. Nutr. Rev. 2012, 70, S10–S13. [Google Scholar] [CrossRef]
- Mahmud, M.R.; Akter, S.; Tamanna, S.K.; Mazumder, L.; Esti, I.Z.; Banerjee, S.; Akter, S.; Hasan, M.R.; Acharjee, M.; Hossain, M.S.; et al. Impact of gut microbiome on skin health: Gut-skin axis observed through the lenses of therapeutics and skin diseases. Gut Microbes 2022, 14, 2096995. [Google Scholar] [CrossRef]
- De Pessemier, B.; Grine, L.; Debaere, M.; Maes, A.; Paetzold, B.; Callewaert, C. Gut–skin axis: Current knowledge of the interrelationship between microbial dysbiosis and skin conditions. Microorganisms 2021, 9, 353. [Google Scholar] [CrossRef] [PubMed]
- Glatthardt, T.; Lima, R.D.; de Mattos, R.M.; Ferreira, R.B.R. Microbe interactions within the skin microbiome. Antibiotics 2024, 13, 49. [Google Scholar] [CrossRef]
- Townsend, E.C.; Kalan, L.R. The dynamic balance of the skin microbiome across the lifespan. Biochem. Soc. Trans. 2023, 51, 71–86. [Google Scholar] [CrossRef]
- Cundell, A.M. Microbial ecology of the human skin. Microb. Ecol. 2018, 76, 113–120. [Google Scholar] [CrossRef]
- Nguyen, U.T.; Kalan, L.R. Forgotten fungi: The importance of the skin mycobiome. Curr. Opin. Microbiol. 2022, 70, 102235. [Google Scholar] [CrossRef]
- Hannigan, G.D.; Meisel, J.S.; Tyldsley, A.S.; Zheng, Q.; Hodkinson, B.P.; SanMiguel, A.J.; Minot, S.; Bushman, F.D.; Grice, E.A. The human skin double-stranded DNA virome: Topographical and temporal diversity, genetic enrichment, and dynamic associations with the host microbiome. mBio 2015, 6, e01578-15. [Google Scholar] [CrossRef]
- Natarelli, N.; Gahoonia, N.; Sivamani, R.K. Bacteriophages and the microbiome in dermatology: The role of the phageome and a potential therapeutic strategy. Int. J. Mol. Sci. 2023, 24, 2695. [Google Scholar] [CrossRef]
- Chen, Y.E.; Fischbach, M.A.; Belkaid, Y. Skin microbiota-host interactions. Nature 2018, 553, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Carmona-Cruz, S.; Orozco-Covarrubias, L.; Sáez de Ocariz, M. The human skin microbiome in selected cutaneous diseases. Front. Cell. Infect. Microbiol. 2022, 12, 834135. [Google Scholar] [CrossRef] [PubMed]
- Bowe, W.P.; Logan, A.C. Acne vulgaris, probiotics and the gut-brain-skin axis—Back to the future? Gut Pathog. 2011, 3, 1. [Google Scholar] [CrossRef]
- Shah, K.R.; Boland, C.R.; Patel, M.; Thrash, B.; Menter, A. Cutaneous manifestations of gastrointestinal disease: Part I. J. Am. Acad. Dermatol. 2013, 68, e1–e21. [Google Scholar] [CrossRef]
- Thrash, B.; Patel, M.; Shah, K.R. Cutaneous manifestations of gastrointestinal disease: Part II. J. Am. Acad. Dermatol. 2013, 68, e1–e33. [Google Scholar] [CrossRef] [PubMed]
- Guet-Revillet, H.; Jais, J.P.; Ungeheuer, M.N.; Coignard-Biehler, H.; Duchatelet, S.; Delage, M.; Lam, T.; Hovnanian, A.; Lortholary, O.; Nassif, X.; et al. The microbiological landscape of anaerobic infections in hidradenitis suppurativa: A prospective metagenomic study. Clin. Infect. Dis. 2017, 65, 282–291. [Google Scholar] [CrossRef]
- O’Neill, C.A.; Monteleone, G.; McLaughlin, J.; Paus, R. The gut-skin axis in health and disease: A paradigm with therapeutic implications. BioEssays 2016, 38, 1167–1176. [Google Scholar] [CrossRef]
- Vaughn, A.R.; Notay, M.; Clark, A.K.; Sivamani, R.K. Skin-gut axis: The relationship between intestinal bacteria and skin health. World J. Dermatol. 2017, 6, 52–58. [Google Scholar] [CrossRef]
- Cianciulli, A.; Calvello, R.; Porro, C.; Lofrumento, D.D.; Panaro, M.A. Inflammatory skin diseases: Focus on the role of suppressors of cytokine signaling (SOCS) proteins. Cells 2024, 13, 505. [Google Scholar] [CrossRef]
- Baba, H.; Masuyama, A.; Yoshimura, C.; Aoyama, Y.; Takano, T.; Ohki, K. Oral intake of Lactobacillus helveticus-fermented milk whey decreased transepidermal water loss and prevented the onset of sodium dodecylsulfate-induced dermatitis in mice. Biosci. Biotechnol. Biochem. 2010, 74, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Philippe, D.; Blum, S.; Benyacoub, J. Oral Lactobacillus paracasei improves skin barrier function recovery and reduces local skin inflammation. Eur. J. Dermatol. 2011, 21, 279–280. [Google Scholar] [CrossRef] [PubMed]
- Gueniche, A.; Philippe, D.; Bastien, P.; Reuteler, G.; Blum, S.; Castiel-Higounenc, I.; Breton, L.; Benyacoub, J. Randomised double-blind placebo-controlled study of the effect of Lactobacillus paracasei NCC 2461 on skin reactivity. Benef. Microbes 2014, 5, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Borrego-Ruiz, A.; Borrego, J.J. An updated overview on the relationship between human gut microbiome dysbiosis and psychiatric and psychological disorders. Prog. Neuropsychopharmacol. Biol. Psychiatry 2024, 128, 110861. [Google Scholar] [CrossRef] [PubMed]
- Forbes, J.D.; Van Domselaar, G.; Bernstein, C.N. The gut microbiota in immune-mediated inflammatory diseases. Front. Microbiol. 2016, 7, 1081. [Google Scholar] [CrossRef]
- Salem, I.; Ramser, A.; Isham, N.; Ghannoum, M.A. The gut microbiome as a major regulator of the gut-skin axis. Front. Microbiol. 2018, 9, 1459. [Google Scholar] [CrossRef]
- Schwarz, A.; Bruhs, A.; Schwarz, T. The short-chain fatty acid sodium butyrate functions as a regulator of the skin immune system. J. Investig. Dermatol. 2017, 1, 855–864. [Google Scholar] [CrossRef]
- Gallitano, S.M.; Berson, D.S. How acne bumps cause the blues: The influence of acne vulgaris on self-esteem. Int. J. Womens Dermatol. 2018, 4, 12–17. [Google Scholar] [CrossRef]
- Morshed, A.S.M.; Noor, T.; Uddin Ahmed, M.A.; Mili, F.S.; Ikram, S.; Rahman, M.; Ahmed, S.; Uddin, M.B. Understanding the impact of acne vulgaris and associated psychological distress on self-esteem and quality of life via regression modeling with CADI, DLQI, and WHOQoL. Sci. Rep. 2023, 13, 21084. [Google Scholar] [CrossRef]
- Vasam, M.; Korutla, S.; Bohara, R.A. Acne vulgaris: A review of the pathophysiology, treatment, and recent nanotechnology based advances. Biochem. Biophys. Rep. 2023, 36, 101578. [Google Scholar] [CrossRef]
- Deng, Y.; Wang, H.; Zhou, J.; Mou, Y.; Wang, G.; Xiong, X. Patients with acne vulgaris have a distinct gut microbiota in comparison with healthy controls. Acta Derm. Venereol. 2018, 98, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.M.; Zhao, H.J.; Guo, D.Y.; Zhu, P.Q.; Zhang, C.L.; Jiang, W. Gut microbiota alterations in moderate to severe acne vulgaris patients. J. Dermatol. 2018, 45, 1166–1171. [Google Scholar] [CrossRef]
- Thompson, K.G.; Rainer, B.M.; Antonescu, C.; Florea, L.; Mongodin, E.F.; Kang, S.; Chien, A.L. Minocycline and its impact on microbial dysbiosis in the skin and gastrointestinal tract of acne patients. Ann. Dermatol. 2020, 32, 21–30. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, L.; Chen, L.; Zhou, L.; Xiong, X.; Deng, Y. Gender-specific differences in gut microbiota composition associated with microbial metabolites for patients with acne vulgaris. Ann. Dermatol. 2021, 33, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Arrones, O.M.; Serrano-Villar, S.; Perez-Brocal, V.; Saceda-Corralo, D.; Morales-Raya, C.; Rodrigues-Barata, R.; Moya, A.; Jaen-Olasolo, P.; Vano-Galvan, S. Analysis of the gut microbiota in alopecia areata: Identification of bacterial biomarkers. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 400–405. [Google Scholar] [CrossRef]
- Juhasz, M.; Chen, S.; Khosrovi-Eghbal, A.; Ekelem, C.; Landaverde, Y.; Baldi, P.; Mesinkovska, N.A. Characterizing the skin and gut microbiome of alopecia areata patients. SKIN J. Cutan. Med. 2020, 4, 23–30. [Google Scholar] [CrossRef]
- Brzychcy, K.; Dróżdż, I.; Skoczylas, S.; Płoszaj, T.; Sobolewska-Sztychny, D.; Skibińska, M.; Narbutt, J.; Lesiak, A. Gut microbiota in alopecia areata. Postep. Derm. Alergol. 2022, 39, 1162–1170. [Google Scholar] [CrossRef]
- Song, H.; Yoo, Y.; Hwang, J.; Na, Y.C.; Kim, H.S. Faecalibacterium prausnitzii subspecies-level dysbiosis in the human gut microbiome underlying atopic dermatitis. J. Allergy Clin. Immunol. 2016, 137, 852–860. [Google Scholar] [CrossRef]
- Nylund, L.; Nermes, M.; Isolauri, E.; Salminen, S.; de Vos, W.M.; Satokari, R. Severity of atopic disease inversely correlates with intestinal microbiota diversity and butyrate-producing bacteria. Allergy 2015, 70, 241–244. [Google Scholar] [CrossRef]
- Lee, E.; Lee, S.Y.; Kang, M.J.; Kim, K.; Won, S.; Kim, B.J.; Choi, K.Y.; Kim, B.S.; Cho, H.J.; Kim, Y.; et al. Clostridia in the gut and onset of atopic dermatitis via eosinophilic inflammation. Ann. Allergy Asthma Immunol. 2016, 117, 91–92.e1. [Google Scholar] [CrossRef]
- Reddel, S.; Del Chierico, F.; Quagliariello, A.; Giancristoforo, S.; Vernocchi, P.; Russo, A.; Fiocchi, A.; Rossi, P.; Putignani, L.; El Hachem, M. Gut microbiota profile in children affected by atopic dermatitis and evaluation of intestinal persistence of a probiotic mixture. Sci. Rep. 2019, 9, 4996. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.M.; Lee, S.Y.; Kang, M.J.; Kim, B.S.; Lee, M.J.; Jung, S.S.; Yoon, J.S.; Cho, H.J.; Lee, E.; Yang, S.I.; et al. Imbalance of gut Streptococcus, Clostridium, and Akkermansia determines the natural course of atopic dermatitis in infant. Allergy Asthma Immunol. Res. 2020, 12, 322–337. [Google Scholar] [CrossRef] [PubMed]
- Nekrasova, A.I.; Kalashnikova, I.G.; Bobrova, M.M.; Korobeinikova, A.V.; Bakoev, S.Y.; Ashniev, G.A.; Petryaikina, E.S.; Nekrasov, A.S.; Zagainova, A.V.; Lukashina, M.V.; et al. Characteristics of the gut microbiota in regard to atopic dermatitis and food allergies of children. Biomedicines 2024, 12, 553. [Google Scholar] [CrossRef] [PubMed]
- Ryguła, I.; Pikiewicz, W.; Kaminiów, K. Impact of diet and nutrition in patients with acne vulgaris. Nutrients 2024, 16, 1476. [Google Scholar] [CrossRef]
- Melnik, B.C. Linking diet to acne metabolomics, inflammation, and comedogenesis: An update. Clin. Cosmet. Investig. Dermatol. 2015, 8, 371–388. [Google Scholar] [CrossRef]
- Mounessa, J.; Caravaglio, J.V.; Domozych, R.; Chapman, S.; Dellavalle, R.P.; Dunnick, C.A.; Norris, D. Commonly prescribed medications associated with alopecia. J. Am. Acad. Dermatol. 2023, 88, 1326–1337.e2. [Google Scholar] [CrossRef] [PubMed]
- Oiwoh, S.O.; Enitan, A.O.; Adegbosin, O.T.; Akinboro, A.O.; Onayemi, E.O. Androgenetic alopecia: A review. Niger. Postgrad. Med. J. 2024, 31, 85–92. [Google Scholar] [CrossRef]
- Sibbald, C. Alopecia areata: An updated review for 2023. J. Cutan. Med. Surg. 2023, 27, 241–259. [Google Scholar] [CrossRef]
- Nair, L.; Dai, Z.; Christiano, A.M. Gut microbiota plays a role in the development of alopecia areata. J. Investig. Dermatol. 2017, 137, 112. [Google Scholar] [CrossRef]
- Rebello, D.; Wang, E.; Yen, E.; Lio, P.A.; Kelly, C.R. Hair growth in two alopecia patients after fecal microbiota transplant. ACG Case Rep. J. 2017, 4, e107. [Google Scholar] [CrossRef]
- Simakou, T.; Butcher, J.P.; Reid, S.; Henriquez, F.L. Alopecia areata: A multifactorial autoimmune condition. J. Autoimmun. 2019, 98, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Migacz-Gruszka, K.; Branicki, W.; Obtulowicz, A.; Pirowska, M.; Gruszka, K.; Wojas-Pelc, A. What’s new in the pathophysiology of alopecia areata? The possible contribution of skin and gut microbiome in the pathogenesis of alopecia—Big opportunities, big challenges, and novel perspectives. Int. J. Trichology 2019, 11, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.; Sangwan, A. Dietary protein deficit and deregulated autophagy: A new clinico-diagnostic perspective in pathogenesis of early aging, skin, and hair disorders. Indian Dermatol. Online J. 2019, 10, 115–124. [Google Scholar] [CrossRef]
- Pham, C.T.; Romero, K.; Almohanna, H.M.; Griggs, J.; Ahmed, A.; Tosti, A. The role of diet as an adjuvant treatment in scarring and nonscarring alopecia. Skin Appendage Disord. 2020, 6, 88–96. [Google Scholar] [CrossRef]
- Afshari, M.; Kolackova, M.; Rosecka, M.; Čelakovská, J.; Krejsek, J. Unraveling the skin; a comprehensive review of atopic dermatitis, current understanding, and approaches. Front. Immunol. 2024, 15, 1361005. [Google Scholar] [CrossRef] [PubMed]
- Lönndahl, L.; Abdelhadi, S.; Holst, M.; Lonne-Rahm, S.B.; Nordlind, K.; Johansson, B. Psychological stress and atopic dermatitis: A focus group study. Ann. Dermatol. 2023, 35, 342–347. [Google Scholar] [CrossRef]
- Sandhu, J.K.; Wu, K.K.; Bui, T.L.; Armstrong, A.W. Association between atopic dermatitis and suicidality: A systematic review and meta-analysis. JAMA Dermatol. 2019, 155, 178–187. [Google Scholar] [CrossRef]
- Hu, C.; van Meel, E.R.; Medina-Gomez, C.; Kraaij, R.; Barroso, M.; Kiefte-de Jong, J.; Radjabzadeh, D.; Pasmans, S.; de Jong, N.W.; de Jongste, J.C.; et al. A population-based study on associations of stool microbiota with atopic diseases in school-age children. J. Allergy Clin. Immunol. 2021, 148, 612–620. [Google Scholar] [CrossRef]
- Lee, S.Y.; Lee, E.; Park, Y.M.; Hong, S.J. Microbiome in the gut-skin axis in atopic dermatitis. Allergy Asthma Immunol. Res. 2018, 10, 354–362. [Google Scholar] [CrossRef]
- Fang, Z.; Li, L.; Zhang, H.; Zhao, J.; Lu, W.; Chen, W. Gut microbiota, probiotics, and their interactions in prevention and treatment of atopic dermatitis: A review. Front. Immunol. 2021, 12, 720393. [Google Scholar] [CrossRef]
- Belkaid, Y.; Hand, T.W. Role of the microbiota in immunity and inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef] [PubMed]
- Moniaga, C.S.; Tominaga, M.; Takamori, K. An altered skin and gut microbiota are involved in the modulation of itch in atopic dermatitis. Cells 2022, 11, 3930. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, B.E.; Leung, D.Y.M. Pathophysiology of atopic dermatitis: Clinical implications. Allergy Asthma Proc. 2019, 40, 84–92. [Google Scholar] [CrossRef]
- Guo, X.; Li, J.; Tang, R.; Zhang, G.; Zeng, H.; Wood, R.J.; Liu, Z. High fat diet alters gut microbiota and the expression of Paneth cell-antimicrobial peptides preceding changes of circulating inflammatory cytokines. Mediators Inflamm. 2017, 2017, 9474896. [Google Scholar] [CrossRef]
- Devereux, G.; Seaton, A. Diet as a risk factor for atopy and asthma. J. Allergy Clin. Immunol. 2005, 115, 1109–1118. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Chaudhary, S.M.; Khungar, N.; Aulakh, S.K.; Idris, H.; Singh, A.; Sharma, K. Dietary influences on skin health in common dermatological disorders. Cureus 2024, 16, e55282. [Google Scholar] [CrossRef] [PubMed]
- Borrego-Ruiz, A. Una revisión crítica sobre la influencia de la dieta vegetariana en la salud mental. Rev. Esp. Nutr. Comun. 2024, 30. Available online: https://www.renc.es/imagenes/auxiliar/files/RENC-D-24-0027.pdf (accessed on 5 August 2024).
- Baldwin, H.; Tan, J. Effects of diet on acne and its response to treatment. Am. J. Clin. Dermatol. 2021, 22, 55–65. [Google Scholar] [CrossRef]
- Stewart, T.J.; Bazergy, C. Hormonal and dietary factors in acne vulgaris versus controls. Dermatoendocrinol. 2018, 10, e1442160. [Google Scholar] [CrossRef]
- Dréno, B. What is new in the pathophysiology of acne, an overview. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 8–12. [Google Scholar] [CrossRef]
- Flores-Balderas, X.; Peña-Peña, M.; Rada, K.M.; Alvarez-Alvarez, Y.Q.; Guzmán-Martín, C.A.; Sánchez-Gloria, J.L.; Huang, F.; Ruiz-Ojeda, D.; Morán-Ramos, S.; Springall, R.; et al. Beneficial effects of plant-based diets on skin health and inflammatory skin diseases. Nutrients 2023, 15, 2842. [Google Scholar] [CrossRef] [PubMed]
- Suppiah, T.S.S.; Sundram, T.K.M.; Tan, E.S.S.; Lee, C.K.; Bustami, N.A.; Tan, C.K. Acne vulgaris and its association with dietary intake: A Malaysian perspective. Asia Pac. J. Clin. Nutr. 2018, 27, 1141–1145. [Google Scholar] [CrossRef] [PubMed]
- Aalemi, A.K.; Anwar, I.; Chen, H. Dairy consumption and acne: A case control study in Kabul, Afghanistan. Clin. Cosmet. Investig. Dermatol. 2019, 12, 481–487. [Google Scholar] [CrossRef]
- Sanchez-Pellicer, P.; Navarro-Moratalla, L.; Núñez-Delegido, E.; Ruzafa-Costas, B.; Agüera-Santos, J.; Navarro-López, V. Acne, microbiome, and probiotics: The gut-skin axis. Microorganisms 2022, 10, 1303. [Google Scholar] [CrossRef]
- Meixiong, J.; Ricco, C.; Vasavda, C.; Ho, B.K. Diet and acne: A systematic review. JAAD Int. 2022, 7, 95–112. [Google Scholar] [CrossRef]
- Fusano, M. Veganism in acne, atopic dermatitis, and psoriasis: Benefits of a plant-based diet. Clin. Dermatol. 2023, 41, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.; Douglas, S.C.; Hall, J.M. Endocrine disrupting chemicals, hormone receptors, and acne vulgaris: A connecting hypothesis. Cells 2021, 10, 1439. [Google Scholar] [CrossRef]
- Riyanto, P.; Subchan, P.; Lelyana, R. Advantage of soybean isoflavone as antiandrogen on acne vulgaris. Dermatoendocrinology 2015, 7, e1063751. [Google Scholar] [CrossRef]
- Lee, H.; Sim, N.; Fotouhi, A.; Daveluy, S. Vegan diet in dermatology: A review. J. Clin. Med. 2023, 12, 5800. [Google Scholar] [CrossRef]
- Neufingerl, N.; Eilander, A. Nutrient intake and status in adults consuming plant-based fiets compared to meat-eaters: A systematic review. Nutrients 2021, 14, 29. [Google Scholar] [CrossRef]
- Guo, E.L.; Katta, R. Diet and hair loss: Effects of nutrient deficiency and supplement use. Dermatol. Pract. Concept. 2017, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.H.; Chu, N.F.; Chang, C.W.; Wang, S.L.; Yang, H.C.; Chu, C.M.; Chang, C.T.; Lin, M.H.; Chien, W.C.; Su, S.L.; et al. Androgenic alopecia is associated with less dietary soy, lower blood vanadium and rs1160312 1 polymorphism in Taiwanese communities. PLoS ONE 2013, 8, e79789. [Google Scholar] [CrossRef] [PubMed]
- English, R.S., Jr.; Barazesh, J.M. Self-assessments of standardized scalp massages for androgenic alopecia: Survey results. Dermatol. Ther. 2019, 9, 167–178. [Google Scholar] [CrossRef]
- Greenberg, S.A. Diet and skin: A primer. Cutis 2020, 106, E31–E32. [Google Scholar] [CrossRef]
- Kouda, K.; Tanaka, T.; Kouda, M.; Takeuchi, H.; Takeuchi, A.; Nakamura, H.; Takigawa, M. Low-energy diet in atopic dermatitis patients: Clinical findings and DNA damage. J. Physiol. Anthropol. Appl. Hum. Sci. 2000, 19, 225–228. [Google Scholar] [CrossRef]
- Tanaka, T.; Kouda, K.; Kotani, M.; Takeuchi, A.; Tabei, T.; Masamoto, Y.; Nakamura, H.; Takigawa, M.; Suemura, M.; Takeuchi, H.; et al. Vegetarian diet ameliorates symptoms of atopic dermatitis through reduction of the number of peripheral eosinophils and of PGE2 synthesis by monocytes. J. Physiol. Anthropol. Appl. Hum. Sci. 2001, 20, 353–361. [Google Scholar] [CrossRef]
- Zhang, J.; Loman, L.; Oldhoff, M.; Schuttelaar, M.L.A. Association between moderate to severe atopic dermatitis and lifestyle factors in the Dutch general population. Clin. Exp. Dermatol. 2022, 47, 1523–1535. [Google Scholar] [CrossRef]
- Sakkas, H.; Bozidis, P.; Touzios, C.; Kolios, D.; Athanasiou, G.; Athanasopoulou, E.; Gerou, I.; Gartzonika, C. Nutritional status and the influence of the vegan diet on the gut microbiota and human health. Medicina 2020, 56, 88. [Google Scholar] [CrossRef] [PubMed]
- Tomova, A.; Bukovsky, I.; Rembert, E.; Yonas, W.; Alwarith, J.; Barnard, N.D.; Kahleova, H. The effects of vegetarian and vegan diets on gut microbiota. Front. Nutr. 2019, 6, 47. [Google Scholar] [CrossRef]
- Borrego-Ruiz, A.; Borrego, J.J. Influence of the vegetarian diet on the human intestinal microbiome [Influencia de la dieta vegetariana en el microbioma intestinal humano]. Nutr. Clín. Diet. Hosp. 2024, 4, 149–157. [Google Scholar] [CrossRef]
- Khan, A.; Adalsteinsson, J.; Whitaker-Worth, D.L. Atopic dermatitis and nutrition. Clin. Dermatol. 2022, 40, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, C.; Echeverría, G.; Villarreal, G.; Martínez, X.; Ferreccio, C.; Rigotti, A. Introducing plant-based Mediterranean diet as a lifestyle medicine approach in Latin America: Opportunities within the Chilean context. Front. Nutr. 2021, 8, 680452. [Google Scholar] [CrossRef] [PubMed]
- Mansilla-Polo, M.; Piquero-Casals, J.; Morgado-Carrasco, D. Popular diets and skin effects: A narrative review [Dietas populares y su impacto en la piel. Una revisión narrativa]. Actas Dermosifiliogr. 2024, 115, 374–386. [Google Scholar] [CrossRef]
- Delgado-Lista, J.; Alcala-Diaz, J.F.; Torres-Peña, J.D.; Quintana Navarro, G.M.; Fuentes, F.; Garcia-Rios, A.; Ortiz-Morales, A.M.; Gonzalez-Requero, A.I.; Perez-Caballero, A.I.; Yubero-Serrano, E.M.; et al. Long-term secondary prevention of cardiovascular disease with a Mediterranean diet and a low-fat diet (CORDIOPREV): A randomized controlled trial. Lancet 2022, 399, 1876–1885. [Google Scholar] [CrossRef]
- Kaluza, J.; Lozynska, K.; Rudzinska, J.; Granda, D.; Sicinska, E.; Szmidt, M.K. Mediterranean-style diet and other determinants of well-being in omnivorous, vegetarian, and vegan women. Nutrients 2023, 15, 725. [Google Scholar] [CrossRef]
- Barrea, L.; Donnarumma, M.; Cacciapuoti, S.; Muscogiuri, G.; De Gregorio, L.; Blasio, C.; Savastano, S.; Colao, A.; Fabbrocini, G. Phase angle and Mediterranean diet in patients with acne: Two easy tools for assessing the clinical severity of disease. J. Transl. Med. 2021, 19, 171. [Google Scholar] [CrossRef]
- Ah-Thiane, L.; Nguyen, J.M.; Khammari, A.; Dréno, B. Lifestyle habits and impact of the Mediterranean diet on facial acne severity in French women: A case-control study. Int. J. Womens Dermatol. 2022, 8, e017. [Google Scholar] [CrossRef] [PubMed]
- Bertolani, M.; Rodighiero, E.; Saleri, R.; Pedrazzi, G.; Bertoli, S.; Leone, A.; Feliciani, C.; Lotti, T.; Satolli, F. The influence of Mediterranean diet in acne pathogenesis and the correlation with insulin-like growth factor-1 serum levels: Implications and results. Dermatol. Rep. 2022, 14, 9143. [Google Scholar] [CrossRef]
- Wei, G.; Martirosyan, D. Hair loss: A review of the role of food bioactive compounds. Bioact. Compod. Health Dis. 2019, 2, 94–125. [Google Scholar] [CrossRef]
- Fortes, C.; Mastroeni, S.; Mannooranparampil, T.; Abeni, D.; Panebianco, A. Mediterranean diet: Fresh herbs and fresh vegetables decrease the risk of androgenetic alopecia in males. Arch. Dermatol. Res. 2018, 310, 71–76. [Google Scholar] [CrossRef]
- Antonogeorgos, G.; Mandrapylia, M.; Liakou, E.; Koutsokera, A.; Drakontaeidis, P.; Thanasia, M.; Ellwood, P.; García-Marcos, L.; Sardeli, O.; Priftis, K.N.; et al. Hierarchical analysis of Mediterranean dietary pattern on atopic diseases’ prevalence in adolescence: The Greek Global Asthma Network study. Allergol. Immunopathol. 2022, 50, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Bédard, A.; Northstone, K.; Henderson, A.J.; Shaheen, S.O. Mediterranean diet during pregnancy and childhood respiratory and atopic outcomes: Birth cohort study. Eur. Respir. J. 2020, 55, 1901215. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Swanson, K.S.; Gibson, G.R.; Hutkins, R.; Reimer, R.A.; Reid, G.; Verbeke, K.; Scott, K.P.; Holscher, H.D.; Azad, M.B.; Delzenne, N.M.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 687–701. [Google Scholar] [CrossRef] [PubMed]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef]
- Al-Ghazzewi, F.; Tester, R. Impact of prebiotics and probiotics on skin health. Benef. Microbes 2014, 5, 99–107. [Google Scholar] [CrossRef]
- Lizardo, M.P.; Tavaria, F.K. Probiotic growth in skin-like conditions. AIMS Microbiol. 2022, 8, 388–402. [Google Scholar] [CrossRef]
- Nakatsuji, T.; Chen, T.H.; Narala, S.; Chun, K.A.; Two, A.M.; Yun, T.; Shafiq, F.; Kotol, P.F.; Bouslimani, A.; Melnik, A.V.; et al. Antimicrobials from human skin commensal bacteria protect against Staphylococcus aureus and are deficient in atopic dermatitis. Sci. Transl. Med. 2017, 9, eaah4680. [Google Scholar] [CrossRef]
- Lizardo, M.; Magalhães, R.M.; Tavaria, F.K. Probiotic adhesion to skin keratinocytes and underlying mechanisms. Biology 2022, 11, 1372. [Google Scholar] [CrossRef]
- Paetzold, B.; Willis, J.R.; Pereira de Lima, J.; Knödlseder, N.; Brüggemann, H.; Quist, S.R.; Gabaldón, T.; Güell, M. Skin microbiome modulation induced by probiotic solutions. Microbiome 2019, 7, 95. [Google Scholar] [CrossRef]
- Abdi, A.; Oroojzadeh, P.; Valivand, N.; Sambrani, R.; Lotfi, H. Immunological aspects of probiotics for improving skin diseases: Influence on the gut-brain-skin axis. Biochem. Biophys. Res. Commun. 2024, 702, 149632. [Google Scholar] [CrossRef] [PubMed]
- Benyacoub, J.; Bosco, N.; Blanchard, C.; Demont, A.; Philippe, D.; Castiel-Higounenc, I.; Guéniche, A. Immune modulation property of Lactobacillus paracasei NCC2461 (ST11) strain and impact on skin defences. Benef. Microbes 2014, 5, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Poutahidis, T.; Kearney, S.M.; Levkovich, T.; Qi, P.; Varian, B.J.; Lakritz, J.R.; Ibrahim, Y.M.; Chatzigiagkos, A.; Alm, E.J.; Erdman, S.E. Microbial symbionts accelerate wound healing via the neuropeptide hormone oxytocin. PLoS ONE 2013, 8, e78898. [Google Scholar] [CrossRef] [PubMed]
- Ghoshal, U.C.; Shukla, R.; Ghoshal, U. Small intestinal bacterial overgrowth and irritable bowel syndrome: A bridge between functional organic dichotomy. Gut Liver 2017, 11, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Roszkowska, P.; Klimczak, E.; Ostrycharz, E.; Rączka, A.; Wojciechowska-Koszko, I.; Dybus, A.; Cheng, Y.H.; Yu, Y.H.; Mazgaj, S.; Hukowska-Szematowicz, B. Small intestinal bacterial overgrowth (SIBO) and twelve groups of related diseases-current state of knowledge. Biomedicines 2024, 12, 1030. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.Y.; Chi, C.C. Rosacea, germs, and bowels: A review on gastrointestinal comorbidities and gut-skin axis of rosacea. Adv. Ther. 2021, 38, 1415–1424. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Ko, Y.; Park, Y.K.; Kim, N.I.; Ha, W.K.; Cho, Y. Dietary effect of lactoferrin-enriched fermented milk on skin surface lipid and clinical improvement of acne vulgaris. Nutrition 2010, 26, 902–909. [Google Scholar] [CrossRef] [PubMed]
- Colletti, A.; Pellizzato, M.; Cicero, A.F. The possible role of probiotic supplementation in inflammation: A narrative review. Microorganisms 2023, 11, 2160. [Google Scholar] [CrossRef]
- Jung, G.W.; Tse, J.E.; Guiha, I.; Rao, J. Prospective, randomized, open-label trial comparing the safety, efficacy, and tolerability of an acne treatment regimen with and without a probiotic supplement and minocycline in subjects with mild to moderate acne. J. Cutan. Med. Surg. 2013, 17, 114–122. [Google Scholar] [CrossRef]
- Fabbrocini, G.; Bertona, M.; Picazo, Ó.; Pareja-Galeano, H.; Monfrecola, G.; Emanuele, E. Supplementation with Lactobacillus rhamnosus SP1 normalises skin expression of genes implicated in insulin signalling and improves adult acne. Benef. Microbes 2016, 7, 625–630. [Google Scholar] [CrossRef]
- Majeed, M.; Majeed, S.; Nagabhushanam, K.; Mundkur, L.; Rajalakshmi, H.R.; Shah, K.; Beede, K. Novel topical application of a postbiotic, LactoSporin®, in mild to moderate acne: A randomized, comparative clinical study to evaluate its efficacy, tolerability and safety. Cosmetics 2020, 7, 70. [Google Scholar] [CrossRef]
- Cui, H.; Guo, C.; Wang, Q.; Feng, C.; Duan, Z. A pilot study on the efficacy of topical lotion containing anti-acne postbiotic in subjects with mild -to -moderate acne. Front. Med. 2022, 9, 1064460. [Google Scholar] [CrossRef] [PubMed]
- Ma’or, Z.; Temmerman, R.; Zhang, X. Topical application of synbiotic Bacillus preparations positively affects skin (micro) biology. J. Cosmet. Dermatol. Sci. Appl. 2023, 13, 107–123. [Google Scholar] [CrossRef]
- Podrini, C.; Schramm, L.; Marianantoni, G.; Apolinarska, J.; McGuckin, C.; Forraz, N.; Milet, C.; Desroches, A.L.; Payen, P.; D’Aguanno, M.; et al. Topical administration of Lactiplantibacillus plantarum (SkinDuoTM) serum improves anti-acne properties. Microorganisms 2023, 11, 417. [Google Scholar] [CrossRef]
- Rybak, I.; Haas, K.N.; Dhaliwal, S.K.; Burney, W.A.; Pourang, A.; Sandhu, S.S.; Maloh, J.; Newman, J.W.; Crawford, R.; Sivamani, R.K. Prospective placebo-controlled assessment of spore-based probiotic supplementation on sebum production, skin barrier function, and acne. J. Clin. Med. 2023, 12, 895. [Google Scholar] [CrossRef]
- Levkovich, T.; Poutahidis, T.; Smillie, C.; Varian, B.J.; Ibrahim, Y.M.; Lakritz, J.R.; Alm, E.J.; Erdman, S.E. Probiotic bacteria induce a ‘glow of health’. PLoS ONE 2013, 8, e53867. [Google Scholar] [CrossRef]
- Horii, Y.; Kaneda, H.; Fujisaki, Y.; Fuyuki, R.; Nakakita, Y.; Shigyo, T.; Nagai, K. Effect of heat-killed Lactobacillus brevis SBC8803 on cutaneous arterial sympathetic nerve activity, cutaneous blood flow and transepidermal water loss in rats. J. Appl. Microbiol. 2014, 116, 1274–1281. [Google Scholar] [CrossRef]
- Ogawa, M.; Saiki, A.; Matsui, Y.; Tsuchimoto, N.; Nakakita, Y.; Takata, Y.; Nakamura, T. Effects of oral intake of heat-killed Lactobacillus brevis SBC8803 (SBL88™) on dry skin conditions: A randomized, double-blind, placebo-controlled study. Exp. Ther. Med. 2016, 12, 3863–3872. [Google Scholar] [CrossRef]
- Xie, W.R.; Yang, X.Y.; Xia, H.H.; Wu, L.H.; He, X.X. Hair regrowth following fecal microbiota transplantation in an elderly patient with alopecia areata: A case report and review of the literature. World J. Clin. Cases 2019, 7, 3074–3081. [Google Scholar] [CrossRef]
- Park, D.W.; Lee, H.S.; Shim, M.S.; Yum, K.J.; Seo, J.T. Do Kimchi and Cheonggukjang probiotics as a functional food improve androgenetic alopecia? A clinical pilot study. World J. Mens Health 2020, 38, 95–102. [Google Scholar] [CrossRef]
- Rinaldi, F.; Trink, A.; Pinto, D. Efficacy of postbiotics in a PRP-like cosmetic product for the treatment of alopecia area celsi: A randomized double-blinded parallel-group study. Dermatol. Ther. 2020, 10, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.H.; Lin, Y.H.; Chan, S.T.; Lin, Y.K.; Chiang, C.F. Lactiplantibacillus plantarum TCI999 probiotic promoted hair growth and regulated gut microbiome: Double-blind, placebo-controlled trial. J. Probiot. Health 2022, 10, 285. [Google Scholar] [CrossRef]
- Navarro-Belmonte, M.R.; Aguado-García, Á.; Sánchez-Pellicer, P.; Núñez-Delegido, E.; Navarro-Moratalla, L.; Martínez-Villaescusa, M.; García-Navarro, A.; Navarro-López, V. The effect of an oral probiotic mixture on clinical evolution and the gut and skin microbiome in patients with alopecia areata: A randomized clinical trial. Cosmetics 2024, 11, 119. [Google Scholar] [CrossRef]
- Rusu, E.; Enache, G.; Cursaru, R.; Alexescu, A.; Radu, R.; Onila, O.; Cavallioti, T.; Rusu, F.; Posea, M.; Jinga, M.; et al. Prebiotics and probiotics in atopic dermatitis. Exp. Ther. Med. 2019, 18, 926–931. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, H.Y.; Lee, S.Y.; Seo, J.H.; Lee, E.; Hong, S.J. Clinical efficacy and mechanism of probiotics in allergic diseases. Korean J. Pediatr. 2013, 56, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Wang, I.J.; Wang, J.Y. Children with atopic dermatitis show clinical improvement after Lactobacillus exposure. Clin. Exp. Allergy 2015, 45, 779–787. [Google Scholar] [CrossRef]
- Blanchet-Réthoré, S.; Bourdès, V.; Mercenier, A.; Haddar, C.H.; Verhoeven, P.O.; Andres, P. Effect of a lotion containing the heat-treated probiotic strain Lactobacillus johnsonii NCC 533 on Staphylococcus aureus colonization in atopic dermatitis. Clin. Cosmet. Investigat. Dermatol. 2017, 10, 249–257. [Google Scholar] [CrossRef]
- Wu, Y.J.; Wu, W.F.; Hung, C.W.; Ku, M.S.; Liao, P.F.; Sun, H.L.; Lu, K.H.; Sheu, J.N.; Lue, K.H. Evaluation of efficacy and safety of Lactobacillus rhamnosus in children aged 4-48 months with atopic dermatitis: An 8-week, double-blind, randomized, placebo-controlled study. J. Microbiol. Immunol. Infect. 2017, 50, 684–692. [Google Scholar] [CrossRef]
- Ibáñez, M.D.; Rodríguez Del Río, P.; González-Segura Alsina, D.; Villegas Iglesias, V. Effect of synbiotic supplementation on children with atopic dermatitis: An observational prospective study. Eur. J. Pediatr. 2018, 177, 1851–1858. [Google Scholar] [CrossRef]
- Myles, I.A.; Earland, N.J.; Anderson, E.D.; Moore, I.N.; Kieh, M.D.; Williams, K.W.; Saleem, A.; Fontecilla, N.M.; Welch, P.A.; Darnell, D.A.; et al. First-in-human topical microbiome transplantation with Roseomonas mucosa for atopic dermatitis. JCI Insight 2018, 3, e120608. [Google Scholar] [CrossRef]
- Navarro-López, V.; Ramírez-Boscá, A.; Ramón-Vidal, D.; Ruzafa-Costas, B.; Genovés-Martínez, S.; Chenoll-Cuadros, E.; Carrión-Gutiérrez, M.; Horga de la Parte, J.; Prieto-Merino, D.; Codoñer-Cortés, F.M. Effect of oral administration of a mixture of probiotic strains on SCORAD index and use of topical steroids in young patients with moderate atopic dermatitis: A randomized clinical trial. JAMA Dermatol. 2018, 154, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Dissanayake, E.; Tani, Y.; Nagai, K.; Sahara, M.; Mitsuishi, C.; Togawa, Y.; Suzuki, Y.; Nakano, T.; Yamaide, F.; Ohno, H.; et al. Skin care and synbiotics for prevention of atopic dermatitis or food allergy in newborn infants: A 2 × 2 factorial, randomized, non-treatment controlled trial. Int. Arch. Allergy Immunol. 2019, 180, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.H.; Yoon, W.; Lee, S.Y.; Shin, H.S.; Lim, M.Y.; Nam, Y.D.; Yoo, Y. Effects of Lactobacillus pentosus in children with allergen-sensitized atopic dermatitis. J. Korean Med. Sci. 2020, 35, e128. [Google Scholar] [CrossRef] [PubMed]
- Climent, E.; Martinez-Blanch, J.F.; Llobregat, L.; Ruzafa-Costas, B.; Carrión-Gutiérrez, M.Á.; Ramírez-Boscá, A.; Prieto-Merino, D.; Genovés, S.; Codoñer, F.M.; Ramón, D.; et al. Changes in gut microbiota correlates with response to treatment with probiotics in patients with atopic dermatitis. A post hoc analysis of a clinical trial. Microorganisms 2021, 9, 854. [Google Scholar] [CrossRef]
- Noll, M.; Jäger, M.; Lux, L.; Buettner, C.; Axt-Gadermann, M. Improvement of atopic dermatitis by synbiotic baths. Microorganisms 2021, 9, 527. [Google Scholar] [CrossRef]
- Carucci, L.; Nocerino, R.; Paparo, L.; De Filippis, F.; Coppola, S.; Giglio, V.; Cozzolino, T.; Valentino, V.; Sequino, G.; Bedogni, G.; et al. Therapeutic effects elicited by the probiotic Lacticaseibacillus rhamnosus GG in children with atopic dermatitis. The results of the ProPAD trial. Pediatr. Allergy Immunol. 2022, 33, e13836. [Google Scholar] [CrossRef]
- de Andrade, P.D.S.M.A.; Maria E Silva, J.; Carregaro, V.; Sacramento, L.A.; Roberti, L.R.; Aragon, D.C.; Carmona, F.; Roxo-Junior, P. Efficacy of probiotics in children and adolescents with atopic dermatitis: A randomized, double-blind, placebo-controlled study. Front. Nutr. 2022, 8, 833666. [Google Scholar] [CrossRef]
- Wang, Y.; Choy, C.T.; Lin, Y.; Wang, L.; Hou, J.; Tsui, J.C.C.; Zhou, J.; Wong, C.H.; Yim, T.K.; Tsui, W.K.; et al. Effect of a novel E3 probiotics formula on the gut microbiome in atopic dermatitis patients: A pilot study. Biomedicines 2022, 10, 2904. [Google Scholar] [CrossRef] [PubMed]
- Colombo, D.; Rigoni, C.; Cantù, A.; Carnevali, A.; Filippetti, R.; Franco, T.; Grassi, A.; Loi, C.; Mazzotta, A.; Patroi, I.; et al. Probiotics and prebiotics orally assumed as disease modifiers for stable mild atopic dermatitis: An Italian real-life, multicenter, retrospective, observational study. Medicina 2023, 59, 2080. [Google Scholar] [CrossRef]
- Yakupu, A.; Aimaier, R.; Yuan, B.; Chen, B.; Cheng, J.; Zhao, Y.; Peng, Y.; Dong, J.; Lu, S. The burden of skin and subcutaneous diseases: Findings from the global burden of disease study 2019. Front. Public Health 2023, 11, 1145513. [Google Scholar] [CrossRef]
- Borrego-Ruiz, A.; Borrego, J.J. Microbial dysbiosis in the skin microbiome and its psychological consequences. Microorganisms 2024, 12, 1908. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Hu, X.; Yao, J.; Cao, W.; Zou, Z.; Wang, L.; Qin, H.; Zhong, D.; Li, Y.; Xue, P.; et al. The role of short-chain fatty acids in inflammatory skin diseases. Front. Microbiol. 2023, 13, 1083432. [Google Scholar] [CrossRef] [PubMed]
- Nagata, H.; Takagi, N.; Inoue, S.; Mizutani, Y. Nicotine affects tight junction barriers via alpha 7 nicotine-like acetylcholine receptor in keratinocytes. J. Dermatol. Sci. 2021, 103, 183–185. [Google Scholar] [CrossRef]
- Cevikbas, F.; Braz, J.M.; Wang, X.; Solorzano, C.; Sulk, M.; Buhl, T.; Steinhoff, M.; Basbaum, A.I. Synergistic antipruritic effects of gamma aminobutyric acid A and B agonists in a mouse model of atopic dermatitis. J. Allergy Clin. Immunol. 2017, 140, 454–464.e2. [Google Scholar] [CrossRef]
- Langan, E.A.; Lisztes, E.; Bíró, T.; Funk, W.; Kloepper, J.E.; Griffiths, C.E.; Paus, R. Dopamine is a novel, direct inducer of catagen in human scalp hair follicles in vitro. Br. J. Dermatol. 2013, 168, 520–525. [Google Scholar] [CrossRef]
- Vaccaro, R.; Casini, A.; Severi, C.; Lamazza, A.; Pronio, A.; Palma, R. Serotonin and melatonin in human lower gastrointestinal tract. Diagnostics 2023, 13, 204. [Google Scholar] [CrossRef]
- Baroni, L.; Rizzo, G.; Galchenko, A.V.; Zavoli, M.; Serventi, L.; Battino, M. Health benefits of vegetarian diets: An insight into the main topics. Foods 2024, 13, 2398. [Google Scholar] [CrossRef] [PubMed]
- Cena, H.; Calder, P.C. Defining a healthy diet: Evidence for the role of contemporary dietary patterns in health and disease. Nutrients 2020, 12, 334. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Cacciapuoti, S.; Megna, M.; Verde, L.; Marasca, C.; Vono, R.; Camajani, E.; Colao, A.; Savastano, S.; Fabbrocini, G.; et al. The effect of the ketogenic diet on acne: Could it be a therapeutic tool? Crit. Rev. Food Sci. Nutr. 2024, 64, 6850–6869. [Google Scholar] [CrossRef]
- Roster, K.; Xie, L.; Nguyen, T.; Lipner, S.R. Impact of ketogenic and low-glycemic diets on inflammatory skin conditions. Cutis 2024, 113, 75–80. [Google Scholar] [CrossRef]
- Verde, L.; Frias-Toral, E.; Cacciapuoti, S.; Simancas-Racines, D.; Megna, M.; Caiazzo, G.; Potestio, L.; Maisto, M.; Tenore, G.C.; Colao, A.; et al. Very low-calorie ketogenic diet (VLCKD): A therapeutic nutritional tool for acne? J. Transl. Med. 2024, 22, 322. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, C.V.; Antiga, E.; Lulli, M. Oral and topical probiotics and postbiotics in skincare and dermatological therapy: A concise review. Microorganisms 2023, 11, 1420. [Google Scholar] [CrossRef] [PubMed]
- Suez, J.; Zmora, N.; Segal, E.; Elinav, E. The pros, cons, and many unknowns of probiotics. Nat. Med. 2019, 25, 716–729. [Google Scholar] [CrossRef]
- Lee, G.R.; Maarouf, M.; Hendricks, A.J.; Lee, D.E.; Shi, V.Y. Topical probiotics: The unknowns behind their rising popularity. Dermatol. Online J. 2019, 25, 13030/qt2v83r5wk. [Google Scholar] [CrossRef]
- Ayichew, T.; Belete, A.; Alebachew, T.; Tsehaye, H.; Berhanu, H.; Minwuyelet, A. Bacterial probiotics their importances and limitations: A review. J. Nutr. Health Sci. 2017, 4, 202. [Google Scholar] [CrossRef]
- da Silva Vale, A.; de Melo Pereira, G.V.; de Oliveira, A.C.; de Carvalho Neto, D.P.; Herrmann, L.W.; Karp, S.G.; Soccol, V.T.; Soccol, C.R. Production, formulation, and application of postbiotics in the treatment of skin conditions. Fermentation 2023, 9, 264. [Google Scholar] [CrossRef]
- Ming, Z.; Han, L.; Bao, M.; Zhu, H.; Qiang, S.; Xue, S.; Liu, W. Living bacterial hydrogels for accelerated infected wound healing. Adv. Sci. 2021, 8, e2102545. [Google Scholar] [CrossRef]
- Wu, J.H.; Cohen, B.A. The stigma of skin disease. Curr. Opin. Pediatr. 2019, 31, 509–514. [Google Scholar] [CrossRef]
- Chernyshov, P.V.; Tomas-Aragones, L.; Manolache, L.; Pustisek, N.; Darlenski, R.; Marron, S.E.; Koumaki, D.; Pochynok, T.V.; Szepietowski, J.C.; Wala-Zielinska, K.; et al. Bullying in persons with skin diseases. J. Eur. Acad. Dermatol. Venereol. 2024, 38, 752–760. [Google Scholar] [CrossRef]
- Borrego-Ruiz, A.; Fernández, S. Humiliation and its relationship with bullying victimization: A narrative review. Psychol. Soc. Ed. 2024, 16, 42–51. [Google Scholar] [CrossRef]
- Homayoon, D.; Hiebler-Ragger, M.; Zenker, M.; Weger, W.; Unterrainer, H.; Aberer, E. Relationship between skin shame, psychological distress and quality of life in patients with psoriasis: A pilot study. Acta Derm. Venereol. 2020, 100, adv00205. [Google Scholar] [CrossRef] [PubMed]
- Borrego-Ruiz, A.; Borrego, J.J. Psychobiotics: A new perspective on the treatment of stress, anxiety, and depression. Anxiety Stress 2024, 30, 79–93. [Google Scholar] [CrossRef]
| Study/Country | Skin Disease | Sequencing Method | Changes in GM |
|---|---|---|---|
| Deng et al. [31]/China | Acne vulgaris. | 16S rRNA and NGS sequencing. | Increased: Bacteroidota members. Decreased: α-diversity, Lachnospiraceae and Ruminococcaceae. |
| Yan et al. [32]/China | Acne vulgaris. | 16S rRNA sequencing. | Increased: Pseudomonadota members. Decreased: Allobaculum, Bifidobacterium, Butyricicoccus, Coprobacillus, and Lactobacillus. |
| Thompson et al. [33]/USA | Acne. | 16S rRNA sequencing. | Increased: Bacteroidota members. Decreased: Lactobacillus iners, Lacticaseibacillus zeae and Bifidobacterium animalis. |
| Huang et al. [34]/China | Acne vulgaris. | V3-V4 regions of 16S rRNA sequencing. | Increased (males): Bacillota members. Decreased (males): Aerococcus, Alkaliphilus, Bacillus, Bilophila, Blautia, Butyricicoccus, Carnobacterium, Enterococcus, Exiguobacterium, Faecalibacterium, Gemmiger, Lachnospiraceae incertae sedis, Lactococcus, Lysinibacillus, Oceanobacillus, Paenibacillus, Pseudomonas and Ruminococcus. Increased (females): Clostridium sensu stricto. Decreased (females): Odoribacter and Oscillibacter. |
| Moreno-Arrones et al. [35]/Spain | Alopecia universalis. | 16S rRNA sequencing. | Increased: Bacteroides eggerthii, Clostridiales vadin BB60 group, Erysipelotrichaceae, Holdemania filiformis, Lachnospiraceae, Parabacteroides distasonis, and P. johnsonii. |
| Juhasz et al. [36]/USA | Alopecia areata. | 16S rRNA and ITS sequencing. | Decreased: Clostridia group. |
| Brzychcy et al. [37]/Poland | Alopecia areata. | V3-V4 regions of 16S rRNA sequencing. | Increased: Bifidobacterium, Eubacterium, Lachnoclostridium, and Streptococcus. |
| Song et al. [38]/Korea | Atopic dermatitis. | 16S rRNA sequencing. | Increased: Faecalibacterium prausnitzii. |
| Nylund et al. [39]/Finland | Atopic dermatitis. | 16S rRNA microarray. | Increased: Coprococcus eutactus. Decreased: Microbial diversity. |
| Lee et al. [40]/Korea | Atopic dermatitis. | 16S rRNA pyrosequencing. | Increased: Clostridia group. |
| Reddel et al. [41]/Italy | Atopic dermatitis. | 16S rRNA metagenomic analysis. | Increased: Bacteroides, Faecalibacterium, Oscillospira, Parabacteroides and Sutterella. Decreased: Bifidobacterium, Blautia, Coprococcus, Eubacterium, and Propionibacterium. |
| Park et al. [42]/Korea | Atopic dermatitis. | Whole metagenomic sequencing. | Increased: Streptococcus. Decreased: Akkermansia and Clostridium. |
| Nekrasova et al. [43]/Russia | Atopic dermatitis. | 16S rRNA and NGS sequencing. | Increased: Members of the families Erysipelotrichaceae, Pasteurellaceae, Ruminococcaceae and Sutterellaceae. Decreased: Members of the family Barnesiellaceae. |
| Study/Country | Intervention | Treatment | Via of Administration | Clinical Assessment | Main Findings |
|---|---|---|---|---|---|
| Fabbrocini et al. [120]/Italy | N = 20 acne patients (average age: 33.7). 12 weeks. A pilot randomized, double-blind, placebo controlled study. | Probiotic: Lacticaseibacillus rhamnosus SP-1. | Oral. | IGA-AS. | Probiotic normalized skin expression of genes involved in insulin signaling and improved the appearance of adult acne. |
| Majeed et al. [121]/India | N = 68 acne patients (age: 18–25). Four weeks. Pilot study. | Postbiotic: LactoSporin (extracellular filtrate of Bacillus coagulans MTCC 5856). | Topic. | VISIA. | Efficacy of LactoSporin was similar to that of benzoyl peroxide in reducing sebaceous secretion and the greasy nature of the skin. Presented anti-microbial activity against C. acnes. |
| Cui et al. [122]/China | N = 22 acne patients (age: >16). Twenty-one days. Randomized open labeled clinical study. | Postbiotic: Heat-inactivated Lactiplantibacillus plantarum VH Probi E15. | Topic. | GAAS. | Anti-acne lotion significantly improvement in acne lesions after 4 weeks of treatment. |
| Ma’or et al. [123]/Israel | N = 31 women volunteers (average age: 23). Four weeks. Clinical trial. | Synbiotic: Spores of Bacillus subtilis, B. licheniformis, B. megaterium, and B. amyloliquefaciens (probiotics), with inulin (prebiotic). | Topic. | Acne-QoL, IGA-AS, RBX. | Significant reduction in IGA dermatologist score of acne severity. Reduction in the number of acne lesions. Improved Acne-QoL scores. |
| Podrini et al. [124]/Italy | Skin cell cultures (skin models) of 8-mm diameter. | Probiotic: Lactiplantibacillus plantarum LP01. | Topic. | In vitro study. | Anti-acne serum with the probiotic mimics the over-production of lipids, has anti-inflammatory properties, and improves acne symptoms. |
| Rybak et al. [125]/USA | N = 25 acne patients. Four weeks. Prospective, single-blinded, placebo-controlled study. | Probiotics: Spores of Bacillus subtilis HU58, B. licheniformis, B. clausii, B. indicus HU36, and B. coagulans. | Oral. | GAAS. | Probiotic supplementation increased the circulating acetate/propionate ratio, and resulted in a decreasing facial sebum and increased TEWL. Patients with acne showed improvement in total lesions and non-inflammatory lesions. |
| Study/Country | Intervention | Treatment | Via of Administration | Main Findings |
|---|---|---|---|---|
| Rebello et al. [50]/USA | N = 1 alopecic male of 34 years-old with infection of Clostridioides difficile. N = 1 alopecic male of 20 years-old with Crohn’s disease. Case report. | Fecal Microbiota Transplantation (FMT). | Colonoscopy. | At follow-up of 8 weeks, hair growth on head, face, and arms of patient 1. After FMT, the patient had regrowth of hair in several sites of his body. |
| Xie et al. [129]/China | N = 1 alopecic male of 86 years-old with a sigmoid colon carcinoma with diarrhea for 6 months. Case report. | Fecal Microbiota Transplantation (FMT). | Colonoscopy. | Diarrhea symptoms remarkably improved one month after FMT. New hair growth in the affected region of his scalp. |
| Park et al. [130]/Korea | N = 46 patients with alopecia (average age: 46.5 [males] and 44.2 [females]). Four weeks. Clinical pilot study. | Synbiotic: Leuconostoc holzapfelii, Leuconostoc mesenteroides, and Latilactobacillus (formerly Lactobacillus) sakei (probiotics), with Hasou extract + Korean black soybean extract (prebiotics). | Oral. | Synbiotic promoted hair growth and reversed loss without adverse gastrointestinal effects. |
| Rinaldi et al. [131]/Italy | N = 160 patients with alopecia areata (age: 18–60). Three months. Randomized double-blinded parallel-group study. | Postbiotic: Plantaricin A and Apilactobacillus (formerly Lactobacillus) kunkeei ferment product. | Topic. | Efficacy of bioactive peptide on the severity of alopecia compared to control group. |
| Liang et al. [132]/Taiwan | N = 50 adults with hair loss (age: >20). Twelve weeks. Double-blind, placebo-controlled study. | Probiotic: Lactiplantibacillus plantarum TC1999. | Oral. | Probiotic increased mitochondrial activity and hair cell growth. Improved gut microbiome. |
| Navarro-Belmonte et al. [133]/Spain | N = 26 alopecic patients (age: >18). Twenty-four weeks. Randomized, double-blind, two-arms, pilot clinical trial. | Probiotics: Lacticaseibacillus rhamnosus CECT 30580 and Bifidobacterium longum CECT 30616. | Oral. | Probiotic mixture appeared to improve the course of alopecia areata. Skin microbiota of scalp lesions was modified after probiotic treatment. |
| Study/Country | Intervention | Treatment | Via of Administration | Clinical Assessment | Main Findings |
|---|---|---|---|---|---|
| Wang and Wang [136]/Taiwan | N = 220 AD patients (age: 8–18). Four months. Prospective randomized, double-blind, placebo-controlled study. | Probiotics: Lacticaseibacillus paracasei and Limosilactobacillus fermentum. | Oral. | SCORAD, FDLQI, CDLQI. | Children who received probiotic mixture showed lower SCORAD scores compared to the placebo group. |
| Blanchet-Rethore et al. [137]/Germany | N = 31 AD patients. Three weeks. Open label multicenter study. | Postbiotic: Heat-treated Lactobacillus johnsonii NCC 533. | Topic. | SCORAD. | The application of the lotion with the postbiotic to the lesions of patients with AD controlled Staphylococcus aureus colonization and was associated with local clinical improvement. |
| Wu et al. [138]/Taiwan | N = 30 AD patients (age: 4–48 months). Eight weeks. Two center, randomized, double-blind, placebo controlled study. | Probiotic: Lacticaseibacillus rhamnosus MP108. | Oral. | SCORAD, IDQLQ. | Probiotic was effective in reducing symptoms of AD after 8 weeks of treatment. |
| Ibañez et al. [139]/Spain | N = 320 children (average age: 5.1). Eight weeks. Observational prospective study. | Synbiotic: Lacticaseibacilluscasei LC5, L. rhamnosus LR5, Lactiplantibacillus plantarum LP3, and Bifidobacterium lactis BL3 (probiotics). Fructooligosaccharides (FOS), galactooligosaccharides (GOS), with biotin (prebiotics). | Oral. | SCORAD, VAS. | SCORAD index and VAS score for pruritus decreased after synbiotic treatment. |
| Myles et al. [140]/USA | N = 15 AD patients (10 adults and 5 children). 10 weeks. Open label phase I. | Topical microbiome transplantation: Roseomonas mucosa. | Topic. | SCORAD. | Treatment with R. mucosa was associated with significant decreases in measures of disease severity. There were no adverse events on treatment application. |
| Navarro-López et al. [141]/Spain | N = 25 AD patients (age: 4–17; average age: 9.3). 12 weeks. Double-blind, placebo-controlled study. | Probiotics: Bifidobacterium animalis subsp. lactis CECT 8145, B. longum CECT 7347 and L. casei CECT 9104. | Oral. | SCORAD. | The mixture of probiotics was effective in reducing SCORAD index and in reducing the use of topical steroids in patients with moderate AD. |
| Dissanayake et al. [142]/Japan | N = 605 pregnant women (age: 24–32) and 549 babies (age: 0–6 months). Follow-up: 4 years. 2 × 2 factorial randomized controlled trial. | Synbiotic: Bifibobacterium bifidum OLB6378 (probiotic), with FOS (prebiotic). | Oral. | EASI. | Neither skin care nor the synbiotic showed any effect on reducing the development of AD and food allergens at 1 year of age. |
| Ahn et al. [143]/Korea | N = 124 AD patients (age:2–13). Twelve weeks. Double-blinded, placebo controlled randomized study. | Probiotic: Lactiplantibacillus pentosus. | Oral. | SCORAD. | Improved symptoms were recorded both in the probiotic and placebo groups, but SCORAD index for the probiotic group was significantly improved compared to those for the placebo group in allergen-sensitized AD. |
| Climent et al. [144]/Spain | N = 50 AD patients (age: 4–17). Twelve weeks. Double-blind, placebo controlled randomized study. | Probiotics: B. animalis subsp. lactis CECT 8145, B. longum CECT 7347 and L. casei CECT 9104. | Oral. | SCORAD. | Probiotic mixture treatment showed a significant improvement in SCORAD index. The treatment modulated the gut microbiome with significant changes in the genera Faecalibacterium and Bacteroides. |
| Noll et al. [145]/Germany | N = 22 AD patients. Fourteen days. Three bath groups (synbiotic, prebiotic, and control). Double-blind, randomized study. | Synbiotic: Bifibobacterium breve ATCC 15698, B. animalis subsp. lactis ATCC 27536, L. casei ATCC 393, L. plantarum ATCC 14917, L. rhamnosus ATCC 53103, and Lactobacillus gasseri ATCC 33323 (probiotics), with maltodextrin, inulin, and apple pectin (prebiotics). | Topic. | SCORAD, QoL. | Significantly reduced SCORAD over time of AD patients after daily synbiotic or prebiotic baths. Synbiotic baths improved pruritus and skin dryness. Improved QoL indices. |
| Carucci et al. [146]/Italy | N = 100 AD patients (age: 6–36 months). Twelve weeks. Double-blind, randomized controlled study. | Probiotic: L. rhamnosus GG. | Oral. | SCORAD, IDQoL, ProPAD. | Beneficial effects on disease severity and quality of life were obtained with the probiotic treatment. |
| De Andrade et al. [147]/Brazil | N = 60 AD patients (age: 6 months–19 years). Six–twelve months. Double-blind, randomized, placebo-controlled clinical trial. | Probiotics: B. animalis subsp. lactis HN019, L. rhamnosus HN001, Lacticaseibacillus paracasei Lep57, and Lactobacillus acidophilus NCFM. | Oral. | SCORAD. | Children and adolescent with AD presented a significant positive clinical response after 6 months with the probiotic cocktail treatment. |
| Wang et al. [148]/Hong Kong-China | N = 41 AD patients (age: 18–73; average age: 47). Eight weeks. Pilot study. | Mixture of probiotics, prebiotics and postbiotics (E3 preparation): B. animalis subsp. lactis GKK2, B. bifidum GKB2, L. rhamnosus GG, L. paracasei GK56, L. acidophilus GK47, L. casei GKC1, and Lactobacillus lactis subsp. lactis GKL2 (probiotics), with FOS, GOS, inulin (prebiotics), and with heat-inactivated L. plantarum (postbiotic). | Oral. | EASI. | EASI of the participants was significantly lower after the E3 treatment. |
| Colombo et al. [149]/Italy | N = 144 AD patients (average age: 25.1). Twelve weeks. Multicenter, retrospective observational study. | Synbiotic: B. animalis subsp. lactis BSO1, L. plantarum LP14, and L. rhamnosus LR05 (probiotics), with FOS and vitamin B2 (prebiotics). | Oral. | SCORAD, EASI, TIS. | Pruritus and AD-related lesions (erythema, edema, papules, and excoriation) exhibited significant clinical improvement. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borrego-Ruiz, A.; Borrego, J.J. Nutritional and Microbial Strategies for Treating Acne, Alopecia, and Atopic Dermatitis. Nutrients 2024, 16, 3559. https://doi.org/10.3390/nu16203559
Borrego-Ruiz A, Borrego JJ. Nutritional and Microbial Strategies for Treating Acne, Alopecia, and Atopic Dermatitis. Nutrients. 2024; 16(20):3559. https://doi.org/10.3390/nu16203559
Chicago/Turabian StyleBorrego-Ruiz, Alejandro, and Juan J. Borrego. 2024. "Nutritional and Microbial Strategies for Treating Acne, Alopecia, and Atopic Dermatitis" Nutrients 16, no. 20: 3559. https://doi.org/10.3390/nu16203559
APA StyleBorrego-Ruiz, A., & Borrego, J. J. (2024). Nutritional and Microbial Strategies for Treating Acne, Alopecia, and Atopic Dermatitis. Nutrients, 16(20), 3559. https://doi.org/10.3390/nu16203559






