Effects of Specially Designed Energy-Restricted Diet on Anthropometric Parameters and Cardiometabolic Risk in Overweight and Obese Adults: Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Dietary Intervention and Diet Composition
- -
- days 1–21 => 3 consecutive cycles;
- -
- day 22 => rest day, i.e., a day without the restriction of nutrient intake;
- -
- day 23 => a day of the minimum intake;
- -
- days 24–30 => another cycle of seven days.
2.3. Measurements
2.3.1. Anthropometry and Body Composition
2.3.2. Biochemical Analysis
2.4. Questionnaire
2.5. Statistical Analysis
3. Results
3.1. Subjects
3.2. Body Composition and Anthropometric Parameters
3.3. Biochemical Parameters and Cardiometabolic Risk Factors
3.4. Markers of Inflammation and Markers of Protein Metabolism
3.5. Satisfaction Questionnaire
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aaseth, J.; Ellefsen, S.; Alehagen, U.; Sundfør, T.M.; Alexander, J. Diets and drugs for weight loss and health in obesity–An update. Biomed. Pharmacother. 2021, 140, 111789. [Google Scholar] [CrossRef] [PubMed]
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in underweight and obesity from 1990 to 2022: A pooled analysis of 3663 population-representative studies with 222 million children, adolescents, and adults. Lancet 2024, 403, 1027–1050. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schutte, S.; Esser, D.; Siebelink, E.; Michielsen, C.J.; Daanje, M.; Matualatupauw, J.C.; Boshuizen, H.C.; Mensink, M.; Afman, L.A.; Wageningen Belly Fat Study Team. Diverging metabolic effects of 2 energy-restricted diets differing in nutrient quality: A 12-week randomized controlled trial in subjects with abdominal obesity. Am. J. Clin. Nutr. 2022, 116, 132–150. [Google Scholar] [CrossRef] [PubMed]
- Cioffi, I.; Evangelista, A.; Ponzo, V.; Ciccone, G.; Soldati, L.; Santarpia, L.; Contaldo, F.; Pasanisi, F.; Ghigo, E.; Bo, S. Intermittent versus continuous energy restriction on weight loss and cardiometabolic outcomes: A systematic review and meta-analysis of randomized controlled trials. J. Transl. Med. 2018, 16, 371. [Google Scholar] [CrossRef]
- Hołowko, J.; Michalczyk, M.M.; Zając, A.; Czerwińska-Rogowska, M.; Ryterska, K.; Banaszczak, M.; Jakubczyk, K.; Stachowska, E. Six weeks of calorie restriction improves body composition and lipid profile in obese and overweight former athletes. Nutrients 2019, 11, 1461. [Google Scholar] [CrossRef]
- Magkos, F.; Fraterrigo, G.; Yoshino, J.; Luecking, C.; Kirbach, K.; Kelly, S.C.; de Las Fuentes, L.; He, S.; Okunade, A.L.; Patterson, B.W. Effects of moderate and subsequent progressive weight loss on metabolic function and adipose tissue biology in humans with obesity. Cell Metab. 2016, 23, 591–601. [Google Scholar] [CrossRef]
- Ludwig, D.S.; Ebbeling, C.B. The carbohydrate-insulin model of obesity: Beyond “calories in, calories out”. JAMA Intern. Med. 2018, 178, 1098–1103. [Google Scholar] [CrossRef] [PubMed]
- Astrup, A.; Raben, A.; Geiker, N. The role of higher protein diets in weight control and obesity-related comorbidities. Int. J. Obes. 2015, 39, 721–726. [Google Scholar] [CrossRef]
- Pavlidou, E.; Papadopoulou, S.K.; Fasoulas, A.; Mantzorou, M.; Giaginis, C. Clinical evidence of low-carbohydrate diets against obesity and diabetes mellitus. Metabolites 2023, 13, 240. [Google Scholar] [CrossRef]
- Al-Reshed, F.; Sindhu, S.; Al Madhoun, A.; Bahman, F.; AlSaeed, H.; Akhter, N.; Malik, M.Z.; Alzaid, F.; Al-Mulla, F.; Ahmad, R. Low carbohydrate intake correlates with trends of insulin resistance and metabolic acidosis in healthy lean individuals. Front. Public Health 2023, 11, 1115333. [Google Scholar] [CrossRef]
- Hall, K.D.; Guo, J. Obesity energetics: Body weight regulation and the effects of diet composition. Gastroenterology 2017, 152, 1718–1727.e1713. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Sun, L.; Mu, Z. Effects of different weight loss dietary interventions on body mass index and glucose and lipid metabolism in obese patients. Medicine 2023, 102, e33254. [Google Scholar] [CrossRef] [PubMed]
- Prins, P.J.; Noakes, T.D.; Buxton, J.D.; Welton, G.L.; Raabe, A.S.; Scott, K.E.; Atwell, A.D.; Haley, S.J.; Esbenshade, N.J.; Abraham, J. High fat diet improves metabolic flexibility during progressive exercise to exhaustion (VO2max testing) and during 5 km running time trials. Biol. Sport 2023, 40, 465. [Google Scholar]
- Elaine, A.Y.; Le, N.A.; Stein, A.D. Measuring postprandial metabolic flexibility to assess metabolic health and disease. J. Nutr. 2021, 151, 3284–3291. [Google Scholar]
- Dulloo, A.G.; Schutz, Y. Adaptive thermogenesis in resistance to obesity therapies: Issues in quantifying thrifty energy expenditure phenotypes in humans. Curr. Obes. Rep. 2015, 4, 230–240. [Google Scholar] [CrossRef]
- Anton, S.D.; Moehl, K.; Donahoo, W.T.; Marosi, K.; Lee, S.A.; Mainous, A.G., III; Leeuwenburgh, C.; Mattson, M.P. Flipping the metabolic switch: Understanding and applying the health benefits of fasting. Obesity 2018, 26, 254–268. [Google Scholar] [CrossRef]
- Wang, D.; Benito, P.J.; Rubio-Arias, J.Á.; Ramos-Campo, D.J.; Rojo-Tirado, M.A. Exploring factors of adherence to weight loss interventions in population with overweight/obesity: An umbrella review. Obes. Rev. 2024, 25, e13783. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.A.; Sypniewski, C.; Bensadon, B.A.; McLaren, C.; Donahoo, W.T.; Sibille, K.T.; Anton, S. Determinants of adherence in time-restricted feeding in older adults: Lessons from a pilot study. Nutrients 2020, 12, 874. [Google Scholar] [CrossRef]
- Berthoud, H.R.; Seeley, R.J.; Roberts, S.B. Physiology of energy intake in the weight-reduced state. Obesity 2021, 29, S25–S30. [Google Scholar] [CrossRef]
- Trujillo-Garrido, N.; Santi-Cano, M.J. Motivation and limiting factors for adherence to weight loss interventions among patients with obesity in primary care. Nutrients 2022, 14, 2928. [Google Scholar] [CrossRef]
- Zhang, Y.; Gu, Y.a.; Wang, N.; Zhao, Q.; Ng, N.; Wang, R.; Zhou, X.; Jiang, Y.; Wang, W.; Zhao, G. Association between anthropometric indicators of obesity and cardiovascular risk factors among adults in Shanghai, China. BMC Public Health 2019, 19, 1035. [Google Scholar] [CrossRef] [PubMed]
- Golabi, S.; Ajloo, S.; Maghsoudi, F.; Adelipour, M.; Naghashpour, M. Associations between traditional and non-traditional anthropometric indices and cardiometabolic risk factors among inpatients with type 2 diabetes mellitus: A cross-sectional study. J. Int. Med. Res. 2021, 49, 03000605211049960. [Google Scholar] [CrossRef] [PubMed]
- Lampignano, L.; Zupo, R.; Donghia, R.; Guerra, V.; Castellana, F.; Murro, I.; Di Noia, C.; Sardone, R.; Giannelli, G.; De Pergola, G. Cross-sectional relationship among different anthropometric parameters and cardio-metabolic risk factors in a cohort of patients with overweight or obesity. PLoS ONE 2020, 15, e0241841. [Google Scholar] [CrossRef] [PubMed]
- Lazzer, S.; D’Alleva, M.; Isola, M.; De Martino, M.; Caroli, D.; Bondesan, A.; Marra, A.; Sartorio, A. Cardiometabolic index (CMI) and visceral adiposity index (VAI) highlight a higher risk of metabolic syndrome in women with severe obesity. J. Clin. Med. 2023, 12, 3055. [Google Scholar] [CrossRef]
- Petrovic, A. Diet Composition for Body Weight Reduction. R.S. Patent 63407, 30 June 2022. [Google Scholar]
- Mateo-Gallego, R.; Marco-Benedí, V.; Perez-Calahorra, S.; Bea, A.M.; Baila-Rueda, L.; Lamiquiz-Moneo, I.; de Castro-Orós, I.; Cenarro, A.; Civeira, F. Energy-restricted, high-protein diets more effectively impact cardiometabolic profile in overweight and obese women than lower-protein diets. Clin. Nutr. 2017, 36, 371–379. [Google Scholar] [CrossRef]
- Lohman, T.; Roche, A.; Martorell, R. Anthropometric Standardization Reference Manual; Human Kinetic Books: Champagne, IL, USA, 1991. [Google Scholar]
- Heyward, V. ASEP methods recommendation: Body composition assessment. J. Exerc. Physiol. 2001, 4, 1–12. [Google Scholar]
- Choudhary, M.K.; Eräranta, A.; Koskela, J.; Tikkakoski, A.J.; Nevalainen, P.I.; Kähönen, M.; Mustonen, J.; Pörsti, I. Atherogenic index of plasma is related to arterial stiffness but not to blood pressure in normotensive and never-treated hypertensive subjects. Blood Press. 2019, 28, 157–167. [Google Scholar] [CrossRef]
- Mambrini, S.P.; Menichetti, F.; Ravella, S.; Pellizzari, M.; De Amicis, R.; Foppiani, A.; Battezzati, A.; Bertoli, S.; Leone, A. Ultra-processed food consumption and incidence of obesity and cardiometabolic risk factors in adults: A systematic review of prospective studies. Nutrients 2023, 15, 2583. [Google Scholar] [CrossRef]
- Michalczyk, M.M.; Maszczyk, A.; Stastny, P. The effects of low-energy moderate-carbohydrate (MCD) and mixed (MixD) diets on serum lipid profiles and body composition in middle-aged men: A randomized controlled parallel-group clinical trial. Int. J. Environ. Res. Public Health 2020, 17, 1332. [Google Scholar] [CrossRef]
- Seidelmann, S.B.; Claggett, B.; Cheng, S.; Henglin, M.; Shah, A.; Steffen, L.M.; Folsom, A.R.; Rimm, E.B.; Willett, W.C.; Solomon, S.D. Dietary carbohydrate intake and mortality: A prospective cohort study and meta-analysis. Lancet Public Health 2018, 3, e419–e428. [Google Scholar] [CrossRef]
- Volek, J.S.; Gómez, A.L.; Kraemer, W.J. Fasting lipoprotein and postprandial triacylglycerol responses to a low-carbohydrate diet supplemented with n-3 fatty acids. J. Am. Coll. Nutr. 2000, 19, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Nordmann, A.J.; Nordmann, A.; Briel, M.; Keller, U.; Yancy, W.S.; Brehm, B.J.; Bucher, H.C. Effects of low-carbohydrate vs low-fat diets on weight loss and cardiovascular risk factors: A meta-analysis of randomized controlled trials. Arch. Intern. Med. 2006, 166, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Eapen, D.J.; Kalra, G.L.; Rifai, L.; Eapen, C.A.; Merchant, N.; Khan, B.V. Raising HDL-C in women. Int. J. Women’s Health 2010, 1, 181–191. [Google Scholar] [CrossRef][Green Version]
- Knopp, R.H.; Paramsothy, P.; Retzlaff, B.M.; Fish, B.; Walden, C.; Dowdy, A.; Tsunehara, C.; Aikawa, K.; Cheung, M.C. Gender differences in lipoprotein metabolism and dietary response: Basis in hormonal differences and implications for cardiovascular disease. Curr. Atheroscler. Rep. 2005, 7, 472–479. [Google Scholar] [CrossRef]
- Most, J.; Redman, L.M. Impact of calorie restriction on energy metabolism in humans. Exp. Gerontol. 2020, 133, 110875. [Google Scholar] [CrossRef]
- Beaulieu, K.; Casanova, N.; Oustric, P.; Turicchi, J.; Gibbons, C.; Hopkins, M.; Varady, K.; Blundell, J.; Finlayson, G. Matched weight loss through intermittent or continuous energy restriction does not lead to compensatory increases in appetite and eating behavior in a randomized controlled trial in women with overweight and obesity. J. Nutr. 2020, 150, 623–633. [Google Scholar] [CrossRef]
- Blundell, J.E.; Finlayson, G. Is susceptibility to weight gain characterized by homeostatic or hedonic risk factors for overconsumption? Physiol. Behav. 2004, 82, 21–25. [Google Scholar] [CrossRef]
- Pesta, D.H.; Samuel, V.T. A high-protein diet for reducing body fat: Mechanisms and possible caveats. Nutr. Metab. 2014, 11, 53. [Google Scholar] [CrossRef]
- Trumbo, P.; Schlicker, S.; Yates, A.A.; Poos, M. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. J. Am. Diet. Assoc. 2002, 102, 1621–1631. [Google Scholar] [CrossRef]
- Galbreath, M.; Campbell, B.; La Bounty, P.; Bunn, J.; Dove, J.; Harvey, T.; Hudson, G.; Gutierrez, J.L.; Levers, K.; Galvan, E. Effects of adherence to a higher protein diet on weight loss, markers of health, and functional capacity in older women participating in a resistance-based exercise program. Nutrients 2018, 10, 1070. [Google Scholar] [CrossRef]
- Hunter, G.R.; McCarthy, J.P.; Bamman, M.M. Effects of resistance training on older adults. Sports Med. 2004, 34, 329–348. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Lee, J.; Bae, W.; Choi, J.; Kim, H.; Cho, B. Efficacy of low-calorie, partial meal replacement diet plans on weight and abdominal fat in obese subjects with metabolic syndrome: A double-blind, randomised controlled trial of two diet plans-one high in protein and one nutritionally balanced. Int. J. Clin. Pract. 2009, 63, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Dodevska, M.S.; Sobajic, S.S.; Dragicevic, V.D.; Stankovic, I.; Ivanovic, N.D.; Djordjevic, B.I. The impact of diet and fibre fractions on plasma adipocytokine levels in prediabetic adults. Nutrients 2021, 13, 487. [Google Scholar] [CrossRef] [PubMed]
- Myhrstad, M.C.; Tunsjø, H.; Charnock, C.; Telle-Hansen, V.H. Dietary fiber, gut microbiota, and metabolic regulation—Current status in human randomized trials. Nutrients 2020, 12, 859. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Carbohydrate Intake for Adults and Children: WHO Guideline; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- O’Keefe, S.J. The association between dietary fibre deficiency and high-income lifestyle-associated diseases: Burkitt’s hypothesis revisited. Lancet Gastroenterol. Hepatol. 2019, 4, 984–996. [Google Scholar] [CrossRef]
- Djordjevic, B.; Ivanovic, N. Precise Nutrition and Metabolic Syndrome, Remodeling the Microbiome with Polyphenols, Probiotics, and Postbiotics. In Advances in Precision Nutrition, Personalization and Healthy Aging; Springer International Publishing: Cham, Switzerland, 2022; pp. 145–178. [Google Scholar]
- Petrovičova, O.D.; Djuričić, I.; Ivanović, N.; Dabetić, N.; Dodevska, M.; Ilić, T. Dietary interventions in obesity: A narrative review. Arch. Pharm. 2024, 74, 281–297. [Google Scholar]
- Brinkworth, G.D.; Noakes, M.; Buckley, J.D.; Keogh, J.B.; Clifton, P.M. Long-term effects of a very-low-carbohydrate weight loss diet compared with an isocaloric low-fat diet after 12 mo. Am. J. Clin. Nutr. 2009, 90, 23–32. [Google Scholar] [CrossRef]
- Sharman, M.J.; Gómez, A.L.; Kraemer, W.J.; Volek, J.S. Very low-carbohydrate and low-fat diets affect fasting lipids and postprandial lipemia differently in overweight men. J. Nutr. 2004, 134, 880–885. [Google Scholar] [CrossRef]
- Dodevska, M.; Kukic Markovic, J.; Sofrenic, I.; Tesevic, V.; Jankovic, M.; Djordjevic, B.; Ivanovic, N.D. Similarities and differences in the nutritional composition of nuts and seeds in Serbia. Front. Nutr. 2022, 9, 1003125. [Google Scholar] [CrossRef]
- WHO. Saturated Fatty Acid and Trans-Fatty Acid Intake for Adults and Children: WHO Guideline; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- Alhassan, S.; Kim, S.; Bersamin, A.; King, A.; Gardner, C. Dietary adherence and weight loss success among overweight women: Results from the A TO Z weight loss study. Int. J. Obes. 2008, 32, 985–991. [Google Scholar] [CrossRef]
- Kroeger, C.M.; Trepanowski, J.F.; Klempel, M.C.; Barnosky, A.; Bhutani, S.; Gabel, K.; Varady, K.A. Eating behavior traits of successful weight losers during 12 months of alternate-day fasting: An exploratory analysis of a randomized controlled trial. Nutr. Health 2018, 24, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Westphal-Nardo, G.; Chaput, J.-P.; Faúndez-Casanova, C.; Fernandes, C.A.M.; de Andrade Gonçalves, E.C.; Utrila, R.T.; Oltramari, K.; Grizzo, F.M.F.; Nardo-Junior, N. Exploring new tools for risk classification among adults with several degrees of obesity. Int. J. Environ. Res. Public Health 2023, 20, 6263. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Feng, Y.; Li, S.; Ma, Y.; Lin, J.; Wan, J.; Zhao, M. The atherogenic index of plasma (AIP) is a predictor for the severity of coronary artery disease. Front. Cardiovasc. Med. 2023, 10, 1140215. [Google Scholar] [CrossRef] [PubMed]
- Dobiasova, M. AIP--Atherogenic index of plasma as a significant predictor of cardiovascular risk: From research to practice. Vnitr. Lek. 2006, 52, 64–71. [Google Scholar]
- Gibson, A.A.; Sainsbury, A. Strategies to improve adherence to dietary weight loss interventions in research and real-world settings. Behav. Sci. 2017, 7, 44. [Google Scholar] [CrossRef]


| Min Intake | Medium Intake | Max Intake | |
|---|---|---|---|
| Carbohydrates | |||
| %EI | 20–35% * | 40–49% * | 50–65% * |
| g/kg BW | 0.9–1.3 | 1.9–2.8 | 2.9–3.6 |
| Proteins | |||
| %EI | 30–40% | 25–30% | 15–25% |
| g/kg BW | 1.7–1.9 | 1.2–1.4 | 0.8–1.2 |
| Fats | |||
| %EI | 30–35% | 25–30% | 15–20% |
| g/kg BW | 0.6–0.85 | 0.5–0.6 | ≤0.5 |
| CH/P ratio in %EI | 0.5–1 | 1.3–1.8 | 2–2.75 |
| Energy Deficiency | 200 kcal up to RMR | 200 kcal up to RMR | 200 kcal up to RMR |
| Parameters/ Category | All Participants | Change | ≤40 Years | Change | > 40 Years | Change | Male | Change | Female | Change |
|---|---|---|---|---|---|---|---|---|---|---|
| BW, kg | ||||||||||
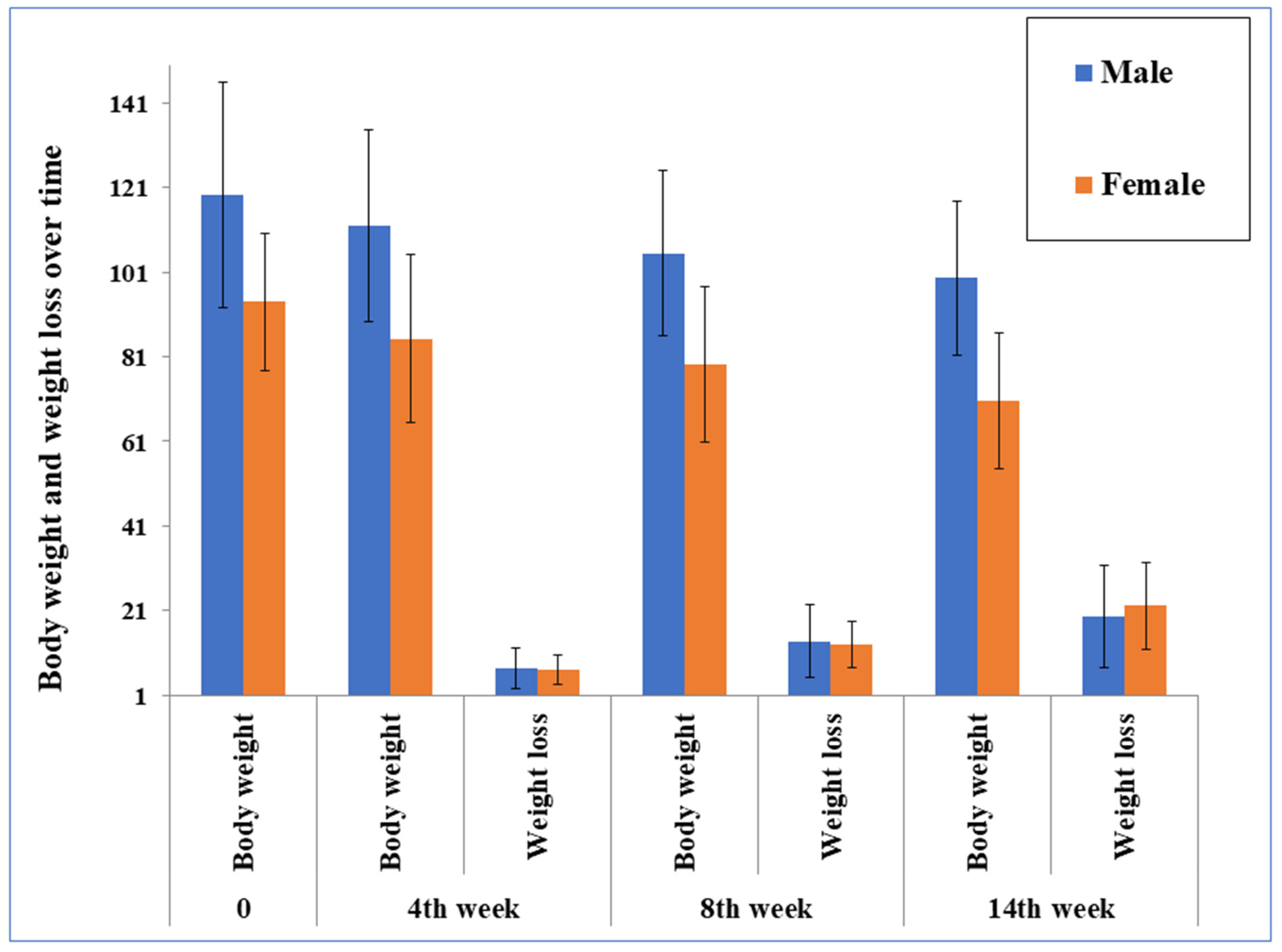
| Value at the baseline | 100.1 (93.5–106.8) | −16.2 (−19.4 to −12.3) * | 101.3 (90.2–112.4) | −19.4 (−24.9 to 13.9) *;† | 98.9 (90.9–107.1) | −13.0 (16.5 to 9.5) * | 115.6 (105.9–125.3) | −16.9 (−22.4 to −11.6) * | 85.9 (79.6–92.3) | −15.4 (−19.5 to −11.4) * |
| Value at the end | 83.9 (79.1–88.8) | 81.9 (74.3–89.4) | 85.9 (79.4–92.5) | 98.7 (29.8–104.6) | 70.5 (66.6–74.4) | |||||
| BMI, kg/m2 | ||||||||||
| Value at the baseline | 32.9 (30.9–34.9) | −6.1 (−7.6 to −4.5) * | 31.9 (29.0–34.7) | −6.0 (−7.9 to 4.2) * | 33.9 (29.1–34.9) | −6.3 (−8.7 to −3.9) * | 34.1 (31.8–36.5) | −5.0 (−6.6 to 3.6) *;‡ | 31.9 (28.7–35.1) | −7.2 (−9.7 to 4.7) * |
| Value at the end | 26.8 (25.8–27.8) | 25.9 (26.3–28.9) | 27.6 (26.3–28.9) | 29.1 (27.9–30.3) | 24.7 (23.5–25.9) | |||||
| WC, cm | ||||||||||
| Value at the baseline | 108.5 (104.9–112.1) | −12.3 (−14.6 to −10.0) * | 107.9 (102.1–113.6) | −13.5 (−17.3 to −9.6) * | 109.1 (104.4–113.8) | −11.2 (−14.0 to −8.3) * | 111.4 (107.0–115.8) | 12.0 (−14.8 to −9.1) * | 105.8 (100.1–111.4) | −12.5 (−16.3 to −8.8) * |
| Value at the end | 96.2 (93.9–98.5) | 94.4 (91.1–97.6) | 97.9 (94.7–101.1) | 99.4 (96.6–102.3) | 93.2 (89.9–96.5) | |||||
| HC, cm | ||||||||||
| Value at the baseline | 114.8 (111.4–118.1) | −10.9 (−12.9 to −8.9) * | 114.6 (109.3–119.9) | −11.6 (−15.0 to −8.2) * | 114.9 (110.5–119.3) | −10.2 (−12.6 to −7.8) * | 118.0 (112.8–123.0) | −11.4 (−14.6 to −8.2) * | 111.8 (107.5–116.1) | −10.4. (−13.1 to −7.7) * |
| Value at the end | 103.9 (101.8–105.9) | 103.0 (100.1–105.8) | 104.7 (101.6–107.8) | 106.5 (103.4–109.7) | 101.4 (98.8–103.9) | |||||
| BF, % | ||||||||||
| Value at the baseline | 37.8 (35.3–40.3) | −7.2 (−9.4 to −5.1) * | 38.7 (34.3–39.6) | −9.2 (−13.2 to −5.3) * | 36.9 (34.3–39.6) | −5.3 (−7.3 to −3.3) * | 33.7 (29.9–37.4) | −5.9 (−9.9 to −1.8) ** | 41.6 (38.7–44.5) | −8.5 (−10.6 to −6.4) * |
| Value at the end | 30.6 (28.9–32.2) | 29.5 (27.1–31.8) | 31.6 (29.4–33.9) | 27.8 (25.9–29.7) | 33.1 (30.8–35.5) | |||||
| SMM, % | ||||||||||
| Value at the baseline | 28.1 (26.9–29.3) | 2.6 (1.7 to 3.4) * | 28.2 (26.3–30.2) | 3.0 (1.7 to 4.3) * | 28.0 (26.5–29.6) | 2.2 (1.0 to 3.4) ** | 31.2 (29.5–32.8) | 2.1 (0.6 to 3.7) ** | 25.4 (24.2–26.5) | 2.9 (2.0 to 3.9) * |
| Value at the end | 30.7 (29.7–31.7) | 30.2 (29.7–32.7) | 30.2 (28.8–31.6) | 33.3 (32.2.−34.4) | 28.3 (27.2–29.4) | |||||
| Water, % | ||||||||||
| Value at the baseline | 47.0 (45.4–48.6) | 4.2 (2.8 to 5.6) * | 48.5 (46.3–50.7) | 4.5 (2.4 to 6.4) * | 45.6 (43.3–47.9) | 3.8 (1.9 to 5.8) * | 48.3 (46.2–50.4) | 3.4 (1.9 to 4.9) * | 45.9 (42.4–48.3) | 4.9 (2.6 to 7.2) * |
| Value at the end | 51.2 (50.4–52.1) | 53.0 (51.9–54.1) | 49.4 (48.6–50.4) | 51.7 (50.6–52.9) | 50.8 (49.5–52.0) | |||||
| WHtR | ||||||||||
| Value at the baseline | 0.62 (0.59 to 0.64) | −0.07 (−0.08 to −0.06) * | 0.61 (0.57 to 0.65) | −0.08 (−0.09 to −0.05) * | 0.63 (0.60 to 0.65) | −0.06 (−0.08 to −0.05) * | 0.61 (0.58 to 0.63) | −0.07 (−0.08 to −0.05) * | 0.63 (0.59 to 0.67) | −0.8 (−0.9 to −0.05) * |
| Value at the end | 0.55 (0.53 to 0.56) | 0.53 (0.52 to 0.55) | 0.56 (0.54 to 0.58) | 0.54 (0.52 to 0.56) | 0.55 (0.53 to 0.58) | |||||
| WHR | ||||||||||
| Value at the baseline | 0.95 (0.93 to 0.96) | −0.02 (−0.03 to −0.01) ** | 0.94 (0.91 to 0.97) | −0.02 (−0.04 to −0.01) ** | 0.95 (0.93 to 0.97) | −0.01 (−0.03 to −0.001) | 0.95 (0.93 to 0.96) | −0.01 (−0.02 to −0.001) *** | 0.95 (0.91 to 0.98) | −0.03 (−0.04 to −0.01) ** |
| Value at the end | 0.93 (0.91 to 0.94) | 0.92 (0.89 to 0.94) | 0.94 (0.91 to 0.96) | 0.93 (0.92 to 0.95) | 0.92 (0.89 to 0.95) | |||||
| Parameters/ Category | All Participants | Change | Male | Change | Female | Change | ≤40 Years | Change | >40 Years | Change |
|---|---|---|---|---|---|---|---|---|---|---|
| Glucose, mmol/L | ||||||||||
| Value at the baseline | 5.4 (5.2 to 5.5) | −0.5 (−0.7 to −0.3) * | 5.6 (5.4 to 5.9) | −0.7 (−0.15 to −0.07) *;†† | 5.1 (4.9 to 5.3) | −0.3 (−0.39 to −0.09) ** | 5.3 (5.0 to 5.5) | −0.4 (−0.6 to 0.1) ** | 5.4 (5.2 to 5.6) | −0.5 (−0.8 to −0.3) * |
| Value at the end | 4.9 (4.7 to 5.0) | 4.9 (4.8 to 5.1) | 4.8 (4.6 to 5.0) | 4.9 (4.7 to 5.1) | 4.9 (4.7 to 5.1) | |||||
| HbA1c, % | ||||||||||
| Value at the baseline | 5.3 (5.2 to 5.4) | −0.2 (−0.5 to −0.1) ** | 5.3 (5.2 to 5.5) | −0.2 (−0.4 to −0.1) *** | 5.3 (5.2 to 5.4) | −0.3 (−0.4 to −0.03) *** | 5.2 (5.1 to 5.4) | −0.1 (−0.3 to −0.002) *** | 5.3 (5.2 to 5.5) | −0.2 (−0.4 to −0.1) ** |
| Value at the end | 5.1 (4.9 to 5.2) | 5.1 (5.0 to 5.2) | 5.0 (4.9 to 5.2) | 5.1 (4.9 to 5.2) | 5.1 (4.9 to 5.2) | |||||
| Total cholesterol, mmol/L | ||||||||||
| Value at the baseline | 5.52 (5.29 to 5.74) | −0.89 (−0.95 to −0.80) * | 5.53 (5.25 to 5.81) | −0.82 (−0.89 to −0.76) * | 5.51 (5.15 to 5.87) | −0.96 (−1.24 to −0.67) * | 5.29 (4.99 to 5.58) | −0.78 (−1.17 to −0.39) * | 5.74 (5.41 to 6.08) | −0.99 (−1.30 to −0.68) * |
| Value at the end | 4.63 (4.44 to 4.82) | 4.71 (4.38 to 5.04) | 4.56 (4.33 to 4.78) | 4.51 (4.21 to 4.80) | 4.75 (4.49 to 5.01) | |||||
| HDL-cholesterol, mmol/L | ||||||||||
| Value at the baseline | 1.32 (1.23 to 1.43) | −0.04 (−0.05 to −0.03) | 1.17 (1.05 to 1.29) | 0.05 (0.01 to 0.17) †† | 1.47 (1.34 to 1.61) | −0.16 (−0.24 to −0.03) *** | 1.34 (1.20 to 1.49) | −0.06 (−0.16 to −0.04) | 1.31 (1.18 to 1.45) | −0.04 (−0.14 to 0.07) |
| Value at the end | 1.28 (1.21 to 1.35) | 1.22 (1.12 to 1.34) | 1.33 (1.24 to 1.42) | 1.28 (1.19 to 1.38) | 1.27 (1.17 to 1.39) | |||||
| LDL-cholesterol, mmol/L | ||||||||||
| Value at the baseline | 3.38 (3.16 to 3.59) | −0.57 (−0.60 to −0.40) * | 3.44 (3.18 to 3.72) | −0.58 (−0.90 to −0.26) ** | 3.32 (2.98 to 3.66) | −0.57 (−0.86 to −0.27) * | 3.23 (2.99 to 3.47) | −0.50 (−0.72 to −0.29) * | 3.53 (3.17 to 3.88) | −0.65 (−1.01 to −0.27) ** |
| Value at the end | 2.81 (2.65 to 2.96) | 2.86 (2.61 to 3.12) | 2.75 (2.55 to 2.95) | 2.73 (2.53 to 2.92) | 2.88 (2.63 to 3.14) | |||||
| Triglycerides, mmol/L | ||||||||||
| Value at the baseline | 2.09 (1.67 to 2.52) | −0.80 (−0.99 to −0.50) * | 2.67 (1.91 to 3.43) | −1.06 (−1.67 to 0.45) ** | 1.56 (1.19 to 1.54) | −0.55 (−0.71 to −0.37) ** | 1.76 (1.18 to 2.35) | −0.39 (−0.88 to −0.08) ‡ | 2.41 (1.79 to 3.04) | −1–19 (−1.68 to −0.69) * |
| Value at the end | 1.29 (0.91 to 1.68) | 1.61 (0.81 to 2.40) | 1.01 (0.85 to 1.18) | 1.36 (0.59 to 2.14) | 1.22 (1.01 to 1.45) | |||||
| AIP | ||||||||||
| Value at the baseline | 0.11 (0.01 to 0.21) | −0.19 (−0.26 to −0.12) * | 0.28 (0.01 to 0.42) | −0.28 (−0.36 to −0.19) *;† | −0.04 (−0.16 to −0.07) | −0.12 (−0.22 to −0.001) | 0.03 (−0.11 to 0.16) | −0.14 (−0.26 to −0.03) *** | 0.18 (0.04 to 0.33) | −0.23 (−0.33 to −0.14) * |
| Value at the end | −0.08 (−0.16 to −0.01) | −0.001 (−0.13 to 0.13) | −0.16 (−0.24 to −0.07) | −0.11 (−0.24 to 0.01) | −0.05 (−0.15 to 0.05) | |||||
| CMI | ||||||||||
| Value at the baseline | 1.09 (0.79 to 1.34) | −0.59 (−0.83 to −0.35) * | 1.41 (0.87 to 1.96) | −0.85 (−1.31 to −0.40) **;† | 0.79 (0.55 to 1.03) | −0.35 (−0.56 to −0.14) ** | 0.81 (0.57 to 1.05) | −0.37 (−0.58 to −0.17) ** | 1.36 (0.83 to 1.88) | −0.80 (−1.23 to −0.36) ** |
| Value at the end | 0.49 (0.42 to 0.58) | 0.56 (0.42 to 0.70) | 0.44 (0.35 to 0.54) | 0.44 (0.33 to 0.54) | 0.56 (0.43 to 0.69) | |||||
| VAI | ||||||||||
| Value at the baseline | 2.5 (1.8 to 3.2) | −1.2 (−1.8 to −0.6) * | 3.7 (2.6 to 4.7) | −1.8 (−2.6 to −0.9) * | ||||||
| Value at the end | 1.3 (1.1 to 1.5) | 1.9 (1.6 to 2.2) | ||||||||
| LAP | ||||||||||
| Value at the baseline | 112 (80 to 144) | −71 (−97 to −44) * | 76 (54 to 99) | −80 (−110 to −49) ** | ||||||
| Value at the end | 41 (32 to 50) | 35 (28 to 43) | ||||||||
| Parameters/ Category | All Participants | Change | Male | Change | Female | Change | ≤40 Years | Change | >40 Years | Change |
|---|---|---|---|---|---|---|---|---|---|---|
| CRP, mg/L | ||||||||||
| Value at the baseline | 4.8 (4.2 to 5.3) | −2.8 (−3.4 to −2.2) * | 4.5 (3.7 to 5.4) | −2.4 (−3.2 to −1.6) * | 4.9 (4.3 to 5.6) | −3.1 (−3.9 to −2.4) * | 5.1 (4.4 to 5.9) | −3.2 (−3.9 to −2.5) * | 4.4 (3.6 to 5.2) | −2.5 (−3.3 to 1.6) * |
| Value at the end | 2.0 (1.6 to 2.3) | 2.1 (1.6 2.7) | 1.8 (1.4 to 2.2) | 1.9 (1.5 to 2.4) | 1.9 (1.5 to 2.5) | |||||
| Fibrinogen, g/L | ||||||||||
| Value at the baseline | 3.1 (2.9 to 3.3) | −0.2 (−0.4 to −0.01) *** | 2.9 (2.7 to 3.2) | 0.1 (−0.2 to 0.3) † | 3.3 (3.1 to 3.5) | −0.4 (−0.8 to −0.1) ** | 3.1 (2.8 to 3.3) | −0.3 (−0.6 to −0.1) | 3.2 (2.9 to 3.4) | −0.2 (−0.5 to 0.1) |
| Value at the end | 2.9 (2.7 to 3.1) | 2.8 (2.7 to 3.3) | 2.9 (2.6 to 3.1) | 2.8 (2.5 to 3.1) | 3.0 (2.7 to 3.3) | |||||
| Sedimentation, mm/h | ||||||||||
| Value at the baseline | 9.3 (8.4 to 10.2) | −5.2 (−6.1 to −4.2) * | 9.0 (7.4 to 10.6) | −5.0 (−6.6 to −3.4) * | 9.6 (8.5 to 10.7) | −5.3 (−6.4 to −4.1) * | 2.3 (8.1 to 10.5) | −5.0 (−6.5 to −3.7) * | 9.3 (7.9 to 10.8) | −5.1 (−6.5 to −3.8) * |
| Value at the end | 4.1 (3.8 to 4.6) | 4.0 (3.5 to 4.5) | 4.3 (3.7 to 4.9) | 4.2 (3.5 to 4.9) | 4.2 (3.7 to 4.6) | |||||
| BUN, mmol/L | ||||||||||
| Value at the baseline | 4.8 (4.5 to 5.2) | −0.1 (−0.7 to 0.7) | 5.2 (4.6 to 5.8) | 0.7 (−0.6 to 2.1) † | 4.5 (4.1 to 4.9) | −0.7 (−1.2 to −0.1) *** | 4.9 (4.5 to 5.3) | −0.7 (−1.2 to 0.2) *** | 4.8 (4.2 to 5.4) | 0.6 (−0.7 to 1.9) |
| Value at the end | 4.7 (4.2 to 5.4) | 5.9 (4.9 to 7.0) | 3.8 (3.4 to 4.3) | 4.2 (3.7 to 4.7) | 5.4 (4.4 to 6.5) | |||||
| Creatinine, μmol/L | ||||||||||
| Value at the baseline | 76.8 (72.6 to 80.9) | −0.5 (−4.7 to 4.7) | 88.5 (83.1 to 93.9) | 2.0 (−7.5 to 11.6) | 66.0 (62.4 to 69.8) | −1.7 (−5.4 to 1.9) | 75.1 (68.6 to 81.5) | −1.9 (−6.3 to 2.5) | 78.4 (72.7 to 84.1) | 1.9 (−6.8 to 10.7) |
| Value at the end | 76.3 (70.9 to 82.6) | 90.5 (81.7 to 99.3) | 64.3 (59.2 to 69.4) | 73.2 (66.0 to 80.3) | 80.3 (70.9 to 89.7) | |||||
| Uric acid, mmol/L | ||||||||||
| Value at the baseline | 335 (310 to 361) | −4 (−26 to 18) | 391 (356 to 426) | −9 (−47 to 29) | 286 (257 to 314) | −1 (−26 to 27) | 340 (302 to 378) | −19 (−47 to 9) | 332 (296 to 368) | 11 (−25 to 46) |
| Value at the end | 331 (312 to 352) | 382 (356 to 407) | 285 (266 to 307) | 321 (289 to 352) | 343 (317 to 369) | |||||
| Total bilirubin, µmol/L | ||||||||||
| Value at the baseline | 12.9 (11.1 to 14.9) | 1 (−0.7 to to 2.6) | 16.0 (12.6 to 19.5) | 1.6 (−1.2 to 4.4) † | 10.1 (8.6 to 11.7) | 0.5 (−1.6 to 2.4) | 13.2 (10.4 to 15.9) | 2.9 (0.5 to 5.4) *** | 12.8 (9.9 to 15.5) | −1.0 (−3.1 to 1.2) |
| Value at the end | 13.9 (11.4 to 16.4) | 17.6 (12.9 to 22.4) | 10.6 (9.1 to 12.1) | 16.1 (11.7 to 20.5) | 11.8 (9.4 to 14.3) | |||||
| Serum iron, µmol/L | ||||||||||
| Value at the baseline | 18.5 (16.9 to 20.0) | −0.9 (−2.8 to 0.8) | 20.2 (18.0 to 22.3) | −1.5 (−4.7 to 1.8) | 16.9 (14.7 to 19.1) | −0.6 (−2.5 to 1.4) | 18.3 (16.3 to 20.3) | 0.3 (−1.8 to 2.5) | 18.6 (16.2 to 21.1) | −2.3 (−5.3 to 0.7) |
| Value at the end | 17.4 (15.9 to 18.9) | 18.7 (16.6 to 20.8) | 16.3 (14.2 to 18.4) | 18.6 (16.4 to 20.8) | 16.3 (14.3 to 18.4) | |||||
| AST, IU/L | ||||||||||
| Value at the baseline | 26 (23 to 29) | −7 (−9 to −4) * | 29 (24 to 33) | −7 (−11 to −2) ** | 23 (19 to 28) | −6.6 (−10.7 to −2.5) ** | 28 (22 to 34) | −10 (−14 to −5) * | 24 (21 to 27) | −4 (−7 to −1) *** |
| Value at the end | 19 (18 to 20) | 22 (19 to 25) | 17 (15 to 19) | 18 (16 to 21) | 20 (18 to 22) | |||||
| ALT, IU/L | ||||||||||
| Value at the baseline | 35 (28 to 42) | −12 (−18 to −7) * | 43 (31 to 54) | −16 (−24 to −6) ** | 28 (21 to 35) | −9.9 (−17.1 to −2.8) ** | 40 (27 to 53) | −18 (−28 to −7) ** | 30 (25 to 36) | −7 (−13 to −2) *** |
| Value at the end | 23 (20 to 25) | 27 (22 to 33) | 18 (16 to 20) | 22 (17 to 28) | 23 (20 to 26) | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrovic, A.; Jovicic, S.; Dodevska, M.; Djordjevic, B.; Milinkovic, N.; Ivanovic, N.D. Effects of Specially Designed Energy-Restricted Diet on Anthropometric Parameters and Cardiometabolic Risk in Overweight and Obese Adults: Pilot Study. Nutrients 2024, 16, 3453. https://doi.org/10.3390/nu16203453
Petrovic A, Jovicic S, Dodevska M, Djordjevic B, Milinkovic N, Ivanovic ND. Effects of Specially Designed Energy-Restricted Diet on Anthropometric Parameters and Cardiometabolic Risk in Overweight and Obese Adults: Pilot Study. Nutrients. 2024; 16(20):3453. https://doi.org/10.3390/nu16203453
Chicago/Turabian StylePetrovic, Ana, Snezana Jovicic, Margarita Dodevska, Brizita Djordjevic, Neda Milinkovic, and Nevena D. Ivanovic. 2024. "Effects of Specially Designed Energy-Restricted Diet on Anthropometric Parameters and Cardiometabolic Risk in Overweight and Obese Adults: Pilot Study" Nutrients 16, no. 20: 3453. https://doi.org/10.3390/nu16203453
APA StylePetrovic, A., Jovicic, S., Dodevska, M., Djordjevic, B., Milinkovic, N., & Ivanovic, N. D. (2024). Effects of Specially Designed Energy-Restricted Diet on Anthropometric Parameters and Cardiometabolic Risk in Overweight and Obese Adults: Pilot Study. Nutrients, 16(20), 3453. https://doi.org/10.3390/nu16203453







