Association of Gut Microbiota Composition with Stunting Incidence in Children under Five in Jakarta Slums
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Protocols and Participants
2.2. Fecal Sample Collection, DNA Extraction, and 16S rRNA Sequencing
2.3. Gut Microbiota Composition
2.4. Nutrient Intake
2.5. Statistical Analysis
3. Results
3.1. Subject Characteristics
3.2. Nutrient Intake
3.3. Other Factors
3.4. Gut Microbiota Composition
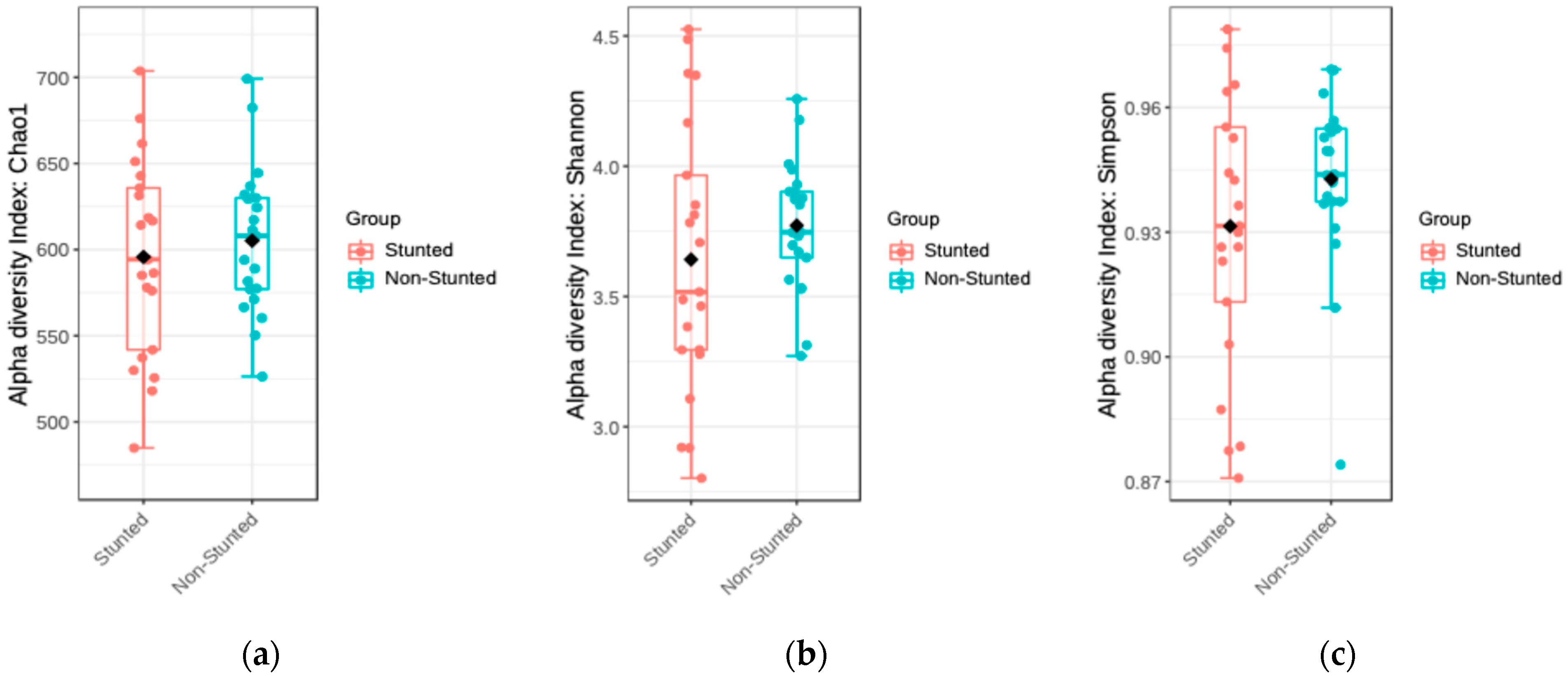
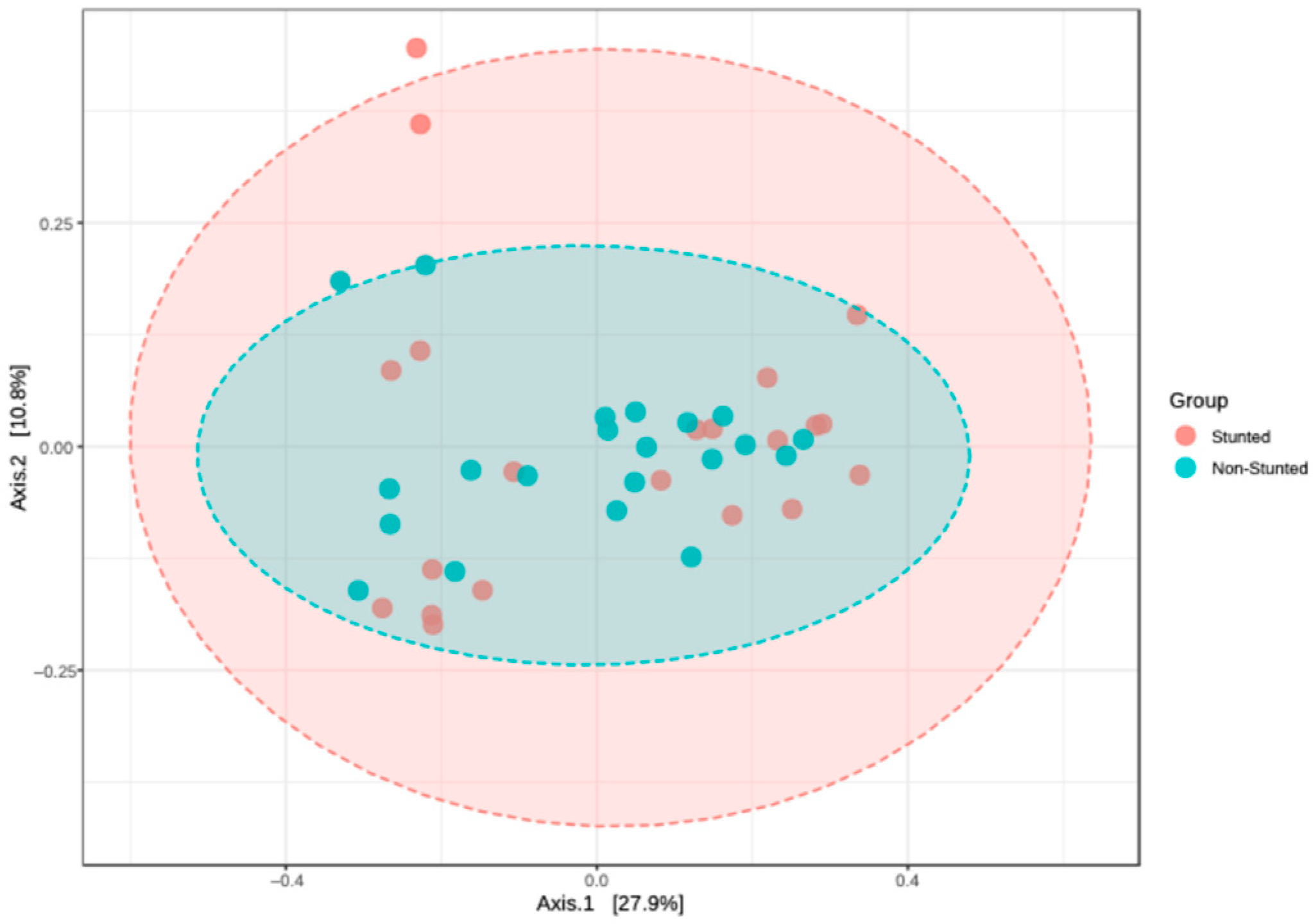
3.4.1. Alpha and Beta Diversity
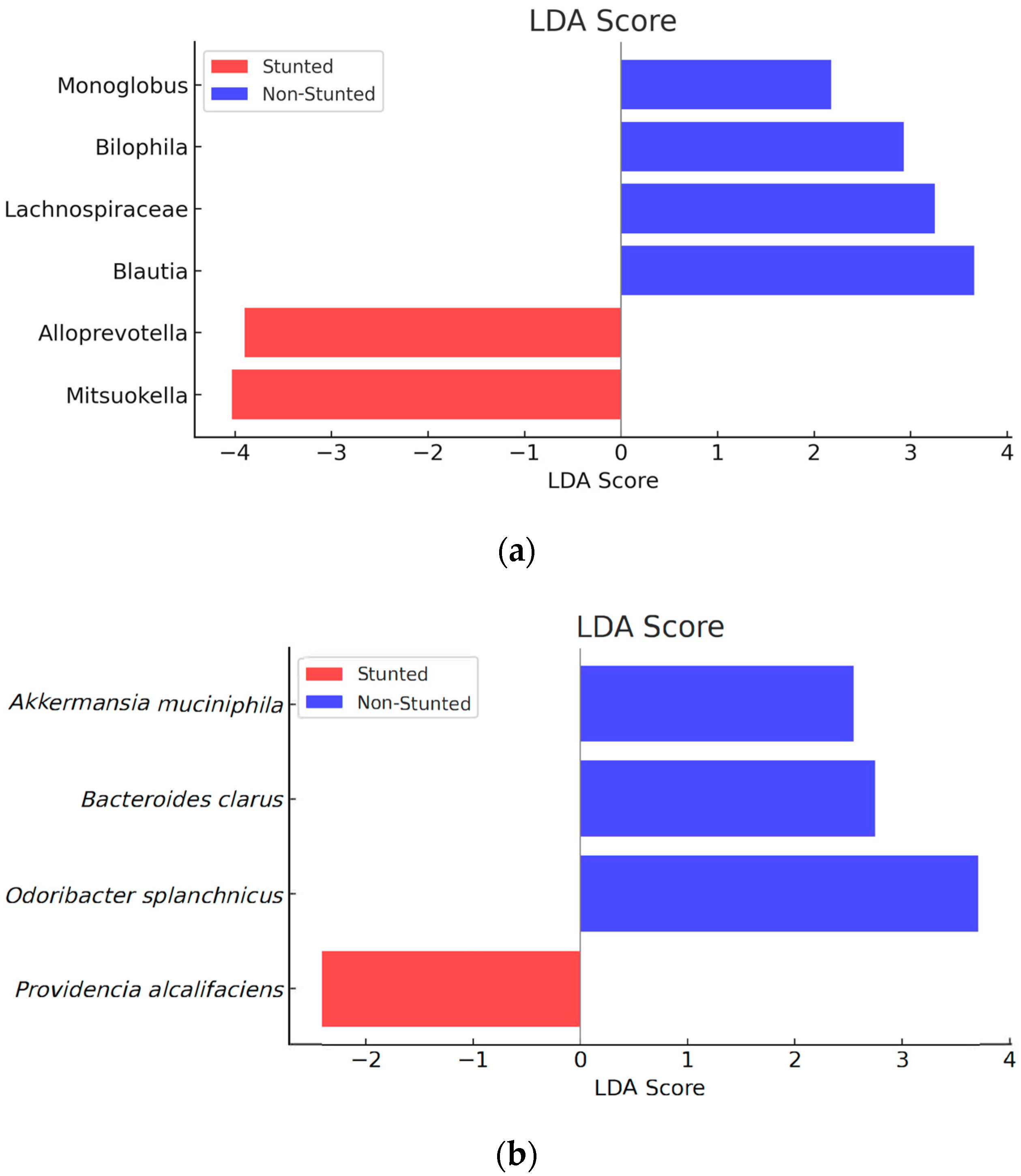
3.4.2. Gut Microbiota Compositions between the Stunted and Non-Stunted Group
3.5. Association between Nutrient Intake and Gut Microbiota Composition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ministry of Public Works and Housing. Profile of a City Without Slum Settlements, DKI Jakarta Province. 2017. Available online: https://pu.go.id/berita/tag/KOTAKU (accessed on 12 December 2022).
- Planning and Financing Division of the DKI Jakarta Health Service. DKI Jakarta Province Health Profile 2017; Central Bureau of Statistics: Jakarta, Indonesia, 2017. Available online: https://dinkes.jakarta.go.id/berita/profil/profil-kesehatan (accessed on 14 April 2020).
- World Health Organization. WHO Child Growth Standards; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- Nutrition Landacape Information System (NLIS). Country Profile Indicators: Interpretation Guide, 2nd edition. Available online: https://www.who.int/publications/i/item/9789241516952 (accessed on 12 December 2022).
- Yadav, P.; Dubey, B.N. Nutritional problems among children in urban slum area. Man India 2017, 97, 349–362. [Google Scholar]
- Islam, M.M.; Sanin, K.I.; Mahfuz, M.; Ahmed, A.S.; Mondal, D.; Haque, R.; Ahmed, T. Risk factors of stunting among children living in an urban slum of Bangladesh: Findings of a prospective cohort study. BMC Public Health 2018, 18, 197. [Google Scholar] [CrossRef]
- Dinh, D.M.; Ramadass, B.; Kattula, D.; Sarkar, R.; Braunstein, P.; Tai, A.; Wankle, C.A.; Hassoun, S.; Kane, A.V.; Naumova, E.N.; et al. Longitudinal Analysis of the Intestinal Microbiota in Persistently Stunted Young Children in South India. PLoS ONE 2016, 11, e0155405. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, T.S.; Gupta, S.S.; Bhattacharya, T.; Yadav, D.; Barik, A.; Chowdhury, A.; Das, B.; Mande, S.S.; Nair, B. Gut Microbiomes of Indian Children of Varying Nutritional Status. PLoS ONE 2014, 9, e95547. [Google Scholar] [CrossRef] [PubMed]
- Monira, S.; Nakamura, S.; Gotoh, K.; Izutsu, K.; Watanabe, H.; Alam, N.; Endtz, H.; Cravioto, A.; Ali, S.; Nakaya, T.; et al. Gut Microbiota of Healthy and Malnourished Children in Bangladesh. Front. Microbiol. 2011, 2, 228. [Google Scholar] [CrossRef]
- Das, B.; Ghosh, T.S.; Kedia, S.; Rampal, R.; Saxena, S.; Bag, S.; Mitra, R.; Dayal, M.; Mehta, O.; Serendranath, A.; et al. Analysis of the Gut Microbiome of Rural and Urban Healthy Indians Living in Sea Level and High-Altitude Areas. Sci. Rep. 2018, 8, 10104. [Google Scholar] [CrossRef]
- De Filippo, C.; Di Paola, M.; Ramazzotti, M.; Albanese, D.; Pieraccini, G.; Banci, E.; Miglietta, F.; Cavalieri, D.; Lionetti, P. Diet, Environments, and Gut Microbiota. A Preliminary Investigation in Children Living in Rural and Urban Burkina Faso and Italy. Front. Microbiol. 2017, 8, 1979. [Google Scholar] [CrossRef]
- Lin, A.; Bik, E.; Costello, E.; Dethlefsen, L.; Haque, R.; Relman, D.; Singh, U. Distinct Distal Gut Microbiome Diversity and Composition in Healthy Children from Bangladesh and the United States. PLoS ONE 2013, 8, e53838. [Google Scholar] [CrossRef]
- De Filippo, C.; Cavalieri, D.; Di Paola, M.; Ramazzotti, M.; Poullet, J.; Massart, S.; Coliini, S.; Pieraccini, G.; Lionetti, P. Impact of Diet in Shaping Gut Microbiota Revealed by A Comparative Study in Children from Europe and Rural Africa. Proc. Natl. Acad. Sci. USA 2010, 107, 14691–14696. [Google Scholar] [CrossRef]
- FAO. Dietary Assessment; Food and Agriculture Organization of The United Nations: Rome, Italy, 2018. [Google Scholar] [CrossRef]
- Ratnayani, R.; Sunardi, D.; Fadilah, F.; Hegar, B. Nutrient intake and stunting in children aged 2-5 years in a slum area of Jakarta. Paediatr. Indones. 2024, 64, 132–138. [Google Scholar] [CrossRef]
- Helmyati, S.; Yuliati, E.; Wisnusanti, S.U.; Maghribi, R.; Juffrie, M. Condition of Digestive Tract Microbiota of Elementary School Children Experiencing Stunting in West Lombok. J. Gizi Dan Pangan 2017, 12, 55–60. [Google Scholar] [CrossRef]
- Zymo Research. DNA/RNA Shield Fecal Collection Tube 2020. Available online: https://zymoresearch.eu/products/dna-rna-shield-fecal-collection-tube (accessed on 30 September 2024).
- Sambrook, J.; Russel, D.W. Molecular Cloning: A Laboratory Manual, 3rd ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2001. [Google Scholar]
- Illumina Inc. Illumina Sequencing Introduction. 2017, pp. 1–8. Available online: http://www.illumina.com/content/dam/illumina-marketing/documents/products/illumina_sequencing_introduction.pdf (accessed on 21 May 2022).
- Ribeiro, R.M.; Souza-basqueira, M.D.; Oliveira, L.C.D.; Salles, F.C.; Pereira, N.B.; Sabino, E.C. An Alternative Storage Method for Characterization of the Intestinal Microbiota Through Next Generation Sequencing. Rev. Inst. Med. Trop. Sao Paulo 2018, 60, e77. [Google Scholar] [CrossRef] [PubMed]
- Berding, K.; Holscher, H.D.; Arthur, A.E.; Donovan, S.M. Fecal Microbiome Composition and Stability in 4- to 8-Year-Old Children Is Associated with Dietary Patterns and Nutrient Intake. J. Nutr. Biochem. 2018, 56, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health. Regulation of the Minister of Health of the Republic of Indonesia Number 28 of 2019 concerning Recommended Dietary Allowance (Angka Kecukupan Gizi, AKG) for Indonesian People 2019. Available online: http://hukor.kemkes.go.id/uploads/produk_hukum/PMK_No__28_Th_2019_ttg_Angka_Kecukupan_Gizi_Yang_Dianjurkan_Untuk_Masyarakat_Indonesia.pdf (accessed on 23 August 2022).
- Gibson, R.S. Development of Nutrient Reference Values. In Principles of Nutritional Assessment, 3rd ed.; 2023; Available online: https://nutritionalassessment.org/nrv/index.html (accessed on 22 October 2022).
- Surono, I.S.; Widiyanti, D.; Kusumo, P.D.; Venema, K. Gut Microbiota Profile of Indonesian Stunted Children and Children with Normal Nutritional Status. PLoS ONE 2021, 16, e0245399. [Google Scholar] [CrossRef]
- Yoh, M.; Matsuyama, J.; Ohnishi, M.; Takagi, K.; Miyagi, H.; Mori, K.; Park, K.; Ono, T.; Honda, T. Importance of Providencia Species as A Major Cause of Travellers’ Diarrhoea. J. Med. Microbiol. 2005, 54, 1077–1082. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.M.; Odoyo, E.; Larson, P.S.; Apondi, E.; Kathiiko, C.; Miringu, G.; Nakashima, M.; Ichinose, Y. First Report of a Foodborne Providencia Alcalifaciens Outbreak in Kenya. Am. J. Trop. Med. Hyg. 2015, 93, 497–500. [Google Scholar] [CrossRef]
- Si, J.; Kang, H.; You, H.J.; Ko, G.P. Revisiting the Role of Akkermansia muciniphila as a Therapeutic bacterium. Gut Microbes 2022, 14, 2078619. [Google Scholar] [CrossRef]
- Nakayama, J.; Watanabe, K.; Jiang, J.; Matsuda, K.; Chao, S.H.; Haryono, P.; La-ongkham, O.; Sarwoko, M.A.; Sujaya, I.S.; Zhao, L.; et al. Diversity in Gut Bacterial Community of School-Age Children in Asia. Nature 2015, 5, 8397. [Google Scholar] [CrossRef]
- Bailén, M.; Bressa, C.; Martínez-López, S.; González-Soltero, R.; Lominchar, M.G.M.; Juan, C.S.; Larrosa, M. Microbiota Features Associated with a High-Fat/Low-Fiber Diet in Healthy Adults. Front. Nutr. 2020, 7, 583608. [Google Scholar] [CrossRef]
- Brinkworth, G.D.; Noakes, M.; Clifton, P.M.; Bird, A.R. Comparative Effects of Very Low-Carbohydrate, High-Fat and High-Carbohydrate, Low-Fat Weight-Loss Diets on Bowel Habit and Faecal Short-Chain Fatty Acids and Bacterial Populations. Br. J. Nutr. 2009, 101, 1493–1502. [Google Scholar] [CrossRef]
- Hiippala, K.; Barreto, G.; Burrello, C.; Diaz-Basabe, A.; Suutarinen, M.; Kainulainen, V.; Bowers, J.R.; Lemmer, D.; Engelthaler, D.M.; Ekhlund, K.K.; et al. Novel Odoribacter splanchnicus Strain and Its Outer Membrane Vesicles Exert Immunoregulatory Effects in vitro. Front. Microbiol. 2020, 11, 575455. [Google Scholar] [CrossRef] [PubMed]
- Barone, M.; D’Amico, F.; Brigidi, P.; Turroni, S. Gut Microbiome–Micronutrient Interaction: The Key to Controlling the Bioavailability of Minerals and Vitamins? BioFactors 2022, 48, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Rusu, I.G.; Suharoschi, R.; Vodnar, D.C.; Pop, C.R.; Socaci, S.A.; Vulturar, R.; Istrati, M.; Morosan, I.; Farcas, A.C.; Kerezsi, D.K.; et al. Iron Supplementation Influence on the Gut Microbiota and Probiotic Intake Effect in Iron Deficiency—A Literature-Based Review. Nutrients 2020, 12, 1993. [Google Scholar] [CrossRef] [PubMed]
- Paganini, D.; Uyoga, M.A.; Kortman, G.A.M.; Cercamondi, C.I.; Winkler, H.C.; Boekhorst, J.; Moretti, D.; Lacroix, C.; Karanja, S.; Zimmermann, M.B. Iron-Containing Micronutrient Powders Modify the Effect of Oral Antibiotics on the Infant Gut Microbiome and Increase Post-Antibiotic Diarrhoea Risk: A Controlled Study in Kenya. Gut 2019, 68, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Penders, J.; Thijs, C.; Vink, C.; Stelma, F.F.; Snijders, B.; Kummeling, I.; van den Brandt, P.A.; Stobberingh, E.E. Factors Influencing the Composition of the Intestinal Microbiota in Early Infancy. Pediatrics 2006, 118, 511–521. [Google Scholar] [CrossRef]
- Palmer, C.; Bik, E.M.; DiGiulio, D.B.; Relman, D.A.; Brown, P.O. Development of the Human Infant Intestinal Microbiota. PLoS Biology 2007, 5, 1556–1573. [Google Scholar] [CrossRef]
- Bezirtzoglou, E.; Tsiotsias, A.; Welling, G.W. Microbiota Profile in Feces of Breast- and Formula-Fed Newborns by Using Fluorescence in situ Hybridization (FISH). Anaerobe 2011, 17, 478–482. [Google Scholar] [CrossRef]
- Roger, L.C.; Costabile, A.; Holland, D.T.; Hoyles, L.; McCartney, A.L. Examination of Faecal Bifidobacterium Populations in Breast- and Formula-Fed Infants During the First 18 Months of Life. Microbiology 2010, 156, 3329–3341. [Google Scholar] [CrossRef]
- Brown, J.; Cairncross, S.; Ensink, J.H.J. Water, Sanitation, Hygiene and Enteric Infections in Children. Arch. Dis. Child. 2013, 98, 629–634. [Google Scholar] [CrossRef]
- Tun, H.M.; Bridgman, S.L.; Chari, R.; Field, C.J.; Guttman, D.S.; Becker, A.B.; Mandhane, P.J.; Turvey, S.E.; Subbarao, P.; Sears, M.R.; et al. Roles of Birth Mode and Infant Gut Microbiota in Intergenerational Transmission of Overweight and Obesity from Mother to Offspring. JAMA Pediatr. 2018, 172, 368–377. [Google Scholar] [CrossRef]
- Blanton, L.V.; Charbonneau, M.R.; Salih, T.; Barratt, M.J.; Venkatesh, S.; Ilkaveya, O.; Subramanian, S.; Manary, M.J.; Trehan, I.; Jorgensen, J.M.; et al. Gut bacteria that prevent growth impairments transmitted by microbiota from malnourished children. Science 1979, 2016, 351. [Google Scholar] [CrossRef] [PubMed]
- Zambruni, M.; Ochoa, T.J.; Somasunderam, A.; Cabada, M.M.; Morales, M.L.; Mitreva, M.; Rosa, B.A.; Acosta, G.J.; Vigo, N.I.; Riveros, M.; et al. Stunting is preceded by intestinal mucosal damage and microbiome changes and is associated with systemic inflammation in a cohort of Peruvian infants. Am. J. Trop. Med. Hyg. 2019, 101, 1009–1017. [Google Scholar] [CrossRef] [PubMed]


| Genus | Standard Deviation | References | N |
|---|---|---|---|
| Bifidobacterium | 0.76 | Helmyati S. et al., 2017 [16] | 9.05 |
| Lactobacillus | 0.96 | Helmyati S. et al., 2017 [16] | 14.45 |
| Enterobacter | 0.73 | Helmyati S. et al., 2017 [16] | 8.35 |
| E Coli | 1.07 | Helmyati S. et al., 2017 [16] | 17.95 |
| Nutrients | 24–47 Months n = 34 | 48–60 Months n = 8 |
|---|---|---|
| Energy (Cal) a | 1350 | 1400 |
| Protein (g) a | 20 | 25 |
| Fat (g) a | 45 | 50 |
| Carbohydrate (g) a | 215 | 220 |
| Fe (mg) b | 5.8 | 6.3 |
| Zinc (mg) b | 3.4 | 4.0 |
| Descriptives | Stunted (n = 21) | Non-Stunted (n = 21) | p-Value 1 |
|---|---|---|---|
| Gender | |||
| Male | 9 (42.9%) | 14 (33.9%) | 0.126 |
| Female | 12 (57.1%) | 7 (66.7%) | |
| Age (month) 1 | 37.95 ± 8.71 | 39.43 ± 10.78 | 0.628 |
| Weight (kg) 1 | 11.47 ± 1.49 | 14.36 ± 2.31 | <0.001 |
| Height (cm) 1 | 85.90 ± 4.88 | 95.83 ± 6.45 | <0.001 |
| HAZ 2 | −2.40 (−3.60–(−2.10)) | −0.60 (−1.00–2.70) | <0.001 |
| Nutrient | Stunted (n = 21) | Non-Stunted (n = 21) | p-Value |
|---|---|---|---|
| Energy (Cal) b [15] | 1043 ± 191 | 1266 ± 178 | 0.003 |
| Carbohydrate (g) a [15] | 142.7 ± 35.2 | 165.4 ± 26.9 | 0.024 |
| Proteins (g) a [15] | 32.2 ± 7.9 | 39.9 ± 8.9 | 0.005 |
| Fat (g) a | 37.3 ± 8.9 | 48.4 ± 11.1 | 0.001 |
| Zn (mg) a | 3.5 ± 0.9 | 4.9 ± 1.4 | 0.001 |
| Fe (mg) b | 6.5 ± 1.9 | 6.6 ± 1.8 | 0.734 |
| Variable | Stunted n (%) | Non-Stunted n (%) | p-Value a |
|---|---|---|---|
| Delivery Mode | |||
| Vaginal | 12 (57.1) | 17 (81.0) | 0.09 |
| Caesarean section | 9 (42.9) | 4 (19.0) | |
| Exclusive Breastfeeding History | |||
| Yes | 15 (71.4) | 13 (61.9) | 0.518 |
| No | 6 (28.6) | 8 (38.1) | |
| History of Illness | |||
| No | 15 (71.4) | 20 (95.2) | 0.041 |
| Yes | 6 (28.6) | 1 (4.8) | |
| Vaccine History | |||
| Complete | 14 (66.7) | 17 (81.0) | 0.298 |
| Incomplete | 7 (33.3) | 4 (19.0) | |
| Source of Drinking Water | |||
| Branded Gallon of Water | 3 (14.3) | 8 (38.1) | 0.083 |
| Refilled Water | 18 (85.7) | 13 (61.9) | |
| Source of Water for Other Activities | |||
| PDAM | 19 (90.5) | 19 (90.5) | 1.00 |
| Well water | 2 (9.5) | 2 (9.5) | |
| Handwashing Before Eating | |||
| Yes | 7 (33.3) | 10 (47.6) | |
| No | 14 (66.7) | 11 (52.4) | 0.351 |
| Handwashing After Defecation | |||
| Yes | 19 (90.5) | 20 (95.2) | 0.554 |
| No | 2 (9.5) | 1 (33.3) |
| Microbiota | Abundance (OTU) | p-Value a | |
|---|---|---|---|
| Stunted | Non-Stunted | ||
| Blautia | 11,550 | 20,755 | 0.016 |
| Lachnospiraceae | 2601 | 6134 | 0.048 |
| Monoglobus | 183 | 484 | 0.030 |
| Bilophila | 10,790 | 12,417 | 0.031 |
| Mitsuokella | 24,469 | 2847 | 0.037 |
| Alloprevotella | 23,952 | 7888 | 0.049 |
| Akkermansia muciniphila | 405 | 1116 | 0.012 |
| Odoribacter_splanchinus | 32,747 | 42,993 | 0.040 |
| Bacteroides clarus | 7772 | 8900 | 0.045 |
| Providencia alcalifaciens | 861 | 353 | 0.023 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ratnayani; Hegar, B.; Sunardi, D.; Fadilah, F.; Gunardi, H.; Fahmida, U.; Vidiawati, D. Association of Gut Microbiota Composition with Stunting Incidence in Children under Five in Jakarta Slums. Nutrients 2024, 16, 3444. https://doi.org/10.3390/nu16203444
Ratnayani, Hegar B, Sunardi D, Fadilah F, Gunardi H, Fahmida U, Vidiawati D. Association of Gut Microbiota Composition with Stunting Incidence in Children under Five in Jakarta Slums. Nutrients. 2024; 16(20):3444. https://doi.org/10.3390/nu16203444
Chicago/Turabian StyleRatnayani, Badriul Hegar, Diana Sunardi, Fadilah Fadilah, Hartono Gunardi, Umi Fahmida, and Dhanasari Vidiawati. 2024. "Association of Gut Microbiota Composition with Stunting Incidence in Children under Five in Jakarta Slums" Nutrients 16, no. 20: 3444. https://doi.org/10.3390/nu16203444
APA StyleRatnayani, Hegar, B., Sunardi, D., Fadilah, F., Gunardi, H., Fahmida, U., & Vidiawati, D. (2024). Association of Gut Microbiota Composition with Stunting Incidence in Children under Five in Jakarta Slums. Nutrients, 16(20), 3444. https://doi.org/10.3390/nu16203444






