Complementary Role of BMI and EOSS in Predicting All-Cause and Cause-Specific Mortality in People with Overweight and Obesity
Abstract
1. Introduction
2. Materials and Methods
2.1. Survey Design and Data Collection
2.2. Clinical Data and Laboratory Tests
2.3. EOSS Stage Classification
2.4. Mortality Ascertainment
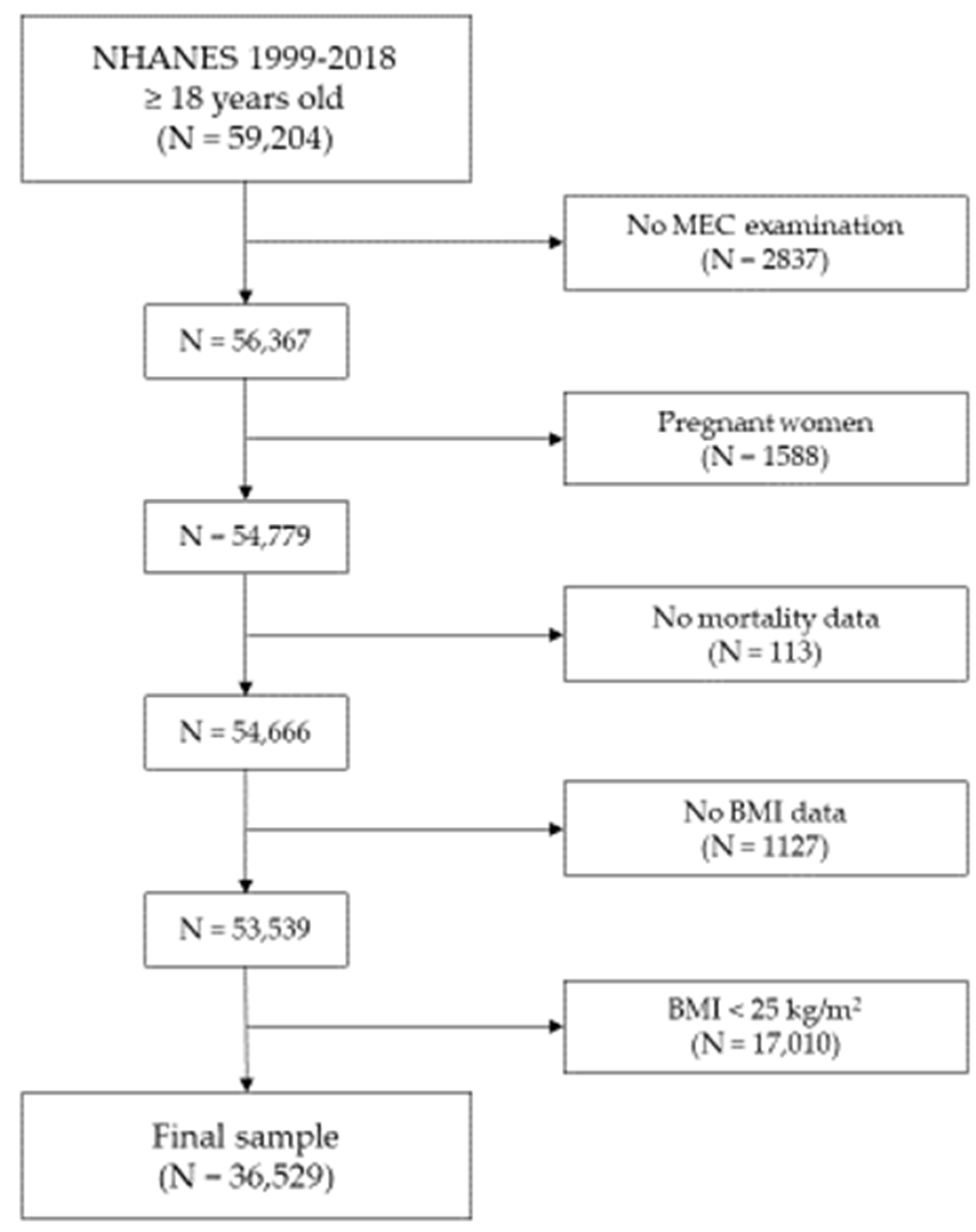
2.5. Sample Selection
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. All-Cause Mortality
3.3. Cause-Specific Mortality
4. Discussion
4.1. All-Cause Mortality
4.2. Cause-Specific Mortality
4.3. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization (WHO). Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 20 May 2024).
- Chooi, Y.C.; Ding, C.; Magkos, F. The epidemiology of obesity. Metabolism 2019, 92, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Neri, D.; Steele, E.M.; Khandpur, N.; Cediel, G.; Zapata, M.E.; Rauber, F.; Marrón-Ponce, J.A.; Machado, P.; da Costa Louzada, M.L.; Andrade, G.C.; et al. NOVA Multi-Country Study Group on Ultra-Processed Foods, Diet Quality and Human Health. Ultraprocessed food consumption and dietary nutrient profiles associated with obesity: A multicountry study of children and adolescents. Obes. Rev. 2022, 23 (Suppl. S1), e13387. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.; Buoncristiano, M.; Nardone, P.; Rito, A.I.; Spinelli, A.; Hejgaard, T.; Kierkegaard, L.; Nurk, E.; Kunešová, M.; Musić Milanović, S.; et al. A Snapshot of European Children’s Eating Habits: Results from the Fourth Round of the WHO European Childhood Obesity Surveillance Initiative (COSI). Nutrients 2020, 12, 2481. [Google Scholar] [CrossRef] [PubMed]
- Nashwan, A.J.; Abdi Hassan, M.; AlBarakat, M.M. Rethinking BMI and Obesity Management: The Transformative Role of Artificial Intelligence. Cureus 2024, 16, e54995. [Google Scholar] [CrossRef]
- Sweatt, K.; Garvey, W.T.; Martins, C. Strengths and Limitations of BMI in the Diagnosis of Obesity: What is the Path Forward? Curr. Obes. Rep. 2024, 13, 584–595. [Google Scholar] [CrossRef]
- Bosy-Westphal, A.; Müller, M.J. Diagnosis of obesity based on body composition-associated health risks-Time for a change in paradigm. Obes. Rev. 2021, 22, e13190. [Google Scholar] [CrossRef]
- Aune, D.; Sen, A.; Prasad, M.; Norat, T.; Janszky, I.; Tonstad, S.; Romundstad, P.; Vatten, L.J. BMI and all cause mortality: Systematic review and non-linear dose-response meta-analysis of 230 cohort studies with 3.74 million deaths among 30.3 million participants. BMJ 2016, 353, i2156. [Google Scholar] [CrossRef]
- Di Angelantonio, E.; Bhupathiraju, S.N.; Wormser, D.; Gao, P.; Kaptoge, S.; De Gonzalez, A.B.; Cairns, B.J.; Huxley, R.; Jackson, C.L.; Joshy, G.; et al. Body-mass index and all-cause mortality: Individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet 2016, 388, 776–786. [Google Scholar] [CrossRef]
- Bhaskaran, K.; dos-Santos-Silva, I.; Leon, D.A.; Douglas, I.J.; Smeeth, L. Association of BMI with overall and cause-specific mortality: A population-based cohort study of 3.6 million adults in the UK. Lancet Diabetes Endocrinol. 2018, 6, 944–953. [Google Scholar] [CrossRef]
- Flegal, K.M.; Kit, B.K.; Orpana, H.; Graubard, B.I. Association of all-cause mortality with overweight and obesity using standard body mass index categories: A systematic review and meta-analysis. JAMA 2013, 309, 71–82. [Google Scholar] [CrossRef]
- Winter, J.E.; MacInnis, R.J.; Wattanapenpaiboon, N.; Nowson, C.A. BMI and all-cause mortality in older adults: A meta-analysis. Am. J. Clin. Nutr. 2014, 99, 875–890. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Cupples, L.A.; Stokes, A.; Liu, C.T. Association of obesity with mortality over 24 years of weight history: Findings from the Framingham Heart Study. JAMA Netw. Open 2018, 1, e184587. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.M.; Kushner, R.F. A proposed clinical staging system for obesity. Int. J. Obes. 2009, 33, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Atlantis, E.; Sahebolamri, M.; Cheema, B.S.; Williams, K. Usefulness of the Edmonton Obesity Staging System for stratifying the presence and severity of weight-related health problems in clinical and community settings: A rapid review of observational studies. Obes. Rev. 2020, 21, e13120. [Google Scholar] [CrossRef]
- Atlantis, E.; Fahey, P.; Williams, K.; Edwards, S.; Samaras, K.; Dugdale, P.; Shi, Z.; Sharma, A.M. Comparing the predictive ability of the Edmonton Obesity Staging System with the body mass index for use of health services and pharmacotherapies in Australian adults: A nationally representative cross-sectional study. Clin. Obes. 2020, 10, e12368. [Google Scholar] [CrossRef]
- Sisay, B.G.; Jima, B.R.; Hassen, H.Y. Association between long-term weight loss and obesity-related complications defined by Edmonton obesity staging system: Analysis from the NHANES (2011–2018). Eur. J. Clin. Nutr. 2024, 78, 43–47. [Google Scholar] [CrossRef]
- Chiappetta, S.; Stier, C.; Weiner, R.A. Members of StuDoQ|MBE of Deutsche Gesellschaft für Allgemein- und Viszeralchirurgie/StuDoQ. The Edmonton Obesity Staging System Predicts Perioperative Complications and Procedure Choice in Obesity and Metabolic Surgery—A German Nationwide Register-Based Cohort Study (StuDoQ|MBE). Obes. Surg. 2019, 29, 3791–3799. [Google Scholar] [CrossRef]
- Skulsky, S.L.; Dang, J.T.; Battiston, A.; Switzer, N.J.; Birch, D.W.; Sharma, A.M.; Karmali, S. Higher Edmonton Obesity Staging System scores are associated with complications following laparoscopic Roux-en-Y gastric bypass. Surg. Endosc. 2020, 34, 3102–3109. [Google Scholar] [CrossRef]
- Kuk, J.L.; Ardern, C.I.; Church, T.S.; Sharma, A.M.; Padwal, R.; Sui, X.; Blair, S.N. Edmonton Obesity Staging System: Association with weight history and mortality risk. Appl. Physiol. Nutr. Metab. 2011, 36, 570–576. [Google Scholar] [CrossRef]
- Padwal, R.S.; Pajewski, N.M.; Allison, D.B.; Sharma, A.M. Using the Edmonton obesity staging system to predict mortality in a population-representative cohort of people with overweight and obesity. Can. Med. Assoc. J. 2011, 183, E1059–E1066. [Google Scholar] [CrossRef]
- Ejima, K.; Xavier, N.A.; Mehta, T. Comparing the Ability of Two Comprehensive Clinical Staging Systems to Predict Mortality: EOSS and CMDS. Obesity 2020, 28, 353–361. [Google Scholar] [CrossRef] [PubMed]
- National Center for Health Statistics. National Health and Nutrition Examination Survey (NHANES) Questionnaires, Datasets, and Related Documentation. Available online: https://wwwn.cdc.gov/nchs/nhanes/Default.aspx (accessed on 14 April 2024).
- Lohman, T.G.; Roche, A.F.; Martorell, R. Anthropometric Standardization Reference Manual; Human Kinetics Books: Champaign, IL, USA, 1988. [Google Scholar]
- National Center for Health Statistics. NCHS—2019 Public-Use Linked Mortality Files. Available online: https://www.cdc.gov/nchs/data-linkage/mortality-public.htm (accessed on 14 April 2024).
- Fine, J.P.; Gray, R.J. A proportional hazards model for the subdistribution of a competing risk. J. Am. Stat. Assoc. 1999, 94, 496–509. [Google Scholar] [CrossRef]
- Donini, L.M.; Pinto, A.; Giusti, A.M.; Lenzi, A.; Poggiogalle, E. Obesity or BMI Paradox? Beneath the Tip of the Iceberg. Front. Nutr. 2020, 7, 53. [Google Scholar] [CrossRef] [PubMed]
- Neeland, I.J.; Ross, R.; Després, J.P.; Matsuzawa, Y.; Yamashita, S.; Shai, I.; Seidell, J.; Magni, P.; International Atherosclerosis Society; International Chair on Cardiometabolic Risk Working Group on Visceral Obesity; et al. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: A position statement. Lancet Diabetes Endocrinol. 2019, 7, 715–725. [Google Scholar] [CrossRef]
- Bowman, K.; Atkins, J.L.; Delgado, J.; Kos, K.; Kuchel, G.A.; Ble, A.; Ferrucci, L.; Melzer, D. Central adiposity and the overweight risk paradox in aging: Follow-up of 130,473 UK Biobank participants. Am. J. Clin. Nutr. 2017, 106, 130–135. [Google Scholar] [CrossRef]
- Drané, M.; Godaert, L. The Obesity Paradox and Mortality in Older Adults: A Systematic Review. Nutrients 2023, 15, 1780. [Google Scholar] [CrossRef]
- Wang, S.; Ren, J. Obesity Paradox in Aging: From Prevalence to Pathophysiology. Prog. Cardiovasc. Dis. 2018, 61, 182–189. [Google Scholar] [CrossRef]
- Liu, C.; Wong, P.Y.; Chung, Y.L.; Chow, S.K.; Cheung, W.H.; Law, S.W.; Chan, J.C.N.; Wong, R.M.Y. Deciphering the “obesity paradox” in the elderly: A systematic review and meta-analysis of sarcopenic obesity. Obes. Rev. 2023, 24, e13534. [Google Scholar] [CrossRef]
- Heymsfield, S.B.; Peterson, C.M.; Thomas, D.M.; Heo, M.; Schuna, J.M., Jr. Why are there race/ethnic differences in adult body mass index-adiposity relationships? A quantitative critical review. Obes. Rev. 2016, 17, 262–275. [Google Scholar] [CrossRef]
- Tinsley, G.M.; Smith-Ryan, A.E.; Kim, Y.; Blue, M.N.M.; Nickerson, B.S.; Stratton, M.T.; Harty, P.S. Fat-free mass characteristics vary based on sex, race, and weight status in US adults. Nutr. Res. 2020, 81, 58–70. [Google Scholar] [CrossRef]
- Campa, F.; Coratella, G.; Cerullo, G.; Noriega, Z.; Francisco, R.; Charrier, D.; Irurtia, A.; Lukaski, H.; Silva, A.M.; Paoli, A. High-standard predictive equations for estimating body composition using bioelectrical impedance analysis: A systematic review. J. Transl. Med. 2024, 22, 515. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.; Varghese, M.; Singer, K. Gender and Sex Differences in Adipose Tissue. Curr. Diab. Rep. 2018, 18, 69. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.M.; Lee, D.H.; Rezende, L.F.M.; Giovannucci, E.L. Different correlation of body mass index with body fatness and obesity-related biomarker according to age, sex and race-ethnicity. Sci. Rep. 2023, 13, 3472. [Google Scholar] [CrossRef] [PubMed]
- Sattar, N. Gender aspects in type 2 diabetes mellitus and cardiometabolic risk. Best Pract. Res. Clin. Endocrinol. Metab. 2013, 27, 501–507. [Google Scholar] [CrossRef]
- Dwivedi, A.K.; Dubey, P.; Cistola, D.P.; Reddy, S.Y. Association between Obesity and Cardiovascular Outcomes: Updated Evidence from Meta-analysis Studies. Curr. Cardiol. Rep. 2020, 22, 25. [Google Scholar] [CrossRef]
- Wang, Z.; Lavikainen, P.; Wikström, K.; Laatikainen, T. Trajectories of Body Mass Index and Risk for Diabetes Complications and All-Cause Mortality in Finnish Type 2 Diabetes Patients. Clin. Epidemiol. 2024, 16, 203–212. [Google Scholar] [CrossRef]
- Heath, L.; Jebb, S.A.; Aveyard, P.; Piernas, C. Obesity, metabolic risk and adherence to healthy lifestyle behaviours: Prospective cohort study in the UK Biobank. BMC Med. 2022, 20, 65. [Google Scholar] [CrossRef]
- Petrelli, F.; Cortellini, A.; Indini, A.; Tomasello, G.; Ghidini, M.; Nigro, O.; Salati, M.; Dottorini, L.; Iaculli, A.; Varricchio, A.; et al. Association of Obesity with Survival Outcomes in Patients with Cancer: A Systematic Review and Meta-analysis. JAMA Netw. Open 2021, 4, e213520. [Google Scholar] [CrossRef]
- Pati, S.; Irfan, W.; Jameel, A.; Ahmed, S.; Shahid, R.K. Obesity and Cancer: A Current Overview of Epidemiology, Pathogenesis, Outcomes, and Management. Cancers 2023, 15, 485. [Google Scholar] [CrossRef]
- Rivera-Izquierdo, M.; Pérez de Rojas, J.; Martínez-Ruiz, V.; Pérez-Gómez, B.; Sánchez, M.J.; Khan, K.S.; Jiménez-Moleón, J.J. Obesity as a Risk Factor for Prostate Cancer Mortality: A Systematic Review and Dose-Response Meta-Analysis of 280,199 Patients. Cancers 2021, 13, 4169. [Google Scholar] [CrossRef]
- Lohmann, A.E.; Soldera, S.V.; Pimentel, I.; Ribnikar, D.; Ennis, M.; Amir, E.; Goodwin, P.J. Association of Obesity with Breast Cancer Outcome in Relation to Cancer Subtypes: A Meta-Analysis. J. Natl. Cancer Inst. 2021, 113, 1465–1475. [Google Scholar] [CrossRef]
| Variable | Patients with Overweight (N = 17,658) | Patients with Class I Obesity (N = 10,644) | Patients with Class II Obesity (N = 4769) | Patients with Class III Obesity (N = 3458) | p-Value |
|---|---|---|---|---|---|
| Age (years) | 47.9 ± 17.1 | 48.3 ± 16.5 | 47.4 ± 16.1 | 45.4 ± 15.3 | <0.001 |
| Sex (%) | <0.001 | ||||
| Female | 43.1 | 47.4 | 58.2 | 65.4 | |
| Male | 56.9 | 52.6 | 41.8 | 34.6 | |
| Race/ethnicity (%) | <0.001 | ||||
| Non-Hispanic White | 68.1 | 66.9 | 65.8 | 62.5 | |
| Non-Hispanic Black | 9.8 | 12.3 | 15.5 | 19.9 | |
| Hispanic | 15.7 | 15.9 | 14.9 | 12.8 | |
| Other | 6.4 | 4.9 | 4.8 | 4.8 | |
| Annual household income (%) | <0.001 | ||||
| ≥75,000 $ | 32.9 | 30.3 | 27.5 | 24.1 | |
| 45,000–74,999 $ | 21.1 | 21.8 | 21.5 | 21.4 | |
| 20,000–44,999 $ | 27.2 | 28.2 | 30.3 | 31.6 | |
| <20,000 $ | 13.6 | 14.8 | 15.6 | 18.6 | |
| Unknown | 5.2 | 4.9 | 5.1 | 4.3 | |
| Education level (%) | <0.001 | ||||
| More than high school graduate | 56.7 | 54.8 | 54.3 | 55.0 | |
| High school graduate | 23.2 | 25.2 | 26.4 | 27.1 | |
| Less than high school graduate | 17.7 | 18.2 | 17.2 | 15.8 | |
| Unknown | 2.4 | 1.8 | 2.1 | 2.1 | |
| Smoking status (%) | <0.001 | ||||
| Never smoker | 52.1 | 52.8 | 53.5 | 56.8 | |
| Former smoker | 26.3 | 26.6 | 28.1 | 24.2 | |
| Current smoker | 19.9 | 19.4 | 17.1 | 17.8 | |
| Unknown | 1.7 | 1.2 | 1.3 | 1.2 | |
| EOSS stage (%) | <0.001 | ||||
| Stage 0/1 | 34.7 | 25.1 | 21.4 | 18.8 | |
| Stage 2 | 54.4 | 61.4 | 62.6 | 62.5 | |
| Stage 3 | 10.9 | 13.5 | 16.0 | 18.7 | |
| Overall Mortality (5348 Deaths) | |||
|---|---|---|---|
| HR | 95% CI | p | |
| BMI class | |||
| Overweight | 1 (reference) | - | - |
| Obesity Class I | 1.06 | 0.98–1.16 | 0.139 |
| Obesity Class II | 1.21 | 1.08–1.36 | 0.001 |
| Obesity Class III | 1.58 | 1.39–1.80 | <0.001 |
| EOSS stage | |||
| 0/1 | 1 (reference) | - | - |
| 2 | 1.38 | 1.16–1.58 | <0.001 |
| 3 | 2.66 | 2.26–3.14 | <0.001 |
| <50 Years | 50 ≤ Years < 70 | ≥70 Years | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | HR | 95% CI | p | |
| BMI class | |||||||||
| Overweight | 1 (ref) | - | - | 1 (ref) | - | - | 1 (ref) | - | - |
| Obesity Class I | 1.01 | 0.75–1.36 | 0.923 | 1.20 | 1.05–1.36 | 0.006 | 1.03 | 0.92–1.15 | 0.548 |
| Obesity Class II | 1.59 | 1.13–2.23 | 0.007 | 1.30 | 1.09–1.56 | 0.004 | 1.14 | 0.97–1.33 | 0.094 |
| Obesity Class III | 1.90 | 1.36–2.64 | <0.001 | 1.75 | 1.40–2.20 | <0.001 | 1.45 | 1.16–1.81 | 0.001 |
| EOSS stage | |||||||||
| 0/1 | 1 (ref) | - | - | 1 (ref) | - | - | 1 (ref) | - | - |
| 2 | 1.54 | 1.15–2.05 | 0.003 | 1.40 | 1.04–1.89 | 0.024 | 1.24 | 0.95–1.64 | 0.110 |
| 3 | 3.07 | 2.10–4.49 | <0.001 | 3.02 | 2.22–4.16 | <0.001 | 2.28 | 1.72–3.02 | <0.001 |
| Males | Females | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | |
| BMI class | ||||||
| Overweight | 1 (ref) | - | - | 1 (ref) | - | - |
| Obesity Class I | 1.13 | 1.01–1.26 | 0.021 | 0.98 | 0.87–1.10 | 0.791 |
| Obesity Class II | 1.37 | 1.17–1.59 | <0.001 | 1.04 | 0.90–1.22 | 0.537 |
| Obesity Class III | 2.03 | 1.62–2.53 | <0.001 | 1.27 | 1.07–1.51 | 0.005 |
| EOSS stage | ||||||
| 0/1 | 1 (ref) | - | - | 1 (ref) | - | - |
| 2 | 1.23 | 0.99–1.53 | 0.054 | 1.57 | 1.21–2.03 | 0.001 |
| 3 | 2.21 | 1.74–2.80 | <0.001 | 3.45 | 2.60–4.58 | <0.001 |
| Non-Hispanic White | Non-Hispanic Black | Hispanic | Other Ethnicity | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | HR | 95% CI | p | HR | 95% CI | p | |
| BMI class | ||||||||||||
| Overweight | 1 (ref) | - | - | 1 (ref) | - | - | 1 (ref) | - | - | 1 (ref) | - | - |
| Obesity Class I | 1.09 | 0.98–1.20 | 0.084 | 0.84 | 0.70–1.01 | 0.069 | 1.11 | 0.91–1.35 | 0.273 | 1.29 | 0.79–2.10 | 0.301 |
| Obesity Class II | 1.21 | 1.06–1.40 | 0.006 | 0.91 | 0.74–1.11 | 0.366 | 1.39 | 1.01–1.93 | 0.043 | 2.29 | 1.12–4.69 | 0.024 |
| Obesity Class III | 1.70 | 1.44–2.00 | <0.001 | 1.11 | 0.91–1.36 | 0.278 | 1.45 | 1.05–1.99 | 0.021 | 1.81 | 0.70–4.68 | 0.220 |
| EOSS stage | ||||||||||||
| 0/1 | 1 (ref) | - | - | 1 (ref) | - | - | 1 (ref) | - | - | 1 (ref) | - | - |
| 2 | 1.30 | 1.06–1.60 | 0.011 | 2.07 | 1.55–2.76 | <0.001 | 1.51 | 1.11–2.06 | 0.008 | 0.71 | 0.31–1.61 | 0.414 |
| 3 | 2.59 | 2.09–3.20 | <0.001 | 4.13 | 2.92–5.84 | <0.001 | 2.60 | 1.90–3.56 | <0.001 | 1.06 | 0.46–2.41 | 0.893 |
| Cardiovascular Mortality (1431 Deaths) | Mortality Due to Malignancies (1243 Deaths) | Cerebrovascular Mortality (288 Deaths) | Mortality Due to DM-Related Complications (219 Deaths) | Mortality from Renal Causes (135 Deaths) | Mortality Due to Other Causes (1012 Deaths) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SHR | 95% CI | p | SHR | 95% CI | p | SHR | 95% CI | p | SHR | 95% CI | p | SHR | 95% CI | p | SHR | 95% CI | p | |
| BMI class | ||||||||||||||||||
| Overweight | 1 (ref) | - | - | 1 (ref) | - | - | 1 (ref) | - | - | 1 (ref) | - | - | 1 (ref) | - | - | 1 (ref) | - | - |
| Obesity Class I | 1.14 | 0.97–1.32 | 0.108 | 1.06 | 0.90–1.26 | 0.487 | 1.16 | 0.83–1.62 | 0.373 | 1.44 | 0.95–2.24 | 0.083 | 1.06 | 0.64–1.73 | 0.827 | 0.95 | 0.83–1.08 | 0.416 |
| Obesity Class II | 1.43 | 1.15–1.77 | 0.001 | 1.23 | 0.99–1.54 | 0.074 | 0.86 | 0.49–1.49 | 0.591 | 1.95 | 1.15–3.46 | 0.014 | 0.97 | 0.47–2.02 | 0.935 | 1.02 | 0.84–1.23 | 0.866 |
| Obesity Class III | 1.97 | 1.52–2.56 | <0.001 | 0.97 | 0.70–1.33 | 0.837 | 0.92 | 0.50–1.69 | 0.800 | 4.29 | 2.68–7.80 | <0.001 | 1.54 | 0.72–3.31 | 0.267 | 1.35 | 1.07–1.71 | 0.011 |
| EOSS stage | ||||||||||||||||||
| 0/1 | 1 (ref) | - | - | 1 (ref) | - | - | 1 (ref) | - | - | 1 (ref) | - | - | 1 (ref) | - | - | 1 (ref) | - | - |
| 2 | 1.60 | 1.09–2.35 | 0.015 | 1.30 | 0.97–1.73 | 0.081 | 1.05 | 0.44–2.48 | 0.914 | 11.93 | 2.77–51.35 | 0.001 | 3.35 | 0.66–16.91 | 0.143 | 1.46 | 1.12–1.89 | 0.004 |
| 3 | 3.63 | 2.44–5.40 | <0.001 | 1.52 | 1.10–2.10 | 0.010 | 1.48 | 0.61–3.57 | 0.388 | 25.57 | 5.75–113.71 | <0.001 | 5.86 | 1.09–31.57 | 0.040 | 2.21 | 1.67–2.91 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bioletto, F.; Ponzo, V.; Goitre, I.; Stella, B.; Rahimi, F.; Parasiliti-Caprino, M.; Broglio, F.; Ghigo, E.; Bo, S. Complementary Role of BMI and EOSS in Predicting All-Cause and Cause-Specific Mortality in People with Overweight and Obesity. Nutrients 2024, 16, 3433. https://doi.org/10.3390/nu16203433
Bioletto F, Ponzo V, Goitre I, Stella B, Rahimi F, Parasiliti-Caprino M, Broglio F, Ghigo E, Bo S. Complementary Role of BMI and EOSS in Predicting All-Cause and Cause-Specific Mortality in People with Overweight and Obesity. Nutrients. 2024; 16(20):3433. https://doi.org/10.3390/nu16203433
Chicago/Turabian StyleBioletto, Fabio, Valentina Ponzo, Ilaria Goitre, Beatrice Stella, Farnaz Rahimi, Mirko Parasiliti-Caprino, Fabio Broglio, Ezio Ghigo, and Simona Bo. 2024. "Complementary Role of BMI and EOSS in Predicting All-Cause and Cause-Specific Mortality in People with Overweight and Obesity" Nutrients 16, no. 20: 3433. https://doi.org/10.3390/nu16203433
APA StyleBioletto, F., Ponzo, V., Goitre, I., Stella, B., Rahimi, F., Parasiliti-Caprino, M., Broglio, F., Ghigo, E., & Bo, S. (2024). Complementary Role of BMI and EOSS in Predicting All-Cause and Cause-Specific Mortality in People with Overweight and Obesity. Nutrients, 16(20), 3433. https://doi.org/10.3390/nu16203433









