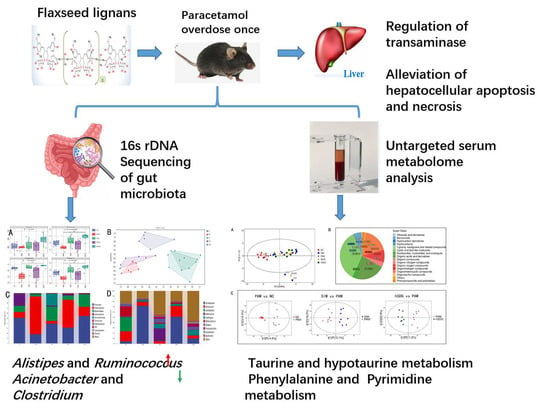
Flaxseed Lignan Alleviates the Paracetamol-Induced Hepatotoxicity Associated with Regulation of Gut Microbiota and Serum Metabolome
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Kits
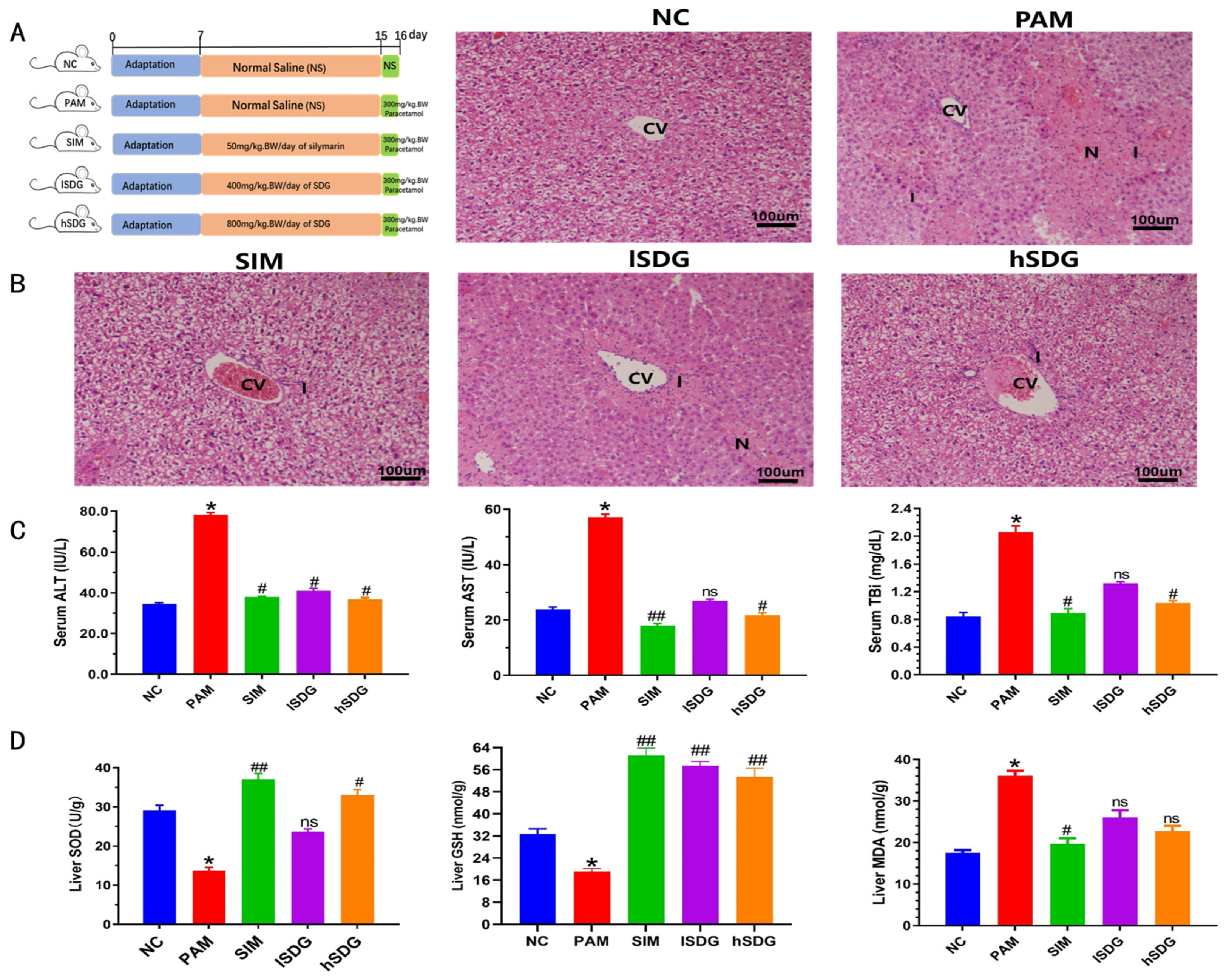
2.2. Animal Experimental Design
2.3. Evaluation of Effect of Paracetamol
2.3.1. Liver Damage
2.3.2. Oxidative Stress
2.3.3. Histopathological Studies
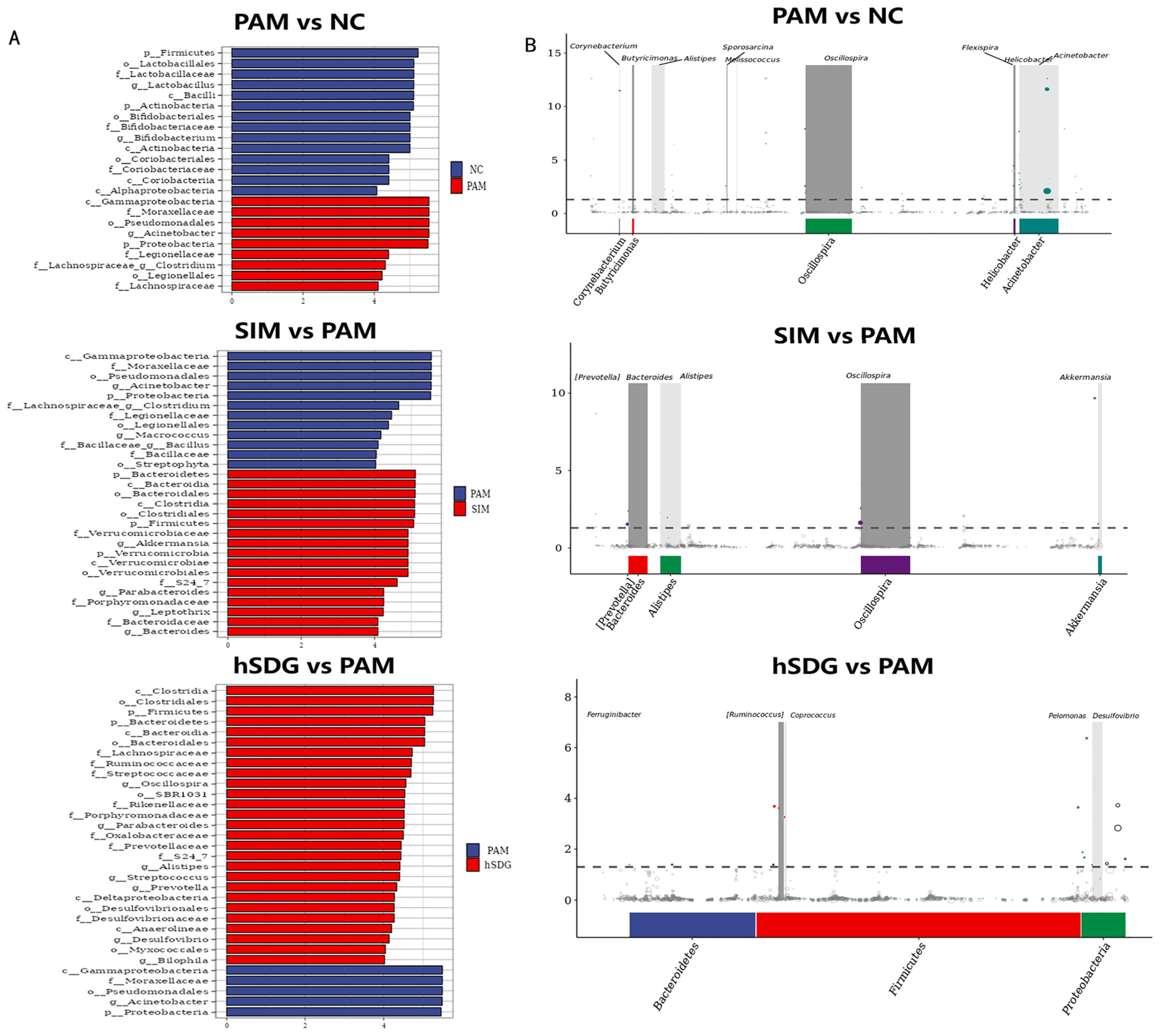
2.3.4. Bioinformatics Analysis of Gut Microbiota
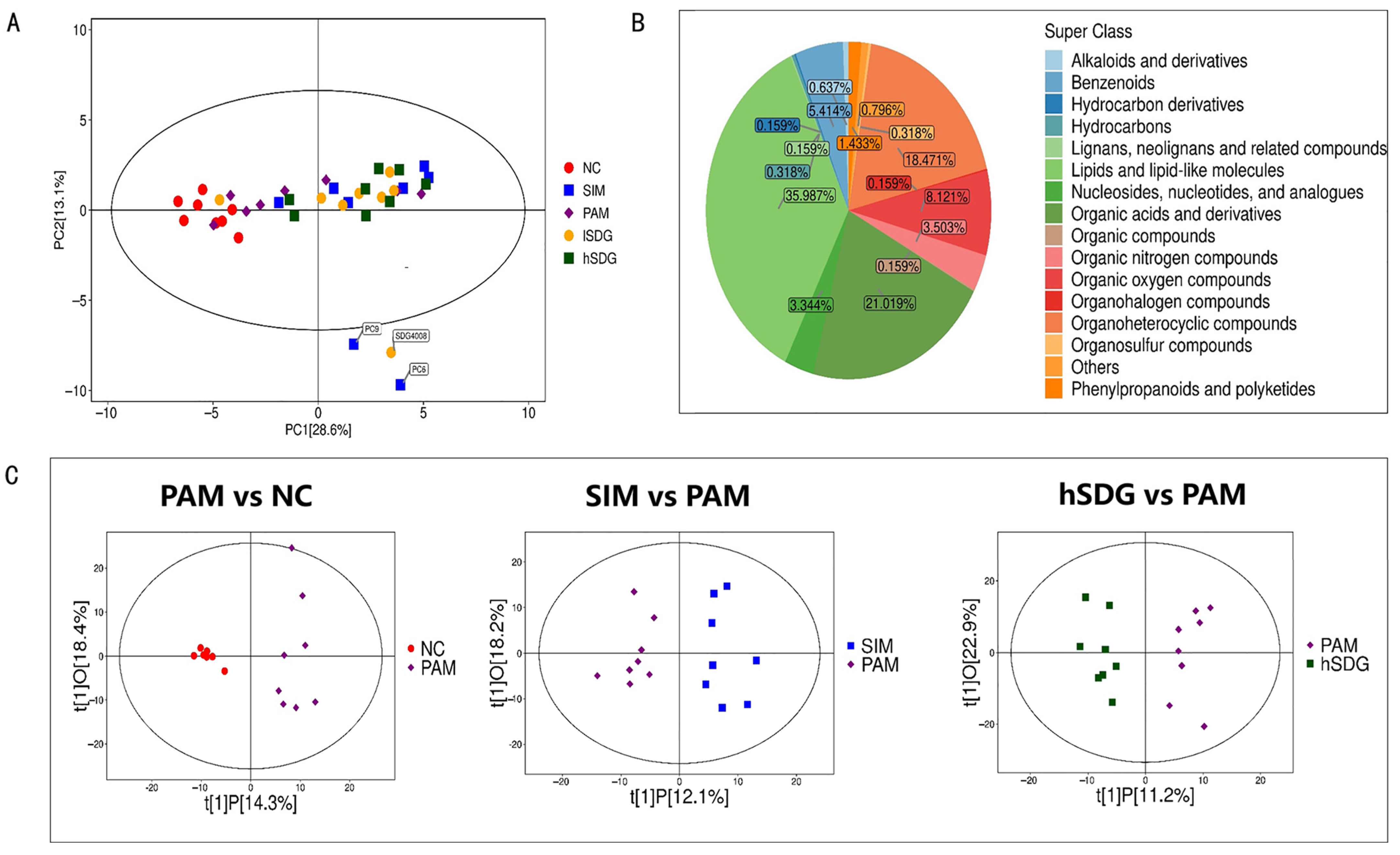
2.3.5. Untargeted Serum Metabolomics Analysis
2.4. Statistical Analysis
3. Results
3.1. Flaxseed Lignan Alleviates Liver Injury Induced by PAM
3.2. Flaxseed Lignan Regulates Gut Microbiota Structure Induced by PAM
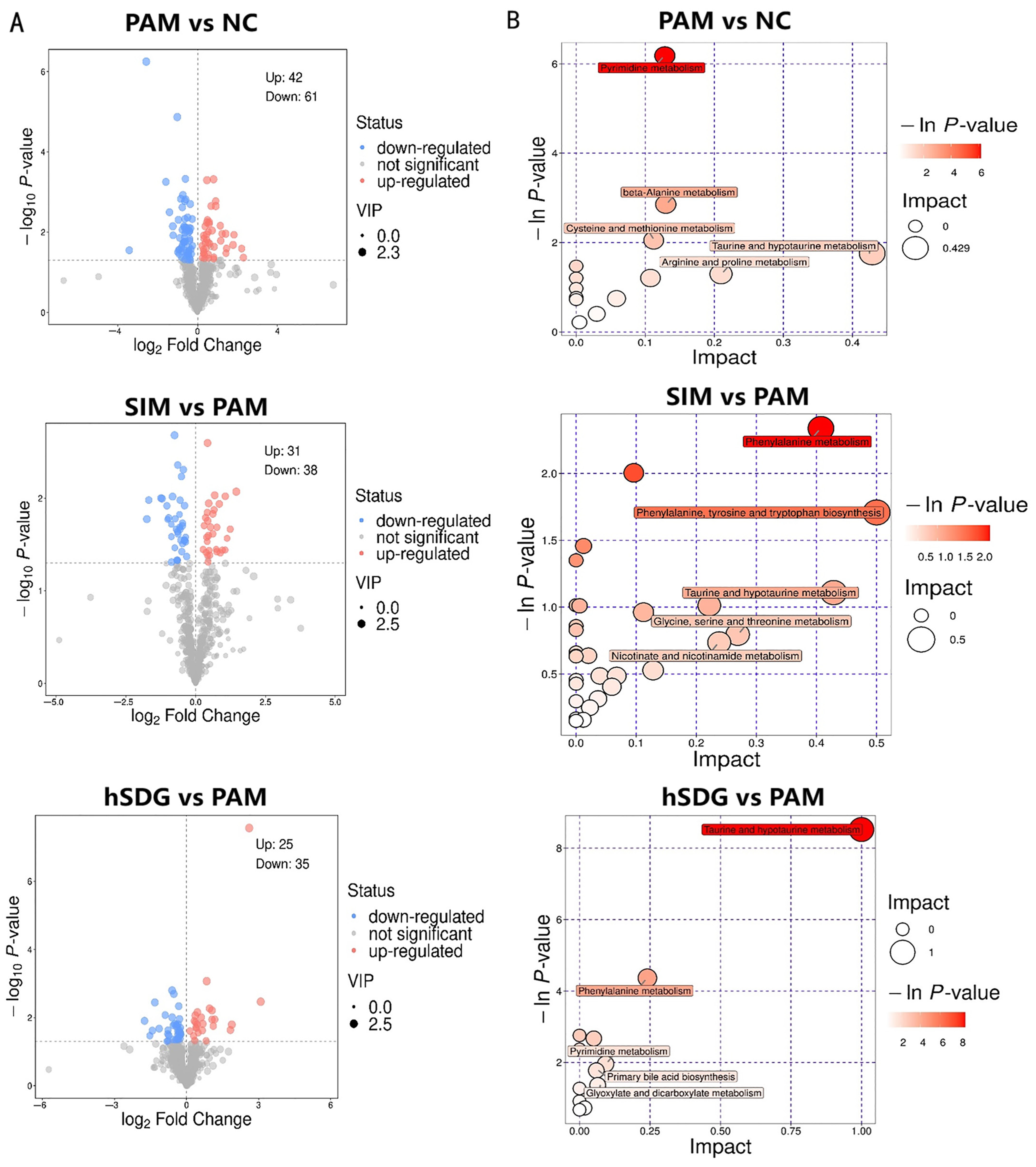
3.3. Flaxseed Lignan Alters Serum Metabolome Composition Induced by PAM
3.4. Regulation of Gut Microbiota Is Associated with Metabolome Alteration
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liao, J.Q.; Lu, Q.X.; Li, Z.Q.; Li, J.T.; Zhao, Q.; Li, J. Acetaminophen-induced liver injury: Molecular mechanism and treatments from natural products. Front. Pharmacol. 2023, 14, 1122632. [Google Scholar] [CrossRef] [PubMed]
- Kaushal, R.; Dave, K.R.; Katyare, S.S. Paracetamol hepatotoxicity and microsomal function. Env. Toxicol. Phar. 1999, 7, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Locigno, R.; Pincemail, J.; Henno, A.; Treusch, G.; Castronovo, V. S-acetyl-glutathione selectively induces apoptosis in human lymphoma cells through a GSH-independent mechanism. Int. J. Oncol. 2002, 20, 69–75. [Google Scholar] [PubMed]
- Saito, C.; Lemasters, J.J.; Jaeschke, H. c-Jun N-terminal kinase modulates oxidant stress and peroxynitrite formation independent of inducible nitric oxide synthase in acetaminophen hepatotoxicity. Toxicol. Appl. Pharm. 2010, 246, 8–17. [Google Scholar] [CrossRef]
- Prozialeck, W.C. Toxicology and Applied Pharmacology. Foreword. Toxicol. Appl. Pharmacol. 2009, 238, 191. [Google Scholar] [CrossRef]
- Jaeschke, H.; McGill, M.R.; Williams, C.D.; Ramachandran, A. Current issues with acetaminophen hepatotoxicity-A clinically relevant model to test the efficacy of natural products. Life Sci. 2011, 88, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Toklu, H.Z.; Sehirli, A.O.; Velioglu-Ogunc, A.; Cetinel, S.; Sener, G. Acetaminophen-induced toxicity is prevented by beta-D-glucan treatment in mice. Eur. J. Pharmacol. 2006, 543, 133–140. [Google Scholar] [CrossRef]
- Ren, Y.S.; Zheng, Y.; Duan, H.; Lei, L.; Deng, X.; Liu, X.Q.; Mei, Z.N.; Deng, X.K. Dandelion polyphenols protect against acetaminophen-induced hepatotoxicity in mice via activation of the Nrf-2/HO-1 pathway and inhibition of the JNK signaling pathway. Chin. J. Nat. Med. 2020, 18, 103–113. [Google Scholar] [CrossRef]
- Mueed, A.; Shibli, S.; Jahangir, M.; Jabbar, S.; Deng, Z.Y. A comprehensive review of flaxseed (Linum usitatissimum L.): Health-affecting compounds, mechanism of toxicity, detoxification, anticancer and potential risk. Crit. Rev. Food Sci. 2022, 63, 11081–11104. [Google Scholar] [CrossRef]
- Wu, Z.Y.; Wu, B.F.; Lv, X.; Xie, Y.; Xu, S.L.; Ma, C.C.; Xu, J.Q.; Tu, X.H.; Wei, F.; Chen, H. Serumal Lipidomics Reveals the Anti-inflammatory Effect of Flax Ligan and Sinapic Acid in High-Fat-Diet-Fed Mice. J. Agric. Food Chem. 2021, 69, 9111–9123. [Google Scholar] [CrossRef]
- Tse, T.J.; Guo, Y.J.; Shim, Y.Y.; Purdy, S.K.; Kim, J.H.; Cho, J.Y.; Alcorn, J.; Reaney, M.J.T. Availability of bioactive flax lignan from foods and supplements. Crit. Rev. Food Sci. 2022, 63, 9843–9858. [Google Scholar] [CrossRef] [PubMed]
- Newairy, A.S.A.; Abdou, H.M. Protective role of flax ligan against lead acetate induced oxidative damage and hyperlipidemia in rats. Food Chem. Toxicol. 2009, 47, 813–818. [Google Scholar] [CrossRef]
- Kim, K.H.; Moon, E.; Kim, S.Y.; Choi, S.U.; Lee, K.R. Lignan constituents of Tilia amurensis and their biological evaluation on antitumor and anti-inflammatory activities. Food Chem. Toxicol. 2012, 50, 3680–3686. [Google Scholar] [CrossRef]
- Coskun, A.B.; Celep, A.G.S. Therapeutic modulation methods of gut microbiota and gut-liver axis. Crit. Rev. Food Sci. 2022, 62, 6505–6515. [Google Scholar] [CrossRef]
- Mueed, A.; Ibrahim, M.; Shibli, S.; Madjirebaye, P.; Deng, Z.Y.; Jahangir, M. The fate of flaxseed-ligan after oral administration: A comprehensive review on its bioavailability, pharmacokinetics, and food design strategies for optimal application. Crit. Rev. Food Sci. 2022. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.L.; Lu, H.C.; Huang, H.Y.; Hwang, G.Y.; Tzen, J.T.C. Sesame Ligan Significantly Alleviate Liver Damage of Rats Caused by Carbon Tetrachloride in Combination with Kava. J. Food Drug Anal. 2010, 18, 249–255. [Google Scholar]
- Jasemine, S.; Srivastava, R.S.; Singh, S.K. Hepatoprotective effect of crude extract and isolated ligan of Justicia simplex Against CCl4-induced hepatotoxicity. Pharm. Biol. 2007, 45, 274–277. [Google Scholar] [CrossRef]
- Leary, S.; Underwood, W.; Anthony, R.; Cartner, S.; Grandin, T.; Greenacre, C.; McCrackin, M.A.; Meyer, B.; Miller, D.; Shearer, J.; et al. The AVMA Guidelines for the Euthanasia of Animals: 2020 edition; American Veterinary Medical Association: Schaumburg, IL, USA, 2020. [Google Scholar]
- Thilagavathi, R.; Begum, S.S.; Varatharaj, S.D.; Balasubramaniam, A.K.; George, J.S.; Selvam, C. Recent insights into the hepatoprotective potential of medicinal plants and plant-derived compounds. Phytother. Res. 2023, 37, 2102–2118. [Google Scholar] [CrossRef]
- De Silva, S.F.; Alcorn, J. Flaxseed Ligan as Important Dietary Polyphenols for Cancer Prevention and Treatment: Chemistry, Pharmacokinetics, and Molecular Targets. Pharm. Base. 2019, 12, 68. [Google Scholar] [CrossRef]
- Senizza, A.; Rocchetti, G.; Mosele, J.I.; Patrone, V.; Callegari, M.L.; Morelli, L.; Lucini, L. Ligan and Gut Microbiota: An Interplay Revealing Potential Health Implications. Molecules 2020, 25, 5709. [Google Scholar] [CrossRef]
- Carraro, J.C.C.; Dantas, M.I.D.; Espeschit, A.C.R.; Martino, H.S.D.; Ribeiro, S.M.R. Flaxseed and Human Health: Reviewing Benefits and Adverse Effects. Food Rev. Int. 2012, 28, 203–230. [Google Scholar] [CrossRef]
- Prasad, K. Effect of chronic administration of lignan complex isolated from flaxseed on the hemopoietic system. Mol. Cell Biochem. 2005, 270, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Billinsky, J.; Glew, R.A.; Cornish, S.M.; Whiting, S.J.; Thorpe, L.U.; Alcorn, J.; Paus-Jenssen, L.; Hadjistavropoulos, T.; Chilibeck, P.D. No evidence of hypoglycemia or hypotension in older adults during 6 months of flax lignan supplementation in a randomized controlled trial: A safety evaluation. Pharm. Biol. 2013, 51, 778–782. [Google Scholar] [CrossRef] [PubMed]
- Dixon, M.F.; Fulker, M.J.; Walker, B.E.; Kelleher, J.; Losowsky, M.S. Serum transaminase levels after experimental paracetamol-induced hepatic necrosis. Gut 1975, 16, 800–807. [Google Scholar] [CrossRef] [PubMed]
- Futter, L.E.; Al-Swayeh, O.A.; Moore, P.K. A comparison of the effect of nitroparacetamol and paracetamol on liver injury. Brit J. Pharmacol. 2001, 132, 10–12. [Google Scholar] [CrossRef]
- Soliman, G.A.; Yusufoglu, H.; Tatli-Cankaya, I.; Abdel-Rahman, R.F.; Anul, S.A.; Akaydin, G. Hepatoprotective activities of Lappula barbata and Plantago holosteum against paracetamol induced liver damage in rats and their in vitro antioxidant effects. Planta Med. 2016, 82, P256. [Google Scholar] [CrossRef]
- Alshawsh, M.A.; Abdulla, M.A.; Ismail, S.; Amin, Z.A. Hepatoprotective Effects of Orthosiphon stamineus Extract on Thioacetamide-Induced Liver Cirrhosis in Rats. Evid.-Based Complement. Altern. Med. 2011, 2011, 103039. [Google Scholar] [CrossRef]
- Bellamakondi, P.K.; Godavarthi, A.; Ibrahim, M. Caralluma umbellata Haw. Protects liver against paracetamol toxicity and inhibits CYP2E1. Bioimpacts. 2018, 8, 23–30. [Google Scholar] [CrossRef]
- Bourdeaux, C.; Bewley, J. Death from paracetamol overdose despite appropriate treatment with N-acetylcysteine. Emerg. Med. J. 2007, 24, 1–2. [Google Scholar] [CrossRef]
- Ahmad, M.M.; Rezk, N.A.; Fawzy, A.; Sabry, M. Protective effects of curcumin and silymarin against paracetamol induced hepatotoxicity in adult male albino rats. Gene 2019, 712, 143966. [Google Scholar] [CrossRef]
- Landete, J.M. Plant and mammalian lignans: A review of source, intake, metabolism, intestinal bacteria and health. Food Res. Int. 2012, 46, 410–424. [Google Scholar] [CrossRef]
- Eeckhaut, E.; Struijs, K.; Possemiers, S.; Vincken, J.P.; De Keukeleire, D.; Verstraete, W. Metabolism of the lignan macromolecule into enterolignans in the gastrointestinal lumen as determined in the simulator of the human intestinal microbial ecosystem. J. Agric. Food Chem. 2008, 56, 4806–4812. [Google Scholar] [CrossRef]
- Fuentealba, C.; Figuerola, F.; Estévez, A.M.; Bastíasc, J.M.; Muñoz, O. Bioaccessibility of lignans from flaxseed (L.) determined by single-batch simulation of the digestive process. J. Sci. Food Agric. 2014, 94, 1729–1738. [Google Scholar] [CrossRef] [PubMed]
- Quartieri, A.; García-Villalba, R.; Amaretti, A.; Raimondi, S.; Leonardi, A.; Rossi, M.; Tomàs-Barberan, F. Detection of novel metabolites of flaxseed lignans in vitro and in vivo. Mol. Nutr. Food Res. 2016, 60, 1590–1601. [Google Scholar] [CrossRef]
- Landete, J.M.; Arqués, J.; Medina, M.; Gaya, P.; de Las Rivas, B.; Muñoz, R. Bioactivation of Phytoestrogens: Intestinal Bacteria and Health. Crit. Rev. Food Sci. 2016, 56, 1826–1843. [Google Scholar] [CrossRef]
- Peirotén, A.; Gaya, P.; Alvarez, I.; Bravo, D.; Landete, J.M. Influence of different lignan compounds on enterolignan production by and strains. Int. J. Food Microbiol. 2019, 289, 17–23. [Google Scholar] [CrossRef]
- Kamal-Eldin, A.; Peerlkamp, N.; Johnsson, P.; Andersson, R.; Andersson, R.E.; Lundgren, L.N.; Åman, P. An oligomer from flaxseed composed of secoisolariciresinoldiglucoside and 3-hydroxy-3-methyl glutaric acid residues. Phytochemistry 2001, 58, 587–590. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Yang, C.; Qiao, Z.X.; Xu, Z.X.; Wang, X.; Deng, Q.C.; Chen, W.C.; Huang, F.H. Algal Oil Rich in Docosahexaenoic Acid Alleviates Intestinal Inflammation Induced by Antibiotics Associated with the Modulation of the Gut Microbiome and Metabolome. J. Agric. Food Chem. 2021, 69, 9124–9136. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Ashafaq, M.; Alshahrani, S.; Bokar, I.A.M.; Siddiqui, R.; Alam, M.I.; Taha, M.M.E.; Almoshari, Y.; Alqahtani, S.S.; Ahmed, R.A.; et al. Hepatoprotective Effect of Curcumin Nano-Lipid Carrier against Cypermethrin Toxicity by Countering the Oxidative, Inflammatory, and Apoptotic Changes in Wistar Rats. Molecules 2023, 28, 881. [Google Scholar] [CrossRef]
- Mar, A.N.; Adr, C.; Dana, M.M.; Mar, R.P.; Cor, L.B.; Emi, C.B.; Anc, D.B. Probiotic Bacillus Spores Protect Against Acetaminophen Induced Acute Liver Injury in Rats. Nutrients 2020, 12, 632. [Google Scholar] [CrossRef]
- Cho, S.Y.; Kim, J.; Lee, J.H.; Sim, J.H.; Cho, D.H.; Bae, I.H.; Lee, H.; Seol, M.A.; Shin, H.M.; Kim, T.J.; et al. Modulation of gut microbiota and delayed immunosenescence as a result of syringaresinol consumption in middle-aged mice. Sci. Rep. 2017, 7, 41667. [Google Scholar] [CrossRef]
- Taibi, A.; Ku, M.; Lin, Z.; Gargari, G.; Kubant, A.; Lepp, D.; Power, K.A.; Guglielmetti, S.; Thompson, L.U.; Comelli, E.M. Discriminatory and cooperative effects within the mouse gut microbiota in response to flaxseed and its oil and lignan components. J. Nutr. Biochem. 2021, 98, 541–594. [Google Scholar] [CrossRef] [PubMed]
- Badger, R.; Aho, K.; Serve, K. Short-term exposure to synthetic flaxseed lignan LGM2605 alters gut microbiota in mice. Microbiologyopen 2021, 10, e1185. [Google Scholar] [CrossRef]
- Corona, G.; Kreimes, A.; Barone, M.; Turroni, S.; Brigidi, P.; Keleszade, E.; Costabile, A. Impact of ligan in oilseed mix on gut microbiome composition and enterolignan production in younger healthy and premenopausal women: An in vitro pilot study. Microb. Cell Fact. 2020, 19, 82. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.Y.; Feng, Y.; Lu, X.J.; Zong, Z.Y. Precise Species Identification for Acinetobacter: A Genome-Based Study with Description of Two Novel Acinetobacter Species. Msystems 2021, 6, 10–1128. [Google Scholar] [CrossRef]
- Nemec, A.; Dijkshoorn, L.; Cleenwerck, I.; De Baere, T.; Janssens, D.; van der Reijden, T.J.K.; Jezek, P.; Vaneechoutte, M. Acinetobacter parvus sp nov., a small-colony-forming species isolated from human clinical specimens. Int. J. Syst. Evol. Microbiol. 2003, 53, 1563–1567. [Google Scholar] [CrossRef]
- Kiu, R.; Caim, S.; Alcon-Giner, C.; Belteki, G.; Clarke, P.; Pickard, D.; Dougan, G.; Hall, L.J. Preterm Infant-Associated Clostridium tertium, Clostridium cadaveris, and Clostridium paraputrificum Strains: Genomic and Evolutionary Insights. Genome Biol. Evol. 2017, 9, 2707–2714. [Google Scholar] [CrossRef]
- Walker, A.; Pfitzner, B.; Harir, M.; Schaubeck, M.; Calasan, J.; Heinzmann, S.S.; Turaev, D.; Rattei, T.; Endesfelder, D.; Zu Castell, W.; et al. Sulfonolipids as novel metabolite markers of Alistipes and Odoribacter affected by high-fat diets. Sci. Rep. 2017, 7, 11047. [Google Scholar] [CrossRef]
- Zhang, T.; Li, Q.Q.; Cheng, L.; Buch, H.; Zhang, F. Akkermansia muciniphila is a promising probiotic. Microb. Biotechnol. 2019, 12, 1109–1125. [Google Scholar] [CrossRef]
- Han, K.H.; Hashimoto, N.; Fukushima, M. Relationships among alcoholic liver disease, antioxidants, and antioxidant enzymes. World J. Gastroentero. 2016, 22, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Bayram, H.M.; Majoo, F.M.; Ozturkcan, A. Polyphenols in the prevention and treatment of non-alcoholic fatty liver disease: An update of preclinical and clinical studies. Clin. Nutr. Espen. 2021, 44, 1–14. [Google Scholar] [CrossRef]
- Yang, Y.P.; Jian, Y.Q.; Cheng, S.W.; Jia, Y.Z.; Liu, Y.B.; Yu, H.H.; Cao, L.; Li, B.; Peng, C.Y.; Choudhary, M.I.; et al. Dibenzocyclooctadiene ligan from Kadsura coccinea alleviate APAP-induced hepatotoxicity via oxidative stress inhibition and activating the Nrf2 pathway in vitro. Bioorg. Chem. 2021, 115, 105277. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.; Shao, Y.H.; Zhu, Z.P.; Yin, X.X.; Shen, B.Y.; Chen, C.; Xu, Y.F.; Shen, J.J.; Li, H.F.; Li, X.N.; et al. Systematically identifying the hepatoprotective ingredients of schisandra lignan extract from pharmacokinetic and pharmacodynamic perspectives. Phytomedicine 2019, 53, 182–192. [Google Scholar] [CrossRef]
- Paulusma, C.C.; Lamers, W.H.; Broer, S.; van de Graaf, S.F.J. Amino acid metabolism, transport and signalling in the liver revisited. Biochem. Pharmacol. 2022, 201, 115074. [Google Scholar] [CrossRef] [PubMed]
- de la Puerta, C.; Arrieta, F.J.; Balsa, J.A.; Botella-Carretero, J.I.; Zamarron, I.; Vazquez, C. Taurine and glucose metabolism: A review. Nutr. Hosp. 2010, 25, 910–919. [Google Scholar] [CrossRef]
- Kunst, C.; Schmid, S.; Michalski, M.; Tumen, D.; Buttenschon, J.; Muller, M.; Gulow, K. The Influence of Gut Microbiota on Oxidative Stress and the Immune System. Biomedicines 2023, 11, 1388. [Google Scholar] [CrossRef]
- Hanafy, M.M.; Lindeque, J.Z.; El-Maraghy, S.A.; Abdel-Hamid, A.H.Z.; Shahin, N.N. Time-based investigation of urinary metabolic markers for Type 2 diabetes: Metabolomics approach for diabetes management. Biofactors 2021, 47, 645–657. [Google Scholar] [CrossRef]
- Glatz, J.F.C.; Luiken, J.J.F.P.; Bonen, A. Membrane Fatty Acid Transporters as Regulators of Lipid Metabolism: Implications for Metabolic Disease. Physiol. Rev. 2010, 90, 367–417. [Google Scholar] [CrossRef]
- Dean, B.; Chang, S.; Doss, G.A.; King, C.; Thomas, P.E. Glucuronidation, oxidative metabolism, and bioactivation of enterolactone in rhesus monkeys. Arch. Biochem. Biophys. 2004, 429, 244–251. [Google Scholar] [CrossRef]
- Drygalski, K.; Berk, K.; Charytoniuk, T.; Ilowska, N.; Lukaszuk, B.; Chabowski, A.; Konstantynowicz-Nowicka, K. Does the enterolactone (ENL) affect fatty acid transporters and lipid metabolism in liver? Nutr. Metab. 2017, 14, 69. [Google Scholar] [CrossRef] [PubMed]
- Puri, P.; Mirshahi, F.; Siddiqui, M.S.; Boyett, S.; Sargeant, C.C.; Joyce, A.; Luketic, V.A.; Sanyal, A.J. Decreased Fecal Microbial Metabolite Enterolactone is a Novel Inflammatory and Oxidative Stress Related Biomarker of Alcoholic Liver Disease. Gastroenterology 2015, 148, S1001. [Google Scholar] [CrossRef]

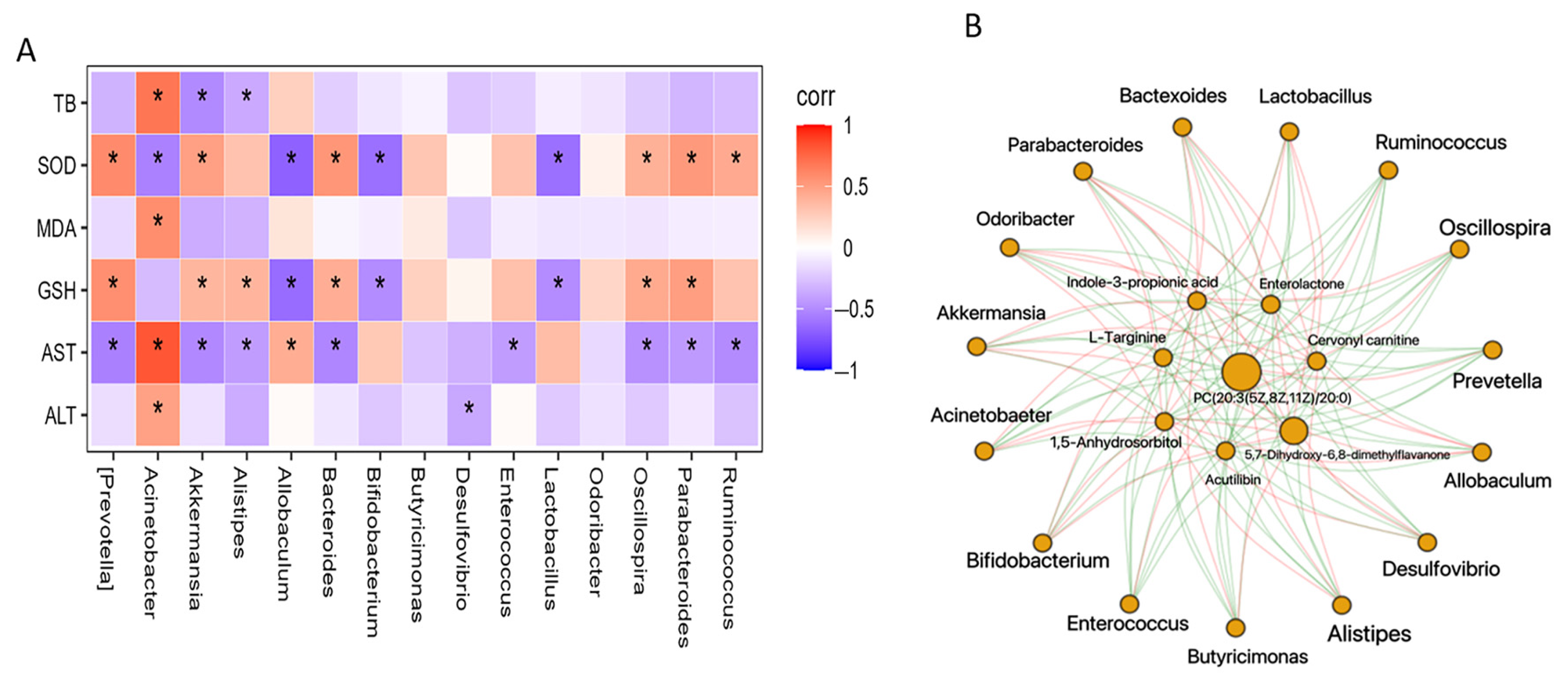
| Metabolite | m/z | RT (min) | Classification | lSDG vs. PAM | hSDG vs. PAM | ||||
|---|---|---|---|---|---|---|---|---|---|
| VIP | FC | Trend | VIP | FC | Trend | ||||
| Indole-3-propionic acid | 188.07 | 93.08 | Organoheterocyclic compounds | 1.81 | 0.407 | ↓ | 1.569 | 0.26 | ↓ |
| 1,5-Anhydrosorbitol | 163.06 | 311.1 | Organic oxygen compounds | 2.55 | 0.464 | ↓ | 1.734 | 0.582 | ↓ |
| PC(20:3(5Z,8Z,11Z)/20:0) | 840.64 | 155.7 | Lipids and lipid-like molecules | 1.8 | 0.484 | ↓ | 1.889 | 0.454 | ↓ |
| Armillaramide | 556.53 | 87.9 | Lipids and lipid-like molecules | 2.17 | 0.616 | ↓ | 1.76 | 0.681 | ↓ |
| Glutamyllysine | 276.16 | 514.5 | Organic acids and derivatives | 1.9 | 0.709 | ↓ | 1.957 | 0.773 | ↓ |
| Threonic acid | 135.03 | 324.8 | Organic oxygen compounds | 2.35 | 0.732 | ↓ | 2.163 | 0.753 | ↓ |
| D-2,3-Dihydroxypropanoic acid | 105.02 | 196.7 | Organic oxygen compounds | 2.41 | 0.74 | ↓ | 1.927 | 0.71 | ↓ |
| N-methyl-L-glutamic acid | 162.08 | 345.7 | Organic acids and derivatives | 2.38 | 0.746 | ↓ | 1.688 | 0.784 | ↓ |
| Imidazoleacetic acid riboside | 259.09 | 367.1 | Nucleosides, nucleotides, and analogs | 1.69 | 0.75 | ↓ | 2.239 | 0.659 | ↓ |
| Santene | 123.12 | 33.86 | Hydrocarbons | 2.57 | 1.209 | ↑ | 2.157 | 1.287 | ↑ |
| Ethylbenzene | 107.09 | 33.85 | Benzenoids | 1.65 | 1.257 | ↑ | 2.183 | 1.366 | ↑ |
| Acutilobin | 329.14 | 147.5 | Phenylpropanoids and polyketides | 2.11 | 1.521 | ↑ | 2.164 | 2.085 | ↑ |
| L-Targinine | 189.13 | 543.5 | Organic acids and derivatives | 2.05 | 1.625 | ↑ | 2.385 | 1.79 | ↑ |
| Cervonyl carnitine | 472.34 | 196.3 | Lipids and lipid-like molecules | 1.79 | 2.984 | ↑ | 1.856 | 2.255 | ↑ |
| 5,7-Dihydroxy-6,8-dimethylflavanone | 285.11 | 25.78 | Phenylpropanoids and polyketides | 2.21 | 3.862 | ↑ | 2.515 | 6.102 | ↑ |
| Enterolactone | 299.13 | 205.6 | Ligan, neoligan, and related compounds | 2.03 | 5.274 | ↑ | 2.394 | 8.512 | ↑ |
| KEGG Pathway | Impact | Changed Metabolite | KEGG No. | VIP | p-Value | Up/Down | |
|---|---|---|---|---|---|---|---|
| NC-PAM | Taurine and hypotaurine metabolism | 0.429 | Taurine | cpd:C00245 | 1.45 | 0.04469 | down |
| Arginine and proline metabolism | 0.222 | Ornithine | cpd:C00077 | 1.90 | 0.00633 | up | |
| L-Aspartic acid | cpd:C00049 | 1.48 | 0.04099 | up | |||
| L-Arginine | cpd:C00062 | 1.85 | 0.00371 | down | |||
| Creatine | cpd:C00300 | 1.54 | 0.02381 | up | |||
| Alanine, aspartate, and glutamate metabolism | 0.193 | L-Aspartic acid | cpd:C00049 | 1.48 | 0.04099 | up | |
| Pyrimidine metabolism | 0.139 | Uridine | cpd:C00299 | 1.55 | 0.01971 | down | |
| Cytidine | cpd:C00475 | 1.68 | 0.01047 | down | |||
| Thymidine | cpd:C00214. | 1.40 | 0.04733 | up | |||
| Dihydrothymine | cpd:C00906. | 1.69 | 0.03190 | up | |||
| Uracil | cpd:C00106 | 1.63 | 0.01816 | down | |||
| Beta-Alanine metabolism | 0.130 | L-Aspartic acid | cpd:C00049 | 1.48 | 0.04099 | up | |
| 3-Aminopropionaldehyde | cpd:C05665 | 1.48 | 0.03089 | up | |||
| Uracil | cpd:C00106 | 1.63 | 0.01816 | down | |||
| hSDG-PAM | Taurine and hypotaurine metabolism | 1.00 | 3-Sulfinoalanine | cpd:C00606 | 2.18 | 0.01294 | down |
| Taurine | cpd:C00245 | 1.93 | 0.02580 | down | |||
| Hypotaurine | cpd:C00519 | 2.13 | 0.01344 | down | |||
| Phenylalanine metabolism | 0.241 | Phenylpyruvic acid | cpd:C00166 | 1.73 | 0.01437 | up | |
| Phenylacetylglycine | cpd:C05598 | 1.70 | 0.04416 | down | |||
| Pyrimidine metabolism | 0.093 | Cytidine | cpd:C00475 | 1.78 | 0.03234 | down | |
| dUMP | cpd:C00365 | 1.84 | 0.02347 | down | |||
| Primary bile acid biosynthesis | 0.060 | Taurine | cpd:C00245 | 1.93 | 0.02580 | down | |
| Taurochenodesoxycholic acid | cpd:C05465 | 1.62 | 0.03692 | up | |||
| Cysteine and methionine metabolism | 0.051 | 5’-Methylthioadenosine | cpd:C00170 | 1.65 | 0.03000 | down | |
| 3-Sulfinoalanine | cpd:C00606 | 2.18 | 0.01294 | down | |||
| SIM-PAM | Glycine, serine and threonine metabolism | 0.269 | Glycine | cpd:C00037 | 1.51 | 0.03110 | down |
| Nicotinate and nicotinamide metabolism | 0.238 | Niacinamide | cpd:C00153 | 1.97 | 0.01476 | up | |
| Primary bile acid biosynthesis | 0.030 | Glycine | cpd:C00037 | 1.51 | 0.03110 | down | |
| Glycerophospholipid metabolism | 0.023 | Glycerophosphocholine | cpd:C00670 | 1.29 | 0.04526 | up | |
| Pantothenate and CoA biosynthesis | 0.020 | Pantothenic | cpd:C00864 | 2.25 | 0.03816 | down |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, Y.; Xu, Z.; Qiao, Z.; Wang, X.; Yang, C. Flaxseed Lignan Alleviates the Paracetamol-Induced Hepatotoxicity Associated with Regulation of Gut Microbiota and Serum Metabolome. Nutrients 2024, 16, 295. https://doi.org/10.3390/nu16020295
Ren Y, Xu Z, Qiao Z, Wang X, Yang C. Flaxseed Lignan Alleviates the Paracetamol-Induced Hepatotoxicity Associated with Regulation of Gut Microbiota and Serum Metabolome. Nutrients. 2024; 16(2):295. https://doi.org/10.3390/nu16020295
Chicago/Turabian StyleRen, Yongyan, Zhenxia Xu, Zhixian Qiao, Xu Wang, and Chen Yang. 2024. "Flaxseed Lignan Alleviates the Paracetamol-Induced Hepatotoxicity Associated with Regulation of Gut Microbiota and Serum Metabolome" Nutrients 16, no. 2: 295. https://doi.org/10.3390/nu16020295
APA StyleRen, Y., Xu, Z., Qiao, Z., Wang, X., & Yang, C. (2024). Flaxseed Lignan Alleviates the Paracetamol-Induced Hepatotoxicity Associated with Regulation of Gut Microbiota and Serum Metabolome. Nutrients, 16(2), 295. https://doi.org/10.3390/nu16020295