Prevalence and Time Trends of Low Serum B12 Levels and Inadequate B12 Dietary Intake in Lebanese Adults amidst the Food Insecurity Situation: Findings from a Nationally Representative Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Eligibility Criteria
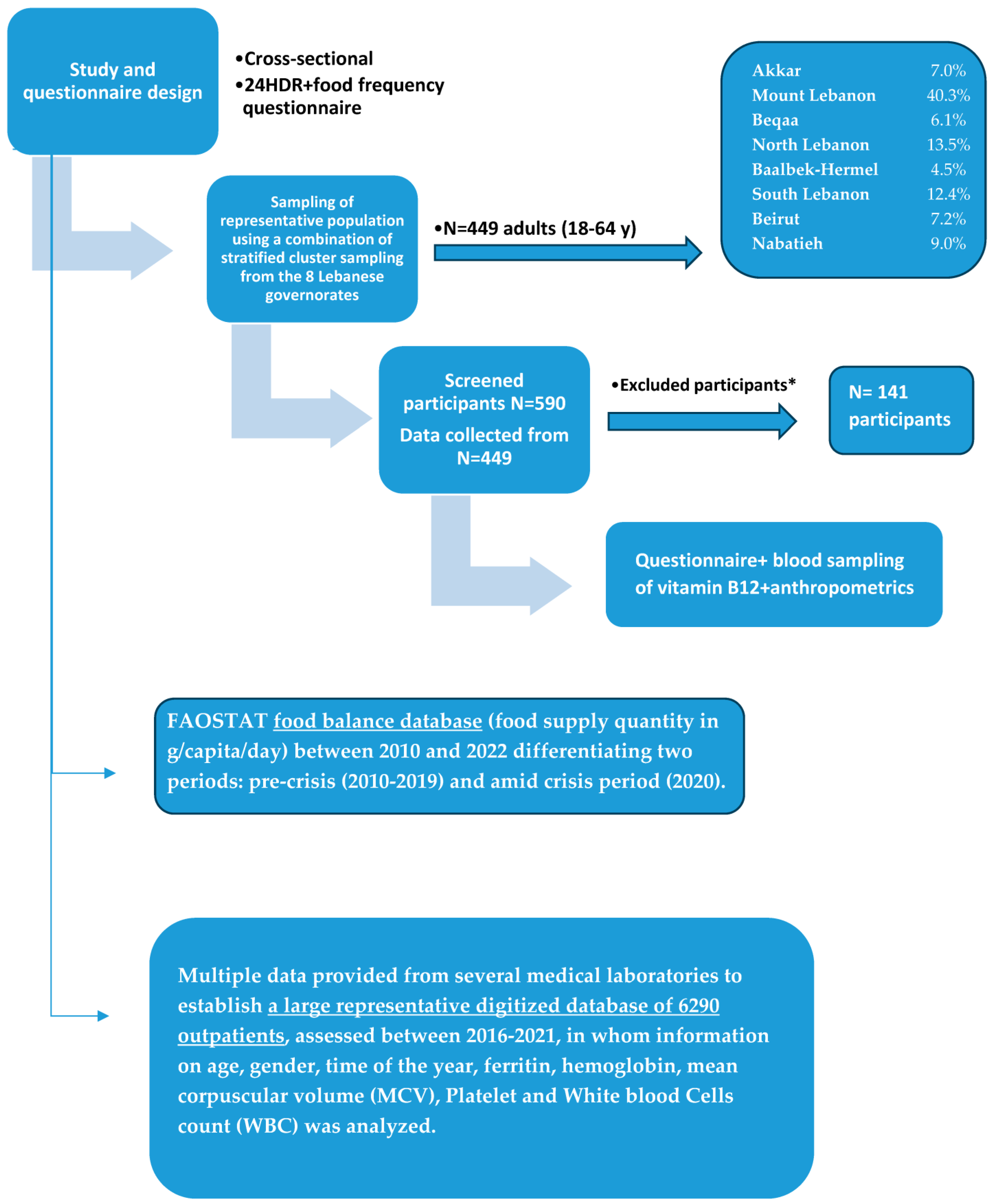
2.2. Sampling Method and Recruitment Process
2.3. Data Collection
2.3.1. Phase 1: Administration of Sociodemographic Questionnaire
2.3.2. Phase 2: Food Frequency Questionnaire and 24 h Recall
2.3.3. Phase 3: Blood Sampling and Anthropometrics
2.4. Time Trends in Consumption of Vitamin B12 Sources and Serum Levels
2.5. Data Management and Statistical Analysis
2.6. Ethical Considerations
3. Results
3.1. Population Characteristics
3.2. Indicators of Household Food Security
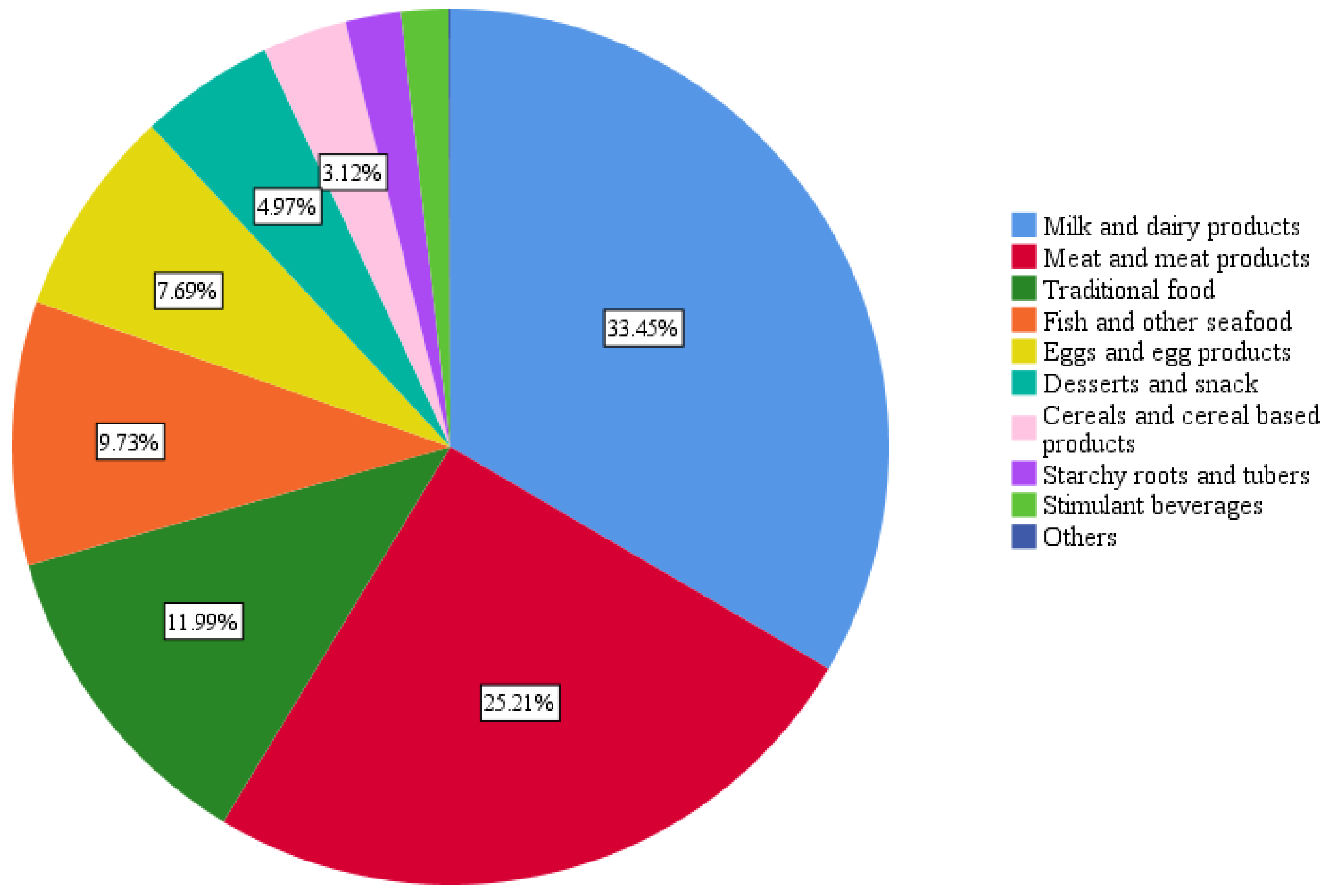
3.3. Population’s Average Food Group Consumption
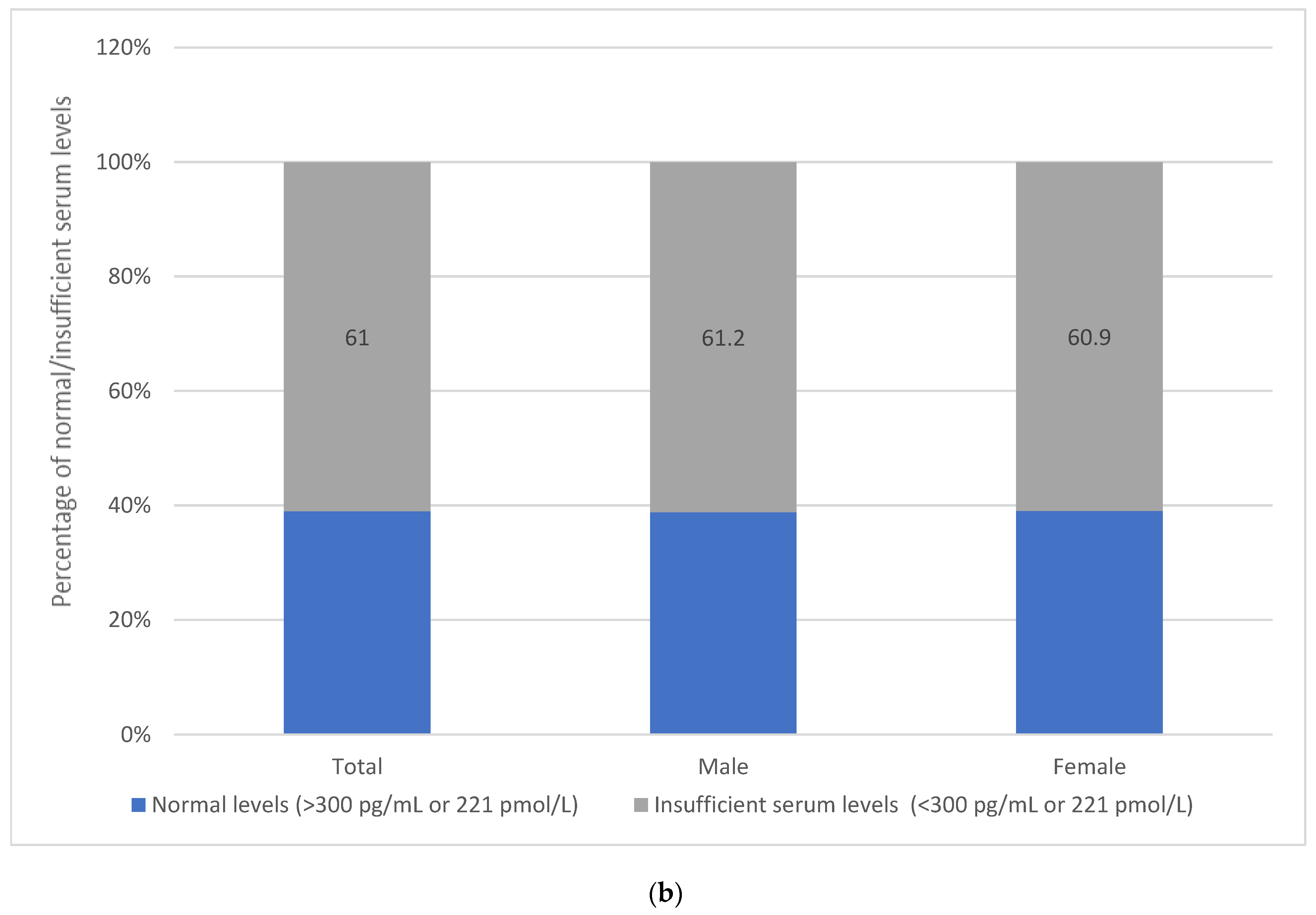
3.4. Assessment of Vitamin B12 Intake and Status
3.5. Association of Household Food Security and Dietary Diversity with Vitamin B12 Intake and Serum Level
3.6. Dietary Sources of Vitamin B12 and Associations with FI
3.7. Determinants of Inadequacy and Insufficient Serum Levels of B12
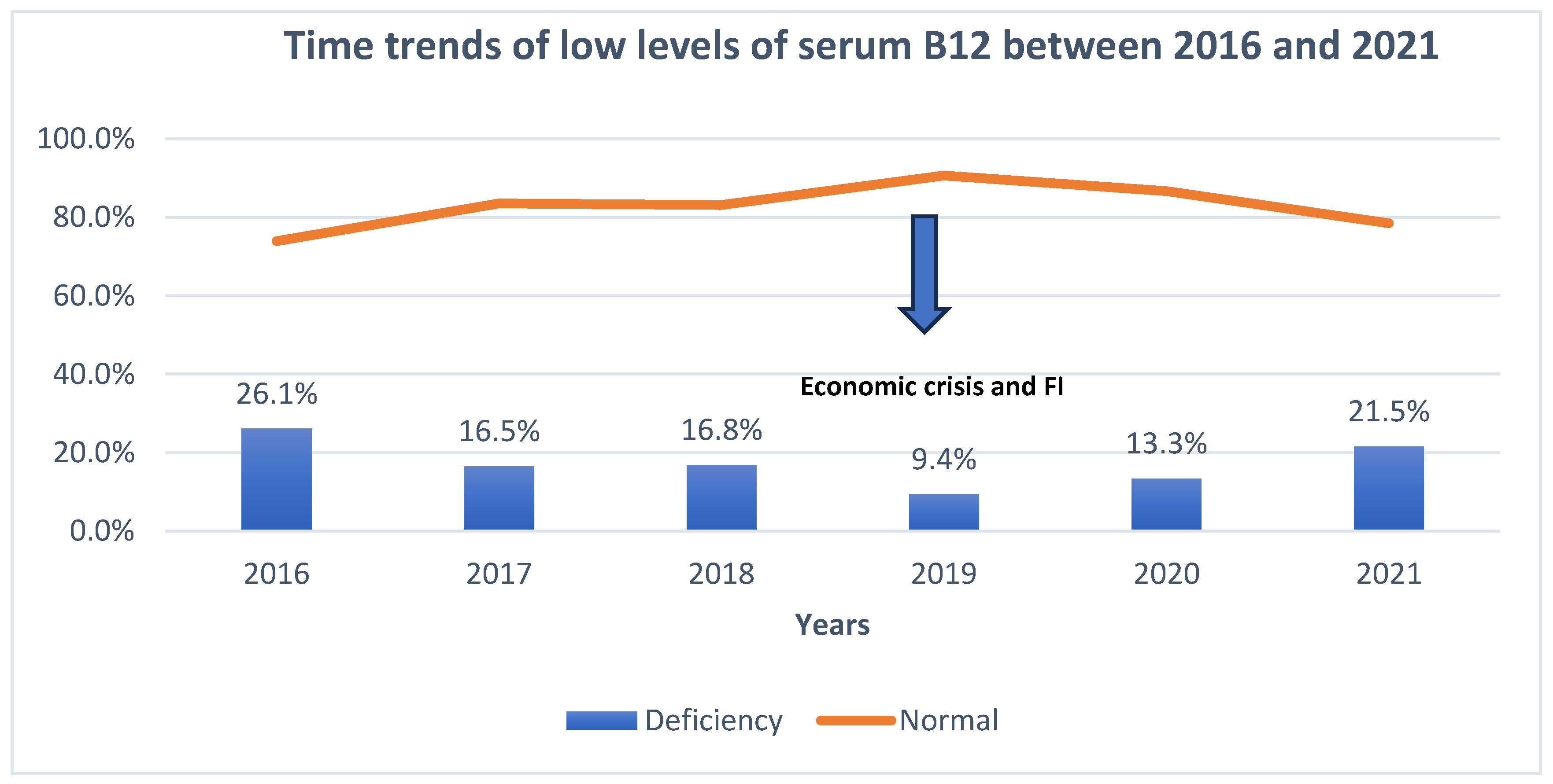
3.8. Time Trends in Vitamin B12 Consumption and Serum Levels before and Amidst the Food Insecurity Situation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Policy Brief: Food Security—Issue 2, June 2006—World|ReliefWeb. Available online: https://reliefweb.int/report/world/policy-brief-food-security-issue-2-june-2006 (accessed on 30 June 2006).
- Ilaboya, I.R.; Atikpo, E.; Omofuma, F.E.; Asekhame, F.F.; Umukoro, L. Causes, Effects and Way Forward to Food Insecurity. Iran. J. Energy Environ. 2012, 3, 180–188. [Google Scholar]
- Assessing the Impact of the Economic and COVID-19 Crises in Lebanon (Round 2)—December 2020—Lebanon|ReliefWeb. Available online: https://reliefweb.int/report/lebanon/assessing-impact-economic-and-covid-19-crises-lebanon-round-2-december-2020 (accessed on 26 January 2021).
- WFP Lebanon—Food Security and Vulnerability Analysis of Lebanese Residents (July 2022)—Lebanon|ReliefWeb. Available online: https://reliefweb.int/report/lebanon/wfp-lebanon-food-security-and-vulnerability-analysis-lebanese-residents-july-2022 (accessed on 23 September 2022).
- Economic: Prices—Dataviz|WFP—VAM. Available online: https://dataviz.vam.wfp.org/economic_explorer/prices?adm0=141 (accessed on 30 May 2023).
- Hoteit, M.; Al-Atat, Y.; Joumaa, H.; Ghali, S.E.; Mansour, R.; Mhanna, R.; Sayyed-Ahmad, F.; Salameh, P.; Al-Jawaldeh, A. Exploring the Impact of Crises on Food Security in Lebanon: Results from a National Cross-Sectional Study. Sustainability 2021, 13, 8753. [Google Scholar] [CrossRef]
- Yazbeck, N.; Mansour, R.; Salame, H.; Chahine, N.B.; Hoteit, M. The Ukraine-Russia War Is Deepening Food Insecurity, Unhealthy Dietary Patterns and the Lack of Dietary Diversity in Lebanon: Prevalence, Correlates and Findings from a National Cross-Sectional Study. Nutrients 2022, 14, 3504. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, F.; Samman, S. Vitamin B12 in health and disease. Nutrients 2010, 2, 299–316. [Google Scholar] [CrossRef] [PubMed]
- Carmel, R. Subclinical cobalamin deficiency. Curr. Opin. Gastroenterol. 2012, 28, 151–158. [Google Scholar] [CrossRef]
- Lam, J.R.; Schneider, J.L.; Zhao, W.; Corley, D.A. Proton pump inhibitor and histamine 2 receptor antagonist use and vitamin B12 deficiency. JAMA 2013, 310, 2435–2442. [Google Scholar] [CrossRef] [PubMed]
- Infante, M.; Leoni, M.; Caprio, M.; Fabbri, A. Long-term metformin therapy and vitamin B12 deficiency: An association to bear in mind. World J. Diabetes 2021, 12, 916–931. [Google Scholar] [CrossRef]
- Linnebank, M.; Moskau, S.; Semmler, A.; Widman, G.; Stoffel-Wagner, B.; Weller, M.; Elger, C.E. Antiepileptic drugs interact with folate and vitamin B12 serum levels. Ann. Neurol. 2011, 69, 352–359. [Google Scholar] [CrossRef]
- Green, R.; Allen, L.H.; Bjørke-Monsen, A.-L.; Brito, A.; Guéant, J.-L.; Miller, J.W.; Molloy, A.M.; Nexo, E.; Stabler, S.; Toh, B.-H.; et al. Vitamin B12 deficiency. Nat. Reviews. Dis. Primers 2017, 3, 17040. [Google Scholar] [CrossRef]
- Allen, L.H. Causes of vitamin B12 and folate deficiency. Food Nutr. Bull. 2008, 29 (Suppl. S2), S20–S34. [Google Scholar] [CrossRef]
- Baik, H.W.; Russell, R.M. Vitamin B12 deficiency in the elderly. Annu. Rev. Nutr. 1999, 19, 357–377. [Google Scholar] [CrossRef] [PubMed]
- Dixon, L.B.; Winkleby, M.A.; Radimer, K.L. Dietary intakes and serum nutrients differ between adults from food-insufficient and food-sufficient families: Third National Health and Nutrition Examination Survey, 1988–1994. J. Nutr. 2001, 131, 1232–1246. [Google Scholar] [CrossRef]
- Champagne, C.M.; Casey, P.H.; Connell, C.L.; Stuff, J.E.; Gossett, J.M.; Harsha, D.W.; McCabe-Sellers, B.; Robbins, J.M.; Simpson, P.M.; Weber, J.L.; et al. Poverty and food intake in rural America: Diet quality is lower in food insecure adults in the Mississippi Delta. J. Am. Diet. Assoc. 2007, 107, 1886–1894. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Sample Size Determination in Health Studies: A Practical Manual; Lwanga, S.K., Lemeshow, S., Eds.; World Health Organization: Geneva, Switzerland, 1991. [Google Scholar]
- World Health Organization. Available online: https://apps.who.int/iris/handle/10665/40062 (accessed on 25 September 2022).
- World Health Organization. WHO Housing and Health Guidelines; World Health Organization: Geneva, Switzerland, 2018; Available online: https://apps.who.int/iris/handle/10665/276001 (accessed on 26 September 2022).
- Sahyoun, N.R.; Nord, M.; Sassine, A.J.; Seyfert, K.; Hwalla, N.; Ghattas, H. Development and validation of an Arab family food security scale. J. Nutr. 2014, 144, 751–757. [Google Scholar] [CrossRef]
- Food Consumption Score—Data Analysis—WFP VAM Resource Centre. Available online: https://resources.vam.wfp.org/data-analysis/quantitative/food-security/food-consumption-score (accessed on 31 May 2023).
- Aoun, C.; Bou Daher, R.; El Osta, N.; Papazian, T.; Khabbaz, L.R. Reproducibility and relative validity of a food frequency questionnaire to assess dietary intake of adults living in a Mediterranean country. PLoS ONE 2019, 14, e0218541. [Google Scholar] [CrossRef] [PubMed]
- Vitamin B12 II Cobas® Elecsys 2010 Modular Analytics E170. Roche Diagnostics GmbH, Sandhofer Strasse 116, D-68305 Mannheim. Available online: www.roche.com (accessed on 31 May 2023).
- FAOSTAT. Available online: https://www.fao.org/faostat/en/#data/FBS24 (accessed on 31 May 2023).
- Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and its Panel on Folate, Other B Vitamins, and Choline. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline; National Academies Press (US): Washington, DC, USA, 1998. Available online: http://www.ncbi.nlm.nih.gov/books/NBK114310/ (accessed on 31 May 2023).
- GEMS/Food—Search. Available online: https://extranet.who.int/gemsfood/Search.aspx (accessed on 31 May 2023).
- Allen, L.H. How common is vitamin B-12 deficiency? Am. J. Clin. Nutr. 2009, 89, 693S–696S. [Google Scholar] [CrossRef] [PubMed]
- de Benoist, B. Conclusions of a WHO Technical Consultation on folate and vitamin B12 deficiencies. Food Nutr. Bull. 2008, 29 (Suppl. S2), S238–S244. [Google Scholar] [CrossRef]
- Lebanon|World Food Programme. Available online: https://www.wfp.org/countries/lebanon (accessed on 31 March 2023).
- Ministry of Economy & Trade. Mini—Basket (Weekly)|Ministry of Economy & Trade, Republic of Lebanon. Available online: http://www.economy.gov.lb//en/services/center-for-pricing-policies/mini---basket-weekly- (accessed on 31 May 2023).
- Kirkpatrick, S.I.; Tarasuk, V. Food insecurity is associated with nutrient inadequacies among Canadian adults and adolescents. J. Nutr. 2008, 138, 604–612. [Google Scholar] [CrossRef]
- Nasreddine, L.; Ayoub, J.J.; Hachem, F.; Tabbara, J.; Sibai, A.M.; Hwalla, N.; Naja, F. Differences in Dietary Intakes among Lebanese Adults over a Decade: Results from Two National Surveys 1997–2008/2009. Nutrients 2019, 11, 1738. [Google Scholar] [CrossRef]
- Azimi, S.; Faramarzi, E.; Sarbakhsh, P.; Ostadrahimi, A.; Somi, M.H.; Ghayour, M. Folate and vitamin B12 status and their relation to hematological indices in healthy adults of Iranians: Azar cohort study. Nutr. Health 2019, 25, 29–36. [Google Scholar] [CrossRef]
- Al-Daghri, N.M.; Al-Othman, A.; Alkharfy, K.M.; Alokail, M.S.; Khan, N.; Alfawaz, H.A.; Aiswaidan, I.A.; Chrousos, G.P. Assessment of selected nutrient intake and adipocytokine profile among Saudi children and adults. Endocr. J. 2012, 59, 1057–1063. [Google Scholar] [CrossRef] [PubMed]
- Gebremedhin, S. Trends in Vitamin B12 Supply and Prevalence of Inadequate Intake in Africa: Regional and Country-Specific Estimates. Food Nutr. Bull. 2021, 42, 467–479. [Google Scholar] [CrossRef] [PubMed]
- Mark, H.E.; Houghton, L.A.; Gibson, R.S.; Monterrosa, E.; Kraemer, K. Estimating dietary micronutrient supply and the prevalence of inadequate intakes from national Food Balance Sheets in the South Asia regiona. Asia Pac. J. Clin. Nutr. 2016, 25, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Lavriša, Ž.; Hristov, H.; Hribar, M.; Žmitek, K.; Kušar, A.; Koroušić Seljak, B.; Gregorič, M.; Blaznik, U.; Gregorič, N.; Zaletel, K.; et al. Dietary Intake and Status of Vitamin B12 in Slovenian Population. Nutrients 2022, 14, 334. [Google Scholar] [CrossRef]
- Instituto Nacional de Estadística y Censos. Encuesta Nacional de Salud, Salud Reproductiva y Nutrición (ENSANUT)-2012; Instituto Nacional de Estadística y Censos: Quito, Ecuador, 2023; Available online: https://www.ecuadorencifras.gob.ec/encuesta-nacional-de-salud-salud-reproductiva-y-nutricion-ensanut-2012/ (accessed on 31 May 2023).
- van Rossum, C.T.; Fransen, H.P.; Verkaik-Kloosterman, J.; Buurma-Rethans, E.J.; Ocké, M.C. Dutch National Food Consumption Survey 2007–2010: Diet of Children and Adults Aged 7 to 69 Years [Report]; Rijksinstituut voor Volksgezondheid en Milieu RIVM: Utrecht, The Netherlands, 2011; Available online: https://rivm.openrepository.com/handle/10029/261553 (accessed on 1 September 2022).
- Bailey, R.L.; Fulgoni, V.L.; Keast, D.R.; Dwyer, J.T. Examination of vitamin intakes among US adults by dietary supplement use. J. Acad. Nutr. Diet. 2012, 112, 657–663.e4. [Google Scholar] [CrossRef]
- NDNS: Results from Years 1 to 4 (Combined). GOV.UK. Available online: https://www.gov.uk/government/statistics/national-diet-and-nutrition-survey-results-from-years-1-to-4-combined-of-the-rolling-programme-for-2008-and-2009-to-2011-and-2012 (accessed on 28 February 2017).
- Aranceta, J.; Serra-Majem, L.; Pérez-Rodrigo, C.; Llopis, J.; Mataix, J.; Ribas, L.; Tojo, R.; Tur, J.A. Vitamins in Spanish food patterns: The eVe Study. Public Health Nutr. 2001, 4, 1317–1323. [Google Scholar] [CrossRef]
- O’Brien, M.M.; Kiely, M.; Harrington, K.E.; Robson, P.J.; Strain, J.J.; Flynn, A. The North/South Ireland Food Consumption Survey: Vitamin intakes in 18-64-year-old adults. Public Health Nutr. 2001, 4, 1069–1079. [Google Scholar] [CrossRef]
- Bangladesh IPC Chronic Food Insecurity Report (June 2022)—Bangladesh|ReliefWeb. Available online: https://reliefweb.int/report/bangladesh/bangladesh-ipc-chronic-food-insecurity-report-june-2022?gclid=Cj0KCQjw4NujBhC5ARIsAF4Iv6fTdOIuat5aKP0eDY5a2pXuKUt-aXbhvMO6DXEUR7EANJl99CprIbEaAsrGEALw_wcB (accessed on 31 May 2023).
- Sivaprasad, M.; Shalini, T.; Balakrishna, N.; Sudarshan, M.; Lopamudra, P.; Suryanarayana, P.; Arlappa, N.; Ravikumar, B.P.; Radhika, M.S.; Reddy, G.B. Status of Vitamin B12 and Folate among the Urban Adult Population in South India. Ann. Nutr. Metab. 2015, 68, 94–100. [Google Scholar] [CrossRef]
- FAO; ECA; AUC. Africa—Regional Overview of Food Security and Nutrition 2021: Statistics and Trends; FAO: Rome, Italy, 2021. [Google Scholar] [CrossRef]
- Australian Health Survey: Nutrition First Results—Foods and Nutrients, 2011–2012 Financial Year|Australian Bureau of Statistics. Available online: https://www.abs.gov.au/statistics/health/health-conditions-and-risks/australian-health-survey-nutrition-first-results-foods-and-nutrients/latest-release (accessed on 5 September 2014).
- Pedersen, A.N.; Fagt, S.; Groth, M.V.; Christensen, T.; Biltoft-Jensen, A.P.; Matthiessen, J.; Andersen, N.L.; Kørup, K.; Hartkopp, H.B.; Ygil, K.H.; et al. Danskernes kostvaner 2003–2008: Hovedresultater. In Danskernes kostvaner 2003—2008; Rapport No. 978-87-92158-67–3; Issues 978-87-92158-67–3; DTU Fødevareinstituttet: Kongens Lyngby, Denmark, 2010. [Google Scholar]
- Planells, E.; Sánchez, C.; Montellano, M.A.; Mataix, J.; Llopis, J. Vitamins B6 and B12 and folate status in an adult Mediterranean population. Eur. J. Clin. Nutr. 2003, 57, 777–785. [Google Scholar] [CrossRef]
- El-Khateeb, M.; Khader, Y.; Batieha, A.; Jaddou, H.; Hyassat, D.; Belbisi, A.; Ajlouni, K. Vitamin B12 deficiency in Jordan: A population-based study. Ann. Nutr. Metab. 2014, 64, 101–105. [Google Scholar] [CrossRef]
- Albalawi, O.; Almubark, R.; Almarshad, A.; Alqahtani, A.S. The Prevalence of Vitamin and Mineral Deficiencies and High Levels of Non-Essential Heavy Metals in Saudi Arabian Adults. Healthcare 2022, 10, 2415. [Google Scholar] [CrossRef]
- Gamble, M.V.; Ahsan, H.; Liu, X.; Factor-Litvak, P.; Ilievski, V.; Slavkovich, V.; Parvez, F.; Graziano, J.H. Folate and cobalamin deficiencies and hyperhomocysteinemia in Bangladesh. Am. J. Clin. Nutr. 2005, 81, 1372–1377. [Google Scholar] [CrossRef] [PubMed]
- Koenig, V.; Stanga, Z.; Zerlauth, M.; Bernasconi, L.; Risch, M.; Huber, A.; Risch, L. Prevalence of vitamin B(12) depletion and deficiency in Liechtenstein. Public Health Nutr. 2014, 17, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Encuesta Nacional de Salud de Panamá archivos. El Blog de Jorge Prosperi. Available online: https://elblogdejorgeprosperi.com/tag/encuesta-nacional-de-salud-de-panama/ (accessed on 31 May 2023).
- Holst-Schumacher, I.; Monge-Rojas, R.; Barrantes-Santamaría, M. Prevalence of mild serum vitamin B(12) deficiency in rural and urban Costa Rican young adults. Rev. Panam. Salud Publica Pan Am. J. Public Health 2007, 22, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, S.M.; McNulty, B.A.; Walton, J.; Flynn, A.; Molloy, A.M.; Scott, J.M.; Nugent, A.P.; Gibney, M.J. Folate and vitamin B12 status in a representative sample of Irish adults. Proc. Nutr. Soc. 2011, 70, E65. [Google Scholar] [CrossRef]
- MacFarlane, A.J.; Greene-Finestone, L.S.; Shi, Y. Vitamin B-12 and homocysteine status in a folate-replete population: Results from the Canadian Health Measures Survey. Am. J. Clin. Nutr. 2011, 94, 1079–1087. [Google Scholar] [CrossRef]
- Bird, J.K.; Murphy, R.A.; Ciappio, E.D.; McBurney, M.I. Risk of Deficiency in Multiple Concurrent Micronutrients in Children and Adults in the United States. Nutrients 2017, 9, 655. [Google Scholar] [CrossRef]
- Castetbon, K.; Vernay, M.; Malon, A.; Salanave, B.; Deschamps, V.; Roudier, C.; Oleko, A.; Szego, E.; Hercberg, S. Dietary intake, physical activity and nutritional status in adults: The French nutrition and health survey (ENNS, 2006–2007). Br. J. Nutr. 2009, 102, 733–743. [Google Scholar] [CrossRef]
- Australian Health Survey: Biomedical Results for Nutrients, 2011–2012|Australian Bureau of Statistics. Available online: https://www.abs.gov.au/statistics/health/health-conditions-and-risks/australian-health-survey-biomedical-results-nutrients/latest-release (accessed on 12 November 2013).
- Lim, H.-S.; Heo, Y.-R. Plasma total homocysteine, folate, and vitamin B12 status in Korean adults. J. Nutr. Sci. Vitaminol. 2002, 48, 290–297. [Google Scholar] [CrossRef]
- Xavier, J.M.; Costa, F.F.; Annichino-Bizzacchi, J.M.; Saad ST, O. High frequency of vitamin B12 deficiency in a Brazilian population. Public Health Nutr. 2010, 13, 1191–1197. [Google Scholar] [CrossRef]
- Alfthan, G.; Laurinen, M.S.; Valsta, L.M.; Pastinen, T.; Aro, A. Folate intake, plasma folate and homocysteine status in a random Finnish population. Eur. J. Clin. Nutr. 2003, 57, 81–88. [Google Scholar] [CrossRef] [PubMed]
- National Research Council (US) Subcommittee on the Tenth Edition of the Recommended Dietary Allowances. Recommended Dietary Allowances, 10th ed.; National Academies Press (US): Washington, DC, USA, 1989. Available online: http://www.ncbi.nlm.nih.gov/books/NBK234932/ (accessed on 10 September 2023).
- Ivers, L.C.; Cullen, K.A. Food insecurity: Special considerations for women. Am. J. Clin. Nutr. 2011, 94, 1740S–1744S. [Google Scholar] [CrossRef] [PubMed]
- Jomaa, L.H.; Naja, F.A.; Kharroubi, S.A.; Diab-El-Harake, M.H.; Hwalla, N.C. Food insecurity is associated with compromised dietary intake and quality among Lebanese mothers: Findings from a national cross-sectional study. Public Health Nutr. 2020, 23, 2687–2699. [Google Scholar] [CrossRef] [PubMed]
- Poverty, Household Food Security, and Nutrition in Rural Pakistan|IFPRI: International Food Policy Research Institute. Available online: https://www.ifpri.org/publication/poverty-household-food-security-and-nutrition-rural-pakistan (accessed on 31 May 2023).
- Soofi, S.; Khan, G.N.; Sadiq, K.; Ariff, S.; Habib, A.; Kureishy, S.; Hussain, I.; Umer, M.; Suhag, Z.; Rizvi, A.; et al. Prevalence and possible factors associated with anaemia, and vitamin B 12 and folate deficiencies in women of reproductive age in Pakistan: Analysis of national-level secondary survey data. BMJ Open 2017, 7, e018007. [Google Scholar] [CrossRef] [PubMed]
- Beitz, R.; Mensink, G.B.M.; Fischer, B.; Thamm, M. Vitamins—Dietary intake and intake from dietary supplements in Germany. Eur. J. Clin. Nutr. 2002, 56, 539–545. [Google Scholar] [CrossRef]
- de Bree, A.; Verschuren, W.M.; Blom, H.J.; Kromhout, D. Association between B vitamin intake and plasma homocysteine concentration in the general Dutch population aged 20–65 y. Am. J. Clin. Nutr. 2001, 73, 1027–1033. [Google Scholar] [CrossRef] [PubMed]
- Zaghloul, S.; Al-Hooti, S.N.; Al-Hamad, N.; Al-Zenki, S.; Alomirah, H.; Alayan, I.; Al-Attar, H.; Al-Othman, A.; Al-Shami, E.; Al-Somaie, M.; et al. Evidence for nutrition transition in Kuwait: Over-consumption of macronutrients and obesity. Public Health Nutr. 2013, 16, 596–607. [Google Scholar] [CrossRef] [PubMed]
- García Closas, R.; Serra Majem, L.; Sabater Sales, G.; Olmos Castellvell, M.; Ribas Barba, L.; Salleras Sanmartí, L.; Grupo de Investigación en Nutrición Comunitaria. Distribution of the serum concentration of vitamin C, folic acid and vitamin B12 in a representative sample of the adult population of Catalonia (Spain). Med. Clin. 2002, 118, 135–141. [Google Scholar] [CrossRef]
- Chittaranjan, Y. Vitamin B12: An Intergenerational Story. Nestle Nutr. Inst. Workshop Ser. 2020, 93, 91–102. [Google Scholar] [CrossRef]
- Hao, L.; Ma, J.; Zhu, J.; Stampfer, M.J.; Tian, Y.; Willett, W.C.; Li, Z. Vitamin B-12 deficiency is prevalent in 35- to 64-year-old Chinese adults. J. Nutr. 2007, 137, 1278–1285. [Google Scholar] [CrossRef]
- Vogiatzoglou, A.; Smith, A.D.; Nurk, E.; Berstad, P.; Drevon, C.A.; Ueland, P.M.; Vollset, S.E.; Tell, G.S.; Refsum, H. Dietary sources of vitamin B-12 and their association with plasma vitamin B-12 concentrations in the general population: The Hordaland Homocysteine Study. Am. J. Clin. Nutr. 2009, 89, 1078–1087. [Google Scholar] [CrossRef] [PubMed]
- Al Khatib, L.; Obeid, O.; Sibai, A.-M.; Batal, M.; Adra, N.; Hwalla, N. Folate deficiency is associated with nutritional anaemia in Lebanese women of childbearing age. Public Health Nutr. 2006, 9, 921–927. [Google Scholar] [CrossRef] [PubMed]
- Dhonukshe-Rutten, R.A.M.; de Vries, J.H.M.; de Bree, A.; van der Put, N.; van Staveren, A.W.; de Groot, L.C.P.G.M. Dietary intake and status of folate and vitamin B12 and their association with homocysteine and cardiovascular disease in European populations. Eur. J. Clin. Nutr. 2009, 63, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Zappacosta, B.; Persichilli, S.; Iacoviello, L.; Di Castelnuovo, A.; Graziano, M.; Gervasoni, J.; Leoncini, E.; Cimino, G.; Mastroiacovo, P. Folate, vitamin B12 and homocysteine status in an Italian blood donor population. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 473–480. [Google Scholar] [CrossRef]
- Guidelines on Food Fortification with Micronutrients. Available online: https://www.who.int/publications-detail-redirect/9241594012 (accessed on 31 May 2023).
- Guideline: Fortification of Wheat Flour with Vitamins and Minerals as a Public Health Strategy. Available online: https://www.who.int/publications-detail-redirect/9789240043398 (accessed on 31 May 2023).


| Variable | Overall (n = 444) | Male (n = 183) | Female (n = 261) | |||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | p-Value | ||
| Age (y) | 34.1 | 12.7 | 33.8 | 12.2 | 34.4 | 13.1 | 0.649 | |
| N | % | N | % | N | % | p-Value | ||
| Age categories a | 18–24 y | 149 | 33.6 | 55 | 36.9 | 94 | 63.1 | 0.005 * |
| 25–39 y | 151 | 34.0 | 78 | 51.7 | 73 | 48.3 | ||
| 40–64 y | 144 | 32.4 | 50 | 34.7 | 94 | 65.3 | ||
| Total | 444 | 183 | 41.2 | 261 | 58.8 | |||
| Residency | Akkar | 31 | 7.0 | 11 | 35.5 | 20 | 64.5 | 0.006 * |
| Mount Lebanon | 179 | 40.3 | 73 | 40.8 | 106 | 59.2 | ||
| Beqaa | 27 | 6.1 | 5 | 18.5 | 22 | 81.5 | ||
| North Lebanon | 60 | 13.5 | 33 | 55.0 | 27 | 45.0 | ||
| Baalbek-Hermel | 20 | 4.5 | 10 | 50.0 | 10 | 50.0 | ||
| South Lebanon | 55 | 12.4 | 30 | 54.5 | 25 | 45.5 | ||
| Beirut | 32 | 7.2 | 10 | 31.3 | 22 | 68.8 | ||
| Nabatieh | 40 | 9.0 | 11 | 27.5 | 29 | 72.5 | ||
| Marital status | Single | 202 | 45.5 | 81 | 40.1 | 121 | 59.9 | 0.128 |
| Married | 223 | 50.2 | 97 | 43.5 | 126 | 56.5 | ||
| Widowed | 7 | 1.6 | 0 | 0.0 | 7 | 100.0 | ||
| Divorced | 12 | 2.7 | 5 | 41.7 | 7 | 58.3 | ||
| Household crowding index | No crowding | 165 | 37.2 | 79 | 47.9 | 86 | 52.1 | 0.036 * |
| Crowding | 279 | 62.8 | 104 | 37.3 | 175 | 62.7 | ||
| Number of children | None | 217 | 48.9 | 87 | 40.1 | 130 | 59.9 | 0.124 |
| 1–3 | 159 | 35.8 | 74 | 46.5 | 85 | 53.5 | ||
| More than 3 | 68 | 15.3 | 22 | 32.4 | 46 | 67.6 | ||
| Education level | Illiterate | 3 | 0.7 | 1 | 33.3 | 2 | 66.7 | 1.000 |
| School | 175 | 39.4 | 72 | 41.1 | 103 | 58.9 | ||
| University | 266 | 59.9 | 110 | 41.4 | 156 | 58.6 | ||
| Current occupation | Unemployed | 218 | 49.1 | 40 | 18.3 | 178 | 81.7 | <0.001 * |
| Employed | 226 | 50.9 | 143 | 63.3 | 83 | 36.7 | ||
| Monthly salary change since the start of economic crisis | No impact | 129 | 29.1 | 63 | 48.8 | 66 | 51.2 | <0.001 * |
| Decline in salary | 123 | 27.7 | 51 | 41.5 | 72 | 58.5 | ||
| Increase in salary | 70 | 15.8 | 42 | 60.0 | 28 | 40.0 | ||
| Already have no salary | 122 | 27.5 | 27 | 22.1 | 95 | 77.9 | ||
| Household monthly income | None | 39 | 8.8 | 17 | 43.6 | 22 | 56.4 | <0.001 * |
| <LBP 1.5 million | 58 | 13.1 | 15 | 25.9 | 43 | 74.1 | ||
| ≥LBP 1.5 million | 211 | 47.5 | 79 | 37.4 | 132 | 62.6 | ||
| ≤USD 300 | 92 | 20.7 | 41 | 44.6 | 51 | 55.4 | ||
| >USD 300 | 44 | 9.9 | 31 | 70.5 | 13 | 29.5 | ||
| Variable | Overall (n = 444) | Male (n = 183) | Female (n = 261) | |||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | p-Value | ||
| Weight (kg) | 73.8 | 17.1 | 81.8 | 16.6 | 68.3 | 15.1 | <0.001 * | |
| Height (cm) | 165.3 | 9.4 | 173.5 | 7.0 | 159.5 | 5.9 | <0.001 * | |
| Body mass index (kg/m2) | 27.0 | 5.8 | 27.2 | 5.3 | 26.9 | 6.1 | 0.626 | |
| N | % | N | % | N | % | p-Value | ||
| BMI classification | Underweight | 19 | 4.3 | 4 | 21.1 | 15 | 78.9 | 0.084 |
| Normal | 150 | 33.8 | 57 | 38.0 | 93 | 62.0 | ||
| Overweight and Obese | 275 | 61.9 | 122 | 44.4 | 153 | 55.6 | ||
| Disease status | No disease | 333 | 75.0 | 150 | 45.0 | 183 | 55.0 | 0.005 * |
| Having disease | 111 | 25.0 | 33 | 29.7 | 78 | 70.3 | ||
| Cardiovascular disease | 14 | 12.6 | 5 | 15.2 | 9 | 11.5 | ||
| Diabetes | 3 | 2.7 | 1 | 3.0 | 2 | 2.6 | ||
| Hypertension | 34 | 30.6 | 16 | 48.5 | 18 | 23.1 | ||
| Kidney disease | 4 | 3.6 | 2 | 6.1 | 2 | 2.6 | ||
| Liver disease | 1 | 0.9 | 1 | 3.0 | 0 | 0.0 | 0.004 | |
| Osteoporosis | 14 | 12.6 | 1 | 3.0 | 13 | 16.7 | ||
| Asthma/Respiratory diseases | 12 | 10.8 | 2 | 6.1 | 10 | 12.8 | ||
| Anemia | 36 | 32.4 | 5 | 15.2 | 31 | 39.7 | ||
| Others a | 32 | 28.8 | 6 | 18.2 | 26 | 33.3 | ||
| History of COVID-19 infection | No | 126 | 28.4 | 52 | 41.3 | 74 | 58.7 | 0.296 |
| Yes | 238 | 53.6 | 104 | 43.7 | 134 | 56.3 | ||
| Don’t know | 80 | 18.0 | 27 | 33.8 | 53 | 66.3 | ||
| COVID-19 vaccination | No | 142 | 32.0 | 51 | 35.9 | 91 | 64.1 | 0.123 |
| Yes | 302 | 68.0 | 132 | 43.7 | 170 | 56.3 | ||
| Variable | Overall (n = 444) | Male (n = 183) | Female (n = 261) | |||||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | p-Value | ||
| Food security status | Food-secure | 234 | 52.7 | 102 | 55.7 | 132 | 50.6 | 0.28 |
| Food-insecure | 210 | 47.3 | 81 | 44.3 | 129 | 49.4 | ||
| Number of meals consumed the day before | 2 meals or fewer | 241 | 54.3 | 100 | 41.5 | 141 | 58.5 | 0.923 |
| 3 meals or more | 203 | 45.7 | 83 | 40.9 | 120 | 59.1 | ||
| Evaluation of number of meals reported | Fewer than usual | 107 | 24.1 | 39 | 36.4 | 68 | 63.6 | 0.491 |
| As usual | 330 | 74.3 | 141 | 42.7 | 189 | 57.3 | ||
| More than usual | 7 | 1.6 | 3 | 42.9 | 4 | 57.1 | ||
| Dietary diversity | Low | 250 | 56.3 | 102 | 40.8 | 148 | 59.2 | 0.846 |
| High | 194 | 43.7 | 81 | 41.8 | 113 | 58.2 | ||
| Variable | Vitamin B12 Intake ** | B12 Serum Levels *** | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Adequate | Inadequate | Normal | Insufficient | ||||||||
| N | % | N | % | p-Value | N | % | N | % | p-Value | ||
| Food security status | Food-secure | 140 | 59.8 | 94 | 40.2 | 0.000 * | 101 | 43.2 | 133 | 56.8 | 0.064 |
| Food-insecure | 71 | 33.8 | 139 | 66.2 | 72 | 34.3 | 138 | 65.7 | |||
| Number of meals consumed the day before | 2 meals or fewer | 90 | 37.3 | 151 | 62.7 | 0.000 * | 89 | 36.9 | 152 | 63.1 | 0.379 |
| 3 meals or more | 121 | 59.6 | 82 | 40.4 | 84 | 41.4 | 119 | 58.6 | |||
| Evaluation of number of meals reported | Fewer than usual | 37 | 34.6 | 70 | 65.4 | 0.005 * | 35 | 32.7 | 72 | 67.3 | 0.291 |
| As usual | 171 | 51.8 | 159 | 48.2 | 135 | 40.9 | 195 | 59.1 | |||
| More than usual | 3 | 42.9 | 4 | 57.1 | 3 | 42.9 | 4 | 57.1 | |||
| Dietary diversity | Low | 83 | 33.2 | 167 | 66.8 | 0.000 * | 86 | 34.4 | 164 | 65.6 | 0.031 * |
| High | 128 | 66.0 | 66 | 34.0 | 87 | 44.8 | 107 | 55.2 | |||
| Determinants of B12 Intake (Adequate (Reference) vs. Inadequate) | OR | 95% CI | p-Value | ||
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Sex | Male (Reference) | ||||
| Female | 2.845 | 1.757 | 4.606 | <0.001 * | |
| Current occupation | Employed (Reference) | ||||
| Unemployed | 1.806 | 1.129 | 2.884 | 0.014 * | |
| Food security status | Food-secure (Reference) | ||||
| Food-insecure | 2.145 | 1.381 | 3.333 | 0.001 * | |
| Number of meals consumed the day before | 3 meals or more (Reference) | ||||
| 2 meals or fewer | 1.497 | 0.951 | 2.358 | 0.081 | |
| Dietary diversity | High dietary diversity (Reference) | ||||
| Low dietary diversity | 3.034 | 1.924 | 4.783 | <0.001 * | |
| Determinants of Low Serum B12 Levels (Normal (Reference) vs. Insufficient) | |||||
| Beirut (Reference) | |||||
| North Lebanon | 1.305 | 0.558 | 3.049 | 0.539 | |
| Residency | Mount Lebanon | 0.817 | 0.374 | 1.788 | 0.614 |
| Beqaa | 2.856 | 1.018 | 8.017 | 0.046 * | |
| South Lebanon | 0.736 | 0.321 | 1.689 | 0.470 | |
| Vitamin B12 intake | Adequate (Reference) | ||||
| Inadequate | 1.788 | 1.206 | 2.650 | 0.004 * | |
| Food Groups | Food Supply Quantity (g/Capita/Day) | Food Consumption (g/Day) | |
|---|---|---|---|
| 2010–2019 (Pre-Crisis) | 2020 | 2022 (Current Study) | |
| Eggs | 8.8 | 12.46575 | 22.2 |
| Fish and seafood | 25.05753 | 23.89041 | 11.4 |
| Meat | 89.87945 | 80.79452 | 69.5 |
| Milk and dairy products | 200.1397 | 169.8082 | 184.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoteit, M.; Khadra, R.; Fadlallah, Z.; Mourad, Y.; Chahine, M.; Skaiki, F.; Al Manasfi, E.; Chahine, A.; Poh, O.B.J.; Tzenios, N. Prevalence and Time Trends of Low Serum B12 Levels and Inadequate B12 Dietary Intake in Lebanese Adults amidst the Food Insecurity Situation: Findings from a Nationally Representative Cross-Sectional Study. Nutrients 2024, 16, 226. https://doi.org/10.3390/nu16020226
Hoteit M, Khadra R, Fadlallah Z, Mourad Y, Chahine M, Skaiki F, Al Manasfi E, Chahine A, Poh OBJ, Tzenios N. Prevalence and Time Trends of Low Serum B12 Levels and Inadequate B12 Dietary Intake in Lebanese Adults amidst the Food Insecurity Situation: Findings from a Nationally Representative Cross-Sectional Study. Nutrients. 2024; 16(2):226. https://doi.org/10.3390/nu16020226
Chicago/Turabian StyleHoteit, Maha, Razan Khadra, Zahraa Fadlallah, Youmna Mourad, Mohamad Chahine, Farouk Skaiki, Elham Al Manasfi, Abdulrahman Chahine, Omasyarifa Binti Jamal Poh, and Nikolaos Tzenios. 2024. "Prevalence and Time Trends of Low Serum B12 Levels and Inadequate B12 Dietary Intake in Lebanese Adults amidst the Food Insecurity Situation: Findings from a Nationally Representative Cross-Sectional Study" Nutrients 16, no. 2: 226. https://doi.org/10.3390/nu16020226
APA StyleHoteit, M., Khadra, R., Fadlallah, Z., Mourad, Y., Chahine, M., Skaiki, F., Al Manasfi, E., Chahine, A., Poh, O. B. J., & Tzenios, N. (2024). Prevalence and Time Trends of Low Serum B12 Levels and Inadequate B12 Dietary Intake in Lebanese Adults amidst the Food Insecurity Situation: Findings from a Nationally Representative Cross-Sectional Study. Nutrients, 16(2), 226. https://doi.org/10.3390/nu16020226







