S-Adenosylmethionine (SAMe) for Central Nervous System Health: A Systematic Review
Abstract
1. Introduction
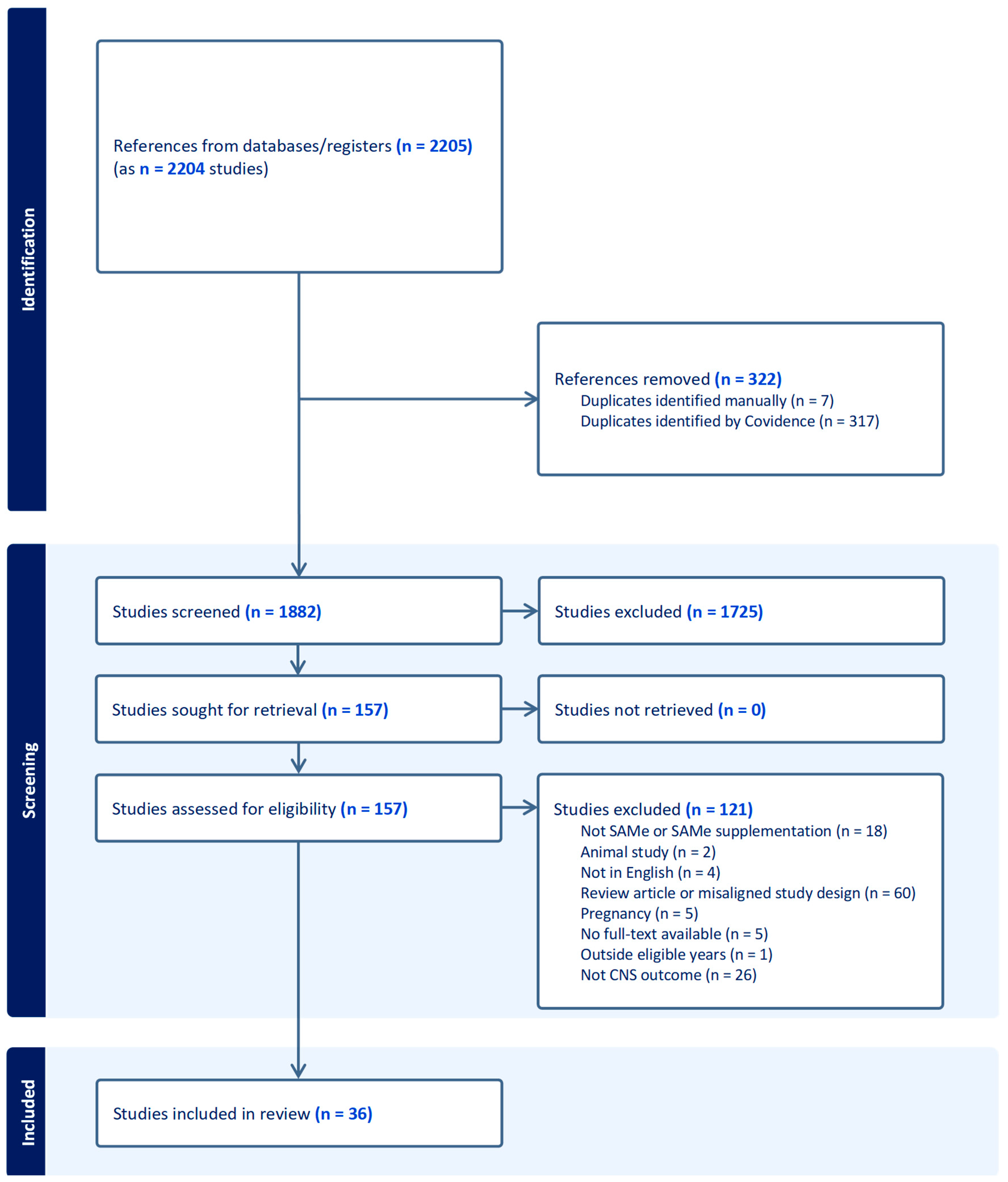
2. Methods
3. Results
Study Characteristics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Mental Disorders. Available online: https://www.who.int/news-room/fact-sheets/detail/mental-disorders (accessed on 22 August 2024).
- Institute for Health Metrics and Evaluation. 2021 Global Burden of Disease Study Results. Available online: https://vizhub.healthdata.org/gbd-results/ (accessed on 22 August 2024).
- World Health Organization. Mental Health and COVID-19: Early Evidence of the Pandemic’s Impact: Scientific Brief. 2 March 2022. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-Sci_Brief-Mental_health-2022.1 (accessed on 22 August 2024).
- Reinert, M.; Fritze, D.; Nguyen, T. The State of Mental Health in America 2024; Mental Health America: Alexandria, VA, USA, 2024. [Google Scholar]
- Limveeraprajak, N.; Nakhawatchana, S.; Visukamol, A.; Siripakkaphant, C.; Suttajit, S.; Srisurapanont, M. Efficacy and acceptability of S-adenosyl-L-methionine (SAMe) for depressed patients: A systematic review and meta- analysis. Prog. Neuropsychopharmacol. Biol. Psychiatry 2024, 132, 110985. [Google Scholar] [CrossRef]
- Sachinvala, N.D.; Teramoto, N.; Stergiou, A. Proposed Neuroimmune Roles of Dimethyl Fumarate, Bupropion, S-Adenosylmethionine, and Vitamin D(3) in Affording a Chronically Ill Patient Sustained Relief from Inflammation and Major Depression. Brain Sci. 2020, 10, 600. [Google Scholar] [CrossRef]
- Cuomo, A.; Beccarini Crescenzi, B.; Bolognesi, S.; Goracci, A.; Koukouna, D.; Rossi, R.; Fagiolini, A. S-Adenosylmethionine (SAMe) in major depressive disorder (MDD): A clinician-oriented systematic review. Ann. Gen. Psychiatry 2020, 19, 50. [Google Scholar] [CrossRef]
- Sharma, A.; Gerbarg, P.; Bottiglieri, T.; Massoumi, L.; Carpenter, L.L.; Lavretsky, H.; Muskin, P.R.; Brown, R.P.; Mischoulon, D. S-Adenosylmethionine (SAMe) for Neuropsychiatric Disorders: A Clinician-Oriented Review of Research. J. Clin. Psychiatry 2017, 78, e656–e667. [Google Scholar] [CrossRef]
- Rambaldi, A.; Gluud, C. S-adenosyl-L-methionine for alcoholic liver diseases. Cochrane Database Syst. Rev. 2006, 1, CD002235. [Google Scholar] [CrossRef]
- Noureddin, M.; Sander-Struckmeier, S.; Mato, J.M. Early treatment efficacy of S-adenosylmethionine in patients with intrahepatic cholestasis: A systematic review. World J. Hepatol. 2020, 12, 46–63. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Hong, Q.N.; Pluye, P.; Fàbregues, S.; Bartlett, G.; Boardman, F.; Cargo, M.; Dagenais, P.; Gagnon, M.P.; Griffiths, F.; Nicolau, B.; et al. Mixed Methods Appraisal Tool (MMAT), Version 2018; Registration of Copyright (#1148552); Canadian Intellectual Property Office, Industry Canada: Gatineau, QC, Canada, 2018. [Google Scholar]
- Abeysundera, H.; Gill, R. Possible SAMe-induced mania. BMJ Case Rep. 2018, 2018, bcr2018224338-bcr. [Google Scholar] [CrossRef]
- Alpert, J.E.; Papakostas, G.; Mischoulon, D.; Worthington, J.J.; Petersen, T.; Mahal, Y.; Burns, A.; Bottiglieri, T.; Nierenberg, A.A.; Fava, M. S-adenosyl-L-methionine (SAMe) as an adjunct for resistant major depressive disorder: An open trial following partial or nonresponse to selective serotonin reuptake inhibitors or venlafaxine. J. Clin. Psychopharmacol. 2004, 24, 661–664. [Google Scholar] [CrossRef]
- Anderson, S.; Panka, J.; Rakobitsch, R.; Tyre, K.; Pulliam, K. Anxiety and Methylenetetrahydrofolate Reductase Mutation Treated With S-Adenosyl Methionine and Methylated B Vitamins. Integr. Med. Clin. J. 2016, 15, 48–52. [Google Scholar]
- Arnold, O.; Saletu, B.; Anderer, P.; Assandri, A.; di Padova, C.; Corrado, M.; Saletu-Zyhlarz, G.M. Double-blind, placebo-controlled pharmacodynamic studies with a nutraceutical and a pharmaceutical dose of ademetionine (SAMe) in elderly subjects, utilizing EEG mapping and psychometry. Eur. Neuropsychopharmacol. 2005, 15, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Bambling, M.; Parham, S.C.; Coulson, S.; Vitetta, L. S-adenosylmethionine (SAMe) and Magnesium Orotate as adjunctives to SSRIs in sub-optimal treatment response of depression in adults: A pilot study. Adv. Integr. Med. 2015, 2, 56–62. [Google Scholar] [CrossRef]
- Carpenter, D.J. St. John’s wort and S-adenosyl methionine as “natural” alternatives to conventional antidepressants in the era of the suicidality boxed warning: What is the evidence for clinically relevant benefit? Altern. Med. Rev. 2011, 16, 17–39. [Google Scholar]
- Chitiva, H.; Audivert, F.; Alvarez, C. Suicide attempt by self-burning associated with ingestion of S-adenosylmethionine: A review of the literature and case report. J. Nerv. Ment. Dis. 2012, 200, 99–101. [Google Scholar] [CrossRef] [PubMed]
- De Berardis, D.; Marini, S.; Serroni, N.; Rapini, G.; Iasevoli, F.; Valchera, A.; Signorelli, M.; Aguglia, E.; Perna, G.; Salone, A.; et al. S-Adenosyl-L-Methionine augmentation in patients with stage II treatment-resistant major depressive disorder: An open label, fixed dose, single-blind study. Sci. World J. 2013, 2013, 204649. [Google Scholar] [CrossRef]
- Di Pierro, F.; Settembre, R. Preliminary results of a randomized controlled trial carried out with a fixed combination of S-adenosyl-L-methionine and betaine versus amitriptyline in patients with mild depression. Int. J. Gen. Med. 2015, 8, 73–78. [Google Scholar] [CrossRef]
- Djokic, G.; Korcok, D.; Djordjevic, V.; Agic, A.; Rankovic, A.; Djukic, D. The effects of S-adenosyl-L-methionine-vitamin B complex on mild and moderate depressive symptoms. Hippokratia 2017, 21, 140–143. [Google Scholar]
- Dolcetta, D.; Parmigiani, P.; Salmaso, L.; Bernardelle, R.; Cesari, U.; Andrighetto, G.; Baschirotto, G.; Nyhan, W.L.; Hladnik, U. Quantitative evaluation of the clinical effects of S-adenosylmethionine on mood and behavior in Lesch-Nyhan patients. Nucleosides Nucleotides Nucleic Acids 2013, 32, 174–188. [Google Scholar] [CrossRef]
- Galizia, I.; Oldani, L.; Macritchie, K.; Amari, E.; Dougall, D.; Jones, T.N.; Lam, R.W.; Massei, G.J.; Yatham, L.N.; Young, A.H. S-adenosyl methionine (SAMe) for depression in adults. Cochrane Database Syst. Rev. 2016, 10, CD011286. [Google Scholar] [CrossRef]
- Green, T.; Steingart, L.; Frisch, A.; Zarchi, O.; Weizman, A.; Gothelf, D. The feasibility and safety of S-adenosyl-L-methionine (SAMe) for the treatment of neuropsychiatric symptoms in 22q11.2 deletion syndrome: A double-blind placebo-controlled trial. J. Neural Transm. 2012, 119, 1417–1423. [Google Scholar] [CrossRef]
- Jaggumantri, S.; Dunbar, M.; Edgar, V.; Mignone, C.; Newlove, T.; Elango, R.; Collet, J.P.; Sargent, M.; Stockler-Ipsiroglu, S.; van Karnebeek, C.D.M. Treatment of Creatine Transporter (SLC6A8) Deficiency with Oral S-Adenosyl Methionine as Adjunct to L-arginine, Glycine, and Creatine Supplements. Pediatr. Neurol. 2015, 53, 360–363.e362. [Google Scholar] [CrossRef]
- Kalman, D.S.; Feldman, S.; Vazquez, R.R.; Krieger, D.R. A Prospective Randomized Double-Blind Study Evaluating UP165 and S-Adenosyl-l-Methionine on Depression, Anxiety and Psychological Well-Being. Foods 2015, 4, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Levkovitz, Y.; Alpert, J.E.; Brintz, C.E.; Mischoulon, D.; Papakostas, G.I. Effects of S-adenosylmethionine augmentation of serotonin-reuptake inhibitor antidepressants on cognitive symptoms of major depressive disorder. Eur. Psychiatry 2012, 27, 518–521. [Google Scholar] [CrossRef] [PubMed]
- Mischoulon, D.; Alpert, J.E.; Arning, E.; Bottiglieri, T.; Fava, M.; Papakostas, G.I. Bioavailability of S-adenosyl methionine and impact on response in a randomized, double-blind, placebo-controlled trial in major depressive disorder. J. Clin. Psychiatry 2012, 73, 843–848. [Google Scholar] [CrossRef]
- Mischoulon, D.; Price, L.H.; Carpenter, L.L.; Tyrka, A.R.; Papakostas, G.I.; Baer, L.; Dording, C.M.; Clain, A.J.; Durham, K.; Walker, R.; et al. A double-blind, randomized, placebo-controlled clinical trial of S-adenosyl-L-methionine (SAMe) versus escitalopram in major depressive disorder. J. Clin. Psychiatry 2014, 75, 370–376. [Google Scholar] [CrossRef]
- Murphy, B.L.; Babb, S.M.; Ravichandran, C.; Cohen, B.M. Oral SAMe in persistent treatment-refractory bipolar depression: A double-blind, randomized clinical trial. J. Clin. Psychopharmacol. 2014, 34, 413–416. [Google Scholar] [CrossRef] [PubMed]
- Olsufka, W.; Abraham, M.-A. Treatment-emergent hypomania possibly associated with over-the-counter supplements. Ment. Health Clin. 2017, 7, 160–163. [Google Scholar] [CrossRef]
- Papakostas, G.I.; Mischoulon, D.; Shyu, I.; Alpert, J.E.; Fava, M. S-adenosyl methionine (SAMe) augmentation of serotonin reuptake inhibitors for antidepressant nonresponders with major depressive disorder: A double-blind, randomized clinical trial. Am. J. Psychiatry 2010, 167, 942–948. [Google Scholar] [CrossRef]
- Peng, T.-R.; Cheng, H.-Y.; Wu, T.-W. S-Adenosylmethionine (SAMe) as an adjuvant therapy for patients with depression: An updated systematic review and meta-analysis. Gen. Hosp. Psychiatry 2024, 86, 118–126. [Google Scholar] [CrossRef]
- Saccarello, A.; Montarsolo, P.; Massardo, I.; Picciotto, R.; Pedemonte, A.; Castagnaro, R.; Brasesco, P.C.; Guida, V.; Picco, P.; Fioravanti, P.; et al. Oral Administration of S-Adenosylmethionine (SAMe) and Lactobacillus Plantarum HEAL9 Improves the Mild-To-Moderate Symptoms of Depression: A Randomized, Double-Blind, Placebo-Controlled Study. Prim. Care Companion CNS Disord. 2020, 22, 23164. [Google Scholar] [CrossRef]
- Sakurai, H.; Carpenter, L.L.; Tyrka, A.R.; Price, L.H.; Papakostas, G.I.; Dording, C.M.; Yeung, A.S.; Cusin, C.; Ludington, E.; Bernard-Negron, R.; et al. Dose increase of S-Adenosyl-Methionine and escitalopram in a randomized clinical trial for major depressive disorder. J. Affect. Disord. 2020, 262, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Sarris, J.; Byrne, G.J.; Bousman, C.; Stough, C.; Murphy, J.; MacDonald, P.; Adams, L.; Nazareth, S.; Oliver, G.; Cribb, L.; et al. Adjunctive S-adenosylmethionine (SAMe) in treating non-remittent major depressive disorder: An 8-week double-blind, randomized, controlled trial. Eur. Neuropsychopharmacol. 2018, 28, 1126–1136. [Google Scholar] [CrossRef] [PubMed]
- Sarris, J.; Byrne, G.J.; Stough, C.; Bousman, C.; Mischoulon, D.; Murphy, J.; Macdonald, P.; Adams, L.; Nazareth, S.; Oliver, G.; et al. Nutraceuticals for major depressive disorder- more is not merrier: An 8-week double-blind, randomised, controlled trial. J. Affect. Disord. 2019, 245, 1007–1015. [Google Scholar] [CrossRef] [PubMed]
- Sarris, J.; Murphy, J.; Stough, C.; Mischoulon, D.; Bousman, C.; MacDonald, P.; Adams, L.; Nazareth, S.; Oliver, G.; Cribb, L.; et al. S-Adenosylmethionine (SAMe) monotherapy for depression: An 8-week double-blind, randomised, controlled trial. Psychopharmacology 2020, 237, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Sarris, J.; Papakostas, G.I.; Vitolo, O.; Fava, M.; Mischoulon, D. S-adenosyl methionine (SAMe) versus escitalopram and placebo in major depression RCT: Efficacy and effects of histamine and carnitine as moderators of response. J. Affect. Disord. 2014, 164, 76–81. [Google Scholar] [CrossRef]
- Sarris, J.; Price, L.H.; Carpenter, L.L.; Tyrka, A.R.; Ng, C.H.; Papakostas, G.I.; Jaeger, A.; Fava, M.; Mischoulon, D. Is S-Adenosyl Methionine (SAMe) for Depression Only Effective in Males? A Re-Analysis of Data from a Randomized Clinical Trial. Pharmacopsychiatry 2015, 48, 141–144. [Google Scholar] [CrossRef]
- Shippy, R.A.; Mendez, D.; Jones, K.; Cergnul, I.; Karpiak, S.E. S-adenosylmethionine (SAM-e) for the treatment of depression in people living with HIV/AIDS. BMC Psychiatry 2004, 4, 38. [Google Scholar] [CrossRef]
- Strous, R.D.; Ritsner, M.S.; Adler, S.; Ratner, Y.; Maayan, R.; Kotler, M.; Lachman, H.; Weizman, A. Improvement of aggressive behavior and quality of life impairment following S-adenosyl-methionine (SAM-e) augmentation in schizophrenia. Eur. Neuropsychopharmacol. 2009, 19, 14–22. [Google Scholar] [CrossRef]
- Targum, S.D.; Cameron, B.R.; Ferreira, L.; MacDonald, I.D. An augmentation study of MSI-195 (S-adenosylmethionine) in Major Depressive Disorder. J. Psychiatr. Res. 2018, 107, 86–96. [Google Scholar] [CrossRef]
- Targum, S.D.; Cameron, B.R.; Ferreira, L.; MacDonald, I.D. Early score fluctuation and placebo response in a study of major depressive disorder. J. Psychiatr. Res. 2020, 121, 118–125. [Google Scholar] [CrossRef]
- Ullah, H.; Di Minno, A.; Esposito, C.; El-Seedi, H.R.; Khalifa, S.A.M.; Baldi, A.; Greco, A.; Santonastaso, S.; Cioffi, V.; Sperandeo, R.; et al. Efficacy of a food supplement based on S-adenosyl methionine and probiotic strains in subjects with subthreshold depression and mild-to-moderate depression: A monocentric, randomized, cross-over, double-blind, placebo-controlled clinical trial. Biomed. Pharmacother. 2022, 156, 113930. [Google Scholar] [CrossRef]
- Kishi, T.; Ikuta, T.; Sakuma, K.; Okuya, M.; Hatano, M.; Matsuda, Y.; Iwata, N. Antidepressants for the treatment of adults with major depressive disorder in the maintenance phase: A systematic review and network meta-analysis. Mol. Psychiatry 2023, 28, 402–409. [Google Scholar] [CrossRef]
- Rao, Y.; Yang, R.; Zhao, J.; Cao, Q. Efficacy and tolerability of antidepressant drugs in treatment of depression in children and adolescents: A network meta-analysis. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2022, 51, 480–490. [Google Scholar] [CrossRef]
- Michalak, J.; Niemeyer, H.; Tschacher, W.; Baumann, N.; Chi Zhang, X.; Adolph, D. Subjective and Objective Measures of Activity in Depressed and Non-depressed Individuals in Everyday Life. J. Exp. Psychopathol. 2022, 13, 20438087221092582. [Google Scholar] [CrossRef]
- Serra-Blasco, M.; Torres, I.J.; Vicent-Gil, M.; Goldberg, X.; Navarra-Ventura, G.; Aguilar, E.; Via, E.; Portella, M.J.; Figuereo, I.; Palao, D.; et al. Discrepancy between objective and subjective cognition in major depressive disorder. Eur. Neuropsychopharmacol. 2019, 29, 46–56. [Google Scholar] [CrossRef]
- Módis, K.; Coletta, C.; Asimakopoulou, A.; Szczesny, B.; Chao, C.; Papapetropoulos, A.; Hellmich, M.R.; Szabo, C. Effect of S-adenosyl-L-methionine (SAM), an allosteric activator of cystathionine-β-synthase (CBS) on colorectal cancer cell proliferation and bioenergetics in vitro. Nitric Oxide 2014, 41, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Scuto, M.; Rampulla, F.; Reali, G.M.; Spanò, S.M.; Trovato Salinaro, A.; Calabrese, V. Hormetic Nutrition and Redox Regulation in Gut-Brain Axis Disorders. Antioxidants 2024, 13, 484. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Hong, S.W.; Kim, M.O.; Kim, H.S.; Jang, J.E.; Leem, J.; Park, I.S.; Lee, K.U.; Koh, E.H. S-adenosyl methionine prevents endothelial dysfunction by inducing heme oxygenase-1 in vascular endothelial cells. Mol. Cells 2013, 36, 376–384. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, R.; Deng, Q.; Wang, W.; Cao, C.; Yu, C.; Li, S.; Shi, L.; Tian, J. S-adenosylmethionine improves cognitive impairment in D-galactose-induced brain aging by inhibiting oxidative stress and neuroinflammation. J. Chem. Neuroanat. 2023, 128, 102232. [Google Scholar] [CrossRef]
- Cordaro, M.; Trovato Salinaro, A.; Siracusa, R.; D’Amico, R.; Impellizzeri, D.; Scuto, M.; Ontario, M.L.; Crea, R.; Cuzzocrea, S.; Di Paola, R.; et al. Hidrox(®) Roles in Neuroprotection: Biochemical Links between Traumatic Brain Injury and Alzheimer’s Disease. Antioxidants 2021, 10, 818. [Google Scholar] [CrossRef]
| Condition | Efficacy Summary | Safety Summary | Dosing Ranges |
|---|---|---|---|
| CNS-Related Signs |
|
|
|
| First Author (Year) Study Design|Location N of Patients|Study Length | Intervention (with Dose) and Comparator | Disease (Sign) | Measurement of Mood/Depression |
|---|---|---|---|
| Abeysundera (2018) [13] Case report|Australia n = 1|2 weeks prior to incident | No dose given | Depression | Differential diagnosis and lab levels |
| Alpert (2004) [14] Open trial|United States n = 30|6 weeks | SSRI/Venlafaxine + SAMe: Initial: 400 mg twice daily At 2 weeks: 800 mg twice daily Comparator: None | Resistant Major Depressive Disorder | HAM-D–17, MADRS, CGI-I, CGI-S, SQ |
| Anderson (2016) [15] Case report|Canada n = 1|~1 month | SAMe: 400 mg twice daily Comparator: None | Anxiety (and hypothyroidism) | Not discussed |
| Arnold (2005) [16] Pharmacodynamic|Europe n = 12|15 days each medication + washout periods | SAMe: 1600 mg/day SAMe: 400 mg/day Comparator: placebo | Mood | EEG mapping and psychometry |
| Bambling (2015) [17] RCT|Australia n = 36|15 weeks | SAMe: 1600 mg/day SAMe: 800 mg/day | Major Depressive Disorder | BDI, ICD-DSM MINI, DASS, SCID, OQ45, WBS, QOLS |
| Carpenter (2011) [18] SR/MA|United States n = 14 studies on SAMe|N/A | SAMe: 500–1050 mg/day | Major Depressive Disorder | Varied |
| Chitiva (2012) [19] Case report|United States n = 1|4 days prior to event | No dose stated | Depression/suicide attempt | Not applicable |
| Cuomo (2020) [7] SR/MA|N/A n = 8 articles (1011 patients)|N/A | SAMe: 200–3200 mg/day | Major Depressive Disorder | Varied |
| De Berardis (2013) [20] Non-randomized experimental|Europe n = 25|8 weeks | Existing medication + SAMe: 800 mg/day | Major Depressive Disorder | HAM-D, CGI-I, SHAPS, SDS |
| Di Pierro (2015) [21] Open-label, randomized, observational|Europe n = 64 (60 completed)|12 months | Betaine 250 mg/day + SAMe: 500 mg/day Comparator: Amitriptyline 75 mg/day | Mild Depression | Zung Self-Rating Depression Scale |
| Djokic (2017) [22] RCT|Europe n = 60|3 months | Vit B complex + SAMe: 200 mg/day Comparator: placebo | Depression (mild to moderate) | HAM-D, CGI-S, CGI-I |
| Dolcetta (2013) [23] Non-randomized experimental|Europe n = 14|12 months | SAMe: 400–1600 mg/day; up to 80 mg/kg, depending on body weight and renal function | Mood Lesch–Nyhan Disease | N/A |
| Galizia (2016) [24] SR/MA|UK n = 8 studies (934 patients)|N/A | SAMe: 200–3200 mg/day | Depression | Varies |
| Green (2012) [25] RCT|Israel n = 12|6 weeks | SAMe: 400 mg/day titrated up to 1600 mg/day (800 mg twice daily) Comparator: placebo | 22q11.2 deletion syndrome: depressive disorder, ADHD, cognitive deficits | Wechsler test: IQ, PANSS, YMRS, CGI-I, CDRS-R, ADHD-RS |
| Jaggumantri (2015) [26] Case report|Canada n = 2|Not described fully | SAMe: 50 mg/kg, with a safe and tolerable dose identified as 17 mg/kg/day | Creatine transporter (SLC6A8) deficiency | MRI Various assessments and questionnaires |
| Kalman (2015) [27] RCT|United States n = 34 in efficacy analysis (out of 42 enrolled)|8 weeks | SAMe: 400 mg/day Comparator: UP165 250 mg/day | Mild depression or anxiety | BDI-II, BAI, SOS-10 |
| Levkovitz (2012) [28] RCT Re-Analysis|United States n = 55|6 weeks | SAMe Weeks 1–2: 800 mg/day Weeks 3–6: 1600 mg/day | Major Depressive Disorder | CPFQ |
| Limveeraprajak (2024) [5] SR/MA|N/A 23 trials (n = 2183)|N/A | SAMe: 200–1600 mg/day | Depressive symptoms | Varied |
| Mischoulon (2012) [29] RCT|United States n = 35|6 weeks | SAMe: 800–1600 mg/day Comparator: placebo | Major Depressive Disorder | HAM-D Plasma SAMe levels |
| Mischoulon (2014) [30] RCT|United States n = 189|12 weeks | SAMe: 1600–3200 mg/day Escitalopram: 10–20 mg/day Comparator: placebo | Major Depressive Disorder | HAM-D |
| Murphy (2014) [31] RCT|United States n = 20 (17 completed)|6 weeks | SAMe: Week 1: 800 mg/day Week 2: 400 mg/day Week 3: 800 mg/day Week 4: 1600 mg (only 3/7 days of week) | Persistent Treatment-Refractory Bipolar Depression | HAM-D, MADRS, YMRS |
| Olsufka (2017) [32] Case Report|United States n = 1|~1 week | SAMe: 400 mg/day for 3 days then increased to 800 mg/day (up to day 10) | Depression | Not applicable |
| Papakostas (2010) [33] RCT|United States n = 73 (55 completed)|6 weeks | Antidepressant + SAMe: 800 mg/day (up to 1600 mg/day) Comparator: antidepressant + placebo | Major Depressive Disorder | HAM-D, CGI-S |
| Peng (2024) [34] SR/MA|Taiwan n = 14 studies (1522 patients)|N/A | SAMe: 200–3200 mg/day | Depression | Varies |
| Saccarello (2020) [35] RCT|Europe n = 89|6 weeks | Lactobacillus plantarum + SAMe: 200 mg/day Comparator: placebo | Mild-to-moderate depression | Zung Self-Rating Depression Scale |
| Sakurai (2020) [36] RCT|United States n = 189|6 weeks | SAMe: 1600 mg/day for 6 weeks (non-responders: 3200 mg/day for 6 weeks) Escitalopram: 10 mg/day Comparator: placebo | Major Depressive Disorder | HAM-D, IDS-SR, CGI-S, CGI-I |
| Sarris (2014) [40] RCT|Australia n = 144|12 weeks | SAMe: 1600–3200 mg/day Escitalopram: 10 mg/day Comparator: placebo | Major Depressive Disorder | HAM-D |
| Sarris (2015) [41] RCT Re-Analysis|United States n = 189|12 weeks | SAMe: 1600–3200 mg/day Escitalopram: 10–20 mg/day Comparator: placebo | Major Depressive Disorder | HAM-D |
| Sarris (2018) [37] RCT|Australia n = 107 (77 completed)|8 weeks | SAMe: 800 mg/day Comparator: placebo | Non-remittent Major Depressive Disorder | MADRS |
| Sarris (2019) [38] RCT|Australia n = 158 | 8 weeks | SAMe 800 mg + folinic acid + Omega-3 fatty acids + 5-HTP + Zinc picolinate + relevant co-factors/day Comparator: placebo | Major Depressive Disorder | MADRS |
| Sarris (2020) [39] RCT|Australia n = 49 (41 completed)|8 weeks | SAMe: 800 mg/day Comparator: placebo | Major Depressive Disorder with mild-to-moderate symptoms | MADRS |
| Shippy (2004) [42] Non-randomized experimental study|United States n = 20 (15 completed)|8 weeks | 1000 μg Vit B12 + 800 mg Folic Acid + SAMe: 400 mg/day (200 mg bid) increased to 1600 mg/day (800 mg bid) Comparator: None | Major Depressive Disorder | HAM-D (Response: ≥50% reduction in scores; Remission: HAM-D ≤ 7) |
| Strous (2009) [43] RCT|Israel n = 18 (15 completed)|8 weeks | SAMe: Week 1: 400 mg/day Weeks 2–8: 800 mg/day Comparator: placebo | Schizophrenia | PANSS, SANS, CGI, OAS, LHA, QLS |
| Targum (2018) [44] RCT|United States n = 234|8 weeks | SAMe:800 mg/day Comparator: placebo | Major Depressive Disorder | HAM-D, MADRS, IDS-SR |
| Targum (2020) [45] RCT (re-analysis)|United States n = 336|8 weeks | SAMe:800 mg/day Comparator: placebo | Major Depressive Disorder | HAM-D, MADRS, IDS-SR, CGI-S |
| Ullah (2022) [46] RCT (Crossover)|Europe n = 80 (65 completed)|3 months each | Crossover between: 200 mg/day SAMe + lactobacillus and placebo | Subthreshold depression Mild-to-moderate depression | HAM-D, PHQ-9 |
| First Author (Year) | Efficacy |
|---|---|
| Alpert (2004) [14] | SAMe: Intent-to-treat analyses based on the HAM-D
|
| Anderson (2016) [15] |
|
| Arnold (2005) [16] |
|
| Bambling (2015) [17] | 1600 mg and 800 mg SAMe were effective:
OQ45 change: significant reduction in functional distress scores [df = 18; p < 0.001] QOL change: significant increase in scores [df = 18; p < 0.001] |
| Carpenter (2011) [18] | Positive Results in Mild-to-Moderate (n = 9 studies):
|
| Cuomo 2020 [7] |
|
| De Berardis (2013) [20] |
|
| Di Pierro (2015) [21] | No improvement for either group at 3 months Effectiveness demonstrated at 6 and 12 months for both groups SAMe vs. amitriptyline:
|
| Djokic (2017) [22] | Significant differences between SAMe and placebo in HAM-D and CGI-S scores at 3 months (p < 0.001) In SAMe:
|
| Dolcetta (2013) [23] | 4 patients tolerated the full dose and demonstrated efficacy:
|
| Galizia (2016) [24] |
|
| Green (2012) [25] |
|
| Jaggumantri (2015) [26] | Patient 1:
|
| Kalman (2015) [27] | SAMe significantly:
|
| Levkovitz (2012) [28] | SAMe:
|
| Limveeraprajak (2024) [5] | SAMe superior to placebo (SMD = −0.58, 95% CI = −0.93 to −0.23, I2 = 68%), even when two trials with a high risk of bias were excluded (SMD = −0.61, 91% CI = −1.05 to −0.17, I2 = 74%)
|
| Mischoulon (2012) [29] | SAMe:
|
| Mischoulon (2014) [30] | SAMe vs. escitalopram vs. placebo:
|
| Murphy (2014) [31] | SAMe vs. placebo:
|
| Papakostas (2010) [33] | SAMe + antidepressant vs. placebo + antidepressant:
|
| Peng (2024) [34] | SAMe vs. placebo:
|
| Saccarello (2020) [35] | SAMe + Lactobacillus plantarum vs. placebo:
|
| Sakurai (2020) [36] | SAMe vs. escitalopram vs. placebo:
|
| Sarris (2014) [40] | SAMe vs. escitalopram vs. placebo:
Remission rates (HAM-D < 7): Significantly different between groups (χ22,102 = 8.57; p = 0.014)
|
| Sarris (2015) [41] | SAMe vs. escitalopram vs. placebo in HAM-D:
|
| Sarris (2018) [37] | SAMe + antidepressant vs. placebo + antidepressant:
|
| Sarris (2019) [38] | Nutraceutical product vs. placebo:
|
| Sarris (2020) [39] | SAMe:
|
| Shippy (2004) [42] |
|
| Strous (2009) [43] | SAMe vs. placebo: Significant improvements in SAMe patients only:
|
| Targum (2018) [44] | SAMe + antidepressant or placebo + antidepressant: No statistically significant treatment differences Note: study did not achieve primary endpoint due to subject selection differences First half of the study participants: favored SAMe
|
| Targum (2020) [45] | MADRS and HAM-D: SAMe was significantly better than placebo (F = 6.39; df = 1; p = 0.012), effect size = 0.404 |
| Ullah (2022) [46] |
|
| First Author (Year) | Safety |
|---|---|
| Abeysundera (2018) [13] | Conclusion was that the patient experienced substance-/medication-induced mood disorder (when adding SAMe to the SSRI) |
| Alpert (2004) [14] | GI and headache side effects were most common No significant changes in weight, folate, B12, or homocysteine levels |
| Arnold (2005) [16] | Good tolerability |
| Bambling (2015) [17] | 10 subjects dropped out |
| Carpenter (2011) [18] |
|
| Chitiva (2012) [19] | Patient attempted suicide after taking SAMe for 4 days |
| Cuomo 2020 [7] | Mild, transient, non-relevant side effects |
| De Berardis (2013) [20] | SAMe was well tolerated Most common adverse events:
|
| Di Pierro (2015) [21] | SAMe group had fewer side effects |
| Dolcetta (2013) [23] | Excess of excitement experienced at lower dosage, which led to discontinuations Increase in anxiety (n = 7) |
| Green (2012) [25] | No manic or psychotic symptoms No significant differences in side effects between groups Most common side effects were GI symptoms |
| Jaggumantri (2015) [26] | Patient 1:
|
| Kalman (2015) [27] | No significant adverse events |
| Limveeraprajak (2024) [5] | Generally well-tolerated |
| Mischoulon (2014) [30] | No significant differences in side effects between groups (p > 0.05) SAMe: GI, stomach discomfort, diarrhea |
| Murphy (2014) [31] | Discontinued after the 800 mg/day SAMe dosage due to brief episode of auditory hallucinations (n = 1) No other issues, including mania |
| Olsufka (2017) [32] | Treatment-emergent hypomania due to use of SAMe
|
| Papakostas (2010) [33] | No serious adverse events |
| Peng (2024) [34] | No significant difference between dropouts due to adverse effects (RR: 0.92, 95% CI: 0.49 to 1.73) |
| Saccarello (2020) [35] | Limited adverse events, which researchers believed were not related to products |
| Sakurai (2020) [36] | 3200 mg/day SAMe:
|
| Sarris (2014) [40] | Well-tolerated No significant adverse events |
| Sarris (2018) [37] | 5 SAMe group withdrawals possibly related to treatment: nausea, heightened anxiety, sleep issues |
| Sarris (2019) [38] |
|
| Sarris (2020) [39] | No significant differences in adverse events between groups (p = 0.53) |
| Shippy (2004) [42] | No dropouts due to side effects |
| Strous (2009) [43] | 3 patients were discontinued from the study due to potential adverse effects of the study medication No significant differences between SAMe and placebo for all adverse events (all p > 0.05) |
| Targum (2018) [44] | High completion rate with 113 SAMe-assigned subjects (95.8%) Predominant adverse events were mild and primarily related to GI tract (<2% of patients) |
| Targum (2020) [45] | SAMe well-tolerated Predominant adverse events were mild and primarily related to GI tract |
| First Author Year | Clear Research Questions | Data Address Question | Total MMAT Score (out of 5) |
|---|---|---|---|
| Abeysundera 2018 [13] | Yes | Can’t Tell a | 4 |
| Alpert 2004 [14] | Yes | Yes | 3 |
| Anderson 2016 [15] | No | No | 3 |
| Arnold 2005 [16] | Yes | Yes | 5 |
| Bambling 2015 [17] | Yes | Yes | 4 |
| Carpenter 2011 [18] | Yes | Yes | 4 |
| Chitiva 2012 [19] | Yes | Can’t Tell a | 4 |
| Cuomo 2020 [7] | Yes | Yes | 5 |
| De Berardis 2013 [20] | Yes | Yes | 5 |
| Di Pierro 2015 [21] | Yes | Yes | 4 |
| Djokic 2017 [22] | Yes | Yes | 5 |
| Dolcetta 2013 [23] | Yes | Yes | 5 |
| Galizia 2016 [24] | Yes | Yes | 5 |
| Green 2012 [25] | Yes | Yes | 4 |
| Jaggumantri 2015 [26] | Yes | Can’t Tell a | 4 |
| Kalman 2015 [27] | Yes | Yes | 5 |
| Levkovitz 2012 [28] | Yes | Yes | 5 |
| Limveeraprajak 2024 [5] | Yes | Yes | 5 |
| Mischoulon 2012 [29] | Yes | Yes | 3 |
| Mischoulon 2014 [30] | Yes | Yes | 5 |
| Murphy 2014 [31] | Yes | Yes | 5 |
| Olsufka 2017 [32] | Yes | Can’t Tell a | 4 |
| Papakostas 2010 [33] | Yes | Yes | 5 |
| Peng 2024 [34] | Yes | Yes | 5 |
| Saccarello 2020 [35] | Yes | Yes | 5 |
| Sakurai 2020 [36] | Yes | Yes | 5 |
| Sarris 2014 [40] | Yes | Yes | 5 |
| Sarris 2015 [41] | Yes | Yes | 5 |
| Sarris 2018 [37] | Yes | Yes | 5 |
| Sarris 2019 [38] | Yes | Yes | 5 |
| Sarris 2020 [36] | Yes | Yes | 5 |
| Shippy 2004 [42] | Yes | Yes | 5 |
| Strous 2009 [43] | Yes | Yes | 4 |
| Targum 2018 [44] | Yes | No b | 3 |
| Targum 2020 [45] | Yes | Yes | 5 |
| Ullah 2022 [46] | Yes | Yes | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baden, K.E.R.; McClain, H.; Craig, E.; Gibson, N.; Draime, J.A.; Chen, A.M.H. S-Adenosylmethionine (SAMe) for Central Nervous System Health: A Systematic Review. Nutrients 2024, 16, 3148. https://doi.org/10.3390/nu16183148
Baden KER, McClain H, Craig E, Gibson N, Draime JA, Chen AMH. S-Adenosylmethionine (SAMe) for Central Nervous System Health: A Systematic Review. Nutrients. 2024; 16(18):3148. https://doi.org/10.3390/nu16183148
Chicago/Turabian StyleBaden, Kyrie Eleyson R., Halley McClain, Eliya Craig, Nathan Gibson, Juanita A. Draime, and Aleda M. H. Chen. 2024. "S-Adenosylmethionine (SAMe) for Central Nervous System Health: A Systematic Review" Nutrients 16, no. 18: 3148. https://doi.org/10.3390/nu16183148
APA StyleBaden, K. E. R., McClain, H., Craig, E., Gibson, N., Draime, J. A., & Chen, A. M. H. (2024). S-Adenosylmethionine (SAMe) for Central Nervous System Health: A Systematic Review. Nutrients, 16(18), 3148. https://doi.org/10.3390/nu16183148







