Effect of Purified Resveratrol Butyrate Ester Monomers against Hypertension after Maternal High-Fructose Intake in Adult Offspring
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Resveratrol Butyrate Esters
2.2. Animal Model
2.3. NO Pathway
2.4. SCFAs and Receptors
2.5. TMA, TMAO, and DMA
2.6. Microbiome Analysis
2.7. Statistics
3. Results
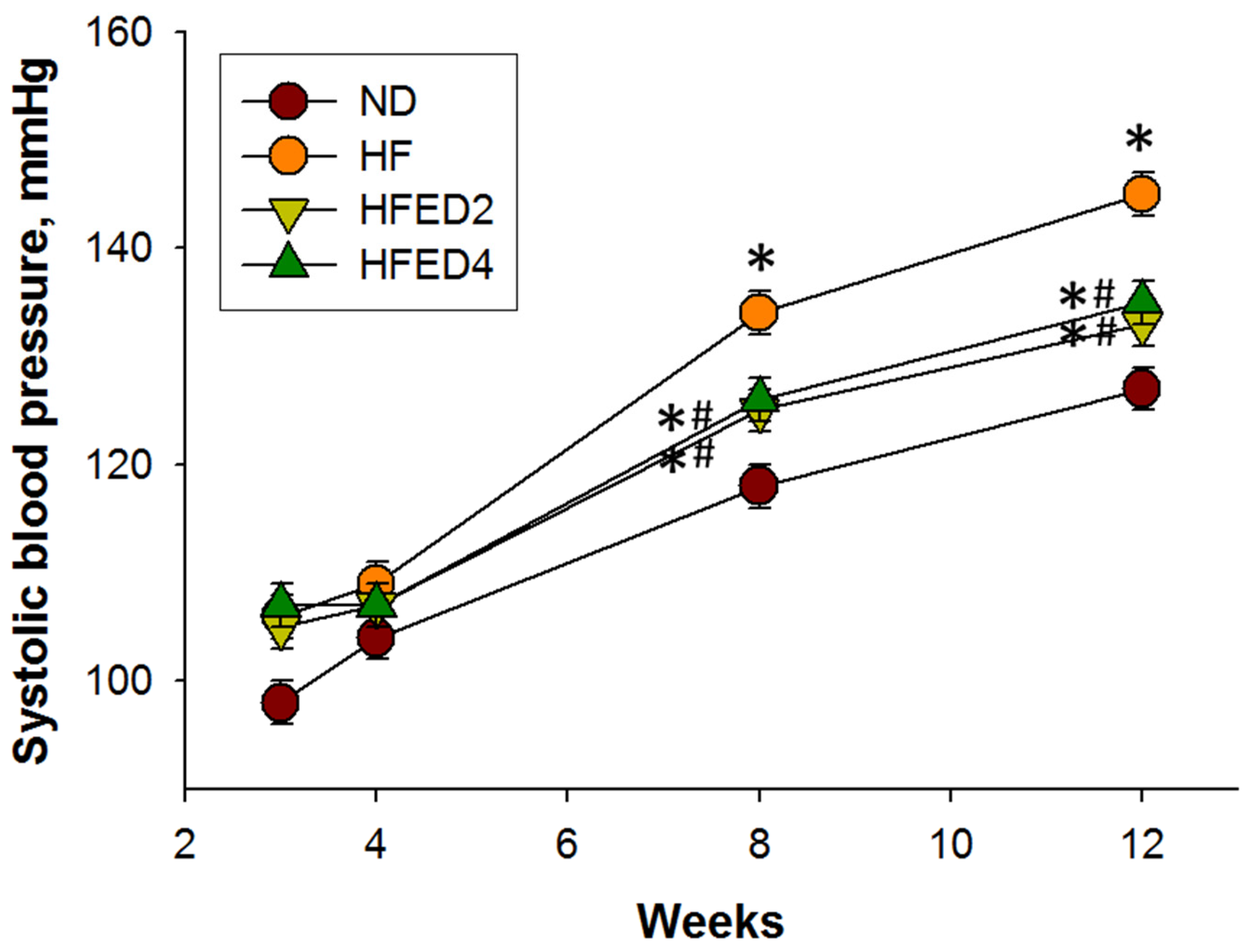
3.1. Body Weight, BP, and Creatinine Level
3.2. NO Pathway
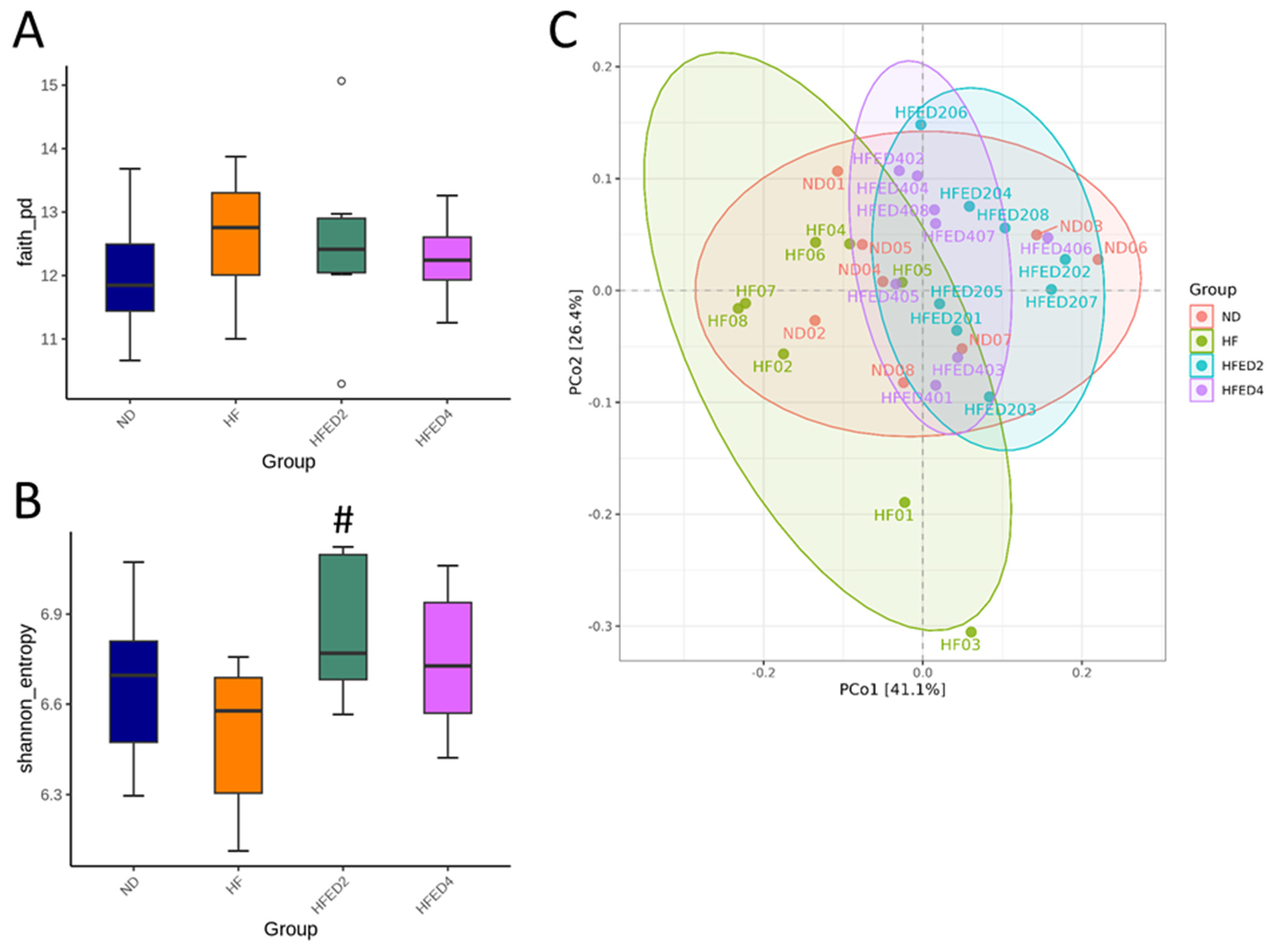
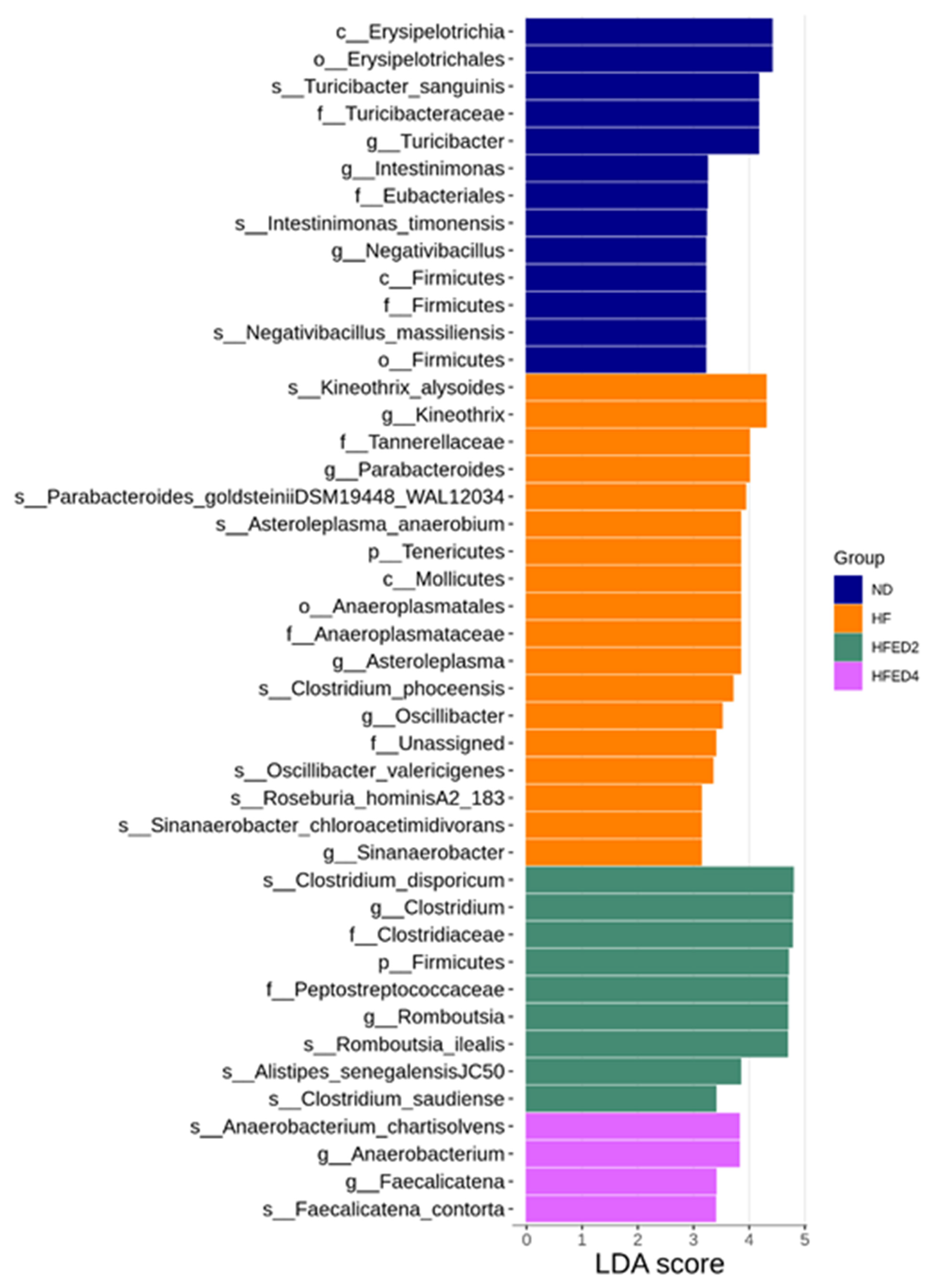
3.3. Gut Microbiota Compositions
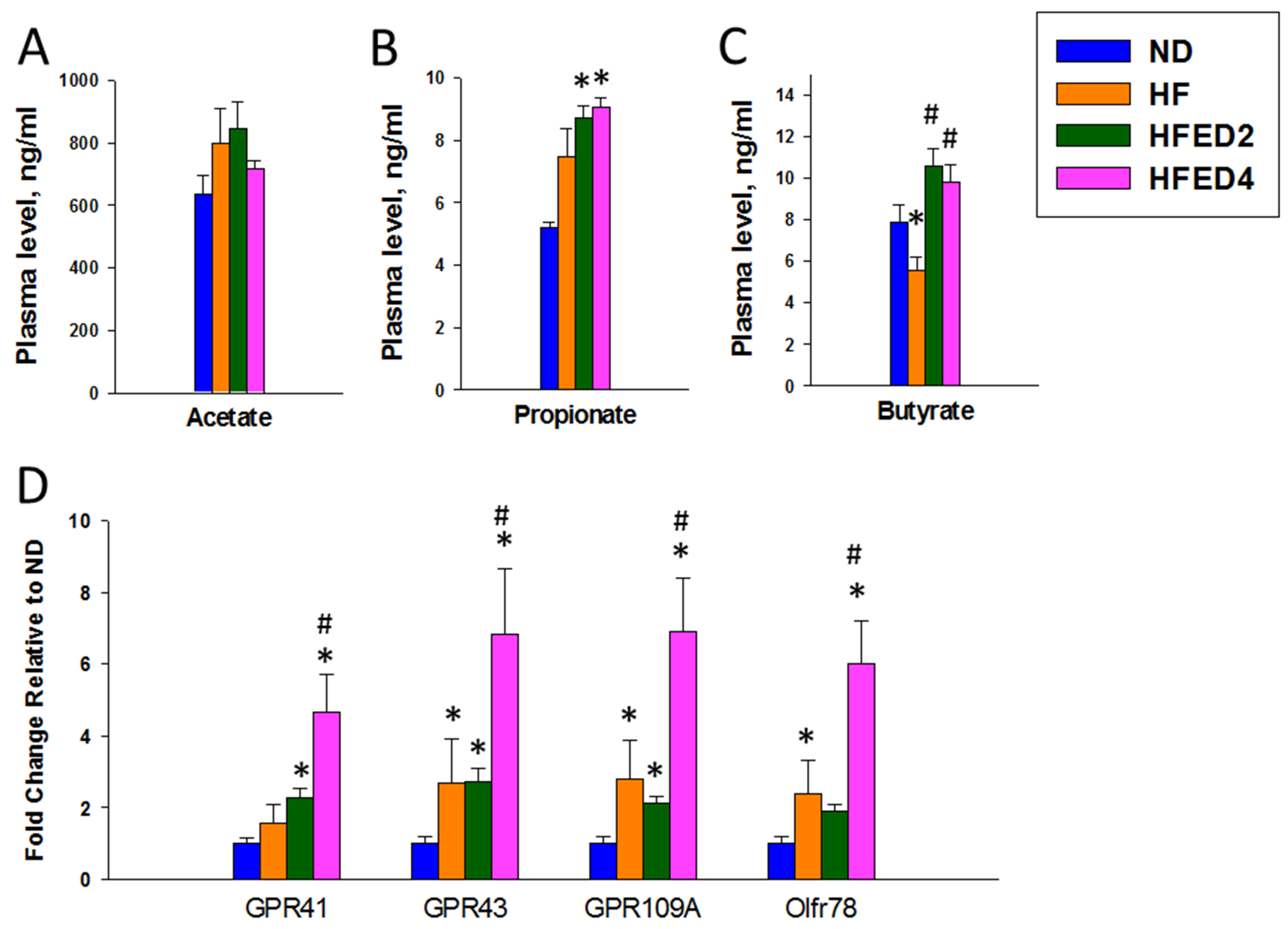
3.4. SCFAs and Their Receptors
3.5. TMAO Metabolic Pathway
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marriott, B.P.; Cole, N.; Lee, E. National estimates of dietary fructose intake increased from 1977 to 2004 in the United States. J. Nutr. 2009, 139, 1228S–1235S. [Google Scholar] [CrossRef] [PubMed]
- Bray, G.A.; Nielsen, S.J.; Popkin, B.M. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am. J. Clin. Nutr. 2004, 79, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Stanhope, K.L. Role of fructose-containing sugars in the epidemics of obesity and metabolic syndrome. Annu. Rev. Med. 2012, 63, 329–343. [Google Scholar] [CrossRef] [PubMed]
- Malik, V.S.; Hu, F.B. The role of sugar-sweetened beverages in the global epidemics of obesity and chronic diseases. Nat. Rev. Endocrinol. 2022, 18, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Cioffi, C.E.; Figueroa, J.; Welsh, J.A. Added Sugar Intake among Pregnant Women in the United States: National Health and Nutrition Examination Survey 2003–2012. J. Acad. Nutr. Diet 2018, 118, 886–895. [Google Scholar] [CrossRef]
- Regnault, T.R.; Gentili, S.; Sarr, O.; Toop, C.R.; Sloboda, D.M. Fructose, pregnancy and later life impacts. Clin. Exp. Pharmacol. Physiol. 2013, 40, 824–837. [Google Scholar] [CrossRef]
- Bromfield, S.; Muntner, P. High blood pressure: The leading global burden of disease risk factor and the need for worldwide prevention programs. Curr. Hypertens. Rep. 2013, 15, 134–136. [Google Scholar] [CrossRef]
- Luyckx, V.A.; Bertram, J.F.; Brenner, B.M.; Fall, C.; Hoy, W.E.; Ozanne, S.E.; Vikse, B.E. Effect of fetal and child health on kidney development and long-term risk of hypertension and kidney disease. Lancet 2013, 382, 273–283. [Google Scholar] [CrossRef]
- Seong, H.Y.; Cho, H.M.; Kim, M.; Kim, I. Maternal High-Fructose Intake Induces Multigenerational Activation of the Renin-Angiotensin-Aldosterone System. Hypertension 2019, 74, 518–525. [Google Scholar] [CrossRef]
- Tain, Y.L.; Chan, J.Y.; Hsu, C.N. Maternal Fructose Intake Affects Transcriptome Changes and Programmed Hypertension in Offspring in Later Life. Nutrients 2016, 8, 757. [Google Scholar] [CrossRef]
- Jia, G.; Hill, M.A.; Sowers, J.R. Maternal Exposure to High Fructose and Offspring Health. Hypertension 2019, 74, 499–501. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Leu, S.; Wu, K.L.; Lee, W.C.; Chan, J.Y. Melatonin prevents maternal fructose intake-induced programmed hypertension in the offspring: Roles of nitric oxide and arachidonic acid metabolites. J. Pineal Res. 2014, 57, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Lee, W.C.; Wu, K.L.H.; Leu, S.; Chan, J.Y.H. Resveratrol Prevents the Development of Hypertension Programmed by Maternal Plus Post-Weaning High-Fructose Consumption through Modulation of Oxidative Stress, Nutrient-Sensing Signals, and Gut Microbiota. Mol. Nutr. Food Res. 2018, 62, e1800066. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Yu, J. Pathophysiological mechanisms of hypertension development induced by fructose consumption. Food Funct. 2022, 13, 1702–1717. [Google Scholar] [CrossRef]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomás-Barberán, F.A. The effects of polyphenols and other bioactives on human health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef]
- Pollack, R.M.; Crandall, J.P. Resveratrol: Therapeutic potential for improving cardiometabolic health. Am. J. Hypertens. 2013, 26, 1260–1268. [Google Scholar] [CrossRef][Green Version]
- Cottart, C.H.; Nivet-Antoine, V.; Laguillier-Morizot, C.; Beaudeux, J.L. Resveratrol bioavailability and toxicity in humans. Mol. Nutr. Food Res. 2010, 54, 7–16. [Google Scholar] [CrossRef]
- Rekha, K.; Venkidasamy, B.; Samynathan, R.; Nagella, P.; Rebezov, M.; Khayrullin, M.; Ponomarev, E.; Bouyahya, A.; Sarkar, T.; Shariati, M.A.; et al. Short-chain fatty acid: An updated review on signaling, metabolism, and therapeutic effects. Crit. Rev. Food Sci. Nutr. 2022, 64, 2461–2489. [Google Scholar] [CrossRef]
- Shih, M.K.; Tain, Y.L.; Cheng, C.M.; Hsu, C.N.; Chen, Y.W.; Huang, H.T.; Chang, C.I.; Hou, C.Y. Separation and Identification of Resveratrol Butyrate Ester Complexes and Their Bioactivity in HepG2 Cell Models. Int. J. Mol. Sci. 2021, 22, 13539. [Google Scholar] [CrossRef]
- Tain, Y.L.; Chang, S.K.C.; Liao, J.X.; Chen, Y.W.; Huang, H.T.; Li, Y.L.; Hou, C.Y. Synthesis of Short-Chain-Fatty-Acid Resveratrol Esters and Their Antioxidant Properties. Antioxidants 2021, 10, 420. [Google Scholar] [CrossRef]
- Huang, P.H.; Chen, D.Q.; Chen, Y.W.; Shih, M.K.; Lee, B.H.; Tain, Y.L.; Hsieh, C.W.; Hou, C.Y. Evaluation of the Feasibility of In Vitro Metabolic Interruption of Trimethylamine with Resveratrol Butyrate Esters and Its Purified Monomers. Molecules 2024, 29, 429. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Chang, C.I.; Hou, C.Y.; Chang-Chien, G.P.; Lin, S.; Hsu, C.N. Dietary Resveratrol Butyrate Monoester Supplement Improves Hypertension and Kidney Dysfunction in a Young Rat Chronic Kidney Disease Model. Nutrients 2023, 15, 635. [Google Scholar] [CrossRef] [PubMed]
- Reckelhoff, J.F. Gender differences in the regulation of blood pressure. Hypertension 2001, 37, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Bode-Böger, S.M.; Scalera, F.; Ignarro, L.J. The L-arginine paradox: Importance of the L-arginine/asymmetrical dimethylarginine ratio. Pharmacol. Ther. 2007, 114, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Moore, B.N.; Pluznick, J.L. Short-Chain Fatty Acid Receptors and Blood Pressure Regulation: Council on Hypertension Mid-Career Award for Research Excellence 2021. Hypertension 2022, 79, 2127–2137. [Google Scholar] [CrossRef] [PubMed]
- Velasquez, M.T.; Ramezani, A.; Manal, A.; Raj, D.S. Trimethylamine N-Oxide: The good, the bad and the unknown. Toxins 2016, 8, 326. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Price, M.N.; Dehal, P.S.; Arkin, A.P. FastTree 2—Approximately maximum-likelihood trees for large alignments. PLoS ONE 2010, 5, e9490. [Google Scholar] [CrossRef]
- Wilcox, C.S. Oxidative stress and nitric oxide deficiency in the kidney: A critical link to hypertension? Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 289, R913–R935. [Google Scholar] [CrossRef]
- DiNatale, J.C.; Crowe-White, K.M. Effects of resveratrol supplementation on nitric oxide-mediated vascular outcomes in hypertension: A systematic review. Nitric Oxide 2022, 129, 74–81. [Google Scholar] [CrossRef]
- Zheng, S.; Feng, Q.; Cheng, J.; Zheng, J. Maternal resveratrol consumption and its programming effects on metabolic health in offspring mechanisms and potential implications. Biosci. Rep. 2018, 38, BSR20171741. [Google Scholar] [CrossRef]
- Lacerda, D.C.; Costa, P.C.T.; de Oliveira, Y.; de Brito Alves, J.L. The effect of resveratrol in cardio-metabolic disorders during pregnancy and offspring outcomes: A review. J. Dev. Orig. Health Dis. 2023, 14, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Oh, W.Y.; Shahidi, F. Antioxidant activity of resveratrol ester derivatives in food and biological model systems. Food Chem. 2018, 261, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Gostimirovic, M.; Rajkovic, J.; Bukarica, A.; Simanovic, J.; Gojkovic-Bukarica, L. Resveratrol and Gut Microbiota Synergy: Preventive and Therapeutic Effects. Int. J. Mol. Sci. 2023, 24, 17573. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Hou, C.Y.; Chang-Chien, G.P.; Lin, S.; Hsu, C.N. Resveratrol Butyrate Ester Supplementation Blunts the Development of Offspring Hypertension in a Maternal Di-2-ethylhexyl Phthalate Exposure Rat Model. Nutrients 2023, 15, 697. [Google Scholar] [CrossRef] [PubMed]
- Baylis, C. Nitric oxide synthase derangements and hypertension in kidney disease. Curr. Opin. Nephrol. Hypertens. 2012, 21, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Suez, J.; Zmora, N.; Segal, E.; Elinav, E. The pros, cons, and many unknowns of probiotics. Nat. Med. 2019, 25, 716–729. [Google Scholar] [CrossRef]
- Verhaar, B.J.H.; Collard, D.; Prodan, A.; Levels, J.H.M.; Zwinderman, A.H.; Bäckhed, F.; Vogt, L.; Peters, M.J.L.; Muller, M.; Nieuwdorp, M.; et al. Associations between gut microbiota, faecal short-chain fatty acids, and blood pressure across ethnic groups: The HELIUS study. Eur. Heart J. 2020, 41, 4259–4267. [Google Scholar] [CrossRef]
- Sun, D.; Xiang, H.; Yan, J.; He, L. Intestinal microbiota: A promising therapeutic target for hypertension. Front. Cardiovasc. Med. 2022, 9, 970036. [Google Scholar] [CrossRef]
- Cheng, Q.; Fan, C.; Liu, F.; Li, Y.; Hou, H.; Ma, Y.; Tan, Y.; Li, Y.; Hai, Y.; Wu, T.; et al. Structural and functional dysbiosis of gut microbiota in Tibetan subjects with coronary heart disease. Genomics 2022, 114, 110483. [Google Scholar] [CrossRef]
- Miyoshi, M.; Sakaki, H.; Usami, M.; Iizuka, N.; Shuno, K.; Aoyama, M.; Usami, Y. Oral administration of tributyrin increases concentration of butyrate in the portal vein and prevents lipopolysaccharide-induced liver injury in rats. Clin. Nutr. 2011, 30, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Pluznick, J.L. Microbial Short-Chain Fatty Acids and Blood Pressure Regulation. Curr. Hypertens. Rep. 2017, 19, 25. [Google Scholar] [CrossRef] [PubMed]
- Romano, K.A.; Vivas, E.I.; Amador-Noguez, D.; Rey, F.E. Intestinal microbiota composition modulates choline bioavailability from diet and accumulation of the proatherogenic metabolite trimethylamine-N-oxide. mBio 2015, 6, e02481. [Google Scholar] [CrossRef] [PubMed]
- Hoyles, L.; Jiménez-Pranteda, M.L.; Chilloux, J.; Brial, F.; Myridakis, A.; Aranias, T.; Magnan, C.; Gibson, G.R.; Sanderson, J.D.; Nicholson, J.K.; et al. Metabolic retroconversion of trimethylamine N-oxide and the gut microbiota. Microbiome 2018, 6, 73. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.N.; Tain, Y.L. Animal Models for DOHaD Research: Focus on Hypertension of Developmental Origins. Biomedicines 2021, 9, 623. [Google Scholar] [CrossRef] [PubMed]
- Semba, R.D.; Ferrucci, L.; Bartali, B.; Urpí-Sarda, M.; Zamora-Ros, R.; Sun, K.; Cherubini, A.; Bandinelli, S.; Andres-Lacueva, C. Resveratrol levels and all-cause mortality in older community-dwelling adults. JAMA Intern. Med. 2014, 174, 1077–1084. [Google Scholar] [CrossRef]


| Groups | ND | HF | HFED2 | HFED4 |
|---|---|---|---|---|
| Body weight (BW) (g) | 298 ± 37 | 273 ± 10 * | 264 ± 7 * | 263 ± 12 * |
| Left kidney weight (KW) (g) | 1.31 ± 0.14 | 1.2 ± 0.05 * | 1.17 ± 0.02 * | 1.22 ± 0.05 * |
| The ratio of KW to BW (g/kg) | 4.4 ± 0.2 | 4.4 ± 0.1 | 4.5 ± 0.1 | 4.7 ± 0.1 |
| Systolic blood pressure (mmHg) | 127 ± 1 | 145 ± 1 * | 133 ± 1 *# | 135 ± 1 *# |
| Diastolic blood pressure (mmHg) | 88 ± 1 | 96 ± 2 * | 87 ± 3 # | 96 ± 2 * |
| Creatinine (μM) | 14.5 ± 0.9 | 14.6 ± 1.4 | 12.9 ± 0.4 | 12.6 ± 0.6 |
| Groups | ND | HF | HFED2 | HFED4 |
|---|---|---|---|---|
| TMA, ng/mL | 3.06 ± 0.48 | 5.94 ± 1.86 | 5.06 ± 1.06 | 3.99 ± 1.13 |
| TMAO, ng/mL | 283.6 ± 19.7 | 266.9 ± 8.11 | 283.9 ± 19.6 | 219.8 ± 13.6 *# |
| DMA, ng/mL | 113.5 ± 9.1 | 135.8 ± 5.8 | 106.2 ± 8.4 # | 130.3 ± 11.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tain, Y.-L.; Hou, C.-Y.; Tzeng, H.-T.; Lin, S.-F.; Chang-Chien, G.-P.; Lee, W.-C.; Wu, K.L.H.; Yu, H.-R.; Chan, J.Y.H.; Hsu, C.-N. Effect of Purified Resveratrol Butyrate Ester Monomers against Hypertension after Maternal High-Fructose Intake in Adult Offspring. Nutrients 2024, 16, 3132. https://doi.org/10.3390/nu16183132
Tain Y-L, Hou C-Y, Tzeng H-T, Lin S-F, Chang-Chien G-P, Lee W-C, Wu KLH, Yu H-R, Chan JYH, Hsu C-N. Effect of Purified Resveratrol Butyrate Ester Monomers against Hypertension after Maternal High-Fructose Intake in Adult Offspring. Nutrients. 2024; 16(18):3132. https://doi.org/10.3390/nu16183132
Chicago/Turabian StyleTain, You-Lin, Chih-Yao Hou, Hong-Tai Tzeng, Shu-Fen Lin, Guo-Ping Chang-Chien, Wei-Chia Lee, Kay L. H. Wu, Hong-Ren Yu, Julie Y. H. Chan, and Chien-Ning Hsu. 2024. "Effect of Purified Resveratrol Butyrate Ester Monomers against Hypertension after Maternal High-Fructose Intake in Adult Offspring" Nutrients 16, no. 18: 3132. https://doi.org/10.3390/nu16183132
APA StyleTain, Y.-L., Hou, C.-Y., Tzeng, H.-T., Lin, S.-F., Chang-Chien, G.-P., Lee, W.-C., Wu, K. L. H., Yu, H.-R., Chan, J. Y. H., & Hsu, C.-N. (2024). Effect of Purified Resveratrol Butyrate Ester Monomers against Hypertension after Maternal High-Fructose Intake in Adult Offspring. Nutrients, 16(18), 3132. https://doi.org/10.3390/nu16183132










