Vitamin D Deficiency Does Not Affect Cognition and Neurogenesis in Adult C57Bl/6 Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Design and Protocol
2.1.1. Animals and Study Design
2.1.2. Diet
2.1.3. BrdU Administration
2.1.4. Tissue Preparation
2.1.5. Immunofluorescence
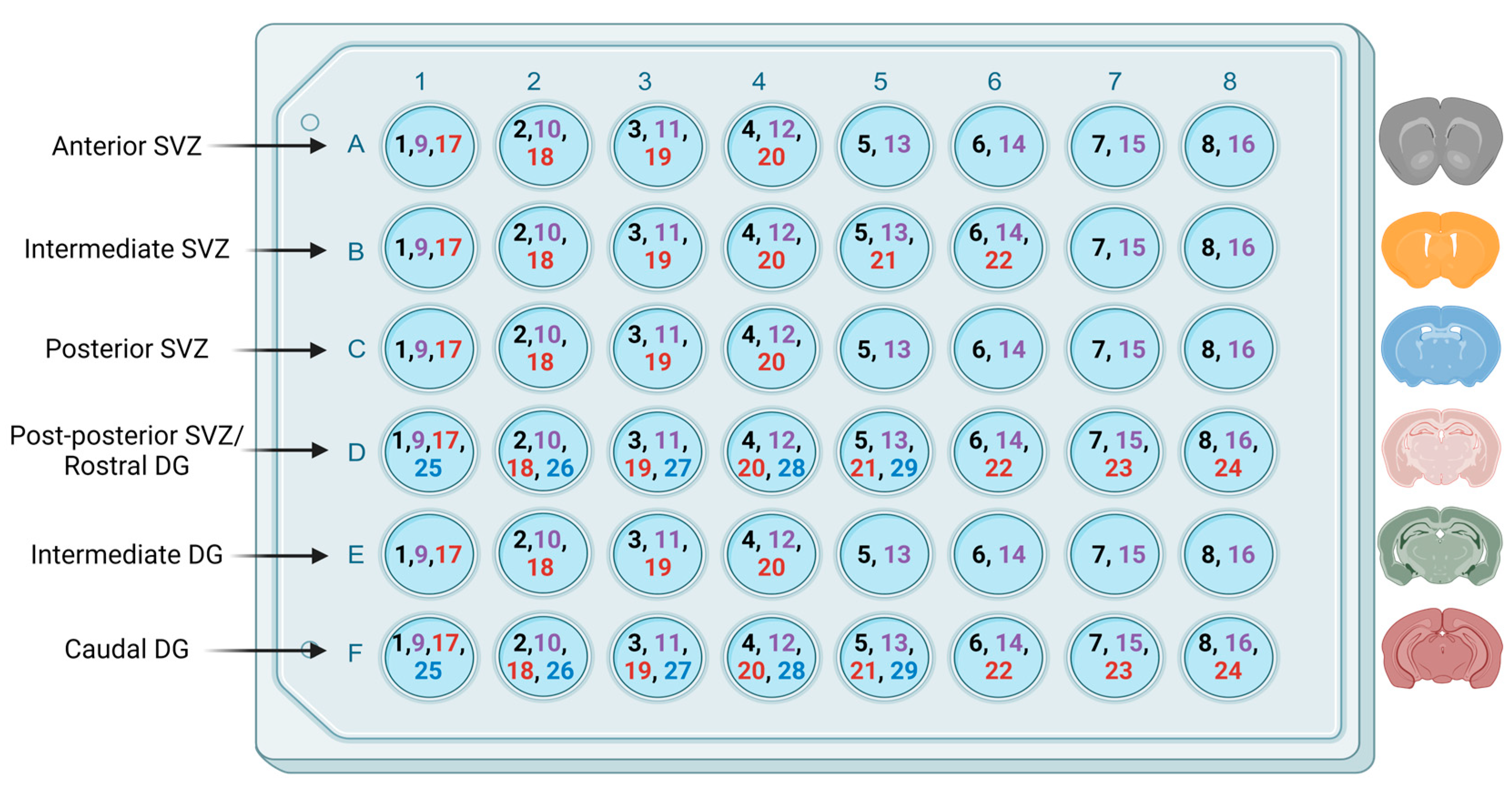
2.1.6. Imaging and Quantification
2.2. Behavioral Tests
2.2.1. Open Field Test
2.2.2. Temporal Order Recognition Test (TOR)
2.3. Statistical Analyses
3. Results
3.1. Vitamin D Deficiency Did Not Alter the Weight of Both Male and Female Mice
3.2. Vitamin D Deficiency and Supplementation Had No Effect on Motor, Cognitive, or Affective Functions in Male or Female Mice
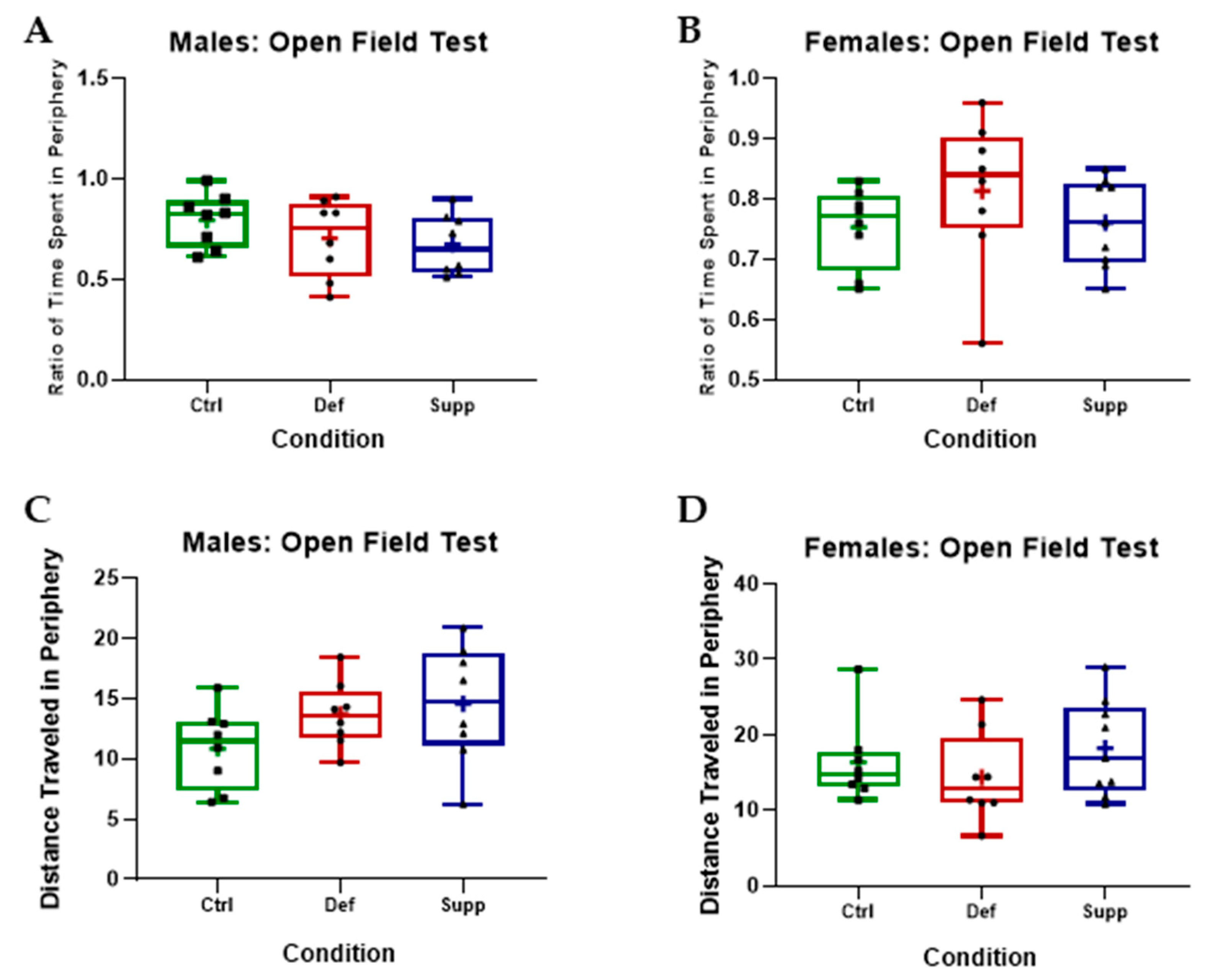
3.2.1. Vitamin D Did Not Affect Anxiety-like Behavior or Locomotion in Male or Female C57Bl/6 Mice
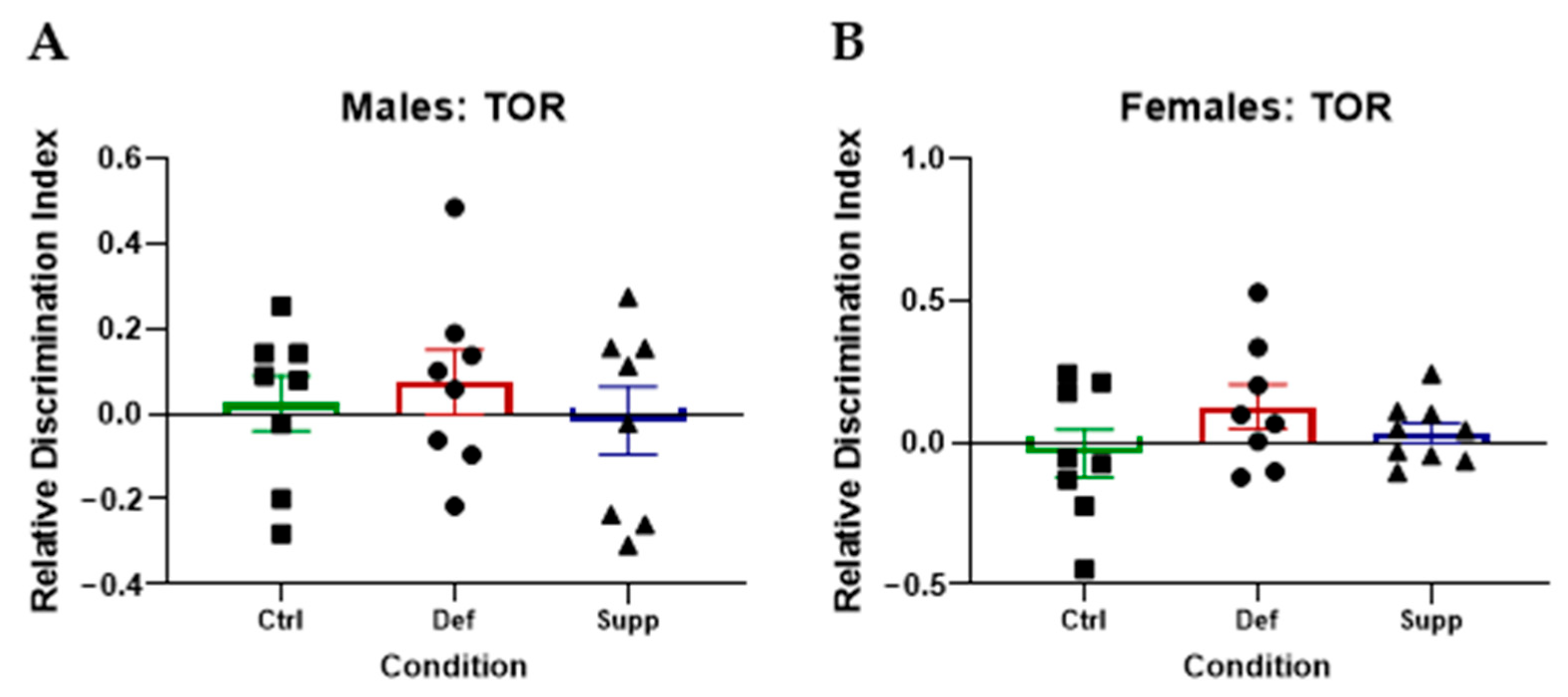
3.2.2. Vitamin D Did Not Affect Hippocampal-Dependent Memory on the TOR in Male or Female C57Bl/6 Mice
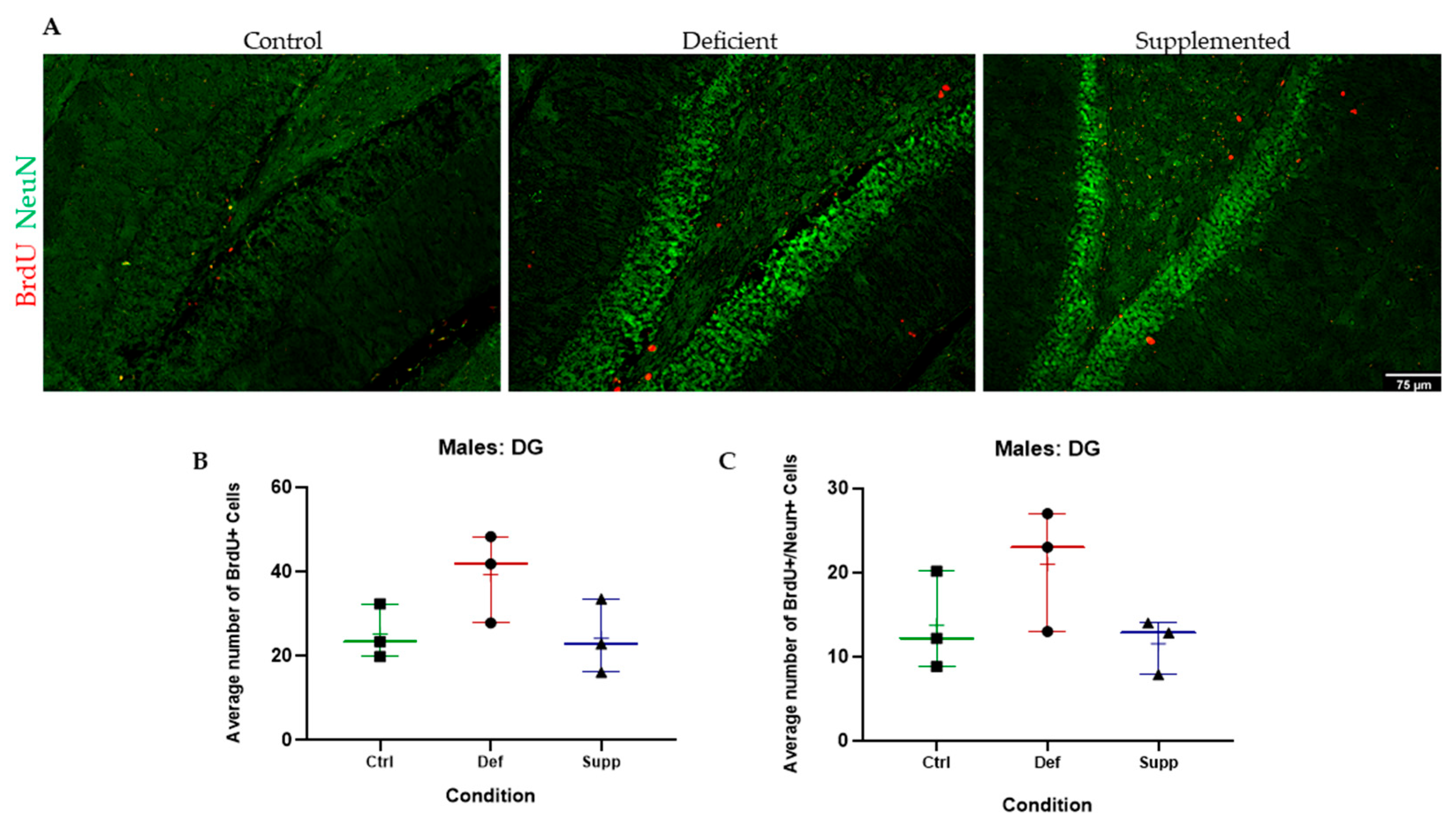
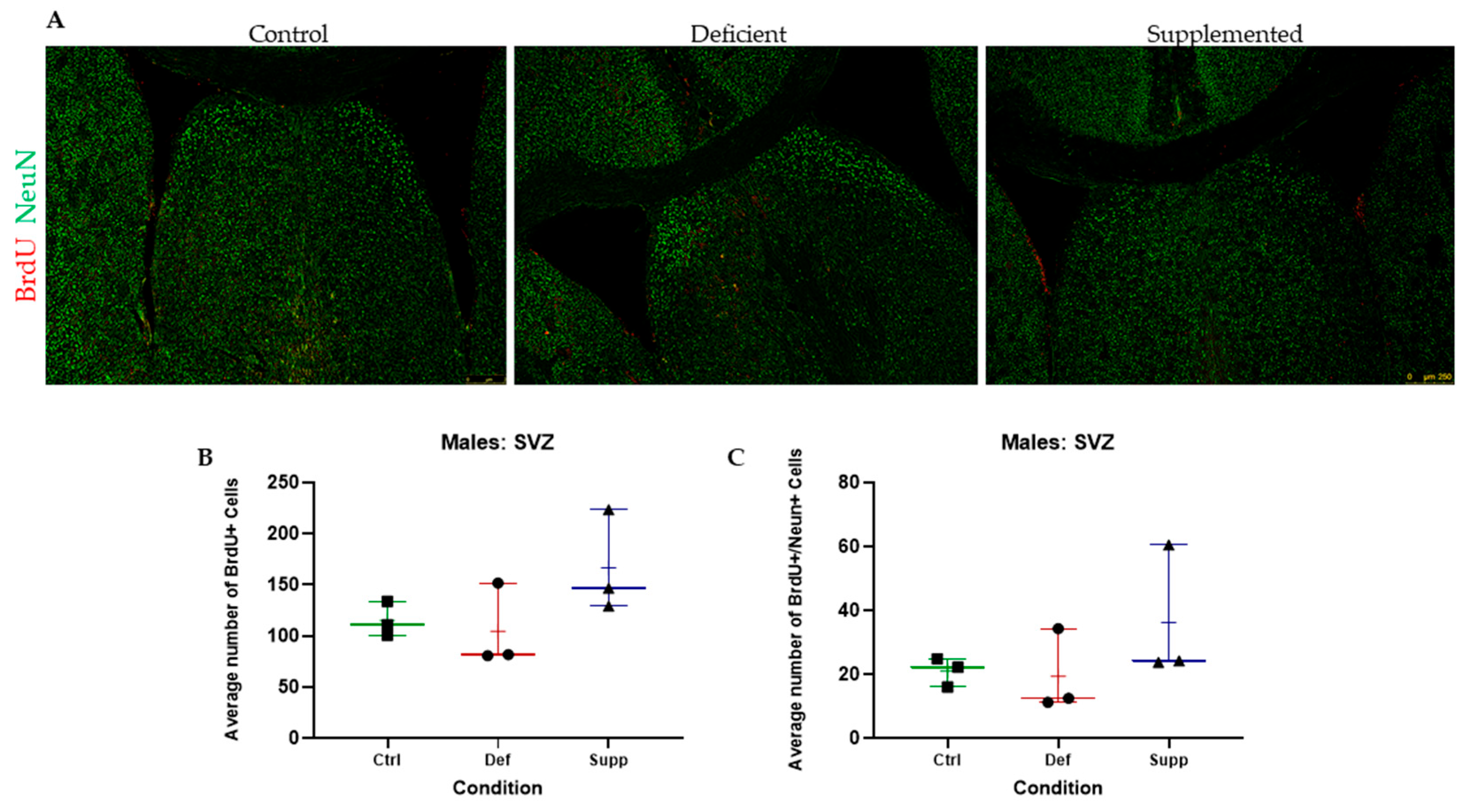
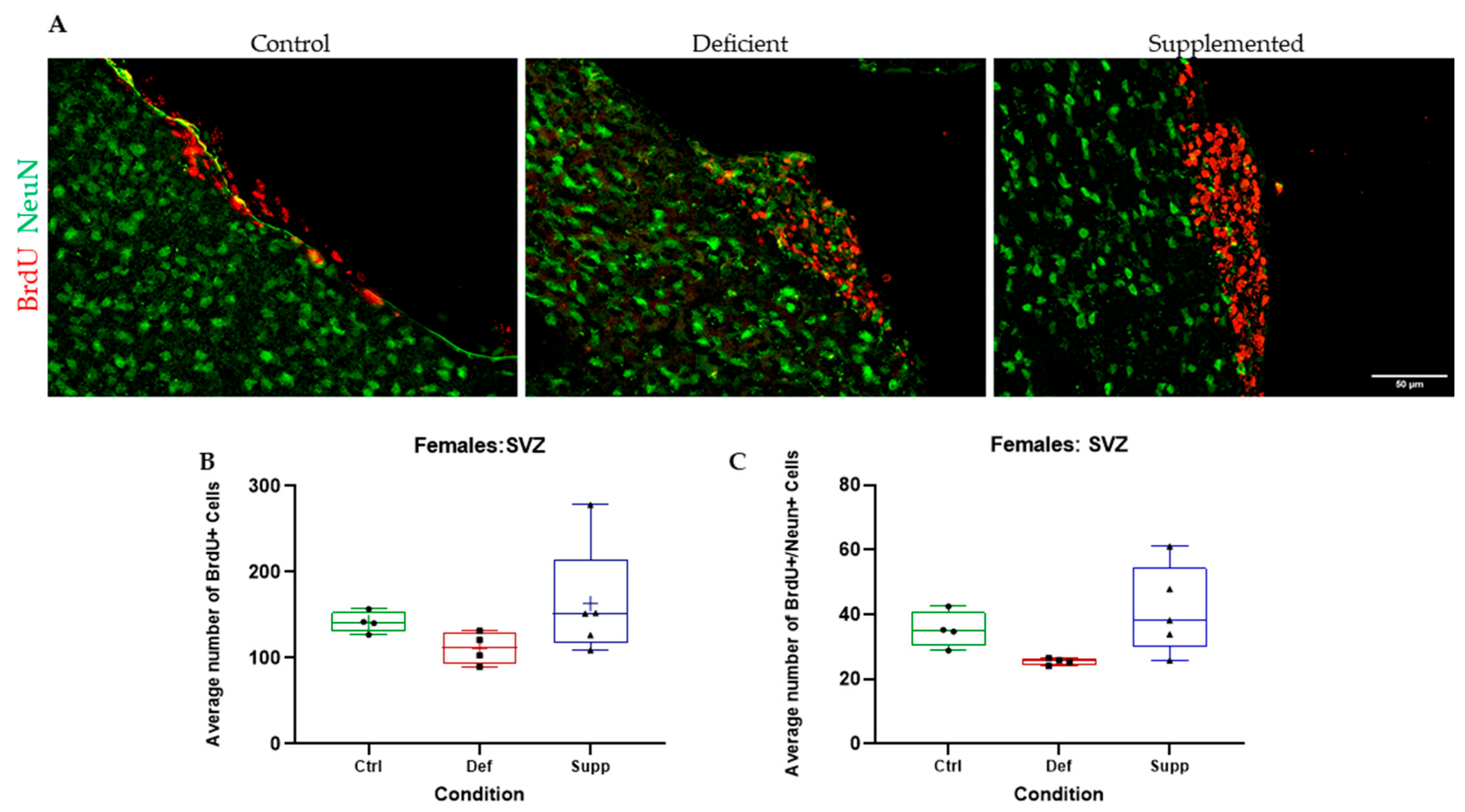
3.3. Vitamin D Deficiency Did Not Alter Neural Stem Cell Proliferation and Differentiation
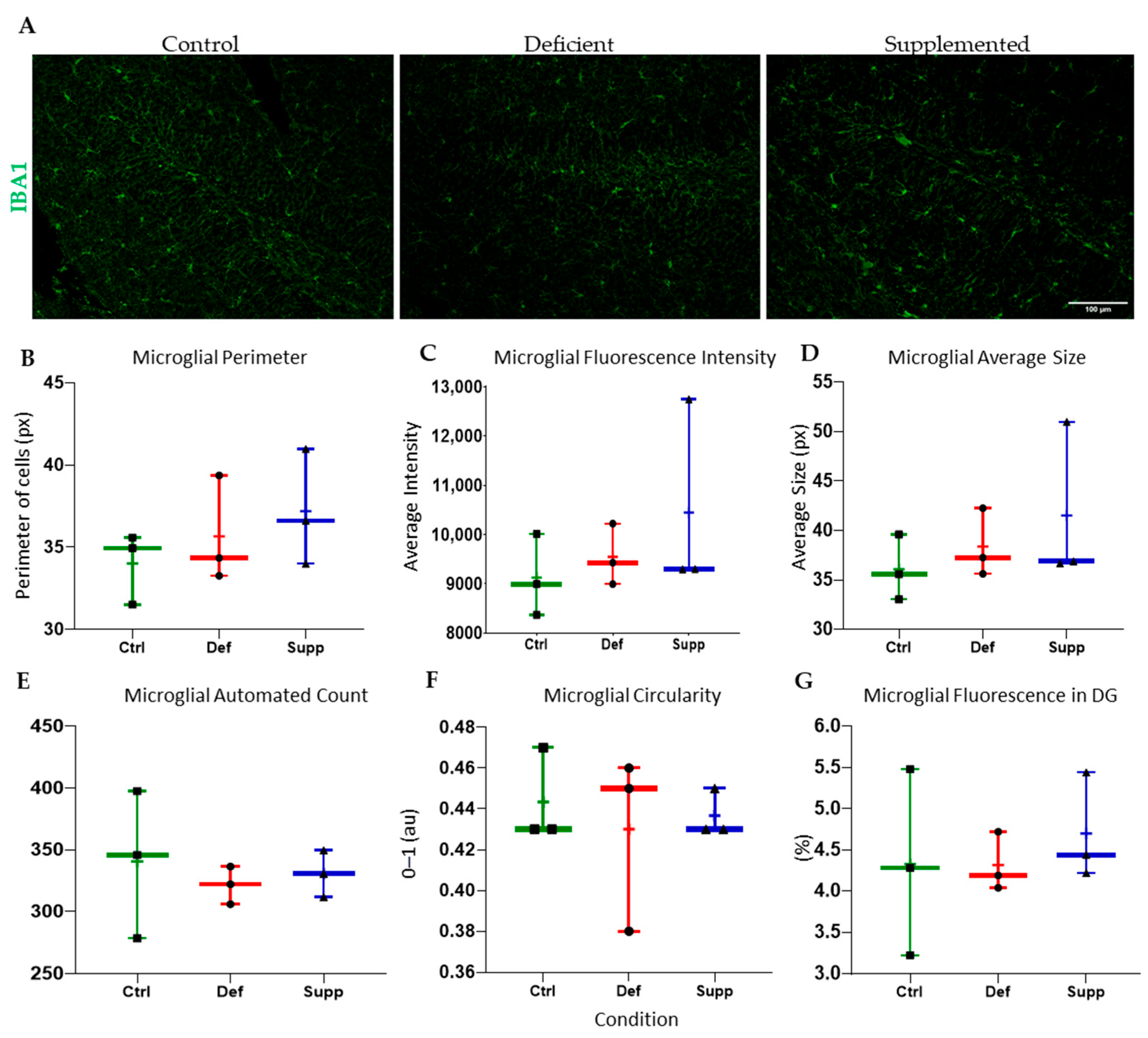
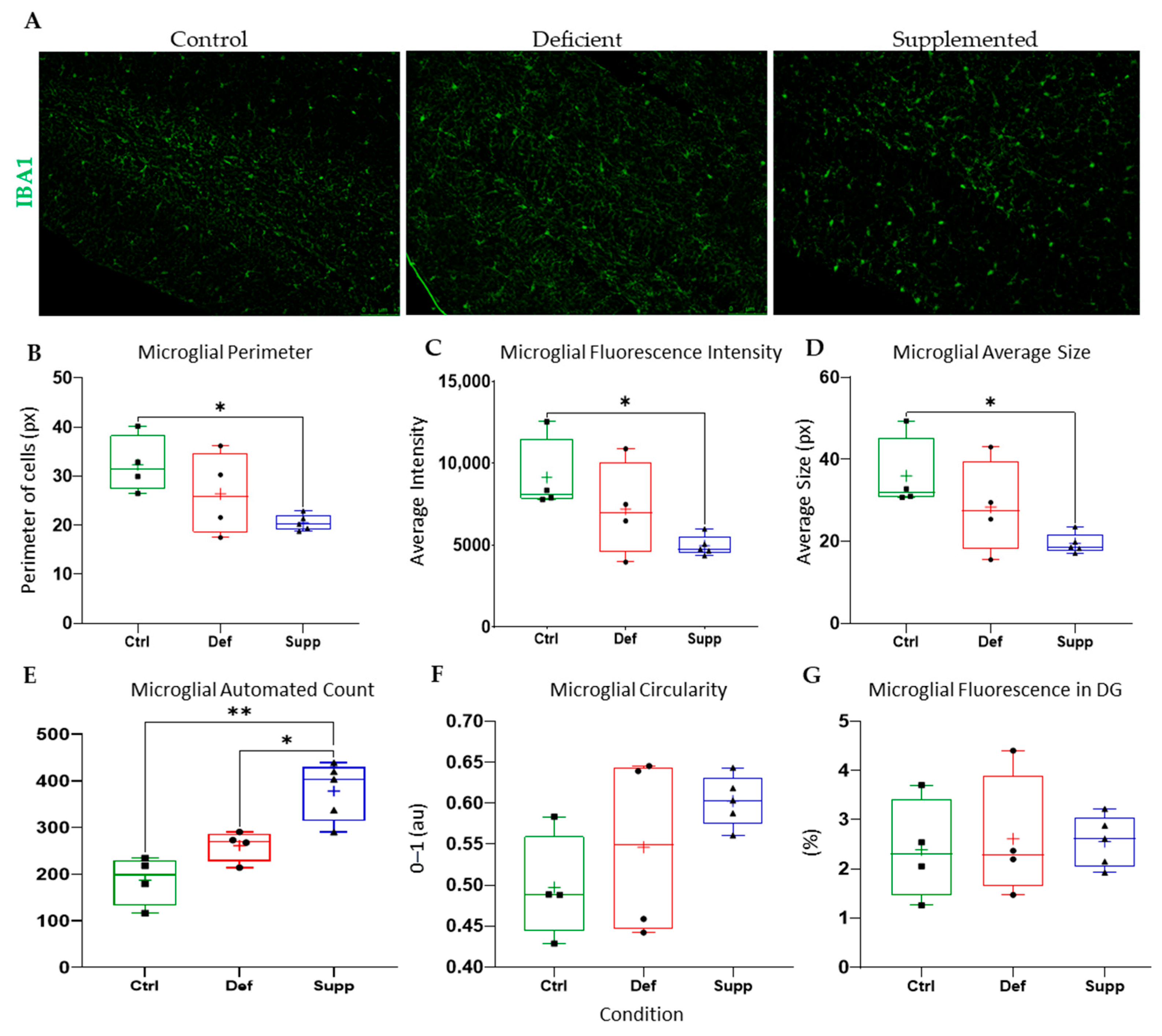
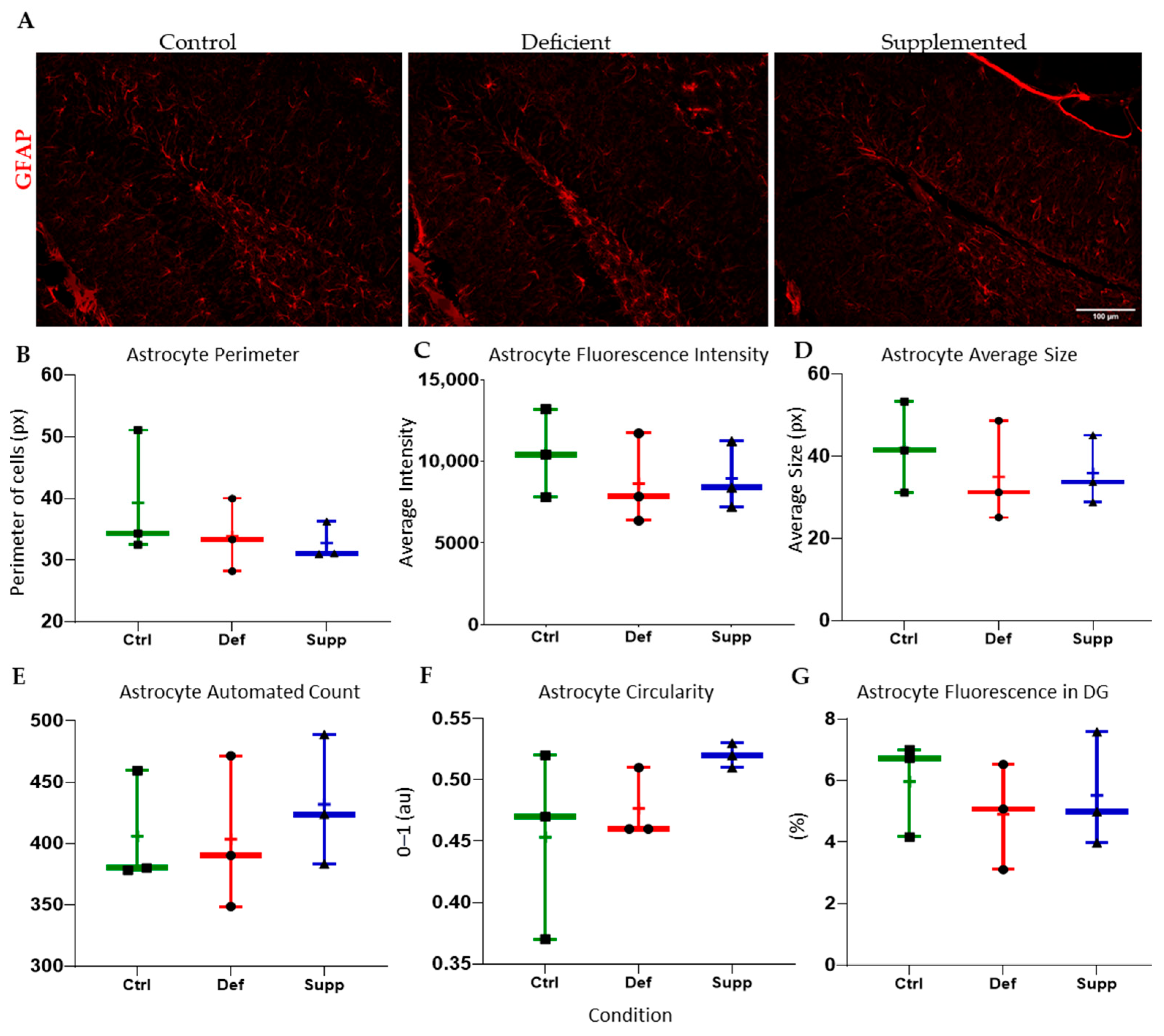
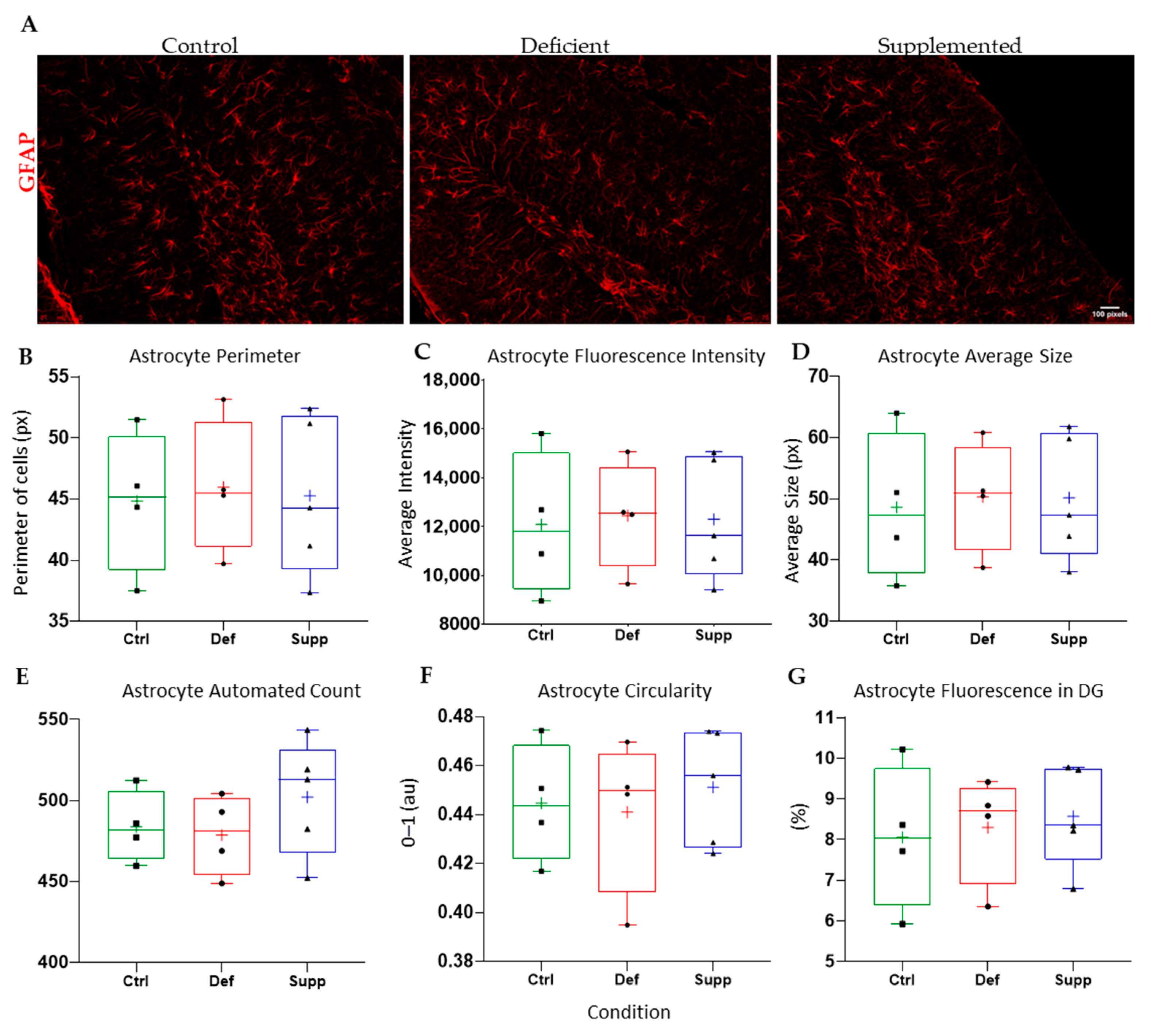
3.4. Vitamin D Affected Microglial Morphology in the DG of Female Mice Only, but Did Not Affect Astrocytes
4. Discussion
4.1. 25(OH)D Serum Levels Indicate Deficiency According to the EFSA Guidelines
4.2. No Effect of Vitamin D on Proliferation, Differentiation, or Behavioral Measures
4.3. Sex-Specific Mircoglial Differences Due to Vitamin D Manipulation
4.4. Future Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Landel, V.; Stephan, D.; Cui, X.; Eyles, D.; Feron, F. Differential expression of vitamin D-associated enzymes and receptors in brain cell subtypes. J. Steroid Biochem. Mol. Biol. 2018, 177, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D and Health: Evolution, Biologic Functions, and Recommended Dietary Intakes for Vitamin D. Vitam. D 2010, 7, 3–33. [Google Scholar] [CrossRef]
- Lips, P.; Cashman, K.D.; Lamberg-Allardt, C.; Bischoff-Ferrari, H.A.; Obermayer-Pietsch, B.; Bianchi, M.L.; Stepan, J.; El-Hajj Fuleihan, G.; Bouillon, R. Current vitamin D status in European and Middle East countries and strategies to prevent vitamin D deficiency: A position statement of the European Calcified Tissue Society. Eur. J. Endocrinol. 2019, 180, P23–P54. [Google Scholar] [CrossRef] [PubMed]
- Amrein, K.; Scherkl, M.; Hoffmann, M.; Neuwersch-Sommeregger, S.; Kostenberger, M.; Tmava Berisha, A.; Martucci, G.; Pilz, S.; Malle, O. Vitamin D deficiency 2.0: An update on the current status worldwide. Eur. J. Clin. Nutr. 2020, 74, 1498–1513. [Google Scholar] [CrossRef]
- Holick, M.F.; Chen, T.C. Vitamin D deficiency: A worldwide problem with health consequences. Am. J. Clin. Nutr. 2008, 87, 1080S–1086S. [Google Scholar] [CrossRef]
- Palacios, C.; Gonzalez, L. Is vitamin D deficiency a major global public health problem? J. Steroid Biochem. Mol. Biol. 2014, 144, 138–145. [Google Scholar] [CrossRef]
- McGrath, J. Does ‘imprinting’ with low prenatal vitamin D contribute to the risk of various adult disorders? Med. Hypotheses 2001, 56, 367–371. [Google Scholar] [CrossRef]
- Pludowski, P.; Holick, M.F.; Pilz, S.; Wagner, C.L.; Hollis, B.W.; Grant, W.B.; Shoenfeld, Y.; Lerchbaum, E.; Llewellyn, D.J.; Kienreich, K.; et al. Vitamin D effects on musculoskeletal health, immunity, autoimmunity, cardiovascular disease, cancer, fertility, pregnancy, dementia and mortality-a review of recent evidence. Autoimmun. Rev. 2013, 12, 976–989. [Google Scholar] [CrossRef]
- Evatt, M.L.; DeLong, M.R.; Kumari, M.; Auinger, P.; McDermott, M.P.; Tangpricha, V.; Parkinson Study Group, D.I. High prevalence of hypovitaminosis D status in patients with early Parkinson disease. Arch. Neurol. 2011, 68, 314–319. [Google Scholar] [CrossRef]
- Fullard, M.E.; Duda, J.E. A Review of the Relationship Between Vitamin D and Parkinson Disease Symptoms. Front. Neurol. 2020, 11, 454. [Google Scholar] [CrossRef]
- Peterson, A.; Mattek, N.; Clemons, A.; Bowman, G.L.; Buracchio, T.; Kaye, J.; Quinn, J. Serum vitamin D concentrations are associated with falling and cognitive function in older adults. J. Nutr. Health Aging 2012, 16, 898–901. [Google Scholar] [CrossRef]
- Suzuki, M.; Yoshioka, M.; Hashimoto, M.; Murakami, M.; Noya, M.; Takahashi, D.; Urashima, M. Randomized, double-blind, placebo-controlled trial of vitamin D supplementation in Parkinson disease. Am. J. Clin. Nutr. 2013, 97, 1004–1013. [Google Scholar] [CrossRef]
- McGrath, J.; Saari, K.; Hakko, H.; Jokelainen, J.; Jones, P.; Jarvelin, M.R.; Chant, D.; Isohanni, M. Vitamin D supplementation during the first year of life and risk of schizophrenia: A Finnish birth cohort study. Schizophr. Res. 2004, 67, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Neriman, A.; Hakan, Y.; Ozge, U. The psychotropic effect of vitamin D supplementation on schizophrenia symptoms. BMC Psychiatry 2021, 21, 309. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.L.; Luo, W.W.; Cheng, X.; Li, Y.; Zhang, Q.Z.; Peng, W.X. Vitamin D deficiency and Schizophrenia in Adults: A Systematic Review and Meta-analysis of Observational Studies. Psychiatry Res. 2020, 288, 112959. [Google Scholar] [CrossRef] [PubMed]
- Anglin, R.E.; Samaan, Z.; Walter, S.D.; McDonald, S.D. Vitamin D deficiency and depression in adults: Systematic review and meta-analysis. Br. J. Psychiatry 2013, 202, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Kouba, B.R.; Torra, A.; Camargo, A.; Rodrigues, A.L.S. The antidepressant-like effect elicited by vitamin D(3) is associated with BDNF/TrkB-related synaptic protein synthesis. Metab. Brain Dis. 2023, 38, 601–611. [Google Scholar] [CrossRef]
- Parker, G.B.; Brotchie, H.; Graham, R.K. Vitamin D and depression. J. Affect. Disord. 2017, 208, 56–61. [Google Scholar] [CrossRef]
- Evatt, M.L.; Delong, M.R.; Khazai, N.; Rosen, A.; Triche, S.; Tangpricha, V. Prevalence of vitamin d insufficiency in patients with Parkinson disease and Alzheimer disease. Arch. Neurol. 2008, 65, 1348–1352. [Google Scholar] [CrossRef]
- Shih, E.J.; Lee, W.J.; Hsu, J.L.; Wang, S.J.; Fuh, J.L. Effect of vitamin D on cognitive function and white matter hyperintensity in patients with mild Alzheimer’s disease. Geriatr. Gerontol. Int. 2020, 20, 52–58. [Google Scholar] [CrossRef]
- Wang, L.; Qiao, Y.; Zhang, H.; Zhang, Y.; Hua, J.; Jin, S.; Liu, G. Circulating Vitamin D Levels and Alzheimer’s Disease: A Mendelian Randomization Study in the IGAP and UK Biobank. J. Alzheimers Dis. 2020, 73, 609–618. [Google Scholar] [CrossRef]
- Darwish, H.; Haddad, R.; Osman, S.; Ghassan, S.; Yamout, B.; Tamim, H.; Khoury, S. Effect of Vitamin D Replacement on Cognition in Multiple Sclerosis Patients. Sci. Rep. 2017, 7, 45926. [Google Scholar] [CrossRef]
- Miclea, A.; Bagnoud, M.; Chan, A.; Hoepner, R. A Brief Review of the Effects of Vitamin D on Multiple Sclerosis. Front. Immunol. 2020, 11, 781. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; He, L.; Liu, L.; Zhu, J.; Jin, T. The efficacy of vitamin D in multiple sclerosis: A meta-analysis. Mult. Scler. Relat. Disord. 2018, 23, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Hooshmand, B.; Lokk, J.; Solomon, A.; Mangialasche, F.; Miralbell, J.; Spulber, G.; Annerbo, S.; Andreasen, N.; Winblad, B.; Cedazo-Minguez, A.; et al. Vitamin D in relation to cognitive impairment, cerebrospinal fluid biomarkers, and brain volumes. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 1132–1138. [Google Scholar] [CrossRef] [PubMed]
- Shea, M.K.; Barger, K.; Dawson-Hughes, B.; Leurgans, S.E.; Fu, X.; James, B.D.; Holland, T.M.; Agarwal, P.; Wang, J.; Matuszek, G.; et al. Brain vitamin D forms, cognitive decline, and neuropathology in community-dwelling older adults. Alzheimers Dement. 2023, 19, 2389–2396. [Google Scholar] [CrossRef]
- Cannell, J.J. Vitamin D and autism, what’s new? Rev. Endocr Metab. Disord. 2017, 18, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Siracusano, M.; Riccioni, A.; Abate, R.; Benvenuto, A.; Curatolo, P.; Mazzone, L. Vitamin D Deficiency and Autism Spectrum Disorder. Curr. Pharm. Des. 2020, 26, 2460–2474. [Google Scholar] [CrossRef]
- Akaltun, I. Trichotillomania Triggered by Vitamin D Deficiency and Resolving Dramatically With Vitamin D Therapy. Clin. Neuropharmacol. 2019, 42, 20–21. [Google Scholar] [CrossRef]
- Titus-Lay, E.; Eid, T.J.; Kreys, T.J.; Chu, B.X.J.; Malhotra, A. Trichotillomania associated with a 25-hydroxy vitamin D deficiency: A case report. Ment. Health Clin. 2020, 10, 38–43. [Google Scholar] [CrossRef]
- Gall, Z.; Szekely, O. Role of Vitamin D in Cognitive Dysfunction: New Molecular Concepts and Discrepancies between Animal and Human Findings. Nutrients 2021, 13, 3672. [Google Scholar] [CrossRef]
- Eyles, D.W.; Burne, T.H.; McGrath, J.J. Vitamin D, effects on brain development, adult brain function and the links between low levels of vitamin D and neuropsychiatric disease. Front. Neuroendocrinol. 2013, 34, 47–64. [Google Scholar] [CrossRef] [PubMed]
- Harvey, L.; Burne, T.; Cui, X.; Mackay-Sim, A.; Eyles, D.; McGrath, J. Vitamin D and the Brain: A Neuropsychiatric Perspective. Clinic Rev Bone Miner Metab 2009, 7, 199–205. [Google Scholar] [CrossRef]
- Mayne, P.E.; Burne, T.H.J. Vitamin D in Synaptic Plasticity, Cognitive Function, and Neuropsychiatric Illness. Trends Neurosci. 2019, 42, 293–306. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.; Bianco, J.I.; McGrath, J.J.; Eyles, D.W. 1,25-Dihydroxyvitamin D3 induces nerve growth factor, promotes neurite outgrowth and inhibits mitosis in embryonic rat hippocampal neurons. Neurosci. Lett. 2003, 343, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Marini, F.; Bartoccini, E.; Cascianelli, G.; Voccoli, V.; Baviglia, M.G.; Magni, M.V.; Garcia-Gil, M.; Albi, E. Effect of 1alpha,25-dihydroxyvitamin D3 in embryonic hippocampal cells. Hippocampus 2010, 20, 696–705. [Google Scholar] [CrossRef]
- Brewer, L.D.; Thibault, V.; Chen, K.C.; Langub, M.C.; Landfield, P.W.; Porter, N.M. Vitamin D hormone confers neuroprotection in parallel with downregulation of L-type calcium channel expression in hippocampal neurons. J. Neurosci. 2001, 21, 98–108. [Google Scholar] [CrossRef]
- Garcion, E.; Sindji, L.; Montero-Menei, C.; Andre, C.; Brachet, P.; Darcy, F. Expression of inducible nitric oxide synthase during rat brain inflammation: Regulation by 1,25-dihydroxyvitamin D3. Glia 1998, 22, 282–294. [Google Scholar] [CrossRef]
- Kroncke, K.D.; Klotz, L.O.; Suschek, C.V.; Sies, H. Comparing nitrosative versus oxidative stress toward zinc finger-dependent transcription. Unique role for NO. J. Biol. Chem. 2002, 277, 13294–13301. [Google Scholar] [CrossRef]
- Eyles, D.; Brown, J.; Mackay-Sim, A.; McGrath, J.; Feron, F. Vitamin d3 and brain development. Neuroscience 2003, 118, 641–653. [Google Scholar] [CrossRef]
- Naveilhan, P.; Neveu, I.; Wion, D.; Brachet, P. 1,25-Dihydroxyvitamin D3, an inducer of glial cell line-derived neurotrophic factor. Neuroreport 1996, 7, 2171–2175. [Google Scholar] [CrossRef] [PubMed]
- Peitl, V.; Silic, A.; Orlovic, I.; Vidrih, B.; Crnkovic, D.; Karlovic, D. Vitamin D and Neurotrophin Levels and Their Impact on the Symptoms of Schizophrenia. Neuropsychobiology 2020, 79, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Wion, D.; MacGrogan, D.; Neveu, I.; Jehan, F.; Houlgatte, R.; Brachet, P. 1,25-Dihydroxyvitamin D3 is a potent inducer of nerve growth factor synthesis. J. Neurosci. Res. 1991, 28, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Masoumi, A.; Goldenson, B.; Ghirmai, S.; Avagyan, H.; Zaghi, J.; Abel, K.; Zheng, X.; Espinosa-Jeffrey, A.; Mahanian, M.; Liu, P.T.; et al. 1alpha,25-dihydroxyvitamin D3 interacts with curcuminoids to stimulate amyloid-beta clearance by macrophages of Alzheimer’s disease patients. J. Alzheimers Dis. 2009, 17, 703–717. [Google Scholar] [CrossRef]
- Mizwicki, M.T.; Liu, G.; Fiala, M.; Magpantay, L.; Sayre, J.; Siani, A.; Mahanian, M.; Weitzman, R.; Hayden, E.Y.; Rosenthal, M.J.; et al. 1alpha,25-dihydroxyvitamin D3 and resolvin D1 retune the balance between amyloid-beta phagocytosis and inflammation in Alzheimer’s disease patients. J. Alzheimers Dis. 2013, 34, 155–170. [Google Scholar] [CrossRef]
- Miller, B.J.; Whisner, C.M.; Johnston, C.S. Vitamin D Supplementation Appears to Increase Plasma Abeta40 in Vitamin D Insufficient Older Adults: A Pilot Randomized Controlled Trial. J. Alzheimers Dis. 2016, 52, 843–847. [Google Scholar] [CrossRef]
- Rcom-H’cheo-Gauthier, A.N.; Meedeniya, A.C.; Pountney, D.L. Calcipotriol inhibits alpha-synuclein aggregation in SH-SY5Y neuroblastoma cells by a Calbindin-D28k-dependent mechanism. J. Neurochem. 2017, 141, 263–274. [Google Scholar] [CrossRef]
- Bellettini-Santos, T.; Garcez, M.L.; Mina, F.; Magnus, N.Q.; Pereira, N.S.; Marques, A.O.; Keller, G.S.; Zabot, G.C.; do Nascimento, N.B.; Medeiros, E.B.; et al. Vitamin D3 improves spatial memory and modulates cytokine levels in aged rats. Metab. Brain Dis. 2023, 38, 1155–1166. [Google Scholar] [CrossRef]
- Briones, T.L.; Darwish, H. Vitamin D mitigates age-related cognitive decline through the modulation of pro-inflammatory state and decrease in amyloid burden. J. Neuroinflammat. 2012, 9, 244. [Google Scholar] [CrossRef]
- Calvello, R.; Cianciulli, A.; Nicolardi, G.; De Nuccio, F.; Giannotti, L.; Salvatore, R.; Porro, C.; Trotta, T.; Panaro, M.A.; Lofrumento, D.D. Vitamin D Treatment Attenuates Neuroinflammation and Dopaminergic Neurodegeneration in an Animal Model of Parkinson’s Disease, Shifting M1 to M2 Microglia Responses. J. Neuroimmune Pharmacol. 2017, 12, 327–339. [Google Scholar] [CrossRef]
- Lima, L.A.R.; Lopes, M.J.P.; Costa, R.O.; Lima, F.A.V.; Neves, K.R.T.; Calou, I.B.F.; Andrade, G.M.; Viana, G.S.B. Vitamin D protects dopaminergic neurons against neuroinflammation and oxidative stress in hemiparkinsonian rats. J. Neuroinflammat. 2018, 15, 249. [Google Scholar] [CrossRef] [PubMed]
- Hirschler, V.; Molinari, C.; Maccallini, G.; Intersimone, P.; Gonzalez, C.D. Vitamin D Levels and Cardiometabolic Markers in Indigenous Argentinean Children Living at Different Altitudes. Glob. Pediatr. Health 2019, 6. [Google Scholar] [CrossRef]
- Bivona, G.; Agnello, L.; Bellia, C.; Iacolino, G.; Scazzone, C.; Lo Sasso, B.; Ciaccio, M. Non-Skeletal Activities of Vitamin D: From Physiology to Brain Pathology. Medicina 2019, 55, 341. [Google Scholar] [CrossRef]
- Cui, X.; Gooch, H.; Petty, A.; McGrath, J.J.; Eyles, D. Vitamin D and the brain: Genomic and non-genomic actions. Mol. Cell. Endocrinol. 2017, 453, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Gooch, H.; Cui, X.; Anggono, V.; Trzaskowski, M.; Tan, M.C.; Eyles, D.W.; Burne, T.H.J.; Jang, S.E.; Mattheisen, M.; Hougaard, D.M.; et al. 1,25-Dihydroxyvitamin D modulates L-type voltage-gated calcium channels in a subset of neurons in the developing mouse prefrontal cortex. Transl. Psychiatry 2019, 9, 281. [Google Scholar] [CrossRef] [PubMed]
- Reichelt, A.C.; Hare, D.J.; Bussey, T.J.; Saksida, L.M. Perineuronal Nets: Plasticity, Protection, and Therapeutic Potential. Trends Neurosci. 2019, 42, 458–470. [Google Scholar] [CrossRef]
- Won, S.; Sayeed, I.; Peterson, B.L.; Wali, B.; Kahn, J.S.; Stein, D.G. Vitamin D prevents hypoxia/reoxygenation-induced blood-brain barrier disruption via vitamin D receptor-mediated NF-kB signaling pathways. PLoS ONE 2015, 10, e0122821. [Google Scholar] [CrossRef]
- Taniura, H.; Ito, M.; Sanada, N.; Kuramoto, N.; Ohno, Y.; Nakamichi, N.; Yoneda, Y. Chronic vitamin D3 treatment protects against neurotoxicity by glutamate in association with upregulation of vitamin D receptor mRNA expression in cultured rat cortical neurons. J Neurosci Res 2006, 83, 1179–1189. [Google Scholar] [CrossRef]
- Shirazi, H.A.; Rasouli, J.; Ciric, B.; Rostami, A.; Zhang, G.X. 1,25-Dihydroxyvitamin D3 enhances neural stem cell proliferation and oligodendrocyte differentiation. Exp. Mol. Pathol. 2015, 98, 240–245. [Google Scholar] [CrossRef]
- Jang, W.; Park, H.H.; Lee, K.Y.; Lee, Y.J.; Kim, H.T.; Koh, S.H. 1,25-dyhydroxyvitamin D3 attenuates L-DOPA-induced neurotoxicity in neural stem cells. Mol. Neurobiol. 2015, 51, 558–570. [Google Scholar] [CrossRef]
- Al-Amin, M.M.; Sullivan, R.K.P.; Kurniawan, N.D.; Burne, T.H.J. Adult vitamin D deficiency disrupts hippocampal-dependent learning and structural brain connectivity in BALB/c mice. Brain Struct. Funct. 2019, 224, 1315–1329. [Google Scholar] [CrossRef] [PubMed]
- Brouwer-Brolsma, E.M.; Schuurman, T.; de Groot, L.C.; Feskens, E.J.; Lute, C.; Naninck, E.F.; Arndt, S.S.; van der Staay, F.J.; Bravenboer, N.; Korosi, A.; et al. No role for vitamin D or a moderate fat diet in aging induced cognitive decline and emotional reactivity in C57BL/6 mice. Behav. Brain Res. 2014, 267, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Byrne, J.H.; Voogt, M.; Turner, K.M.; Eyles, D.W.; McGrath, J.J.; Burne, T.H. The impact of adult vitamin D deficiency on behaviour and brain function in male Sprague-Dawley rats. PLoS ONE 2013, 8, e71593. [Google Scholar] [CrossRef]
- Doiphode, P.; Bhosale, U.A.; Karandikar, Y.S.; Pawar, N.R. Experimental Evaluation of Vitamin-D Supplementation on Memory and Learning Using Different Animal Models in Albino Rat. J. Pharm. Res. Int. 2020, 32, 103–110. [Google Scholar] [CrossRef]
- Groves, N.J.; Bradford, D.; Sullivan, R.K.; Conn, K.A.; Aljelaify, R.F.; McGrath, J.J.; Burne, T.H. Behavioural Effects of Adult Vitamin D Deficiency in BALB/c Mice Are not Associated with Proliferation or Survival of Neurons in the Adult Hippocampus. PLoS ONE 2016, 11, e0152328. [Google Scholar] [CrossRef]
- Groves, N.J.; Burne, T.H. Sex-specific attentional deficits in adult vitamin D deficient BALB/c mice. Physiol. Behav. 2016, 157, 94–101. [Google Scholar] [CrossRef]
- Groves, N.J.; Kesby, J.P.; Eyles, D.W.; McGrath, J.J.; Mackay-Sim, A.; Burne, T.H. Adult vitamin D deficiency leads to behavioural and brain neurochemical alterations in C57BL/6J and BALB/c mice. Behav. Brain Res. 2013, 241, 120–131. [Google Scholar] [CrossRef]
- Groves, N.J.; Zhou, M.; Jhaveri, D.J.; McGrath, J.J.; Burne, T.H.J. Adult vitamin D deficiency exacerbates impairments caused by social stress in BALB/c and C57BL/6 mice. Psychoneuroendocrinology 2017, 86, 53–63. [Google Scholar] [CrossRef]
- Jaeschke, K.N.; Blackmore, D.G.; Groves, N.J.; Al-Amin, M.M.; Alexander, S.; Burne, T.H. Vitamin D Levels Are Not Associated with Hippocampal-Dependent Learning in Young Adult Male C57BL/6J Mice: A Negative Report. J. Psychiatry Brain Sci. 2019, 4, e190008. [Google Scholar] [CrossRef]
- Liang, Q.; Cai, C.; Duan, D.; Hu, X.; Hua, W.; Jiang, P.; Zhang, L.; Xu, J.; Gao, Z. Postnatal Vitamin D Intake Modulates Hippocampal Learning and Memory in Adult Mice. Front. Neurosci. 2018, 12, 141. [Google Scholar] [CrossRef]
- Morello, M.; Landel, V.; Lacassagne, E.; Baranger, K.; Annweiler, C.; Feron, F.; Millet, P. Vitamin D Improves Neurogenesis and Cognition in a Mouse Model of Alzheimer’s Disease. Mol. Neurobiol. 2018, 55, 6463–6479. [Google Scholar] [CrossRef] [PubMed]
- Taghizadeh, M.; Talaei, S.A.; Salami, M. Vitamin D deficiency impairs spatial learning in adult rats. Iran. Biomed. J. 2013, 17, 42–48. [Google Scholar] [CrossRef]
- Carretero-Guillen, A.; Trevino, M.; Gomez-Climent, M.A.; Dogbevia, G.K.; Bertocchi, I.; Sprengel, R.; Larkum, M.E.; Vlachos, A.; Gruart, A.; Delgado-Garcia, J.M.; et al. Dentate gyrus is needed for memory retrieval. Mol. Psychiatry 2024, 1–12. [Google Scholar] [CrossRef]
- National Research Council, Division on Earth, Life Studies; Institute for Laboratory Animal Research; Committee for the Update of the Guide for the Care, and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals, 8th ed.; National Academies Press: Washington, DC, USA, 2011. [Google Scholar] [CrossRef]
- Hubrecht, R.C.; Carter, E. The 3Rs and Humane Experimental Technique: Implementing Change. Animals 2019, 9, 754. [Google Scholar] [CrossRef] [PubMed]
- The Principles of Humane Experimental Technique. Med. J. Aust. 1960, 1, 500. [CrossRef]
- Zimmermann, M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 1983, 16, 109–110. [Google Scholar] [CrossRef]
- Taupin, P. BrdU immunohistochemistry for studying adult neurogenesis: Paradigms, pitfalls, limitations, and validation. Brain Res. Rev. 2007, 53, 198–214. [Google Scholar] [CrossRef]
- Cicero, L.; Fazzotta, S.; Palumbo, V.D.; Cassata, G.; Lo Monte, A.I. Anesthesia protocols in laboratory animals used for scientific purposes. Acta Biomed. 2018, 89, 337–342. [Google Scholar] [CrossRef]
- Flecknell, P. Managing and Monitoring Anaesthesia. In Laboratory Animal Anaesthesia; Academic Press: Cambridge, MA, USA, 2016; pp. 77–108. [Google Scholar] [CrossRef]
- Andersen, B.B.; Gundersen, H.J. Pronounced loss of cell nuclei and anisotropic deformation of thick sections. J. Microsc. 1999, 196, 69–73. [Google Scholar] [CrossRef]
- Paxinos, G.; Franklin, K.B.J. Paxinos and Franklin’s the Mouse Brain in Stereotaxic Coordinates; Elsevier Science: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Falcao, A.M.; Palha, J.A.; Ferreira, A.C.; Marques, F.; Sousa, N.; Sousa, J.C. Unbiased stereological method to assess proliferation throughout the subependymal zone. Methods Mol. Biol. 2013, 1035, 141–152. [Google Scholar] [CrossRef]
- Darwish, B.; Chamaa, F.; Awada, B.; Lawand, N.; Saade, N.E.; Abou Fayad, A.G.; Abou-Kheir, W. Urinary Tract Infections Impair Adult Hippocampal Neurogenesis. Biology 2022, 11, 891. [Google Scholar] [CrossRef] [PubMed]
- Falcao, A.M.; Palha, J.A.; Ferreira, A.C.; Marques, F.; Sousa, N.; Sousa, J.C. Topographical analysis of the subependymal zone neurogenic niche. PLoS ONE 2012, 7, e38647. [Google Scholar] [CrossRef]
- Norden, D.M.; Trojanowski, P.J.; Walker, F.R.; Godbout, J.P. Insensitivity of astrocytes to interleukin 10 signaling following peripheral immune challenge results in prolonged microglial activation in the aged brain. Neurobiol. Aging 2016, 44, 22–41. [Google Scholar] [CrossRef] [PubMed]
- Young, K.; Morrison, H. Quantifying Microglia Morphology from Photomicrographs of Immunohistochemistry Prepared Tissue Using ImageJ. J. Vis. Exp. 2018, 136, e57648. [Google Scholar] [CrossRef]
- Cruz-Sanchez, A.; Dematagoda, S.; Ahmed, R.; Mohanathaas, S.; Odenwald, N.; Arruda-Carvalho, M. Developmental onset distinguishes three types of spontaneous recognition memory in mice. Sci. Rep. 2020, 10, 10612. [Google Scholar] [CrossRef]
- Barker, G.R.; Bird, F.; Alexander, V.; Warburton, E.C. Recognition memory for objects, place, and temporal order: A disconnection analysis of the role of the medial prefrontal cortex and perirhinal cortex. J. Neurosci. 2007, 27, 2948–2957. [Google Scholar] [CrossRef]
- Dere, E.; Huston, J.P.; De Souza Silva, M.A. Integrated memory for objects, places, and temporal order: Evidence for episodic-like memory in mice. Neurobiol. Learn. Mem. 2005, 84, 214–221. [Google Scholar] [CrossRef]
- Hebda-Bauer, E.K.; Pletsch, A.; Darwish, H.; Fentress, H.; Simmons, T.A.; Wei, Q.; Watson, S.J.; Akil, H. Forebrain glucocorticoid receptor overexpression increases environmental reactivity and produces a stress-induced spatial discrimination deficit. Neuroscience 2010, 169, 645–653. [Google Scholar] [CrossRef][Green Version]
- Lueptow, L.M. Novel Object Recognition Test for the Investigation of Learning and Memory in Mice. J. Vis. Exp. 2017, 2017, e55718. [Google Scholar] [CrossRef]
- Byers, S.L.; Wiles, M.V.; Dunn, S.L.; Taft, R.A. Mouse estrous cycle identification tool and images. PLoS ONE 2012, 7, e35538. [Google Scholar] [CrossRef]
- Analyze Particles. Available online: https://imagej.net/ij/docs/menus/analyze.html (accessed on 26 August 2024).
- Mallya, S.M.; Corrado, K.R.; Saria, E.A.; Yuan, F.F.; Tran, H.Q.; Saucier, K.; Atti, E.; Tetradis, S.; Arnold, A. Modeling vitamin D insufficiency and moderate deficiency in adult mice via dietary cholecalciferol restriction. Endocr. Res. 2016, 41, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Belenchia, A.M.; Johnson, S.A.; Kieschnick, A.C.; Rosenfeld, C.S.; Peterson, C.A. Time Course of Vitamin D Depletion and Repletion in Reproductive-age Female C57BL/6 Mice. Comp. Med. 2017, 67, 483–490. [Google Scholar] [PubMed]
- Arneson, W.L.; Arneson, D.L. Current Methods for Routine Clinical Laboratory Testing of Vitamin D Levels. Lab. Med. 2013, 44, e38–e42. [Google Scholar] [CrossRef]
- Abdel-Wareth, L.; Haq, A.; Turner, A.; Khan, S.; Salem, A.; Mustafa, F.; Hussein, N.; Pallinalakam, F.; Grundy, L.; Patras, G.; et al. Total vitamin D assay comparison of the Roche Diagnostics “Vitamin D total” electrochemiluminescence protein binding assay with the Chromsystems HPLC method in a population with both D2 and D3 forms of vitamin D. Nutrients 2013, 5, 971–980. [Google Scholar] [CrossRef]
- Xue, Y.; He, X.; Li, H.D.; Deng, Y.; Yan, M.; Cai, H.L.; Tang, M.M.; Dang, R.L.; Jiang, P. Simultaneous Quantification of 25-Hydroxyvitamin D3 and 24,25-Dihydroxyvitamin D3 in Rats Shows Strong Correlations between Serum and Brain Tissue Levels. Int. J. Endocrinol. 2015, 2015, 296531. [Google Scholar] [CrossRef]
- Feng, Y.; Cheng, G.; Wang, H.; Chen, B. The associations between serum 25-hydroxyvitamin D level and the risk of total fracture and hip fracture. Osteoporos. Int. 2017, 28, 1641–1652. [Google Scholar] [CrossRef]
- Lv, Q.B.; Gao, X.; Liu, X.; Shao, Z.X.; Xu, Q.H.; Tang, L.; Chi, Y.L.; Wu, A.M. The serum 25-hydroxyvitamin D levels and hip fracture risk: A meta-analysis of prospective cohort studies. Oncotarget 2017, 8, 39849–39858. [Google Scholar] [CrossRef]
- Zhao, J.G.; Zeng, X.T.; Wang, J.; Liu, L. Association Between Calcium or Vitamin D Supplementation and Fracture Incidence in Community-Dwelling Older Adults: A Systematic Review and Meta-analysis. JAMA 2017, 318, 2466–2482. [Google Scholar] [CrossRef]
- Kahwati, L.C.; Weber, R.P.; Pan, H.; Gourlay, M.; LeBlanc, E.; Coker-Schwimmer, M.; Viswanathan, M. Vitamin D, Calcium, or Combined Supplementation for the Primary Prevention of Fractures in Community-Dwelling Adults: Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2018, 319, 1600–1612. [Google Scholar] [CrossRef]
- Bolland, M.J.; Grey, A.; Avenell, A. Effects of vitamin D supplementation on musculoskeletal health: A systematic review, meta-analysis, and trial sequential analysis. Lancet Diabetes Endocrinol. 2018, 6, 847–858. [Google Scholar] [CrossRef]
- Bjelakovic, G.; Gluud, L.L.; Nikolova, D.; Whitfield, K.; Krstic, G.; Wetterslev, J.; Gluud, C. Vitamin D supplementation for prevention of cancer in adults. Cochrane Database Syst. Rev. 2014, 2014, CD007469. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, R.; Kunutsor, S.; Vitezova, A.; Oliver-Williams, C.; Chowdhury, S.; Kiefte-de-Jong, J.C.; Khan, H.; Baena, C.P.; Prabhakaran, D.; Hoshen, M.B.; et al. Vitamin D and risk of cause specific death: Systematic review and meta-analysis of observational cohort and randomised intervention studies. BMJ 2014, 348, g1903. [Google Scholar] [CrossRef] [PubMed]
- Theodoratou, E.; Tzoulaki, I.; Zgaga, L.; Ioannidis, J.P. Vitamin D and multiple health outcomes: Umbrella review of systematic reviews and meta-analyses of observational studies and randomised trials. BMJ 2014, 348, g2035. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Pinedo, U.; Cuevas, J.A.; Benito-Martin, M.S.; Moreno-Jimenez, L.; Esteban-Garcia, N.; Torre-Fuentes, L.; Matias-Guiu, J.A.; Pytel, V.; Montero, P.; Matias-Guiu, J. Vitamin D increases remyelination by promoting oligodendrocyte lineage differentiation. Brain Behav. 2020, 10, e01498. [Google Scholar] [CrossRef]
- Shirazi, H.A.; Rasouli, J.; Ciric, B.; Wei, D.; Rostami, A.; Zhang, G.X. 1,25-Dihydroxyvitamin D(3) suppressed experimental autoimmune encephalomyelitis through both immunomodulation and oligodendrocyte maturation. Exp. Mol. Pathol. 2017, 102, 515–521. [Google Scholar] [CrossRef]
- Orme, R.P.; Bhangal, M.S.; Fricker, R.A. Calcitriol imparts neuroprotection in vitro to midbrain dopaminergic neurons by upregulating GDNF expression. PLoS ONE 2013, 8, e62040. [Google Scholar] [CrossRef]
- Xu, S.; Li, J.; Zhai, M.; Yao, X.; Liu, H.; Deng, T.; Cai, H.; Zhang, W.; Zhang, W.; Lou, J.; et al. 1,25-(OH)2D3 protects Schwann cells against advanced glycation end products-induced apoptosis through PKA-NF-kappaB pathway. Life Sci. 2019, 225, 107–116. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhou, R.; Yang, R.; Zhang, Z.; Bai, Y.; Chang, F.; Li, L.; Sokabe, M.; Goltzman, D.; Miao, D.; et al. Abnormal neurogenesis in the dentate gyrus of adult mice lacking 1,25-dihydroxy vitamin D3 (1,25-(OH)2 D3). Hippocampus 2012, 22, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Uthaiah, C.A.; Beeraka, N.M.; Rajalakshmi, R.; Ramya, C.M.; Madhunapantula, S.V. Role of Neural Stem Cells and Vitamin D Receptor (VDR)-Mediated Cellular Signaling in the Mitigation of Neurological Diseases. Mol. Neurobiol. 2022, 59, 4065–4105. [Google Scholar] [CrossRef]
- Pertile, R.A.; Cui, X.; Eyles, D.W. Vitamin D signaling and the differentiation of developing dopamine systems. Neuroscience 2016, 333, 193–203. [Google Scholar] [CrossRef]
- Langub, M.C.; Herman, J.P.; Malluche, H.H.; Koszewski, N.J. Evidence of functional vitamin D receptors in rat hippocampus. Neuroscience 2001, 104, 49–56. [Google Scholar] [CrossRef] [PubMed]
- de la Fuente, A.G.; Errea, O.; van Wijngaarden, P.; Gonzalez, G.A.; Kerninon, C.; Jarjour, A.A.; Lewis, H.J.; Jones, C.A.; Nait-Oumesmar, B.; Zhao, C.; et al. Vitamin D receptor-retinoid X receptor heterodimer signaling regulates oligodendrocyte progenitor cell differentiation. J. Cell Biol. 2015, 211, 975–985. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Mu, M.; Zhang, S.; Li, C.; Tian, K.; Li, Z.; Li, B.; Wang, W.; Cao, H.; Sun, Q.; et al. Vitamin D3 suppresses astrocyte activation and ameliorates coal dust-induced mood disorders in mice. J. Affect. Disord. 2022, 303, 138–147. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, C.; Yu, H.; Hao, L.; Ju, M.; Feng, W.; Guo, Z.; Sun, X.; Fan, Q.; Xiao, R. Vitamin D, Folic Acid and Vitamin B(12) Can Reverse Vitamin D Deficiency-Induced Learning and Memory Impairment by Altering 27-Hydroxycholesterol and S-Adenosylmethionine. Nutrients 2022, 15, 132. [Google Scholar] [CrossRef] [PubMed]
- Gall, Z.; Csudor, A.; Savel, I.G.; Kelemen, K.; Kolcsar, M. Cholecalciferol Supplementation Impacts Behavior and Hippocampal Neuroglial Reorganization in Vitamin D-Deficient Rats. Nutrients 2024, 16, 2326. [Google Scholar] [CrossRef]
- Cekic, M.; Sayeed, I.; Stein, D.G. Combination treatment with progesterone and vitamin D hormone may be more effective than monotherapy for nervous system injury and disease. Front. Neuroendocrinol. 2009, 30, 158–172. [Google Scholar] [CrossRef]
- Monastra, G.; De Grazia, S.; De Luca, L.; Vittorio, S.; Unfer, V. Vitamin D: A steroid hormone with progesterone-like activity. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 2502–2512. [Google Scholar] [CrossRef]
- Kim, C.H. A functional relay from progesterone to vitamin D in the immune system. DNA Cell Biol. 2015, 34, 379–382. [Google Scholar] [CrossRef]
- Tekes, K.; Gyenge, M.; Folyovich, A.; Csaba, G. Influence of neonatal vitamin A or vitamin D treatment on the concentration of biogenic amines and their metabolites in the adult rat brain. Horm. Metab. Res. 2009, 41, 277–280. [Google Scholar] [CrossRef]
- Tekes, K.; Gyenge, M.; Hantos, M.; Csaba, G. Transgenerational hormonal imprinting caused by vitamin A and vitamin D treatment of newborn rats. Alterations in the biogenic amine contents of the adult brain. Brain Dev. 2009, 31, 666–670. [Google Scholar] [CrossRef]
- Harms, L.R.; Cowin, G.; Eyles, D.W.; Kurniawan, N.D.; McGrath, J.J.; Burne, T.H. Neuroanatomy and psychomimetic-induced locomotion in C57BL/6J and 129/X1SvJ mice exposed to developmental vitamin D deficiency. Behav. Brain Res. 2012, 230, 125–131. [Google Scholar] [CrossRef]
- Narvaiz, D.A.; Kwok, E.M.; Hodges, S.L.; Binder, M.S.; Nolan, S.O.; Pranske, Z.J.; Senger, S.; Herrera, R.; Lugo, J.N. Vitamin D supplementation positively affects activity but impairs stimulus response behavior in an age and sex specific manner in C57BL/6 mice. Neurotoxicol. Teratol. 2023, 98, 107180. [Google Scholar] [CrossRef] [PubMed]
- Pertile, R.A.N.; Cui, X.; Hammond, L.; Eyles, D.W. Vitamin D regulation of GDNF/Ret signaling in dopaminergic neurons. FASEB J. 2018, 32, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Nissou, M.F.; Brocard, J.; El Atifi, M.; Guttin, A.; Andrieux, A.; Berger, F.; Issartel, J.P.; Wion, D. The transcriptomic response of mixed neuron-glial cell cultures to 1,25-dihydroxyvitamin d3 includes genes limiting the progression of neurodegenerative diseases. J. Alzheimers Dis. 2013, 35, 553–564. [Google Scholar] [CrossRef] [PubMed]
- Azim, K.; Fiorelli, R.; Zweifel, S.; Hurtado-Chong, A.; Yoshikawa, K.; Slomianka, L.; Raineteau, O. 3-dimensional examination of the adult mouse subventricular zone reveals lineage-specific microdomains. PLoS ONE 2012, 7, e49087. [Google Scholar] [CrossRef]
- Encinas, J.M.; Michurina, T.V.; Peunova, N.; Park, J.H.; Tordo, J.; Peterson, D.A.; Fishell, G.; Koulakov, A.; Enikolopov, G. Division-coupled astrocytic differentiation and age-related depletion of neural stem cells in the adult hippocampus. Cell Stem Cell 2011, 8, 566–579. [Google Scholar] [CrossRef]
- Farzanehfar, P. Comparative review of adult midbrain and striatum neurogenesis with classical neurogenesis. Neurosci. Res. 2018, 134, 1–9. [Google Scholar] [CrossRef]
- Ming, G.L.; Song, H. Adult neurogenesis in the mammalian brain: Significant answers and significant questions. Neuron 2011, 70, 687–702. [Google Scholar] [CrossRef]
- Dityatev, A.; Rusakov, D.A. Molecular signals of plasticity at the tetrapartite synapse. Curr. Opin. Neurobiol. 2011, 21, 353–359. [Google Scholar] [CrossRef]
- Zhan, Y.; Paolicelli, R.C.; Sforazzini, F.; Weinhard, L.; Bolasco, G.; Pagani, F.; Vyssotski, A.L.; Bifone, A.; Gozzi, A.; Ragozzino, D.; et al. Deficient neuron-microglia signaling results in impaired functional brain connectivity and social behavior. Nat. Neurosci. 2014, 17, 400–406. [Google Scholar] [CrossRef]
- Escalada, P.; Ezkurdia, A.; Ramirez, M.J.; Solas, M. Essential Role of Astrocytes in Learning and Memory. Int. J. Mol. Sci. 2024, 25, 1899. [Google Scholar] [CrossRef] [PubMed]
- Hua, F.; Reiss, J.I.; Tang, H.; Wang, J.; Fowler, X.; Sayeed, I.; Stein, D.G. Progesterone and low-dose vitamin D hormone treatment enhances sparing of memory following traumatic brain injury. Horm. Behav. 2012, 61, 642–651. [Google Scholar] [CrossRef]
- Molinari, C.; Morsanuto, V.; Ghirlanda, S.; Ruga, S.; Notte, F.; Gaetano, L.; Uberti, F. Role of Combined Lipoic Acid and Vitamin D3 on Astrocytes as a Way to Prevent Brain Ageing by Induced Oxidative Stress and Iron Accumulation. Oxid. Med. Cell Longev. 2019, 2019, 2843121. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, T.V.; Rychkova, M.P.; Basova, N.E.; Yefimova, M.G. Vitamin D3 Inhibits Phagocytic Activity of Rat Brain Astrocytes in Primary Culture. J. Evol. Biochem. Physiol. 2022, 58, 666–676. [Google Scholar] [CrossRef]
- Moore, M.E.; Piazza, A.; McCartney, Y.; Lynch, M.A. Evidence that vitamin D3 reverses age-related inflammatory changes in the rat hippocampus. Biochem. Soc. Trans. 2005, 33, 573–577. [Google Scholar] [CrossRef]
- Smolders, J.; Schuurman, K.G.; van Strien, M.E.; Melief, J.; Hendrickx, D.; Hol, E.M.; van Eden, C.; Luchetti, S.; Huitinga, I. Expression of vitamin D receptor and metabolizing enzymes in multiple sclerosis-affected brain tissue. J. Neuropathol. Exp. Neurol. 2013, 72, 91–105. [Google Scholar] [CrossRef]
- Spanier, J.A.; Nashold, F.E.; Nelson, C.D.; Praska, C.E.; Hayes, C.E. Vitamin D3-mediated resistance to a multiple sclerosis model disease depends on myeloid cell 1,25-dihydroxyvitamin D3 synthesis and correlates with increased CD4(+) T cell CTLA-4 expression. J. Neuroimmunol. 2020, 338, 577105. [Google Scholar] [CrossRef]
- Alessio, N.; Belardo, C.; Trotta, M.C.; Paino, S.; Boccella, S.; Gargano, F.; Pieretti, G.; Ricciardi, F.; Marabese, I.; Luongo, L.; et al. Vitamin D Deficiency Induces Chronic Pain and Microglial Phenotypic Changes in Mice. Int. J. Mol. Sci. 2021, 22, 3604. [Google Scholar] [CrossRef]
- Koivisto, O.; Hanel, A.; Carlberg, C. Key Vitamin D Target Genes with Functions in the Immune System. Nutrients 2020, 12, 1140. [Google Scholar] [CrossRef]
- Miron, V.E.; Boyd, A.; Zhao, J.W.; Yuen, T.J.; Ruckh, J.M.; Shadrach, J.L.; van Wijngaarden, P.; Wagers, A.J.; Williams, A.; Franklin, R.J.M.; et al. M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat. Neurosci. 2013, 16, 1211–1218. [Google Scholar] [CrossRef]
- Ricca, C.; Aillon, A.; Bergandi, L.; Alotto, D.; Castagnoli, C.; Silvagno, F. Vitamin D Receptor Is Necessary for Mitochondrial Function and Cell Health. Int. J. Mol. Sci. 2018, 19, 1672. [Google Scholar] [CrossRef] [PubMed]
- Green, T.R.F.; Rowe, R.K. Quantifying microglial morphology: An insight into function. Clin. Exp. Immunol. 2024, 216, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Strackeljan, L.; Baczynska, E.; Cangalaya, C.; Baidoe-Ansah, D.; Wlodarczyk, J.; Kaushik, R.; Dityatev, A. Microglia Depletion-Induced Remodeling of Extracellular Matrix and Excitatory Synapses in the Hippocampus of Adult Mice. Cells 2021, 10, 1862. [Google Scholar] [CrossRef] [PubMed]
- Carlberg, C. Vitamin D Signaling in the Context of Innate Immunity: Focus on Human Monocytes. Front. Immunol. 2019, 10, 2211. [Google Scholar] [CrossRef]
- Matkovits, T.; Christakos, S. Ligand occupancy is not required for vitamin D receptor and retinoid receptor-mediated transcriptional activation. Mol. Endocrinol. 1995, 9, 232–242. [Google Scholar] [CrossRef][Green Version]
- Schaller, B.; Andres, R.H.; Huber, A.W.; Meyer, M.; Perez-Bouza, A.; Ducray, A.D.; Seiler, R.W.; Widmer, H.R. Effect of GDNF on differentiation of cultured ventral mesencephalic dopaminergic and non-dopaminergic calretinin-expressing neurons. Brain Res. 2005, 1036, 163–172. [Google Scholar] [CrossRef]
- Seyedi, M.; Gholami, F.; Samadi, M.; Djalali, M.; Effatpanah, M.; Yekaninejad, M.S.; Hashemi, R.; Abdolahi, M.; Chamari, M.; Honarvar, N.M. The Effect of Vitamin D3 Supplementation on Serum BDNF, Dopamine, and Serotonin in Children with Attention-Deficit/Hyperactivity Disorder. CNS Neurol. Disord. Drug Targets 2019, 18, 496–501. [Google Scholar] [CrossRef]
- Long, W.; Fatehi, M.; Soni, S.; Panigrahi, R.; Philippaert, K.; Yu, Y.; Kelly, R.; Boonen, B.; Barr, A.; Golec, D.; et al. Vitamin D is an endogenous partial agonist of the transient receptor potential vanilloid 1 channel. J. Physiol. 2020, 598, 4321–4338. [Google Scholar] [CrossRef]
- Benitez-Angeles, M.; Morales-Lazaro, S.L.; Juarez-Gonzalez, E.; Rosenbaum, T. TRPV1: Structure, Endogenous Agonists, and Mechanisms. Int. J. Mol. Sci. 2020, 21, 3421. [Google Scholar] [CrossRef]
- Kim, H.; Shin, J.-Y.; Lee, Y.-S.; Yun, S.P.; Maeng, H.-J.; Lee, Y. Brain Endothelial P-Glycoprotein Level Is Reduced in Parkinson’s Disease via a Vitamin D Receptor-Dependent Pathway. Int. J. Mol. Sci. 2020, 21, 8538. [Google Scholar] [CrossRef]
- Chow, E.C.; Durk, M.R.; Cummins, C.L.; Pang, K.S. 1Alpha,25-dihydroxyvitamin D3 up-regulates P-glycoprotein via the vitamin D receptor and not farnesoid X receptor in both fxr(−/−) and fxr(+/+) mice and increased renal and brain efflux of digoxin in mice in vivo. J. Pharmacol. Exp. Ther. 2011, 337, 846–859. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.T.; Sun, L.; Sun, C.H. PDIA3-regulted inflammation and oxidative stress contribute to the traumatic brain injury (TBI) in mice. Biochem. Biophys. Res. Commun. 2019, 518, 657–663. [Google Scholar] [CrossRef]
- Yoo, D.Y.; Cho, S.B.; Jung, H.Y.; Kim, W.; Lee, K.Y.; Kim, J.W.; Moon, S.M.; Won, M.H.; Choi, J.H.; Yoon, Y.S.; et al. Protein disulfide-isomerase A3 significantly reduces ischemia-induced damage by reducing oxidative and endoplasmic reticulum stress. Neurochem. Int. 2019, 122, 19–30. [Google Scholar] [CrossRef]
- Gonzalez-Perez, P.; Woehlbier, U.; Chian, R.J.; Sapp, P.; Rouleau, G.A.; Leblond, C.S.; Daoud, H.; Dion, P.A.; Landers, J.E.; Hetz, C.; et al. Identification of rare protein disulfide isomerase gene variants in amyotrophic lateral sclerosis patients. Gene 2015, 566, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Woehlbier, U.; Colombo, A.; Saaranen, M.J.; Perez, V.; Ojeda, J.; Bustos, F.J.; Andreu, C.I.; Torres, M.; Valenzuela, V.; Medinas, D.B.; et al. ALS-linked protein disulfide isomerase variants cause motor dysfunction. EMBO J. 2016, 35, 845–865. [Google Scholar] [CrossRef] [PubMed]
- Coe, H.; Michalak, M. ERp57, a multifunctional endoplasmic reticulum resident oxidoreductase. Int. J. Biochem. Cell Biol. 2010, 42, 796–799. [Google Scholar] [CrossRef] [PubMed]
- Marcinkowska, E. The Vitamin D System in Humans and Mice: Similar but Not the Same. Reports 2020, 3, 1. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doumit, M.; El-Mallah, C.; El-Makkawi, A.; Obeid, O.; Kobeissy, F.; Darwish, H.; Abou-Kheir, W. Vitamin D Deficiency Does Not Affect Cognition and Neurogenesis in Adult C57Bl/6 Mice. Nutrients 2024, 16, 2938. https://doi.org/10.3390/nu16172938
Doumit M, El-Mallah C, El-Makkawi A, Obeid O, Kobeissy F, Darwish H, Abou-Kheir W. Vitamin D Deficiency Does Not Affect Cognition and Neurogenesis in Adult C57Bl/6 Mice. Nutrients. 2024; 16(17):2938. https://doi.org/10.3390/nu16172938
Chicago/Turabian StyleDoumit, Mark, Carla El-Mallah, Alaa El-Makkawi, Omar Obeid, Firas Kobeissy, Hala Darwish, and Wassim Abou-Kheir. 2024. "Vitamin D Deficiency Does Not Affect Cognition and Neurogenesis in Adult C57Bl/6 Mice" Nutrients 16, no. 17: 2938. https://doi.org/10.3390/nu16172938
APA StyleDoumit, M., El-Mallah, C., El-Makkawi, A., Obeid, O., Kobeissy, F., Darwish, H., & Abou-Kheir, W. (2024). Vitamin D Deficiency Does Not Affect Cognition and Neurogenesis in Adult C57Bl/6 Mice. Nutrients, 16(17), 2938. https://doi.org/10.3390/nu16172938







