Investigation of Human Milk as a Biological System in a Multicenter Mother–Infant Cohort: Protocol Design and Cohort Profile of the Phoenix Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Setting
2.2. Participants and Eligibility Criteria
2.3. Data Collection
2.3.1. Anthropometric Assessment
2.3.2. Questionnaires
- Sociodemographic information, maternity, and infant birth
- Maternal dietary intake
- Maternal physical activity and sleep
- Maternal and infant diseases
- Infant skin and respiratory symptoms
- Pediatric quality of life
- Infant Gastrointestinal Symptom Questionnaire
- Infant feeding practice
2.3.3. Digital Diaries
2.3.4. Sample Collection
- Human milk collection
- Maternal fecal sample collection
- Infant fecal sample collection
- Infant saliva collection
2.4. Laboratory Analysis on Biological Samples
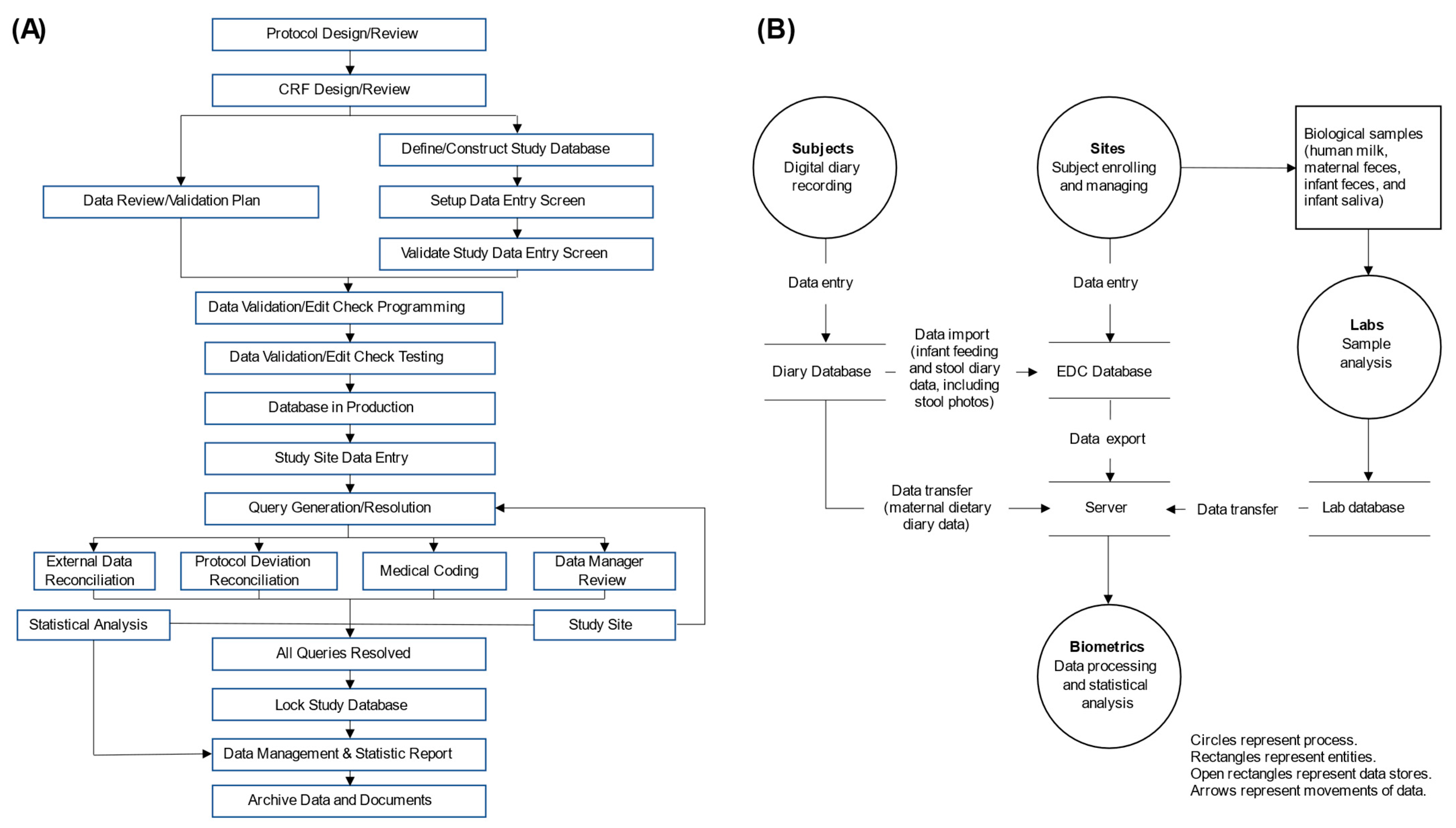
2.5. Data Management and Monitoring
2.6. Quality Control
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization; UNICEF. Global Strategy for Infant and Young Child Feeding; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Victora, C.G.; Bahl, R.; Barros, A.J.D.; Franca, G.V.A.; Horton, S.; Krasevec, J.; Murch, S.; Sankar, M.J.; Walker, N.; Rollins, N.C.; et al. Breastfeeding in the 21st century: Epidemiology, mechanisms, and lifelong effect. Lancet 2016, 387, 475–490. [Google Scholar] [CrossRef] [PubMed]
- Meek, J.Y.L.; Noble, L.; Breastfeeding, S.O. Policy statement: Breastfeeding and the use of human milk. Pediatrics 2022, 150, e2022057989. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, R.; Sinha, B.; Sankar, M.J.; Taneja, S.; Bhandari, N.; Rollins, N.; Bahl, R.; Martines, J. Breastfeeding and maternal health outcomes: A systematic review and meta-analysis. Acta Paediatr. 2015, 104, 96–113. [Google Scholar] [CrossRef] [PubMed]
- Ballard, O.; Morrow, A.L. Human milk composition: Nutrients and bioactive factors. Pediatr. Clin. N. Am. 2013, 60, 49–74. [Google Scholar] [CrossRef] [PubMed]
- Dekker, P.M.; Azad, M.B.; Boeren, S.; Mandhane, P.J.; Moraes, T.J.; Simons, E.; Subbarao, P.; Turvey, S.E.; Saccenti, E.; Hettinga, K.A. The human milk proteome and allergy of mother and child: Exploring associations with protein abundances and protein network connectivity. Front. Immunol. 2022, 13, 977470. [Google Scholar] [CrossRef] [PubMed]
- Biorepository MsMHMR. 2022. Available online: https://mommysmilkresearch.org/ (accessed on 1 August 2024).
- Yin, S.-A.; Yang, Z.-Y. An on-line database for human milk composition in China. Asia Pac. J. Clin. Nutr. 2016, 25, 818–825. [Google Scholar] [CrossRef]
- Yuan, T.; Qi, C.; Dai, X.; Xia, Y.; Sun, C.; Sun, J.; Yu, R.; Zhou, Q.; Jin, Q.; Wei, W.; et al. Triacylglycerol composition of breast milk during different lactation stages. J. Agric. Food Chem. 2019, 67, 2272–2278. [Google Scholar] [CrossRef]
- Ren, X.; Yan, J.; Bi, Y.; Shuttleworth, P.W.; Wang, Y.; Jiang, S.; Wang, J.; Duan, Y.; Lai, J.; Yang, Z. Human milk oligosaccharides are associated with lactation stage and lewis phenotype in a chinese population. Nutrients 2023, 15, 1408. [Google Scholar] [CrossRef]
- Andreas, N.J.; Kampmann, B.; Le-Doare, K.M. Human breast milk: A review on its composition and bioactivity. Early Hum. Dev. 2015, 91, 629–635. [Google Scholar] [CrossRef]
- Gridneva, Z.; George, A.D.; Suwaydi, M.A.; Sindi, A.S.; Jie, M.; Stinson, L.F.; Geddes, D.T. Environmental determinants of human milk composition in relation to health outcomes. Acta Paediatr. 2022, 111, 1121–1126. [Google Scholar] [CrossRef]
- Vinjamuri, A.; Davis, J.C.C.; Totten, S.M.; Wu, L.D.; Klein, L.D.; Martin, M.; A Quinn, E.; Scelza, B.; Breakey, A.; Gurven, M.; et al. Human milk oligosaccharide compositions illustrate global variations in early nutrition. J. Nutr. 2022, 152, 1239–1253. [Google Scholar] [CrossRef] [PubMed]
- Moossavi, S.; Sepehri, S.; Robertson, B.; Bode, L.; Goruk, S.; Field, C.J.; Lix, L.M.; de Souza, R.J.; Becker, A.B.; Mandhane, P.J.; et al. Composition and variation of the human milk microbiota are influenced by maternal and early-life factors. Cell Host Microbe 2019, 25, 324–335.e4. [Google Scholar] [CrossRef] [PubMed]
- Christian, P.; Smith, E.R.; Lee, S.E.; Vargas, A.J.; A Bremer, A.; Raiten, D.J. The need to study human milk as a biological system. Am. J. Clin. Nutr. 2021, 113, 1063–1072. [Google Scholar] [CrossRef]
- Ramani, S.; Stewart, C.J.; Laucirica, D.R.; Ajami, N.J.; Robertson, B.; Autran, C.A.; Shinge, D.; Rani, S.; Anandan, S.; Hu, L.; et al. Human milk oligosaccharides, milk microbiome and infant gut microbiome modulate neonatal rotavirus infection. Nat. Commun. 2018, 9, 5010. [Google Scholar] [CrossRef]
- Yew, W.C.; Young, G.R.; Nelson, A.; Cheung, W.; Stewart, C.J.; Bridge, S.H.; Granger, C.; Berrington, J.E.; Embleton, N.D.; Smith, D.L. The core phageome and its interrelationship with preterm human milk lipids. Cell Rep. 2023, 42, 113373. [Google Scholar] [CrossRef]
- Pannaraj, P.S.; da Costa-Martins, A.G.; Cerini, C.; Li, F.; Wong, S.-S.; Singh, Y.; Urbanski, A.H.; Gonzalez-Dias, P.; Yang, J.; Webby, R.J.; et al. Molecular alterations in human milk in simulated maternal nasal mucosal infection with live attenuated influenza vaccination. Mucosal Immunol. 2022, 15, 1040–1047. [Google Scholar] [CrossRef]
- Donovan, S.M.; Aghaeepour, N.; Andres, A.; Azad, M.B.; Becker, M.; Carlson, S.E.; Järvinen, K.M.; Lin, W.; Lönnerdal, B.; Slupsky, C.M.; et al. Evidence for human milk as a biological system and recommendations for study design—A report from “Breastmilk Ecology: Genesis of Infant Nutrition (BEGIN)” Working Group 4. Am. J. Clin. Nutr. 2023, 117 (Suppl. S1), S61–S86. [Google Scholar] [CrossRef]
- Olga, L.; Petry, C.J.; van Diepen, J.A.; Prentice, P.M.; Hughes, I.A.; Vervoort, J.; Ong, K.K. Extensive Study of Breast Milk and Infant Growth: Protocol of the Cambridge Baby Growth and Breastfeeding Study (CBGS-BF). Nutrients 2021, 13, 2879. [Google Scholar] [CrossRef] [PubMed]
- Gould, J.F.; Yelland, L.N.; A Gibson, R.; McPhee, A.J.; Varghese, J.; Grivell, R.; Makrides, M. Protocol for a multicentre prospective observational study of families with full-term infants on postnatal wards and in the community to capture feeding practices across the first year of life: The Mother Infant Lactation Questionnaire (MILQ) study. BMJ Open 2022, 12, e066355. [Google Scholar] [CrossRef]
- Eow, S.Y.; Gan, W.Y.; Jiang, T.; Loh, S.P.; Lee, L.J.; Chin, Y.S.; Than, L.T.L.; How, K.N.; Thong, P.L.; Liu, Y.; et al. MYBIOTA: A birth cohort on maternal and infant microbiota and its impact on infant health in Malaysia. Front. Nutr. 2022, 9, 994607. [Google Scholar] [CrossRef]
- Poulsen, K.O.; Astono, J.; Jakobsen, R.R.; Uldbjerg, N.; Fuglsang, J.; Nielsen, D.S.; Sundekilde, U.K. Influence of maternal body mass index on human milk composition and associations to infant metabolism and gut colonisation: MAINHEALTH—A study protocol for an observational birth cohort. BMJ Open 2022, 12, e059552. [Google Scholar] [CrossRef] [PubMed]
- Allen, L.H.; Hampel, D.; Shahab-Ferdows, S.; Andersson, M.; Barros, E.; Doel, A.M.; Eriksen, K.G.; Christensen, S.H.; Islam, M.; Kac, G.; et al. The Mothers, Infants, and Lactation Quality (MILQ) Study: A Multi-Center Collaboration. Curr. Dev. Nutr. 2021, 5, nzab116. [Google Scholar] [CrossRef] [PubMed]
- Symington, E.A.; Baumgartner, J.; Malan, L.; Zandberg, L.; Ricci, C.; Smuts, C.M. Nutrition during pregnancy and early development (NuPED) in urban South Africa: A study protocol for a prospective cohort. BMC Pregnancy Childbirth 2018, 18, 308. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Yang, Y.; Li, F.; Shao, Y.; Sun, Z.; Zhong, C.; Fan, P.; Li, Z.; Zhang, M.; Li, X.; et al. Development and validation of a photographic atlas of food portions for accurate quantification of dietary intakes in China. J. Hum. Nutr. Diet. 2021, 34, 604–615. [Google Scholar] [CrossRef]
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.L.; Yngve, A.; Sallis, J.F.; et al. International Physical Activity Questionnaire: 12-Country Reliability and Validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F., III; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Varni, J.W.; Limbers, C.A.; Neighbors, K.; Schulz, K.; Lieu, J.E.C.; Heffer, R.W.; Tuzinkiewicz, K.; Mangione-Smith, R.; Zimmerman, J.J.; Alonso, E.M. The PedsQL™ Infant Scales: Feasibility, internal consistency reliability, and validity in healthy and ill infants. Qual. Life Res. 2010, 20, 45–55. [Google Scholar] [CrossRef]
- Riley, A.W.; Trabulsi, J.; Yao, M.; Bevans, K.B.; DeRusso, P.A. Validation of a parent report questionnaire: The infant gastrointestinal symptom questionnaire. Clin. Pediatr. 2015, 54, 1167–1174. [Google Scholar] [CrossRef]
- Shao, Y.; Luo, H.; Wang, Z.; Chen, M.; Yin, L.; Chen, D.; Ren, J. Validation of an online dietary assessment tool. J. Hyg. Res. 2017, 46, 272–276. [Google Scholar]
- Xiao, F.; Wang, Y.; Ludwig, T.; Li, X.; Chen, S.; Sun, N.; Zheng, Y.; Huysentruyt, K.; Vandenplas, Y.; Zhang, T. Generation and application of a convolutional neural networks algorithm in evaluating stool consistency in diapers. Acta Paediatr. 2023, 112, 1333–1340. [Google Scholar] [CrossRef]
- Gan, J.; Siegel, J.B.; German, J.B. Molecular annotation of food—Towards personalized diet and precision health. Trends Food Sci. Technol. 2019, 91, 675–680. [Google Scholar] [CrossRef]
- Saarela, T.; Kokkonen, J.; Koivisto, M. Macronutrient and energy contents of human milk fractions during the first six months of lactation. Acta Paediatr. 2005, 94, 1176–1181. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Jiang, J.; Lu, M.; Tong, W.; Zhou, R.; Li, J.; Yuan, J.; Wang, F.; Li, D. Human milk microbiota development during lactation and its relation to maternal geographic location and gestational hypertensive status. Gut Microbes 2020, 11, 1438–1449. [Google Scholar] [CrossRef] [PubMed]
- Pärnänen, K.; Karkman, A.; Hultman, J.; Lyra, C.; Bengtsson-Palme, J.; Larsson, D.G.J.; Rautava, S.; Isolauri, E.; Salminen, S.; Kumar, H.; et al. Maternal gut and breast milk microbiota affect infant gut antibiotic resistome and mobile genetic elements. Nat. Commun. 2018, 9, 3891. [Google Scholar] [CrossRef] [PubMed]

| V1 | V2 | V3 | V4 | ||
|---|---|---|---|---|---|
| 1 Month ± 1 Week | 4 Months ± 2 Weeks | 6 Months ± 2 Weeks | 12 Months ± 2 Weeks | ||
| Anthropometric assessment | Maternal height | √ | |||
| Maternal weight | √ | √ | √ | √ | |
| Infant weight, length, and head circumference | √ | √ | √ | √ | |
| Questionnaires | Sociodemographic, maternity, and infant birth | √ | |||
| Maternal dietary intake (FFQ) | √ | √ | √ | √ | |
| Maternal physical activity and sleep | √ | √ | √ | √ | |
| Maternal and infant diseases | √ | √ | √ | √ | |
| Infant skin and respiratory symptoms | √ | √ | √ | √ | |
| Pediatric quality of life (PedsQL) | √ | √ | √ | √ | |
| Infant gastrointestinal symptom questionnaire (IGSQ) | √ | √ | √ | √ | |
| Infant feeding practice | √ | √ | √ | √ | |
| Digital diaries | 24 h maternal dietary intake diary | √ | √ | √ | √ |
| 24 h infant feeding diary | √ | √ | √ | √ | |
| 5-day infant stool frequency and consistency diary | √ | √ | √ | √ | |
| Sample collection | Human milk sample | √ | √ | √ | √ |
| Maternal fecal sample | √ | ||||
| Infant fecal sample | √ | √ | √ | √ | |
| Infant saliva sample | √ |
| Characteristics | Mean ± SD/n (%) |
|---|---|
| Mothers (n = 769) | |
| Age, years | 31.1 ± 3.8 |
| Ethnic groups | |
| Han | 754 (98.0%) |
| Zhuang | 2 (0.3%) |
| Hui | 9 (1.2%) |
| Other | 4 (0.5%) |
| Education | |
| Below primary school | 1 (0.1%) |
| Primary school | 2 (0.3%) |
| Junior high school | 42 (5.5%) |
| Senior high school | 51 (6.6%) |
| Junior college | 194 (25.2%) |
| Bachelor | 344 (44.7%) |
| Postgraduate or above | 135 (17.6%) |
| Occupational status | |
| Employed | 557 (72.4%) |
| Self-employed | 55 (7.2%) |
| Housewife | 120 (15.6%) |
| Student | 5 (0.6%) |
| Other | 32 (4.2%) |
| Infants (n = 769) | |
| Gestational age, weeks | 39.4 ± 1.0 |
| Birth weight, g | 3340.4 ± 372.9 |
| Sex | |
| Male | 390 (50.7%) |
| Female | 379 (49.3%) |
| Delivery mode | |
| Vaginal delivery | 432 (56.2%) |
| Caesarean section | 337 (43.8%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.; Gan, J.; Zeng, G.; Luo, X.; Yang, N.; Zhang, Z.; Sun, Y.; Shen, J.; Wei, W.; Yan, J.; et al. Investigation of Human Milk as a Biological System in a Multicenter Mother–Infant Cohort: Protocol Design and Cohort Profile of the Phoenix Study. Nutrients 2024, 16, 2892. https://doi.org/10.3390/nu16172892
Wu J, Gan J, Zeng G, Luo X, Yang N, Zhang Z, Sun Y, Shen J, Wei W, Yan J, et al. Investigation of Human Milk as a Biological System in a Multicenter Mother–Infant Cohort: Protocol Design and Cohort Profile of the Phoenix Study. Nutrients. 2024; 16(17):2892. https://doi.org/10.3390/nu16172892
Chicago/Turabian StyleWu, Jieshu, Junai Gan, Guo Zeng, Xiaoqin Luo, Nianhong Yang, Zheqing Zhang, Yongye Sun, Jian Shen, Wei Wei, Jingyu Yan, and et al. 2024. "Investigation of Human Milk as a Biological System in a Multicenter Mother–Infant Cohort: Protocol Design and Cohort Profile of the Phoenix Study" Nutrients 16, no. 17: 2892. https://doi.org/10.3390/nu16172892
APA StyleWu, J., Gan, J., Zeng, G., Luo, X., Yang, N., Zhang, Z., Sun, Y., Shen, J., Wei, W., Yan, J., Zhu, J., Ludwig, T., Stahl, B., Zhao, X., & Wang, Z., on behalf of the Phoenix Study Group. (2024). Investigation of Human Milk as a Biological System in a Multicenter Mother–Infant Cohort: Protocol Design and Cohort Profile of the Phoenix Study. Nutrients, 16(17), 2892. https://doi.org/10.3390/nu16172892







